Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor
Abstract
1. Introduction
2. Materials and Methods
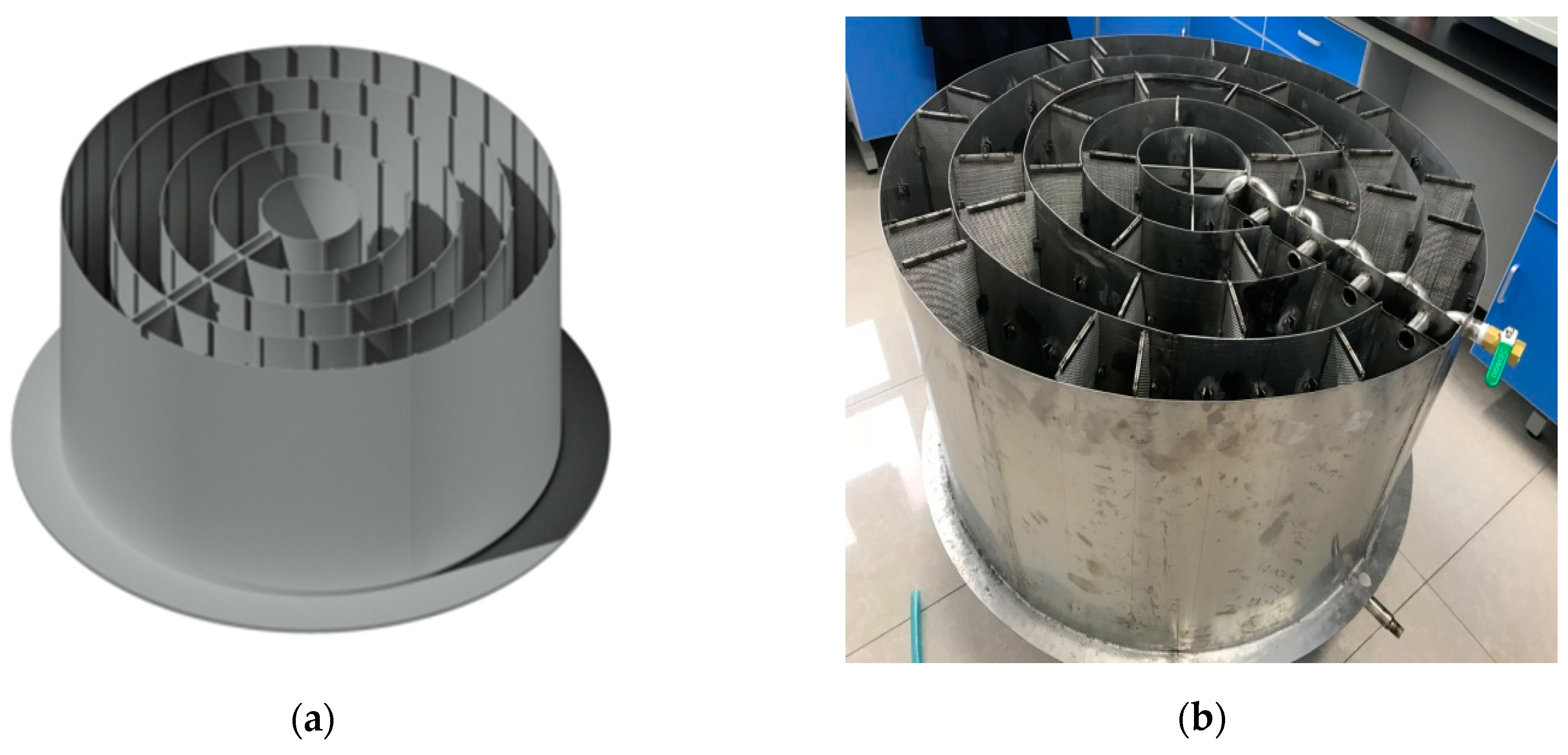
2.1. The Concentric Circular Diversion Wall Adsorption Reactor
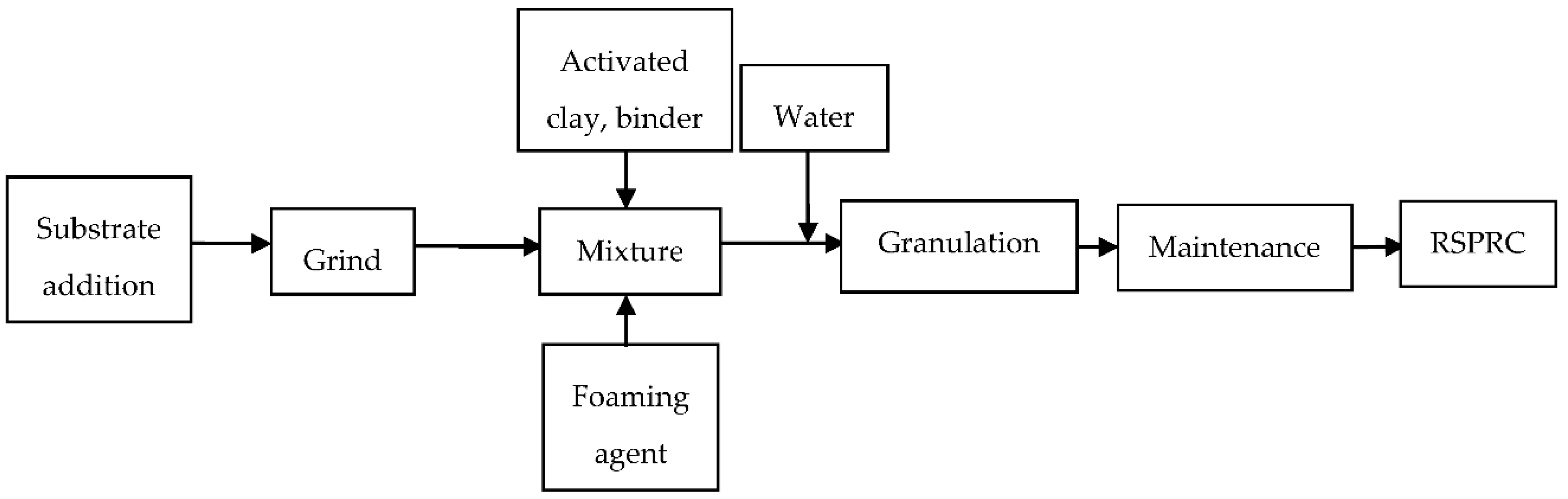
2.2. RSPRC Preparation
2.3. Study on Decolorization and P Removal of RSPRC with Activated Clay
2.4. Study on P Removal Characteristics of RSPRC
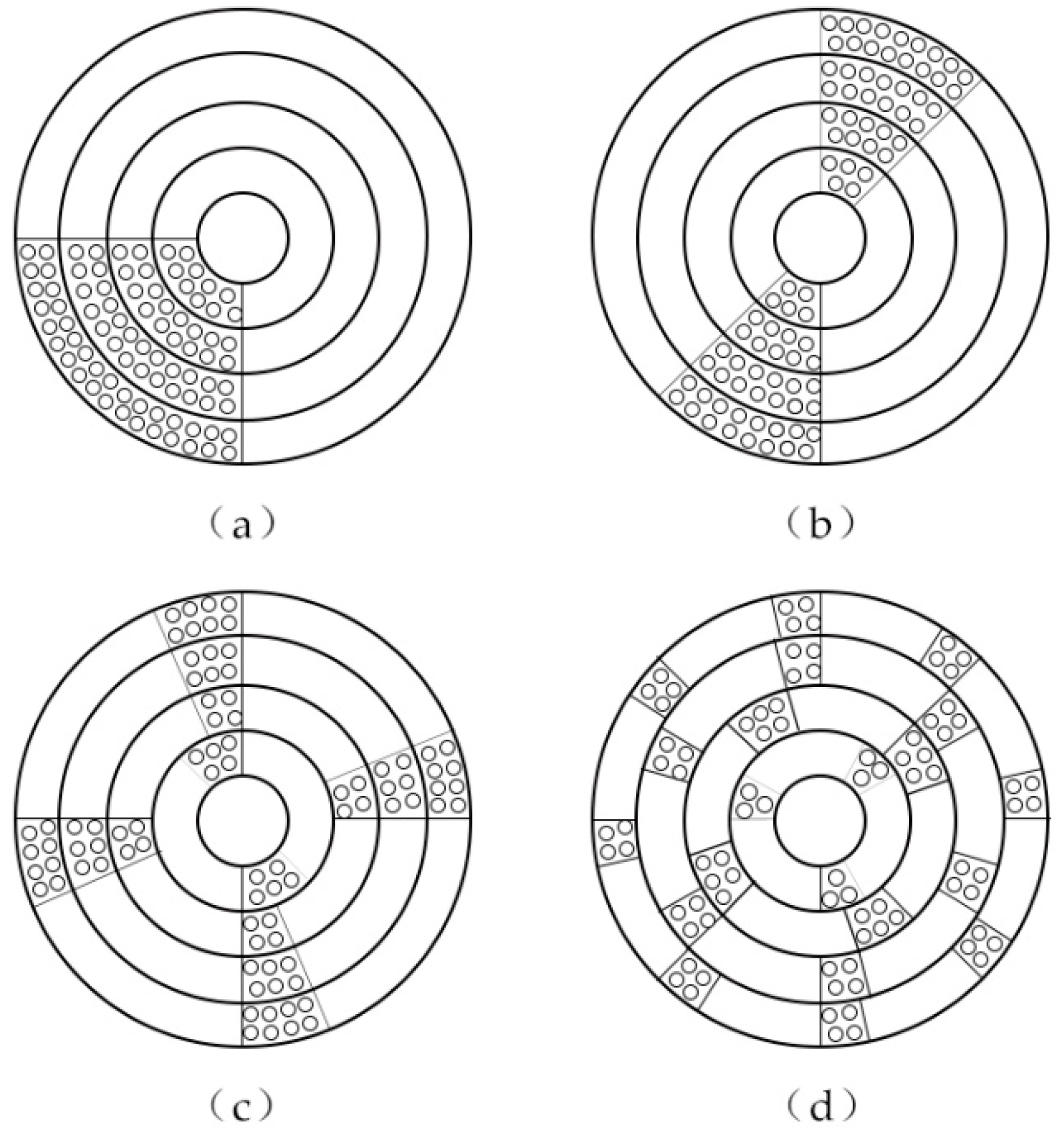
2.5. Study on the Influence of RSPRC Distribution in the Reactor on Phosphorus Removal Effect
2.6. Study on the Influence of RSPRC Dosage in the Reactor on Phosphorus Removal Effect
2.7. Study on the Influence of HRT in the Reactor on Phosphorus Removal Effect
3. Results and Discussion
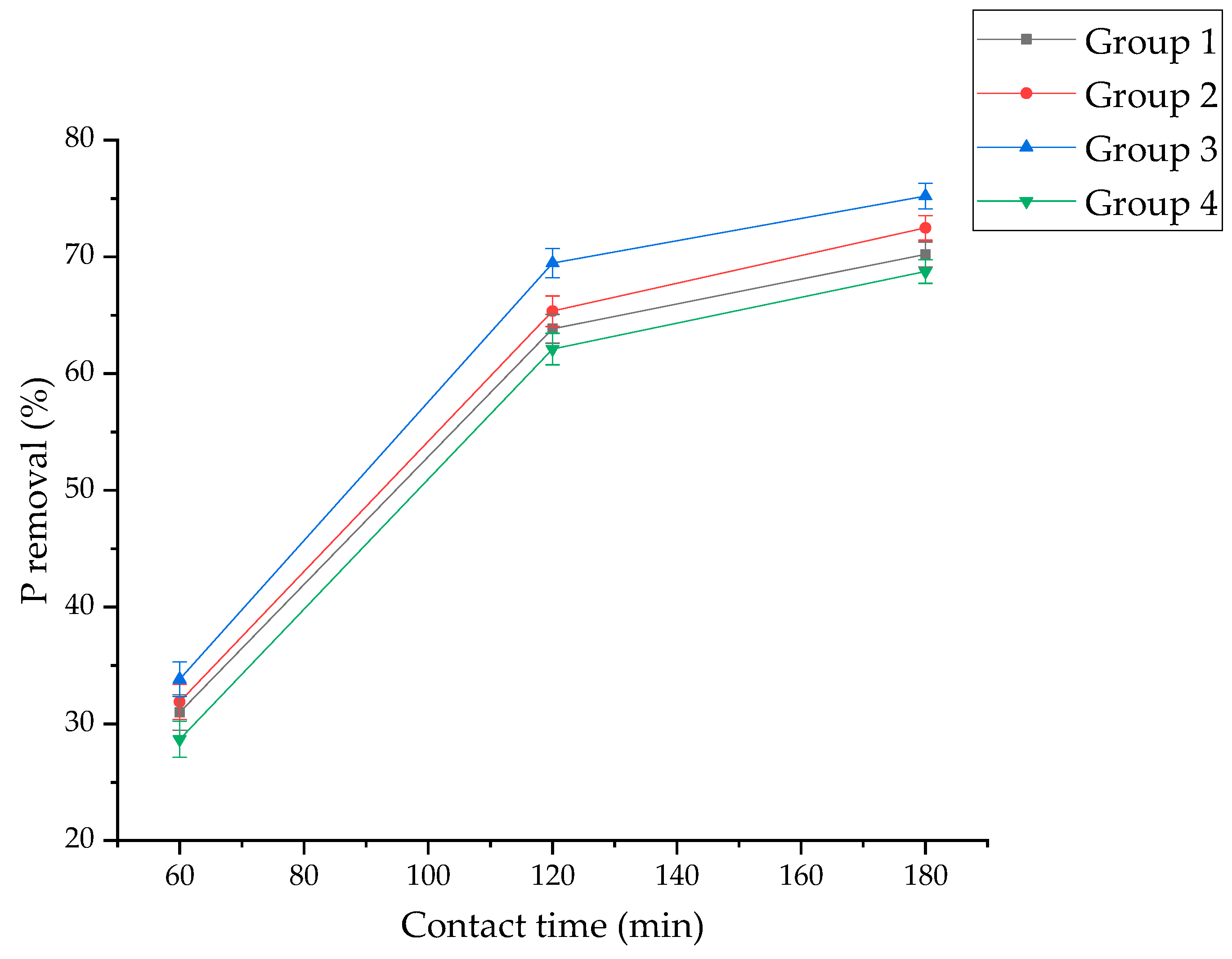
3.1. Effect of Proportion of Activated Clay in RSPRC on Decolorization and Phosphorus Removal
3.2. Characterization of RSPRC
3.3. Phosphorus Removal Characteristics of RSPRC
3.4. RSPRC Adsorption Kinetics
3.5. Effect of RSPRC Distribution in Reactor on Phosphorus Removal
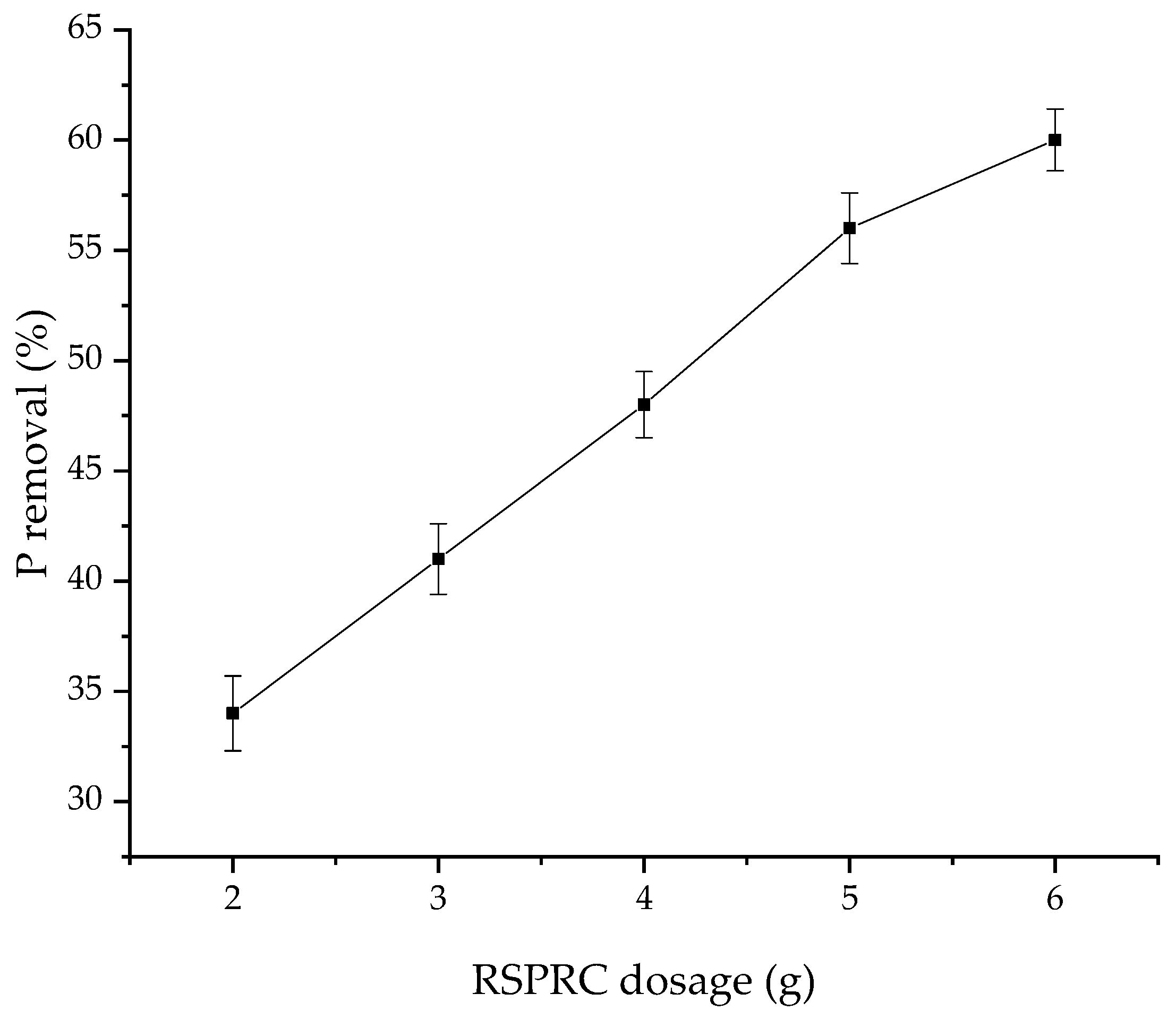
3.6. Effect of RSPRC Dosage in Reactor on Phosphorus Removal
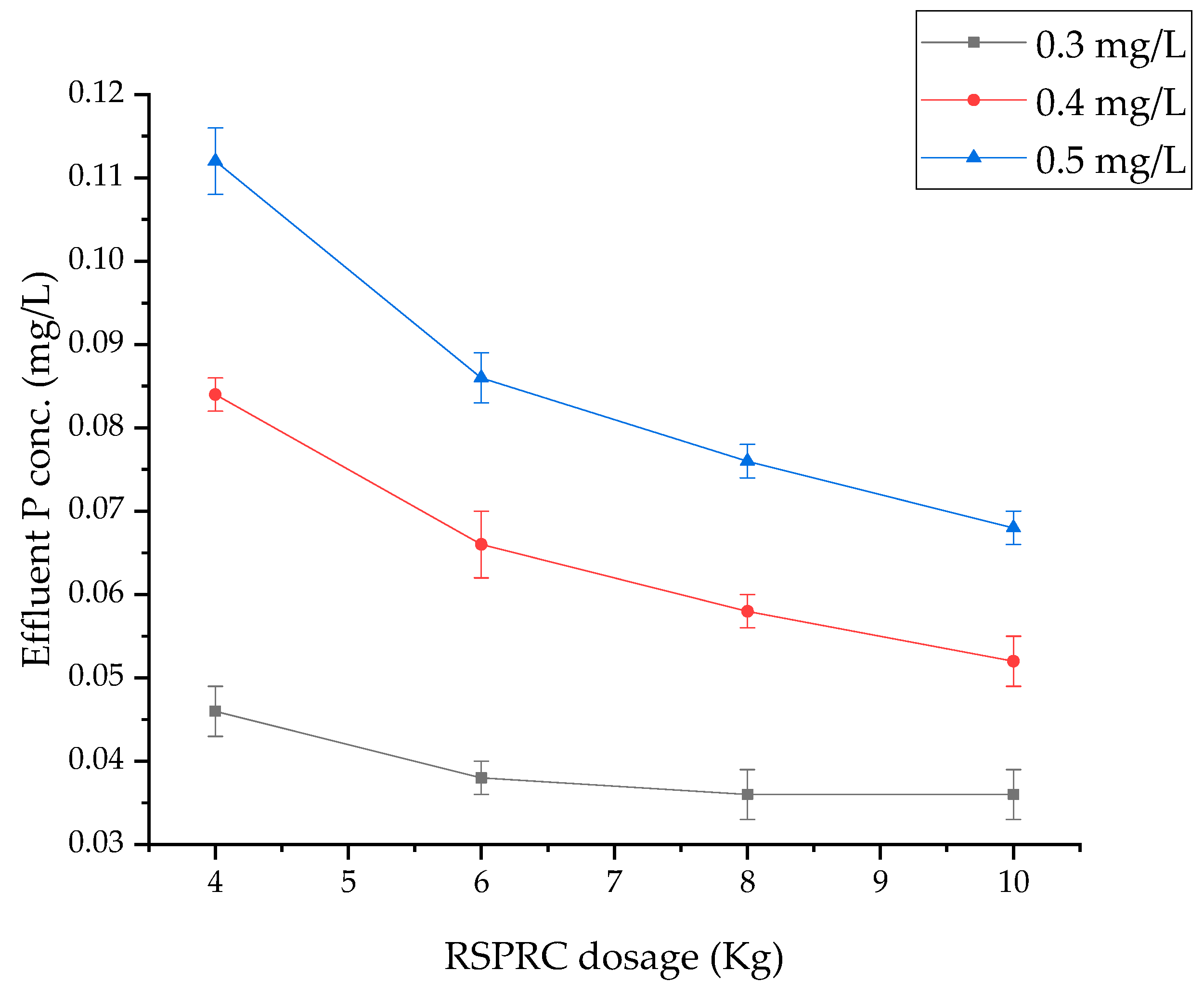
3.7. Effect of HRT in Reactor on Phosphorus Removal
4. Practical Applications and Future Research Perspective
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, A. Estimation of physicochemical characteristics and associated metal contamination risk in the Narmada River, India. Environ. Eng. Res. 2020, 26, 190521. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P.; Taxak, A.K. Analysis of water environment changing trend in Bhagirathi tributary of Ganges in India. Desalin. Water Treat. 2017, 63, 55–62. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P.; Rai, S.P. A novel approach for river health assessment of Chambal using fuzzy modeling, India. Desalin. Water Treat. 2017, 58, 72–79. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Hu, H. Enhanced phosphate removal from wastewater by using in situ generated fresh trivalent Fe composition through the interaction of Fe(II) on CaCO3. J. Environ. Manag. 2018, 221, 38–44. [Google Scholar] [CrossRef]
- Benammar, L.; Menasria, T.; Ayachi, A.; Benounis, M. Phosphate removal using aerobic bacterial consortium and pure cultures isolated from activated sludge. Process Saf. Environ. 2015, 95, 237–246. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Fan, W.; Yuan, L.; Luo, D. The mechanism of biological phosphorus removal under anoxic-aerobic alternation condition with starch as sole carbon source and its biochemical pathway. Biochem. Eng. J. 2018, 132, 90–99. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)(2) treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestari, A.R.; Carvalho, W.A. Use of a La(Ⅲ)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef]
- Mulkerrins, D.; Dobson, A.; Colleran, E. Parameters affecting biological phosphate removal from wastewaters. Environ. Int. 2004, 30, 249–259. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, B.H. Progress of phosphorus wastewater treatment technology. Energy Conserv. Emiss. Reduct. Pet. Petrochem. Ind. 2013, 3, 37–40. (In Chinese) [Google Scholar]
- Bhatnagar, A.; Sillanpaa, M. A review of emerging adsorbents for nitrate removal from water. Biochem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Ahmed, S.; Ashiq, M.N.; Li, D.; Tang, P.; Leroux, F.; Feng, Y. Recent progress on adsorption materials for phosphate removal. Recent Pat. Nanotechnol. 2019, 13, 3–16. [Google Scholar] [CrossRef]
- Lakshmi, V.; Resmi, V.G.; Raju, A.; Deepa, J.P.; Rajan, T.P.D.; Pavithran, C.; Pai, B.C. Concentration dependent pore morphological tuning of kaolin clay foams using sodium dodecyl sulfate as foaming agent. Ceram. Int. 2015, 41, 14263–14269. [Google Scholar] [CrossRef]
- Zhang, W. Progress in the application of bentonite to the adsorption for inorganic pollutants in water pollution control. Ind. Water Treat. 2018, 38, 10–16. (In Chinese) [Google Scholar]
- Wang, T.; Liu, T.; Wu, D.; Li, M.; Chen, J.; Teng, S. Performance of phosphoric acid activated montmorillonite as buffer materials for radioactive waste repository. J. Hazard. Mater. 2010, 173, 335–342. [Google Scholar] [CrossRef]
- Wu, L.L.; Liu, Y.; Wang, S.H.; Zhang, H.; Huang, M. Effects of the particle size and pH value on EPRC in phosphate removal. Saf. Environ. Eng. 2008, 15, 45–48. (In Chinese) [Google Scholar]
- Zhou, J. Study on Phosphorus Removal Characteristics of Perforated EPRC. Master’s Thesis, Southeast University, Nanjing, China, 2010. (In Chinese). [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater; APHA (American Public Health Association): Washington, DC, USA, 1989.
- Gamba, M.; Kovar, P.; Pospisil, M.; Torres Sanchez, R.M. Insight into thiabendazole interaction with montmorillonite and organically modified montmorillonites. Appl. Clay Sci. 2017, 137, 59–68. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Jiang, J. Study on the sorption-desorption-regeneration performance of Ca-, Mg- and CaMg-based layered double hydroxides for removing phosphate from water. Biochem. Eng. J. 2014, 246, 97–105. [Google Scholar] [CrossRef]
- Patra, B.S.; Baliarsingh, N.; Parida, K.M.; Das, J. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar]
- Wang, H.Z.; Song, W.D.; Xing, K.; Zhao, Z.P.; Guo, L.G. Adsorption of tripolyphosphate from aqueous solution by Mg-Al-CO3-layered double hydroxides. Colloids Surf. A 2008, 328, 15–20. [Google Scholar]
- Li, J.; Wu, B.; Zhou, T.; Chai, X. Preferential removal of phosphorus using modified steel slag and cement combination for its implications in engineering applications. Environ. Technol. Innov. 2018, 10, 264–274. [Google Scholar] [CrossRef]
- Shan, H.D.; Lu, S.G.; Bai, S.Q.; Zhu, L. Removal mechanism of phosphate from aqueous solution by fly ash. J. Hazard. Mater. 2009, 161, 95–101. [Google Scholar]
- Agyei, N.M.; Strydom, C.A.; Potgieter, J.H. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cem. Concr. Res. 2002, 32, 1889–1897. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Li, W.; Wang, P.; Su, C. Adsorption of acid and basic dyes by sludge-based activated carbon: Isotherm and kinetic studies. J. Central South. Univ. 2015, 22, 103–113. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Babatunde, A.O. Equilibrium and kinetic analysis of phosphorus adsorption from aqueous solution using waste alum sludge. J. Hazard. Mater. 2010, 184, 746–752. [Google Scholar]
- Meyer, D.; Andres, Y.; Chazarenc, F.; Barca, C.; Gerente, C. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar]
- Liu, Y.; Li, L.; Zhang, S.J.; Wang, S.H. Kinetics and thermodynamics of efficient phosphorus removal by a complex material. Desalin. Water Treat. 2015, 56, 1949–1954. [Google Scholar] [CrossRef]
- Yao, X.D.; Haghseresht, F.; Wang, S.B.; Rudolph, V.; Zhu, Z.H.; Li, L.; Huang, W.W. Phosphate removal from wastewater using red mud. J. Hazard. Mater. 2008, 158, 35–42. [Google Scholar]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Inter. Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, S.M. Phosphate removal from aqueous solution using slag and fly ash. HBRC J. 2013, 9, 270–275. [Google Scholar] [CrossRef]
- Rastas, L.; Hedstrom, A. Methodological aspects of using blast furnace slag for wastewater phosphorus removal. J. Environ. Eng. 2006, 132, 1431–1438. [Google Scholar]
- Molle, P.; Saenz De Miera, L.E.; Blanco, I.; Ansola, G. Basic Oxygen Furnace steel slag aggregates for phosphorus treatment. Evaluation of its potential use as a substrate in constructed wetlands. Water Res. 2016, 89, 355–365. [Google Scholar]
- Senan, P.; Anirudhan, T.S. Adsorption of phosphate ions from water using a novel cellulose-based adsorbent. Chem. Ecol. 2011, 27, 147–164. [Google Scholar]
- Lu, S.G.; Shan, H.D.; Bai, S.Q. Mechanisms of phosphate removal from aqueous solution by blast furnace slag and steel furnace slag. J. Zhejiang Univ. Sci. A 2008, 9, 125–132. [Google Scholar] [CrossRef]
- Pengthamkeerati, P.; Satapanajaru, T.; Chularuengoaksorn, P. Chemical modification of coal fly ash for the removal of phosphate from aqueous solution. Fuel 2008, 87, 2469–2476. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S. Environmental quantification of soil elements in the catchment of hydroelectric reservoirs in India. Hum. Ecolog. Risk Asses 2017, 23, 1202–1218. [Google Scholar] [CrossRef]




| Oxide | CaO | Fe2O3 | Al2O3 | SiO2 | MgO | MnO | SO3 | V2O5 | TiO2 | Na2O | ZnO | CuO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition of fly ash (%) | 1.31 | 4.39 | 45.9 | 44.4 | 0.261 | 0.026 | 0.666 | 0.038 | 1.26 | 0.094 | 0.021 | 0.02 |
| Composition of steel slag (%) | 55.0 | 21.5 | 1.51 | 13.4 | 3.65 | 1.75 | 0.512 | 0.417 | 0.296 | 0.077 | - | - |
| Particle Size of fly ash/mesh | <6 | 6~18 | 18~45 | 45~70 | >70 |
| proportion/% | 7.45 | 12.76 | 42.93 | 23.55 | 11.87 |
| Particle size of steel slag/mesh | <12 | 12~80 | 80~320 | >320 | - |
| proportion/% | 2.49 | 7.68 | 32.40 | 57.36 | - |
| Material | Fly Ash | Steel Slag | Activated Clay | Binder |
|---|---|---|---|---|
| Composition of Group 1 (%) | 78.25 | 11.25 | 5 | 5 |
| Composition of Group 2 (%) | 74.38 | 10.63 | 10 | 5 |
| Composition of Group 3 (%) | 70 | 10 | 15 | 5 |
| Composition of Group 4 (%) | 83.13 | 11.88 | — | 5 |
| Physical Parameter | Grain Size (mm) | Density (g/cm3) | Porosity (%) | Specific Surface Area (m2/g) |
|---|---|---|---|---|
| RSPRC | 5~20 | 1.16 | 30.98 | 2.62~8.56 |
| Initial Concentration (mg·L−1) | qexp (mg·g−1) | Pseudo-First Order Kinetic Model | Pseudo-Second Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| qe (mg·g−1) | K1 (h−1) | R2 | qe (mg·g−1) | K2 (h−1) | R2 | ||
| 0.3 | 0.038 | 0.036 | 0.016 | 0.9246 | 0.039 | 0.301 | 0.9813 |
| 0.5 | 0.068 | 0.064 | 0.012 | 0.9638 | 0.072 | 0.416 | 0.9842 |
| 1.0 | 0.116 | 0.110 | 0.72 | 0.9644 | 0.118 | 0.742 | 0.9954 |
| Experimental Condition | Initial Concentration (mg/L) | Effluent Concentration (mg/L) | Phosphorus Removal Rate (%) |
|---|---|---|---|
| a | 0.50 | 0.34 | 32 |
| b | 0.50 | 0.28 | 44 |
| c | 0.50 | 0.18 | 64 |
| d | 0.50 | 0.08 | 84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, L.; Singh, R.P. Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor. Appl. Sci. 2020, 10, 3629. https://doi.org/10.3390/app10103629
Liu Y, Zhang L, Singh RP. Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor. Applied Sciences. 2020; 10(10):3629. https://doi.org/10.3390/app10103629
Chicago/Turabian StyleLiu, Yan, Limin Zhang, and Rajendra Prasad Singh. 2020. "Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor" Applied Sciences 10, no. 10: 3629. https://doi.org/10.3390/app10103629
APA StyleLiu, Y., Zhang, L., & Singh, R. P. (2020). Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor. Applied Sciences, 10(10), 3629. https://doi.org/10.3390/app10103629






