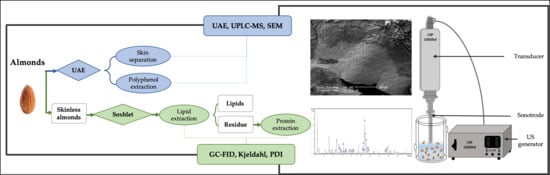
A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyphenols in Almond Skin
2.1.1. Extraction Procedures—UAE, Blanching
2.1.2. Central Composite Design (CCD)
2.1.3. Kinetics
2.1.4. Total Polyphenol Content (TPC)
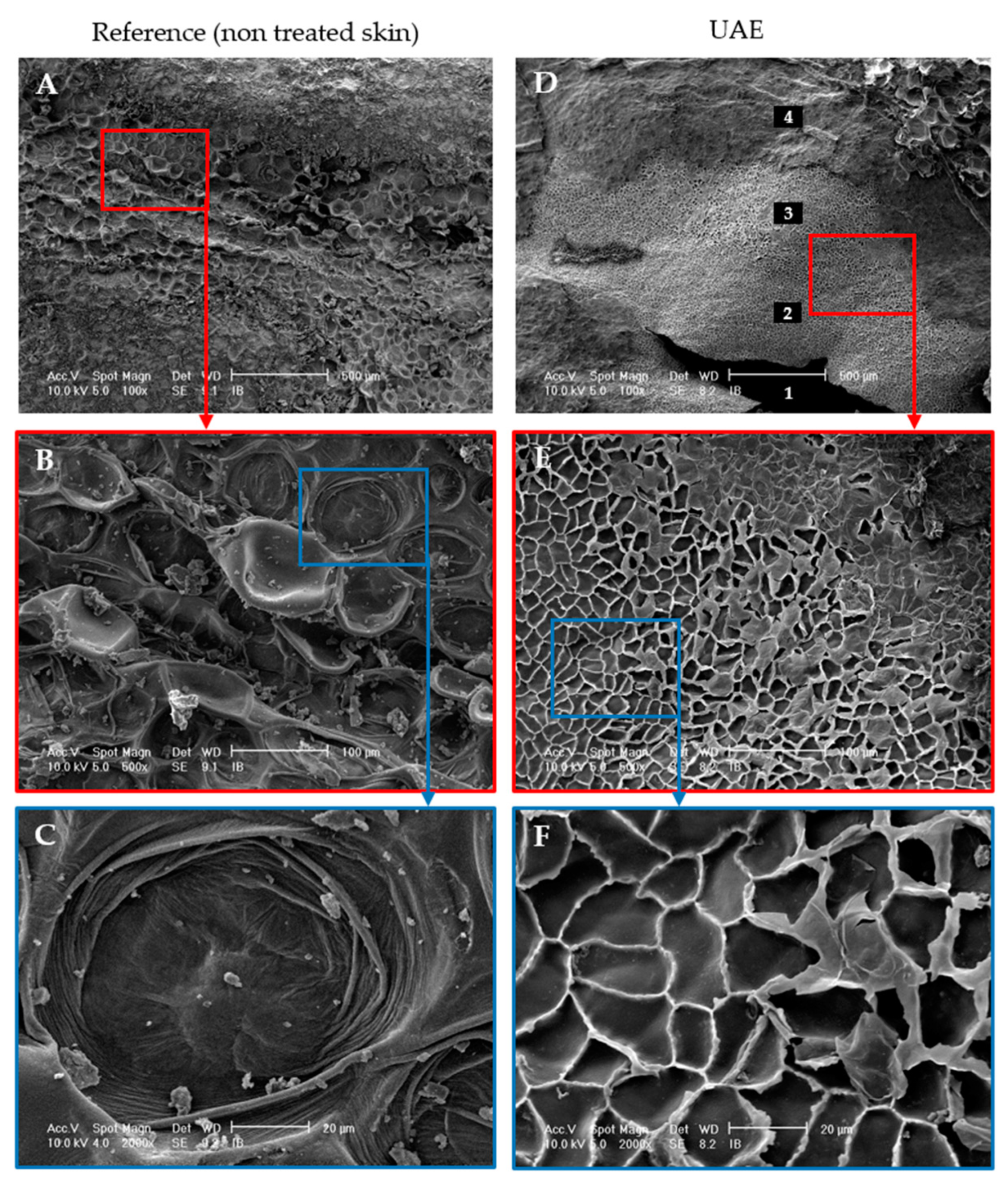
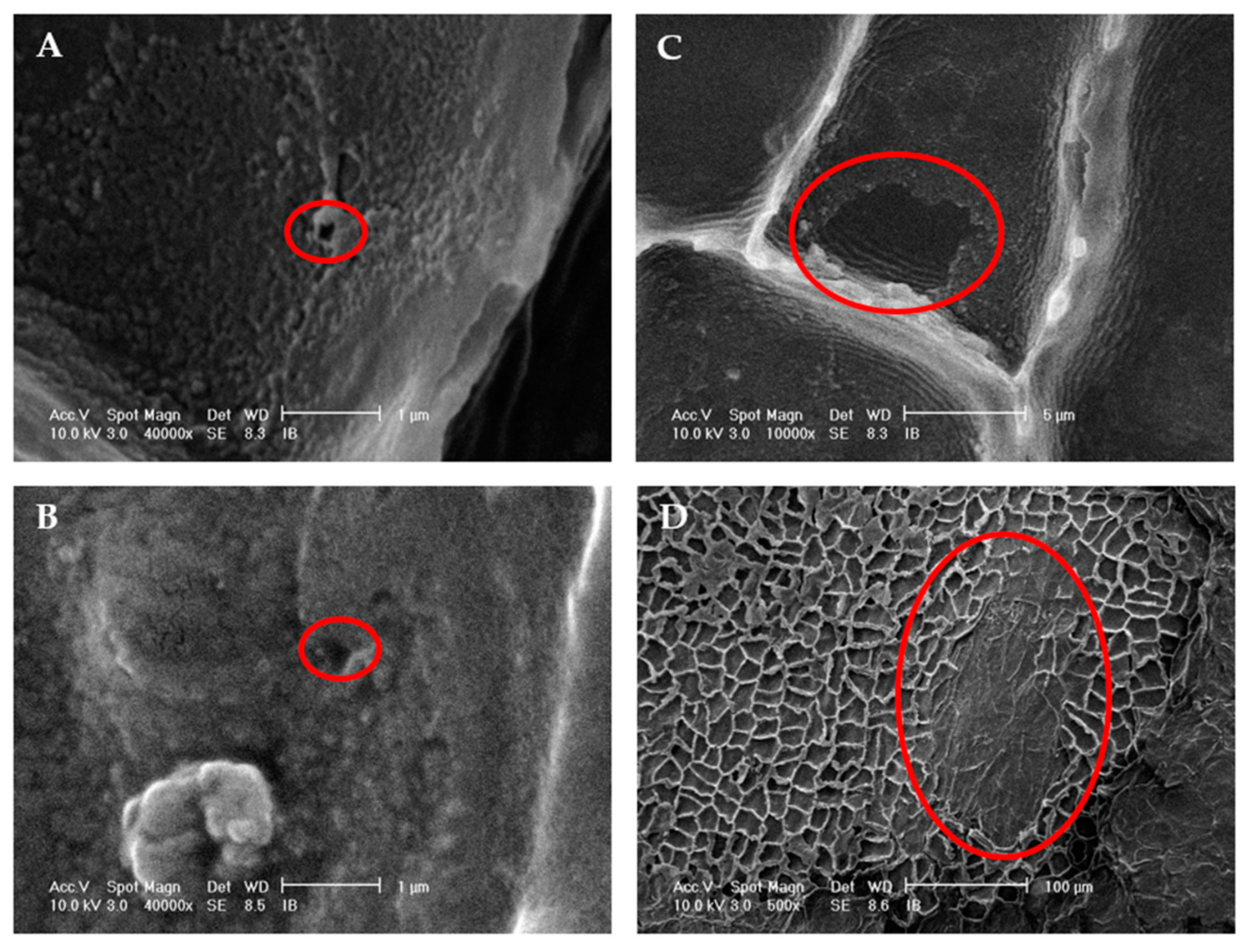
2.1.5. Localized sonication—Scanning Electron Microscopy (SEM)
2.1.6. Ultra-High-Performance Liquid Chromatography—Mass Spectrometry
2.2. Almond Oil
2.3. Almond Proteins
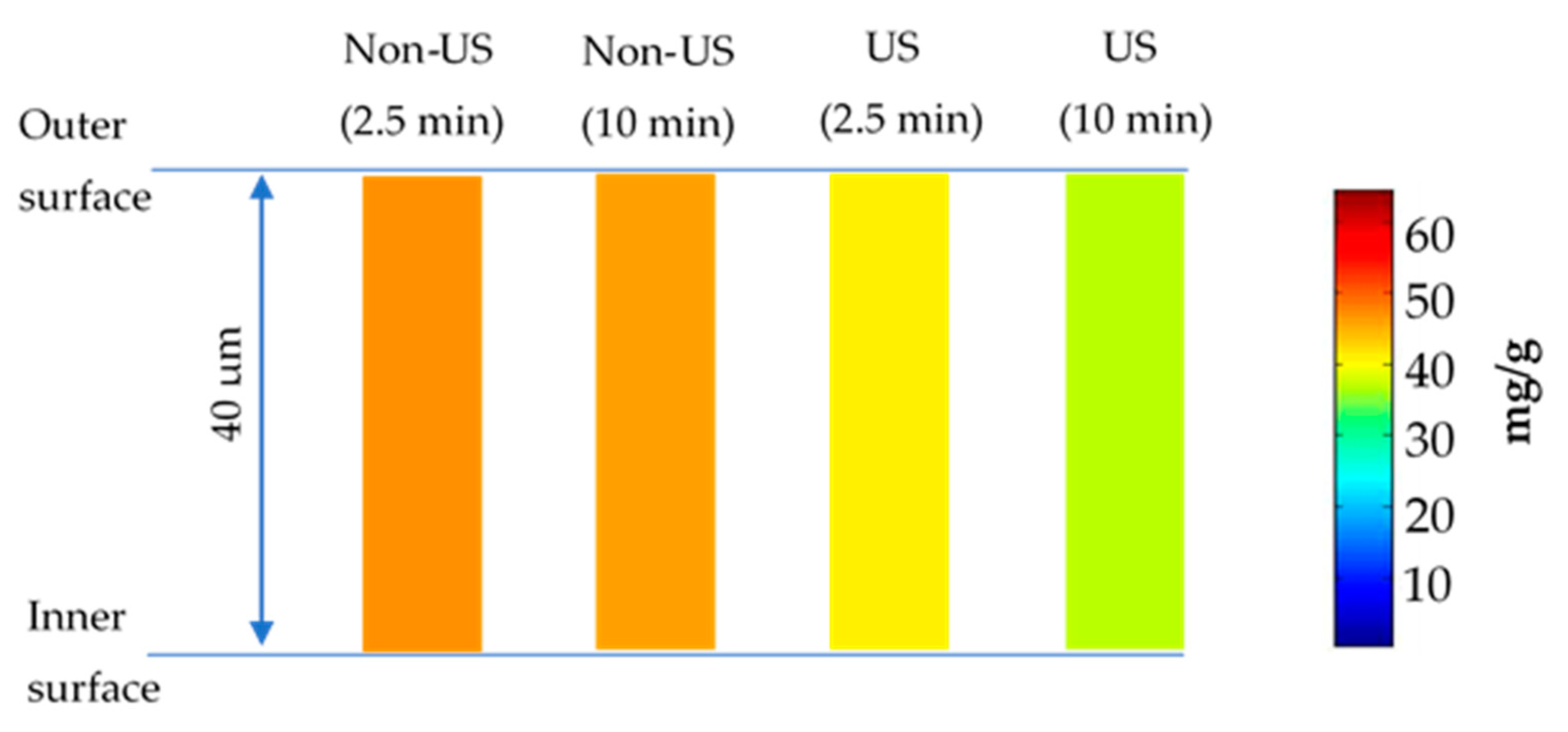
2.4. Diffusion Model
- Almond skin was considered as slab geometry due to the low thickness value (40 μm on average measured by a vernier caliper). Phenolic transfer within almond skin took place in one direction from the inner surface to the outer surface, and the thickness value of almond skin referred to the mass transfer distance [23];
- Phenolics were distributed homogeneously within almond skins before extraction;
- Phenolic diffusivity kept constant during extraction, and phenolic content in almond skin varied with position and time;
- External mass transfer resistance was neglected due to sonication or periodic stirring at each sampling time.
3. Results and Discussions
3.1. Polyphenols
3.1.1. Experimental Design
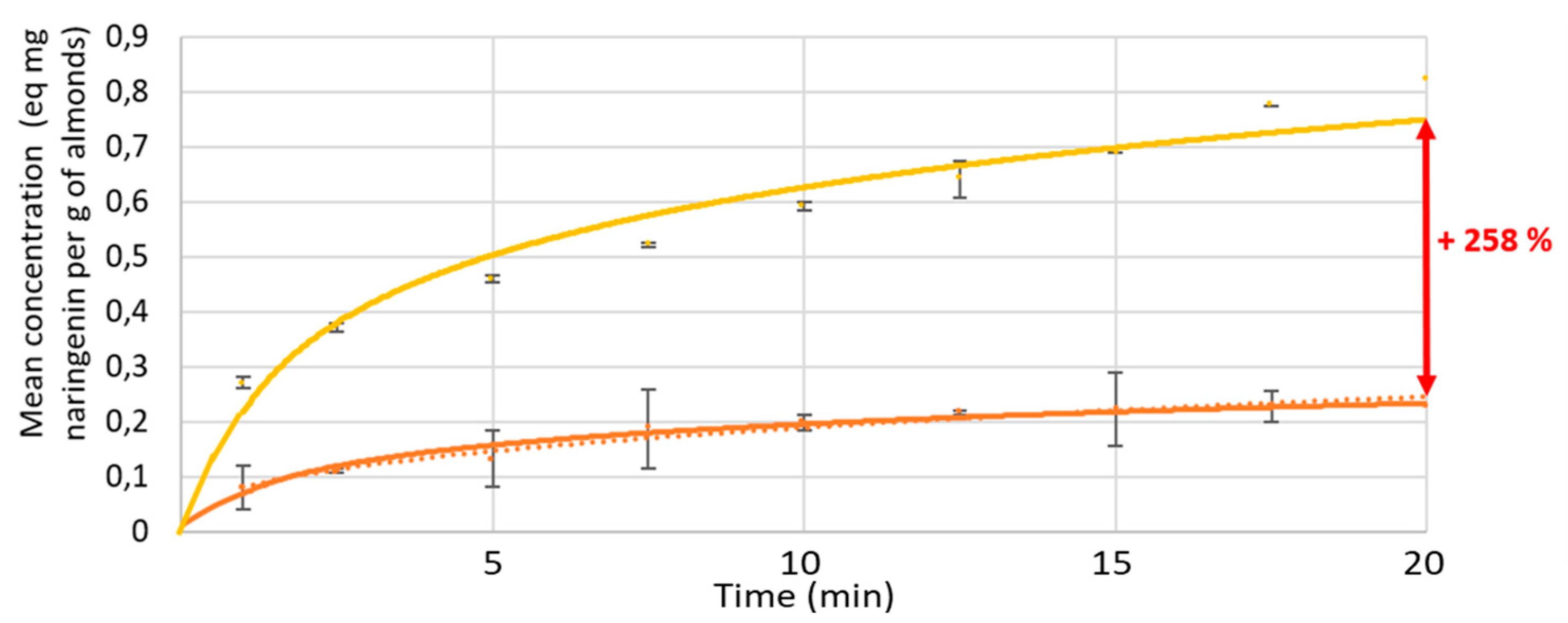
3.1.2. Kinetics
3.1.3. Ultrasound Effect on Almond Skins Microstructure
3.1.4. Quantification of Phenolic Compounds in Almond Skin
3.2. UAE Effect on Almond Oil
3.3. UAE Effect on Almond Protein
3.4. Diffusion Modelling and Numerical Simulation for Polyphenol Extraction
4. Conclusions and Future Trends
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.Y.; Lapsley, K.; Blumberg, J. Perspective A nutrition and health perspective on almonds. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; Trautwein, A.A.; Parker, T.L.; Josse, R.G.; Leiter, L.A.; et al. The Effect of Combining Plant Sterols, Soy Protein, Viscous Fibers, and Almonds in Treating Hypercholesterolemia. Metabolism 2013, 52, 1478–1483. [Google Scholar] [CrossRef]
- Almond Board of California. Waste not; Soil health research utilizing almond coproducts, document # 2017IR0202; Almond Board of California: Modesto, CA, USA, 2017. [Google Scholar]
- Vargas, F.J. Árboles productores de frutos secos: Origen, descripción, distribución y producción. In Frutos Secos, Salud Y Culturas Mediterraneas; Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; Editorial Glosa: Barcelona, Spain, 2005; p. 21. [Google Scholar]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yanling, C.; Shaobo, D.; Xiangyang, L.; Guangwei, H.; Ruan, R. Utilization of almond residues. Int. J. Agric. Biol. Eng. 2010, 3, 1–18. [Google Scholar]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial potential of polyphenols extracted from almond skin. Lett. Appl. Microbiol. 2010, 51, 81–89. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Comprehensive Reviews in Food Science and Food Safety 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Siriwardhana, S.K.W.S.; Shahidi, F. Antiradical, Activity of Extracts of Almond and Its By-products. J. Am. Oil Chem. Soc. 2002, 79, 903–908. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszyńska, A.; Shahidi, F. Antioxidant activity of almond seed extract and its fractions. J. Food Lipids 2005, 12, 344–358. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Lo Turco, V.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fiber from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert Vian, M. Ultrasound assisted extraction of food and natural products: Mechanisms, techniques, combinations, protocols and applications. A Review. Ultrason. Sonochem. 2016, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.D.P.P.; Somathilaka Ranaweera, K.K.D.; Vasantha Rupasinghe, H.P. Effect of Different Cooking Methods on Polyphenols, Carotenoids and Antioxidant Activities of Selected Edible Leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, D.; Janghel, A.; Deo, S.; Raut, P.; Verma, C.; Kumar, S.S.; Agrawal, M.; Amit, N.; Sharma, M.; Giri, T.; et al. Emerging ultrasound assisted extraction (UAE) Techniques as Innovative Green Technologies for the effective extraction of the active phytopharmaceuticals. Res. J. Pharm. Tech. 2015, 8, 14. [Google Scholar] [CrossRef]
- Boukroufa, M.; Sicaire, A.G.; Fine, F.; Larré, C.; Le Goff, A.; Solé Jamault, V.; Rakotomanomana, N.; Chemat, F. Green Sonoextraction of Protein from Oleaginous Press Rapeseed Cake. Molecules 2017, 22, 80. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Cheynier, V.; Labarbe, B.; Moutounet, M. Estimation of Procyanidin Chain Length. Methods Enzymol. 2001, 335, 87–88. [Google Scholar]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; Van Den Broek, L.-A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Van Eys, J.E. Manual of Quality Analyses for Soybean Products in the Feed Industry, 2nd ed.; Soybean Export Council: Fourqueux, France, 2012; pp. 8–9.
- Tao, Y.; Zhang, J.; Jiang, S.; Xu, Y.; Show, P.-L.; Han, Y.; Ye, X.; Ye, M. Contacting ultrasound enhanced hot-air convective drying of garlic slices: Mass transfer modeling and quality evaluation. J. Food Eng. 2018, 235, 79–88. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Pan, M.; Zhong, S.; Wu, Y.; Yang, R.; Han, Y.; Zhou, J. Combined ANFIS and numerical methods to simulate ultrasound-assisted extraction of phenolics from chokeberry cultivated in China and analysis of phenolic composition. Sep. Purif. Technol. 2017, 178, 178–188. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Y.; Han, Y.; Chemat, F.; Li, D.; Show, P.L. Insight into mass transfer during ultrasound-enhanced adsorption/desorption of blueberry anthocyanins on macroporous resins by numerical simulation considering ultrasonic influence on resin properties. Chem. Eng. J. 2020, 380, 122530. [Google Scholar] [CrossRef]
- Dular, M.; Osterman, A. Pit clustering in cavitation erosion. Wear 2008, 265, 811–820. [Google Scholar] [CrossRef]
- Dular, M.; Bacherta, B.; Stoffela, B.; Sirok, B. Relationship between cavitation structures and cavitation damage. Wear 2004, 257, 1176–1184. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Boussetta, N.; Turc, M.; De Taeye, C.; Larondelle, Y.; Lanoisellé, J.L.; Vorobiev, E. Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind. Crop. Prod. 2013, 49, 690–696. [Google Scholar] [CrossRef]
- Sathe, S.K.; Seeram, N.P.; Kshirsagar, H.H.; Heber, D.; Lapsley, K.A. Fatty Acid Composition of California Grown Almonds. J. Food Sci. 2008, 73, C607–C614. [Google Scholar] [CrossRef]
- Özcana, M.M.; Ünver, A.; Erkanb, E.; Arslan, D. Characteristics of some almond kernel and oils. Sci. Hortic. 2011, 127, 330–333. [Google Scholar] [CrossRef]
- Ahmad, Z. The uses and properties of almond oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Ahrens, S.; Venkatachalam, M.; Mistry, A.M.; Karen Lapsley, K.; Sathe, S.K. Almond (Prunus dulcis L.) Protein Quality. Plant Foods Hum. Nutr. 2005, 60, 123–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Jieqiong Zhang, J.; Sheng, W.; Wang, S.; Fu, T.J. Effects of heat and high-pressure treatments on the solubility and immunoreactivity of almond proteins. Food Chem. 2016, 199, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Berger, J. Procédé De Préparation D’un Lait D’amandes, Et Produits Obtenus. Brevet EU n ° EP 0 776 165 B1, 7 January 1999. [Google Scholar]
- Sathe, S.K.; Sze, K.W.C. Thermal aggregation of almond protein isolate. Food Chem. 1997, 59, 95–99. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of Roasting, Blanching, Autoclaving, and Microwave Heating on Antigenicity of Almond (Prunus dulcis L.) Proteins. J. Agric. Food Chem. 2002, 50, 3544–3548. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.K.; Vian, M.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
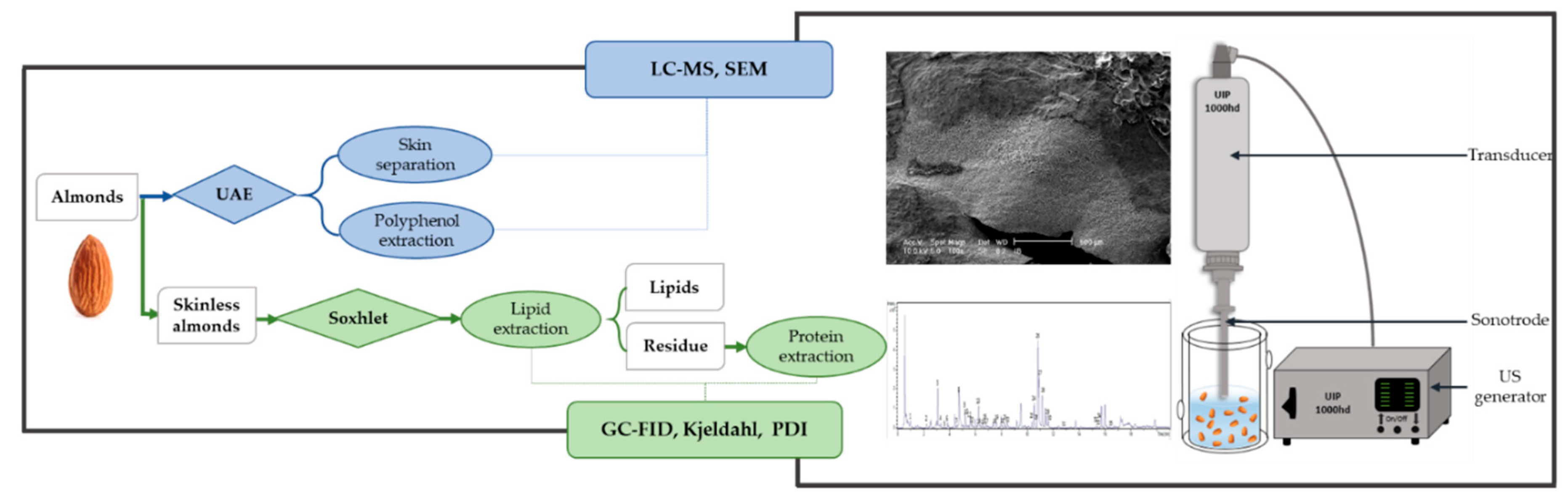
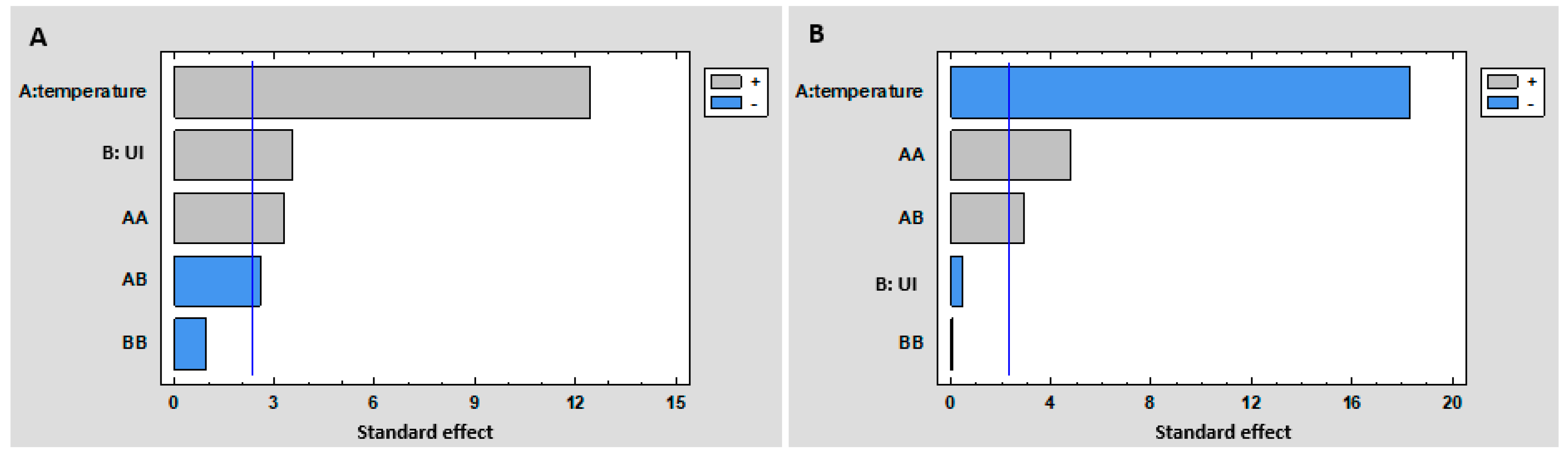
| No. | UI | Temperature | TPC (eq mg Naringenin/g Almonds) | SST (min) |
|---|---|---|---|---|
| 1 | −1 | +1 | 0.061 | 5 |
| 2 | 0 | 0 | 0.039 | 10 |
| 3 | 0 | −α | 0.030 | 10 |
| 4 | 0 | 0 | 0.041 | 11 |
| 5 | +1 | −1 | 0.035 | 17 |
| 6 | +α | 0 | 0.063 | 6 |
| 7 | 0 | 0 | 0.041 | 10 |
| 8 | 0 | 0 | 0.041 | 11 |
| 9 | 0 | 0 | 0.042 | 10 |
| 10 | 0 | +α | 0.046 | 10 |
| 11 | −α | 0 | 0.031 | 20 |
| 12 | +1 | +1 | 0.057 | 7 |
| 13 | 0 | 0 | 0.040 | 10 |
| 14 | −1 | −1 | 0.024 | 20 |
| Sample | NS (µg/100 g of Almond) | NUA (µg/100 g of Almond) | UA (µg/100 g of Almond) | BA (µg/100 g of Almond) |
|---|---|---|---|---|
| Vanillic acid di-hexoside | 1078.8 ± 34.9 | 66.5 ± 5.4 | 423.5 ± 49.4 | 245.0 ± 13.7 |
| Catechin | 648.6 ± 13.8 | 72.7 ± 21.5 | 493,0 ± 245,1 | 335,9 ± 24.1 |
| Epicatechin | 263.9 ± 18.2 | 37.6 ± 10.4 | 152,7 ± 44,7 | 220.0 ± 4.0 |
| (Epi)Catechin-hexoside | 198.4 ± 19.9 | 11.0 ± 3.1 | 136.4 ± 19.9 | 146.9 ± 44.1 |
| Chlorogenic acid | 34.413 ± 2.1 | 5.2 ± 1.5 | 33.3 ± 6.8 | 12.7 ± 0.5 |
| Isorhamnetin | 262.3 ± 2.4 | 9.1 ± 2.0 | 60.7 ± 0.75 | 28.3 ± 2.2 |
| Isorhamnetin-3-O-rutinoside | 2399.9 ± 118.0 | 161.5 ± 36.4 | 957.4 ± 66.7 | 522.5 ± 20.8 |
| Isorhamnetin-3-O-glucoside | 1571.9 ± 62.94 | 87.1 ± 19.9 | 487.3 ± 32.0 | 317.0 ± 15.0 |
| Kaempferol-3-O-glucoside | 72.0 ± 3.5 | 5.9 ± 1.4 | 29.5 ± 3.4 | 23.1 ±1.4 |
| Kaempferol-3-O-rutinoside | 228.9 ± 23.5 | 16.3 ± 3.1 | 105.1 ± 6.5 | 61.6 ± 3.4 |
| Naringenin | 23.8 ± 0.2 | 1.6 ± 0.2 | 10.7 ± 1.5 | 13.2 ± 1.0 |
| Procyanidins | 64636.8 ± 2670.1 | 2438.4 ± 617.4 | 21003.5 ± 3086.2 | 3994.0 ±77.2 |
| Total | 71419.8 | 2912.9 | 23893.0 | 5920.0 |
| 100.0% | 4.1% | 33.5% | 8.3% |
| NA (%) | BA (%) | UA (%) | |
|---|---|---|---|
| C16:0 | 6.069 ± 0.011 | 6.055 ± 0.068 | 5.903 ± 0.007 |
| C16:1 n7 | 0.494 ± 0.001 | 0.522 ± 0.009 | 0.434 ± 0.001 |
| C18:0 | 1.131 ± 0.009 | 1.158 ± 0.000 | 1.206 ± 0.000 |
| C18:1 n9 | 71.475 ± 0.009 | 71.903 ± 0.596 | 72.065 ± 0.012 |
| C18:2 n6 | 20.490 ± 0.010 | 20.590 ± 0.189 | 20.060 ± 0.001 |
| Fresh matter yield (%) | 52.050 ± 0.015 | 52.500 ± 0.002 | 51.680 ± 0.024 |
| Sample | Protein Content (%) | PDI (%) |
|---|---|---|
| NA | 26.127 ± 0.081 | 49.239 ± 0.517 |
| BA | 25.734 ± 0.298 | 39.260 ± 0.579 |
| UA | 25.396 ± 0.155 | 45.804 ± 1.055 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Appl. Sci. 2020, 10, 3628. https://doi.org/10.3390/app10103628
Tabib M, Tao Y, Ginies C, Bornard I, Rakotomanomana N, Remmal A, Chemat F. A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Applied Sciences. 2020; 10(10):3628. https://doi.org/10.3390/app10103628
Chicago/Turabian StyleTabib, Malak, Yang Tao, Christian Ginies, Isabelle Bornard, Njara Rakotomanomana, Adnane Remmal, and Farid Chemat. 2020. "A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality" Applied Sciences 10, no. 10: 3628. https://doi.org/10.3390/app10103628
APA StyleTabib, M., Tao, Y., Ginies, C., Bornard, I., Rakotomanomana, N., Remmal, A., & Chemat, F. (2020). A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Applied Sciences, 10(10), 3628. https://doi.org/10.3390/app10103628