Enzymatic Transesterification of Waste Frying Oil from Local Restaurants in East Colombia Using a Combined Lipase System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment and Characterization of WFO
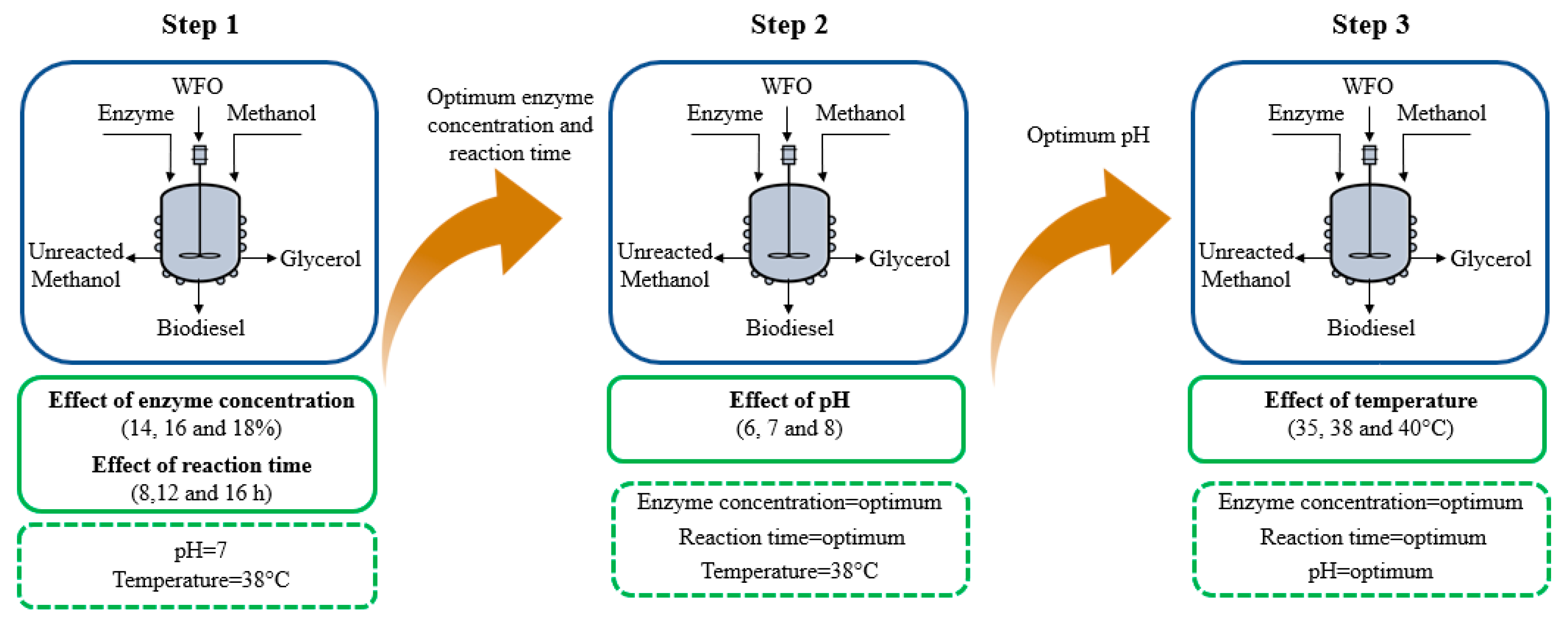
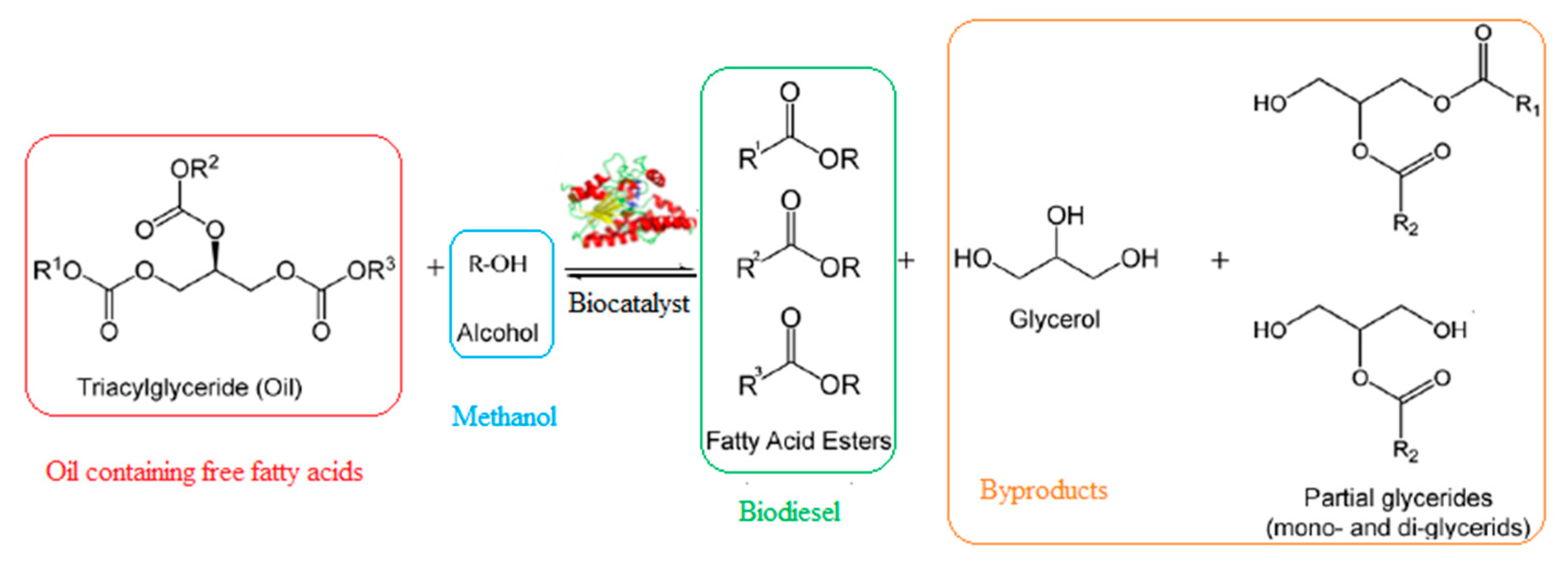
2.3. Biodiesel Production by Enzymatic Transesterification
2.4. Physicochemical Characterization of Biodiesel
3. Results and Discussion
3.1. Physicochemical Characterization of Waste Frying Oil
Fatty Acids Profile
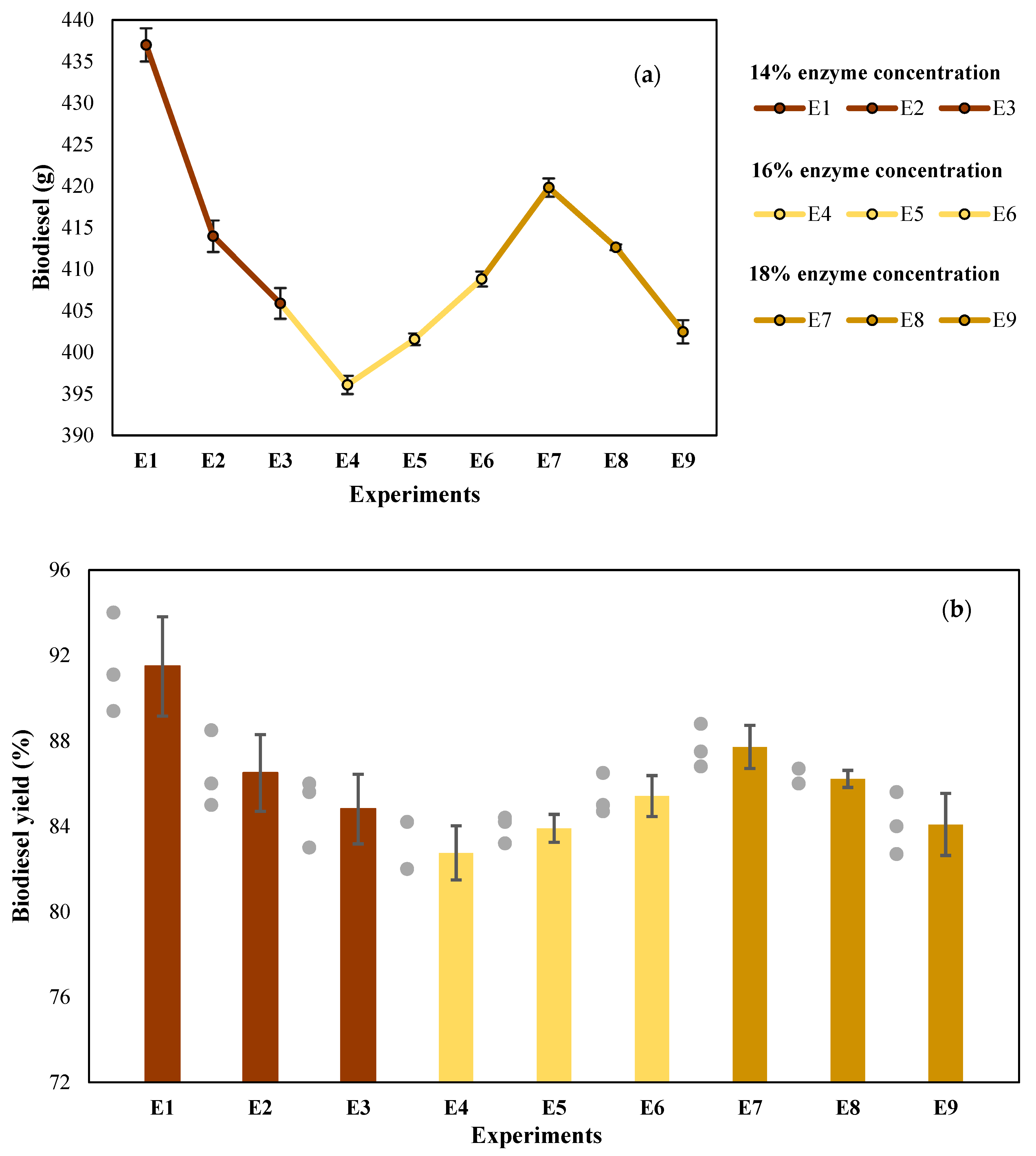
3.2. Biodiesel Production by Enzymatic Transesterification (First Stage)
3.2.1. Physicochemical Characterization of Biodiesel
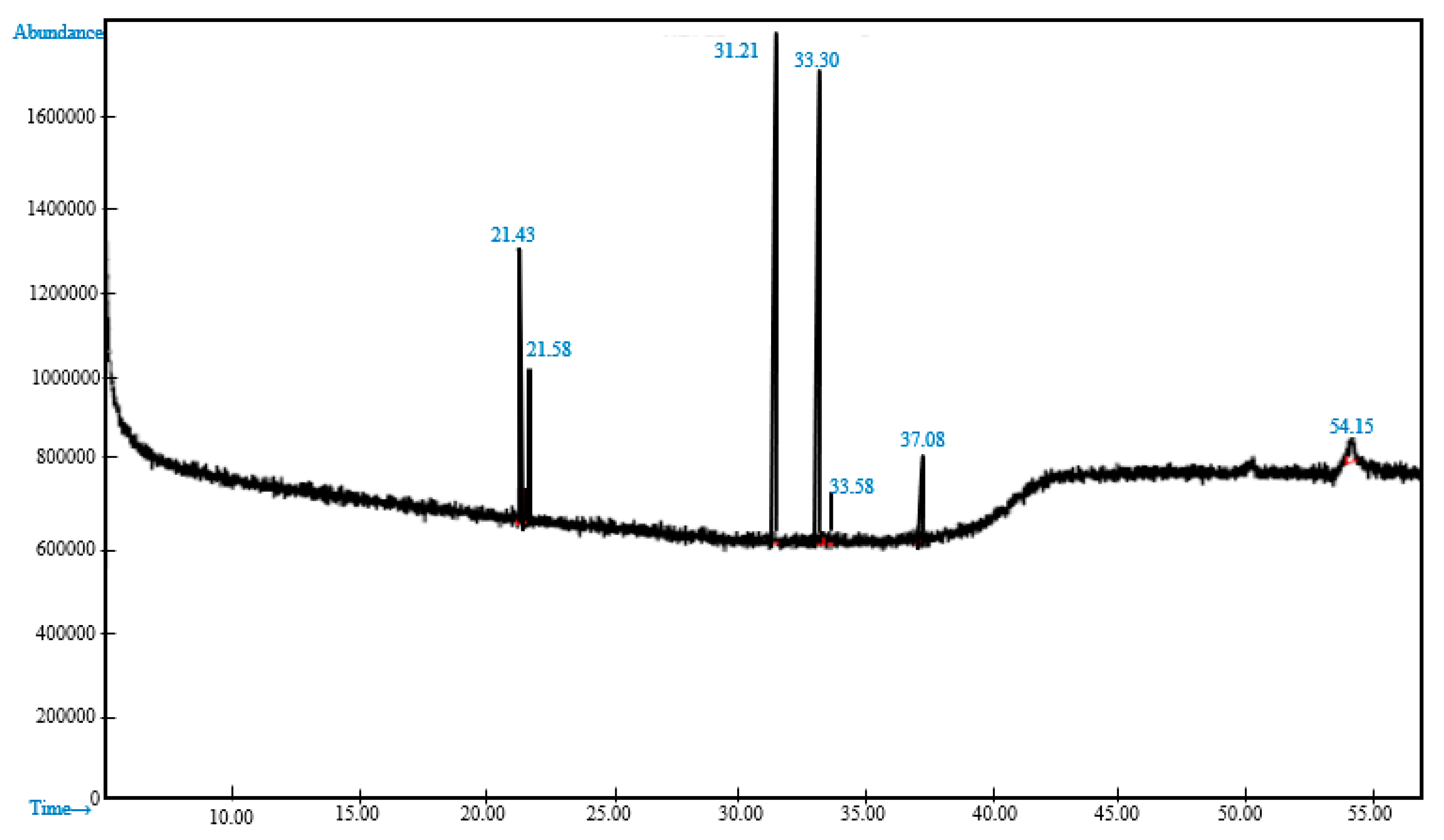
3.2.2. Analysis of Methyl Esters in the Biodiesel
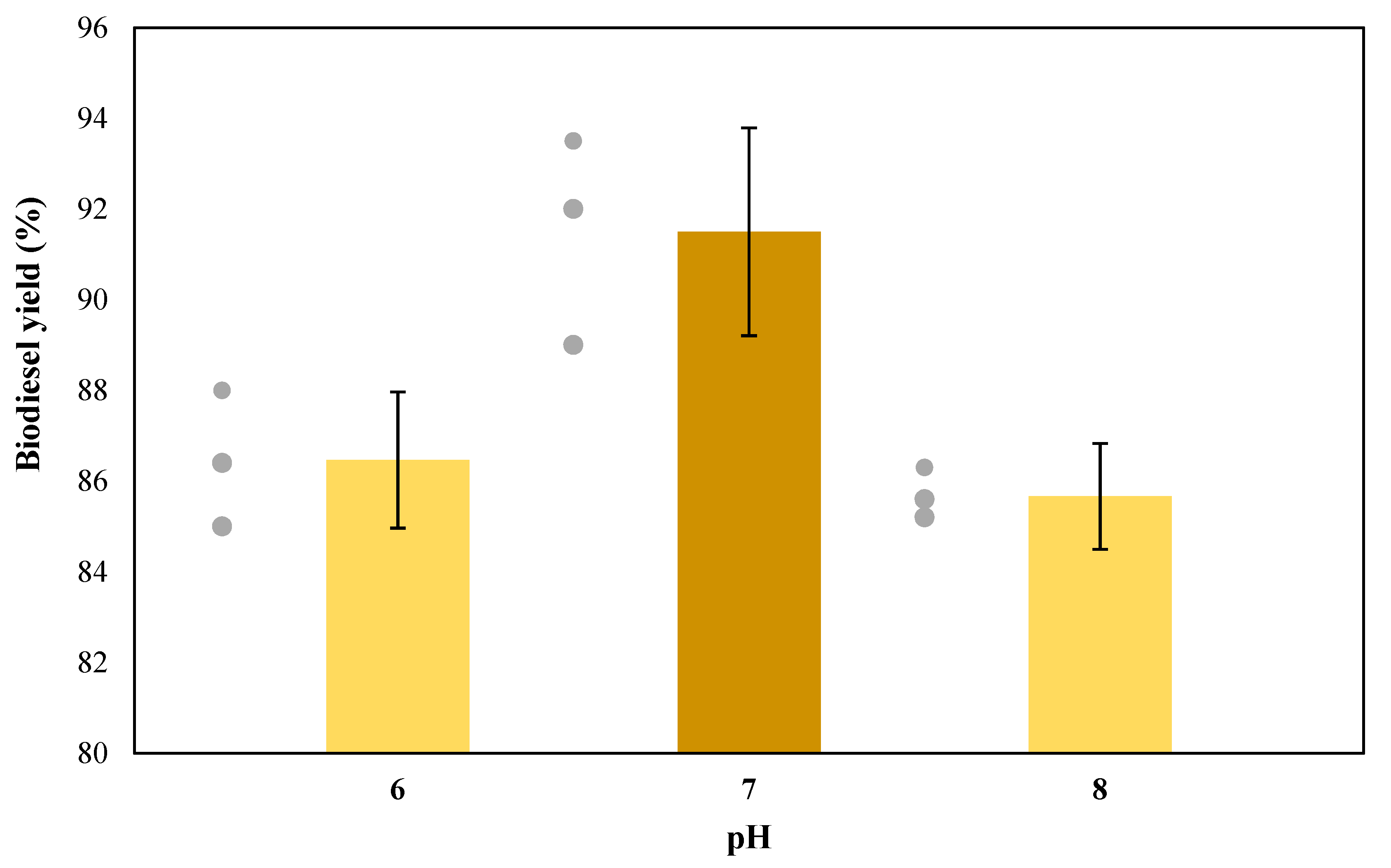
3.3. Effect of pH on Enzymatic Transesterification (Second Stage)
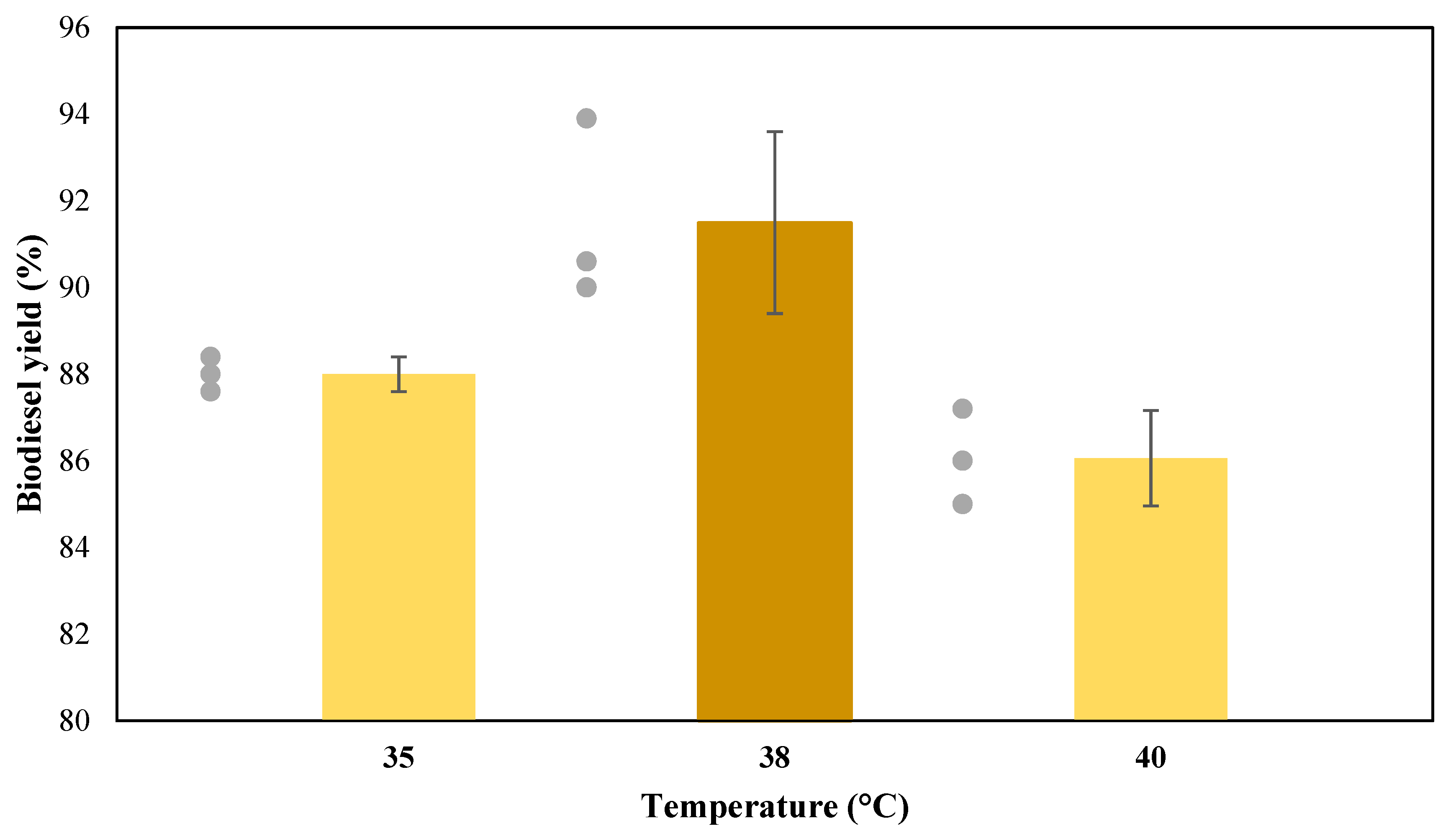
3.4. Effect of Temperature on Enzymatic Transesterification (Third Stage)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. GC-MS Chromatography (Column/Parameters/Conditions)
| Equipment Characteristics | Operating Conditions |
| Model: 6890N Agilent | Carrier gas: Helium |
| Detector: 5973N Agilent | Operating mode: Splitless |
| Column: DB-1MS | Injection volume: 3.0 µL |
| Injector: Split/Splitless | Injector temperature: 250 °C |
| Detector temperature: 320 °C | |
| Initial temperature: 70 °C | |
| Heating rate: 8.00 °C/min | |
| Recording time: 60 min |
References
- Tantawy, A.H.; Abo-riya, M.A.; Abdallah, S.M.; El-dougdoug, W. Novel cationic surfactants based on waste frying oil for cleaning water surface from petroleum films: Synthesis, antimicrobial and surface properties. J. Mol. Liq. 2018, 253, 36–44. [Google Scholar] [CrossRef]
- Yao, K.; Jo-han, N.; Tung, C.; Shiung, S.; Tong, W. Biodiesel process intensification through catalytic enhancement and emerging reactor designs: A critical review. Renew. Sustain. Energy Rev. 2019, 116, 109399. [Google Scholar]
- Gozmen, B.; Uludamar, E.; Özcanli, M. Evaluation of energetic-exergetic and sustainability parameters of biodiesel fuels produced from palm oil and opium poppy oil as alternative fuels in diesel engines. Fuel 2019, 258, 116116. [Google Scholar] [CrossRef]
- Hasan, M.; Rahman, M. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Lit, Z.; Hua, Y.; San, Y.; Kansedo, J.; Mubarak, N.M.; Ghasemi, M.; Omar, M. Life cycle assessment of waste cooking oil for biodiesel production using waste chicken eggshell derived CaO as catalyst via transesterification. Biocatal. Agric. Biotechnol. 2019, 21, 101317. [Google Scholar]
- Murcia Ordoñez, B.; Chaves, L.C.; Rodríguez-Pérez, W.; Andredy Murcia, M.; Alvarado, E.R. Caracterización de biodiesel obtenido de aceite residual de cocina. Rev. Colomb. De Biotecnol. 2013, 15, 61–70. [Google Scholar]
- Bulla Pereira, E.A. Diseño del Proceso de Producción del Biodiesel a Partir de Aceites de Fritura. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2014. [Google Scholar]
- Acevedo, J.C.; Urbina, N.A.; Acevedo, A.Z.; Becerra, L.C.; Arenas, E. Analysis of the biodiesel production yield from waste frying oil. J. Phys. Conf. Ser. 2018, 1126, 012006. [Google Scholar] [CrossRef]
- Dávila Barrera, J.D.; Cortés López, C.C. Obtención de Biodiesel a Partir de Aceite de Fritura; Universidad Libre de Colombia: Bogotá, Colombia, 2017. [Google Scholar]
- Moazeni, F.; Chen, Y.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Panadare, D.; Rathod, V. Applications of Waste Cooking Oil Other Than Biodiesel: A Review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]
- Tan, J.; Zhang, S.; Lu, T.; Li, R.; Zhong, T.; Zhu, X. Design and synthesis of ethoxylated esters derived from waste frying oil as anti-ultraviolet and ef fi cient primary plasticizers for poly (vinyl chloride). J. Clean. Prod. 2019, 229, 1274–1282. [Google Scholar] [CrossRef]
- Acevedo-Paez, J.; Urbina-Suarez, N.; Acevedo-Rodríguez, A.Z.; Becerra-Orozco, L.C.; Mendez-Nuñez, J. El aceite residual de fritura como fuente de energía asequible y poco contaminante. In Semana de Divulgación Científica; Universidad de Santander UDES (Colombia): Santander, Colombia, 2017; pp. 1–8. [Google Scholar]
- Marchini, J.; Teleken, J.; De Cinque, V.; Da Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar]
- Abdelkareem, M.A.; Olabi, A.G. Fuzzy modeling and parameters optimization for the enhancement of biodiesel production from waste frying oil over montmorillonite clay K-30. Sci. Total Environ. 2019, 666, 821–827. [Google Scholar]
- Hussein, A.; Durak, E. Study of the tribological properties the mixture of soybean oil and used (waste) frying oil fatty acid methyl ester under boundary lubrication conditions. Renew. Energy 2020, 145, 1730–1747. [Google Scholar]
- Pascoal, C.; Oliveira, A.; Figueiredo, D.; Assuncao, J. Optimization and kinetic study of ultrasonic-mediated in situ transesterification for biodiesel production from the almonds of Syagrus cearensis. Renew. Energy 2020, 147, 1815–1824. [Google Scholar] [CrossRef]
- Vieira, M.; Gil, B.; da Costa Marques, L.; Soares, A.; Vasconcelos, M. Comparative study of NOx emissions of biodiesel-diesel blends from soybean, palm and waste frying oils using methyl and ethyl transesterification routes. Fuel 2017, 194, 144–156. [Google Scholar] [CrossRef]
- Berrios, M.; Martín, M.A.; Chica, A.F.; Martín, A. Study of esterification and transesterification in biodiesel production from used frying oils in a closed system. Chem. Eng. Res. Des. 2010, 160, 473–479. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. Parametric study of the alkali catalyzed transesterification of waste frying oil for Biodiesel production. Energy Convers. Manag. 2014, 79, 246–254. [Google Scholar] [CrossRef]
- Cordero-ravelo, V.; Schallenberg-rodriguez, J. Biodiesel production as a solution to waste cooking oil (WCO) disposal. Will any type of WCO do for a transesteri fi cation process? A quality assessment. J. Environ. Manag. 2018, 228, 117–129. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinler, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Naccarato, S.; Albo, L.; Paola MGDe Chakraborty, S.; Curcio, S.; Calabrò, V. Enzymatic transesterification of waste vegetable oil to produce biodiesel. Ecotoxicol. Environ. Saf. 2015, 121, 229–235. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-lafuente, R.; Rodrigues, R.C.; Antônio, M.; Ayub, Z. Transesterification of Waste Frying Oil and Soybean Oil by Combi-lipases Under Ultrasound-Assisted Reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Souissi, Y.; Alouini, M.; Mnif, W. Chemical and Biological Investigation of Organic Wastes of Frying Oils and Beef Fats: Valorization for Biodiesel Production. J. Chem. 2018, 2018, 6248047. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Das, T.; Shekher, B.; Rene, E.R.; Verma, B. Biodiesel production from hybrid non-edible oil using bio-support beads immobilized with lipase from Pseudomonas cepacia. Fuel 2019, 255, 115801. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel Production from Waste Cooking Oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Worthington Biochemical Corporation. Introduction to Enzymes. In Manual of Clinical Enzyme Measurements; Worthington Biochemical Corporation: Freehold, NJ, USA, 1972; p. 54. [Google Scholar]
- Rangel-Basto, Y.A.; García-Ochoa, I.E.; Suárez-Gelvez, J.H.; Zuorro, A.; Barajas-Solano, A.F.; Urbina-Suárez, N.A. The Effect of Temperature and Enzyme Concentration in the Transesterification Process of Synthetic Mecroalgae Oil. Chem. Eng. Trans. 2018, 64, 331–336. [Google Scholar]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, e00486. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Husson, F.; Kermasha, S. Optimization of the Hydrolysis of Safflower Oil for the Production of Linoleic Acid, Used as Flavor Precursor. Int. J. Food Sci. 2015, 2015, 594238. [Google Scholar] [CrossRef]
- Park, Y.; Pastore, G.; Almeida, M. Hydrolysis of soybean oil by a combined lipase system. J. Am. Oil Chem. Soc. 2007, 23, 1725–1731. [Google Scholar] [CrossRef]
- Bezbradica, D.; Karalazic, I.; Ognjanovic, N.; Mijin, D.; Siler-Marinkovic, S.; Knezevic, Z. Studies on the specificity of Candida rugosa lipase catalyzed esterification reactions in organic media. J. Serb. Chem. Soc. 2006, 71, 31–41. [Google Scholar] [CrossRef]
- Pedro, K.; Parreira, J.; Correia, I.; Henriques, C.; Langone, M. Enzymatic biodiesel synthesis from acid oil using a lipase mixture. Química Nova 2018, 41, 284–291. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Devanesan, M.G.; Viruthagiri, T.; Sugumar, N. Transesterification of Jatropha oil using immobilized Pseudomonas fluorescens. Afr. J. Biotechnol. 2007, 6, 2497–2501. [Google Scholar]
- Baadhe, R.; Potumarthi, R.; Gupta, V. Lipase-Catalyzed Biodiesel Production: Technical Challenges. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 119–129. [Google Scholar]
- Acevedo-Páez, J.C.; Urbina-Suárez, N.A.; Acevedo-Rodríguez, A.Z.; Becerra-Orozco, L.C. Study of the production of biodiesel by enzymatic and chemical processes from used cooking oil. Aibi Rev. De Investig. Adm. E Ing. 2019, 7, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Paez, J.; Urbina, N.; Acevedo, A.Z.; Becerra, L. Physicochemical study of used frying oil as feedstock for the production of biodiesel. J. Phys. Conf. Ser. 2019, 1388, 8. [Google Scholar] [CrossRef]
- Ramírez Cleves, N.P. Estudio para la Implementación de Aceite de Cocina Residual de una Cadena de Restaurantes Para la Producción de Biodiesel en Bogotá. Bachelor’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2017. [Google Scholar]
- Herrera, J.; Velez, J. Caracterizacion y Aprovechamiento del Aceite Residual de Frituras Para la Obtencion de un Combustible (Biodiesel). Ph.D. Thesis, Universidad Tecnológica de Pereira, Pereira, Colombia, 2008. [Google Scholar]
- Sánchez Moreno, N.B.; Sarmiento Lozano, D.C. Propuesta de instalación de un punto de acopio de aceite de cocina usado en la zona de comidas de la plaza de mercado de Sogamoso “Sogabastos”. Specialization’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2016. [Google Scholar]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Yahida, N.; Ngadi, N.; Jusoh, M.; Amirah, N.; Halim, A. Characterization and parametric study of mesoporous calcium titanate catalyst for transesterification of waste cooking oil into biodiesel. Energy Convers. Manag. 2016, 129, 275–283. [Google Scholar]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.; Rashid, U.; Saiman, M.; Ping, Y.; Alsultan, G.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Fillieres, R.; Benjelloun-Mlayeh, B.; Delmas, M. Ethanolysis of rapeseed oil: Quantitation of ethyl esters, mono-, di- and triglycerides and glycerol by high-performance size-exclusion chromatography. J. Am. Oil Chem. Soc. 1995, 72, 427–432. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, C.; Kang, J.; Park, C.; Tae, B.; Kim, S. Biodiesel Production from Various Oils Under Supercritical Fluid Conditions by Candida antartica Lipase B Using a Stepwise Reaction Method. Biotechnol. Appl. Biochem. 2009, 156, 454–464. [Google Scholar] [CrossRef]
- Uzun, B.; Kılıç, M.; Özbay, N.; Pütün, A.; Pütün, E. Biodiesel production from waste frying oils: Optimization of reaction parameters and determination of fuel properties Bas. Energy 2012, 44, 347–351. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Maceiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M.C. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- Jilse, S.; Chandrasekharan, M.; Arockiasamy, S. A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production. Cogent Eng. 2016, 3, 1–12. [Google Scholar]
- Cavalcanti-oliveira, E.A.; Rufino, P.; Pec, A.; Alexandre, D.; Aranda, G.; Guimar, D.M. Study of Soybean Oil Hydrolysis Catalyzed by Thermomyces lanuginosus Lipase and Its Application to Biodiesel Production via Hydroesterification. Enzym. Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.; Chen, C.; Chang, J. Continuous biodiesel conversion via enzymatic transesterification catalyzed by immobilized Burkholderia lipase in a packed-bed bioreactor. Appl. Energy 2016, 168, 340–350. [Google Scholar] [CrossRef]
- Azócar-Ulloa, L.H. Proceso Enzimático Para la Producción de Metil Ésteres de Ácidos Grasos Utilizando Aceites Residuales de Fritura en Mezcla con Aceite de Raps Como Materia Prima. Ph.D. Thesis, Universidad de la Frontera, Temuco, Chile, 2010. [Google Scholar]
- Regil, R.; De Sandoval, G. Biocatalysis for Biobased Chemicals. Biomolecules 2013, 3, 812–847. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.; Liu, C. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef]
- Lee, S.U.; Jung, K.; Park, G.W.; Seo, C.; Hong, Y.K.; Hong, W.H.; Chang, H.N. Bioprocessing aspects of fuels and chemicals from biomass. Korean J. Chem. Eng. 2012, 29, 831–850. [Google Scholar] [CrossRef]
- Kiss, A.A.; Ignat, R. Enhanced methanol recovery and glycerol separation in biodiesel production – DWC makes it happen. Appl. Energy 2012, 99, 146–153. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Biodiesel from Used Frying Oil. Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Fajar, B.; Sukarno, A. Experimental Study of Additives on Viscosity biodiesel at Low Temperature Experimental Study of Additives on Viscosity biodiesel at Low Temperature. Iop Conf. Ser. Mater. Sci. Eng. 2015, 88, 1–8. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Corrosion mechanism of copper in palm biodiesel. Corros. Sci. 2013, 67, 50–59. [Google Scholar] [CrossRef]
- Haseed, A.; Masjuki, H.; Ann, L.; Fazal, M. Corrosion characteristics ofcopper and leaded bronze in palm biodiesel. Fuel Process. Technol. 2010, 91, 329–334. [Google Scholar]
- Avellaneda Vargas, F.A. Producción y Caracterización de Biodiesel de Palma y de Aceite Reciclado Mediante un Proceso Batch y un Proceso Continuo con un Reactor Helicoidal. Ph.D. Thesis, Universitat Rovira I Virgili, Tarragona, Spain, 2010. [Google Scholar]
- Thi, T.; Bazile, J.; Bessiéres, D. Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges. Energie 2018, 11, 1–14. [Google Scholar]
- Nurdin, M.; Fatma, F.; Natsir, M.; Wibowo, D. Characterization of methyl ester compound of biodiesel from industrial liquid waste of crude palm oil processing. Anal. Chem. Res. 2017, 12, 1–9. [Google Scholar]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Nde, D.B.; Astete, C.; Boldor, D. ESolvent-free, enzyme-catalyzed biodiesel production from mango, neem, and shea oils via response surface methodology. Amb Express 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Charpe, T.W.; Rathod, V.K. Biodiesel production using waste frying oil. Waste Manag. 2011, 31, 85–90. [Google Scholar] [CrossRef]

| Property | Method | Reference |
|---|---|---|
| Density at 25 °C | Gravimetric method | [29] |
| Kinematic viscosity at 40 °C | Laboratory guide for fluids and hydraulic machines UFPS | [29] |
| Acidity index | ICONTEC N 218 * | [38] |
| Moisture content | NTC 287 * | [39] |
| Flash point | Open cup method | [39] |
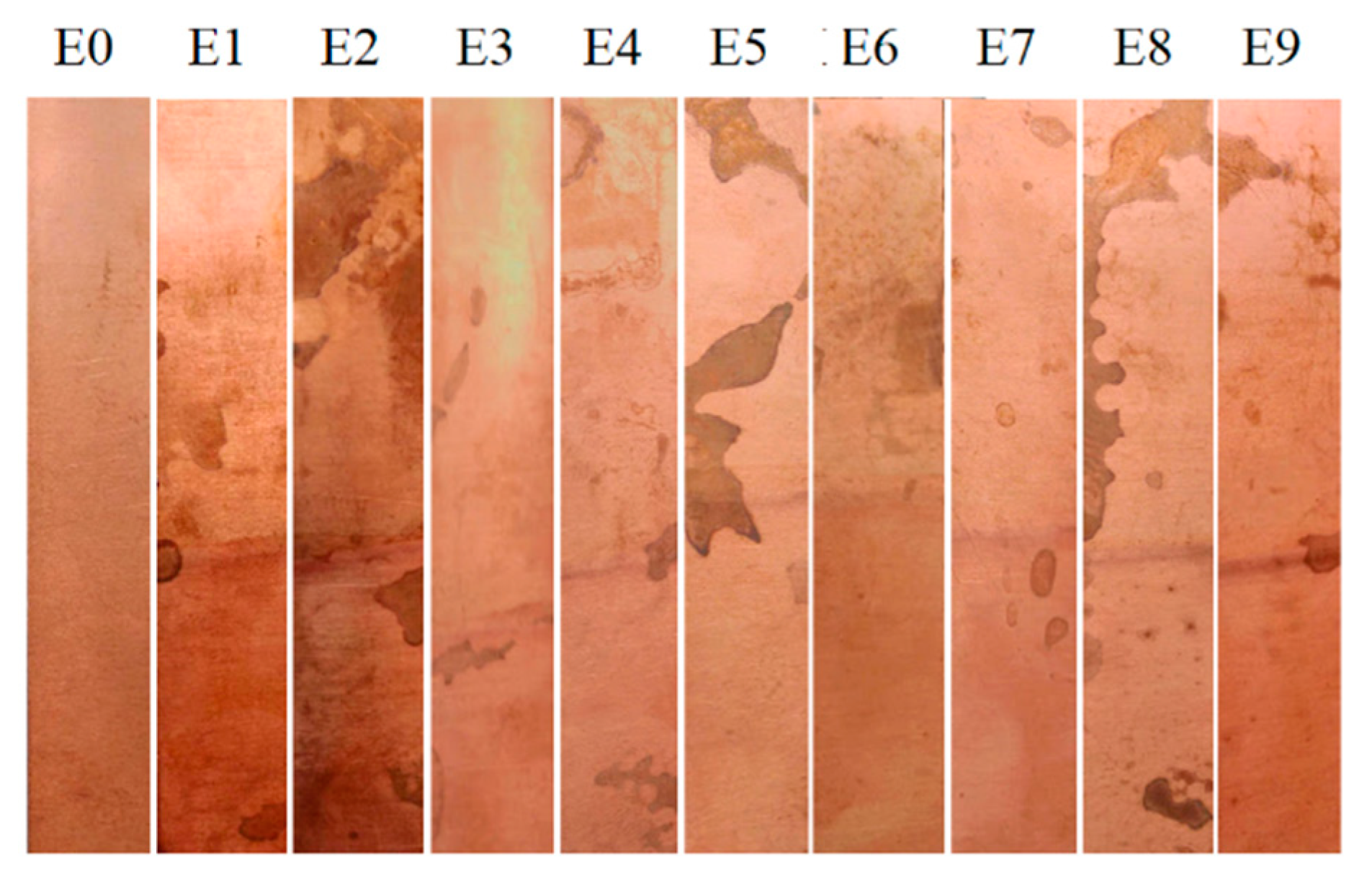
| Copper corrosion test | ASTM D130 * | [29] |
| Properties | Result |
|---|---|
| Density (g/mL) | 0.9572 ± 0.00014 |
| Moisture (mass fraction) | 0.0600 ± 0.016 |
| Acidity index (mg KOH/g) | 2.5624 ± 0.3945 |
| pH | 5.7 ± 0 |
| References | Fatty Acid Composition (%) | |
|---|---|---|
| Palmitic Acid (C16:0) | Oleic Acid (C18:1) | |
| This work | 30.35 | 18.84 |
| Eze et al. [43] | 6.1 | 64.2 |
| Yahya et al. [44] | 34.80 | 53.30 |
| Tacias Pascacio et al. [45] | 17.82 | 40.98 |
| Mansir et al. [46] | 60.1 | 27.20 |
| Enzyme Concentration | 8 h | 12 h | 16 h |
|---|---|---|---|
| 14% | E1 | E2 | E3 |
| 16% | E4 | E5 | E6 |
| 18% | E7 | E8 | E9 |
| Exp. | Density (g/mL) | Moisture Content (%) | Acidity Index (mg KOH/g Sample) | Flash Point (°C) | Kinematic Viscosity (mm2/s) |
|---|---|---|---|---|---|
| E1 | 0.922 ± 0.0041 | 0.279 ± 0.01 | 2.84 ± 0.28 | 252.5 ± 5 | 10.53 ± 0.04 |
| E2 | 0.925 ± 0.0042 | 0.381 ± 0.06 | 3.38 ± 0.69 | 240.0 ± 0 | 9.29 ± 0.07 |
| E3 | 0.928 ± 0.0013 | 0.256 ± 0.04 | 4.61 ± 1.17 | 250.0 ± 0 | 9.66 ± 0.98 |
| E4 | 0.921 ± 0.0021 | 0.111 ± 0.10 | 3.65 ± 0.55 | 257.5 ± 5 | 10.06 ± 0.09 |
| E5 | 0.929 ± 0.0008 | 0.239 ± 0.098 | 3.49 ± 0.82 | 242.5 ± 5 | 9.58 ± 0.90 |
| E6 | 0.929 ± 0.0048 | 0.315 ± 0.05 | 3.79 ± 0.49 | 227.5 ± 5 | 8.66 ± 0.27 |
| E7 | 0.923 ± 0.0006 | 0.137 ± 0.13 | 3.41 ± 0.35 | 242.5 ± 5 | 8.59 ± 0.098 |
| E8 | 0.922 ± 0.0008 | 0.199 ± 0.12 | 2.07 ± 0.20 | 252.5 ± 5 | 9.44 ± 0.20 |
| E9 | 0.925 ± 0.0033 | 0.499 ± 0.29 | 4.59 ± 0.33 | 227.5 ± 5 | 7.08 ± 0.86 |
| FAME | Retention Time | Composition (%) |
|---|---|---|
| Methyl palmitate | 31.21 | 20.25 |
| Methyl linolelaidate | 21.43 | 2.80 |
| Methyl oleate | 33.30 | 17.91 |
| Methyl tridecanoate | 21.58 | 1.90 |
| Reference | Rangel et al. [29] | Acevedo et al. [8] | Acevedo et al. [8] | This Work |
|---|---|---|---|---|
| Oil type | Seaweed oil | WFO | WFO | WFO |
| Catalyst | Enzymatic | Enzymatic | Alkaline | Enzymatic |
| Conditions | 10% of lipase enzyme XX split | 5% of lipase enzyme XX 25 split | 1% of KOH | 14% of lipase enzyme XX 25 split |
| Oil-alcohol 1:3 | Oil-Alcohol 1:3 | Oil-Alcohol 1:6 | Oil-Alcohol 1:3 | |
| 33 °C | 38 °C | 60 °C | 38 °C | |
| 6 h | 3 h | 70 min | 8 h | |
| Yield | 85.49% | 50% | 89.40% | 87.11% |
| FAMEs Composition (%) | Composition (%) | |
|---|---|---|
| 35 °C | 40 °C | |
| Methyl palmitate | 25.4 | 26.4 |
| Methyl linolelaidate | 0 | 0 |
| Methyl oleate | 17.40 | 20.30 |
| Methyl tridecanoate | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Vela, M.A.; Acevedo-Páez, J.C.; Urbina-Suárez, N.; Rangel Basto, Y.A.; González-Delgado, Á.D. Enzymatic Transesterification of Waste Frying Oil from Local Restaurants in East Colombia Using a Combined Lipase System. Appl. Sci. 2020, 10, 3566. https://doi.org/10.3390/app10103566
Ferreira Vela MA, Acevedo-Páez JC, Urbina-Suárez N, Rangel Basto YA, González-Delgado ÁD. Enzymatic Transesterification of Waste Frying Oil from Local Restaurants in East Colombia Using a Combined Lipase System. Applied Sciences. 2020; 10(10):3566. https://doi.org/10.3390/app10103566
Chicago/Turabian StyleFerreira Vela, Mary Angélica, Juan C. Acevedo-Páez, Nestor Urbina-Suárez, Yeily Adriana Rangel Basto, and Ángel Darío González-Delgado. 2020. "Enzymatic Transesterification of Waste Frying Oil from Local Restaurants in East Colombia Using a Combined Lipase System" Applied Sciences 10, no. 10: 3566. https://doi.org/10.3390/app10103566
APA StyleFerreira Vela, M. A., Acevedo-Páez, J. C., Urbina-Suárez, N., Rangel Basto, Y. A., & González-Delgado, Á. D. (2020). Enzymatic Transesterification of Waste Frying Oil from Local Restaurants in East Colombia Using a Combined Lipase System. Applied Sciences, 10(10), 3566. https://doi.org/10.3390/app10103566








