Ultra-Sensitive Hydrogen Peroxide Sensor Based on Peroxiredoxin and Fluorescence Resonance Energy Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Protein Expression and Purification
2.3. SDS-PAGE
2.4. Fluorescence Measurement
2.5. Selectivity Test
2.6. Glucose Detection
3. Results and Discussion
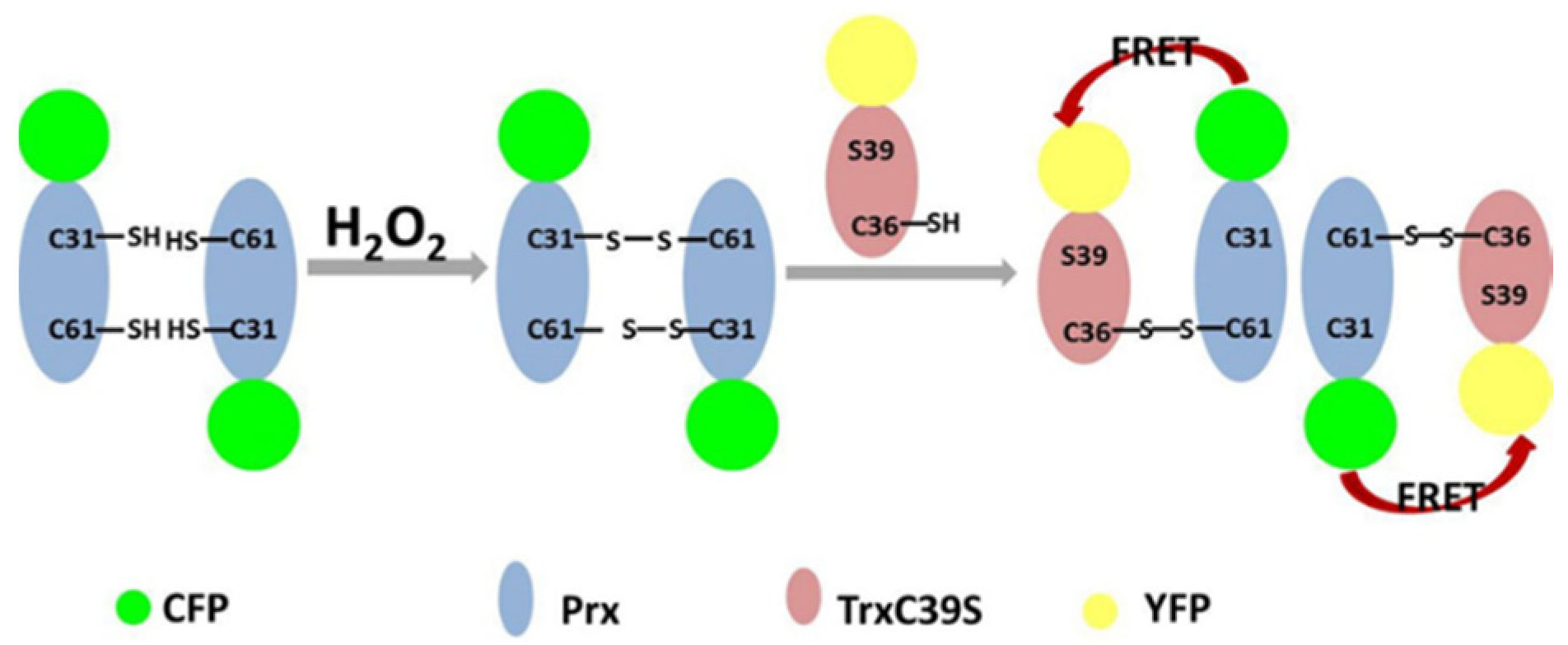
3.1. Construction of the Proposed H2O2 Probe
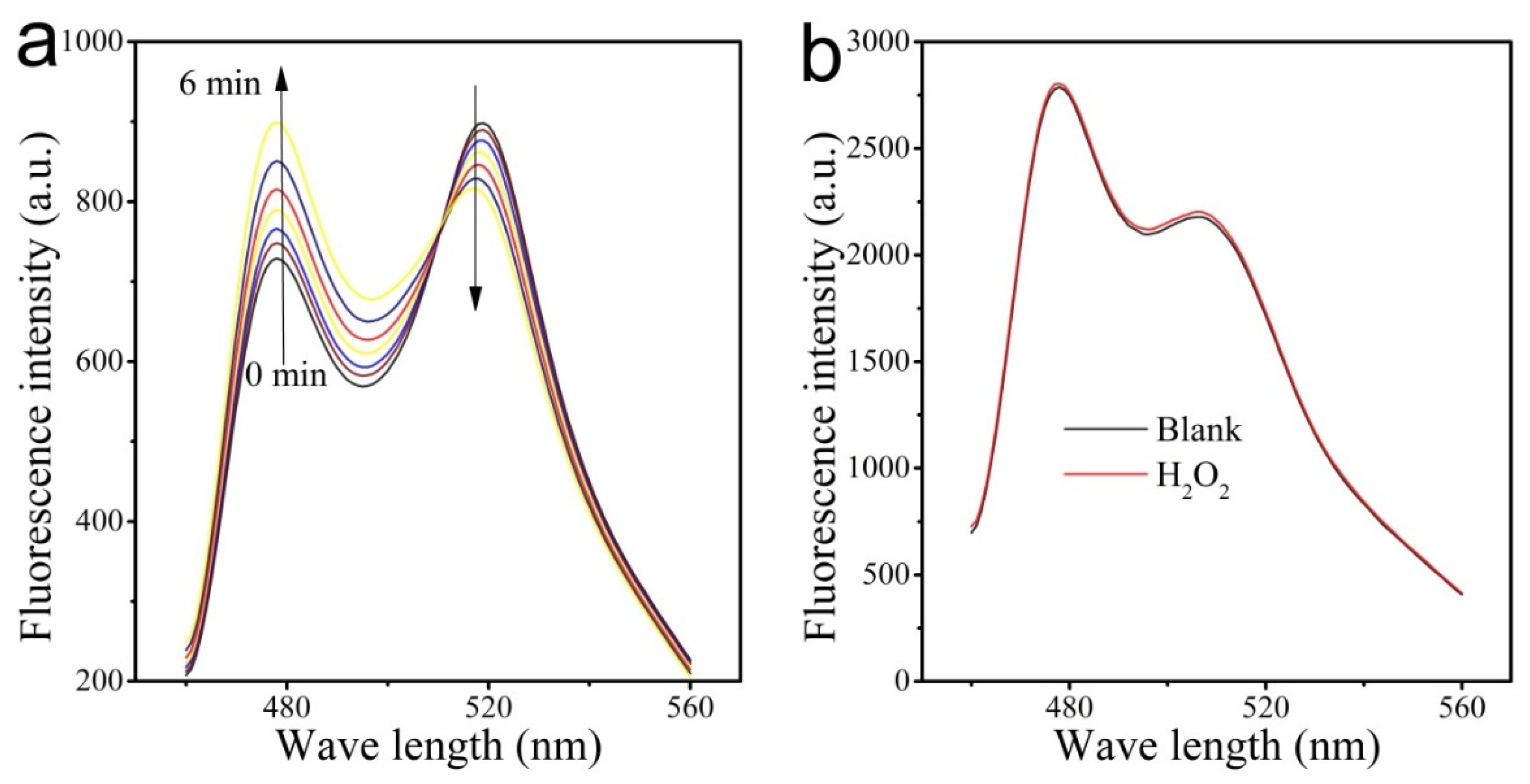
3.2. FRET Signals Respond to H2O2 Concentration as a Result of CFP-Prx/mTrx-YFP Conjugation through Disulfide Bond
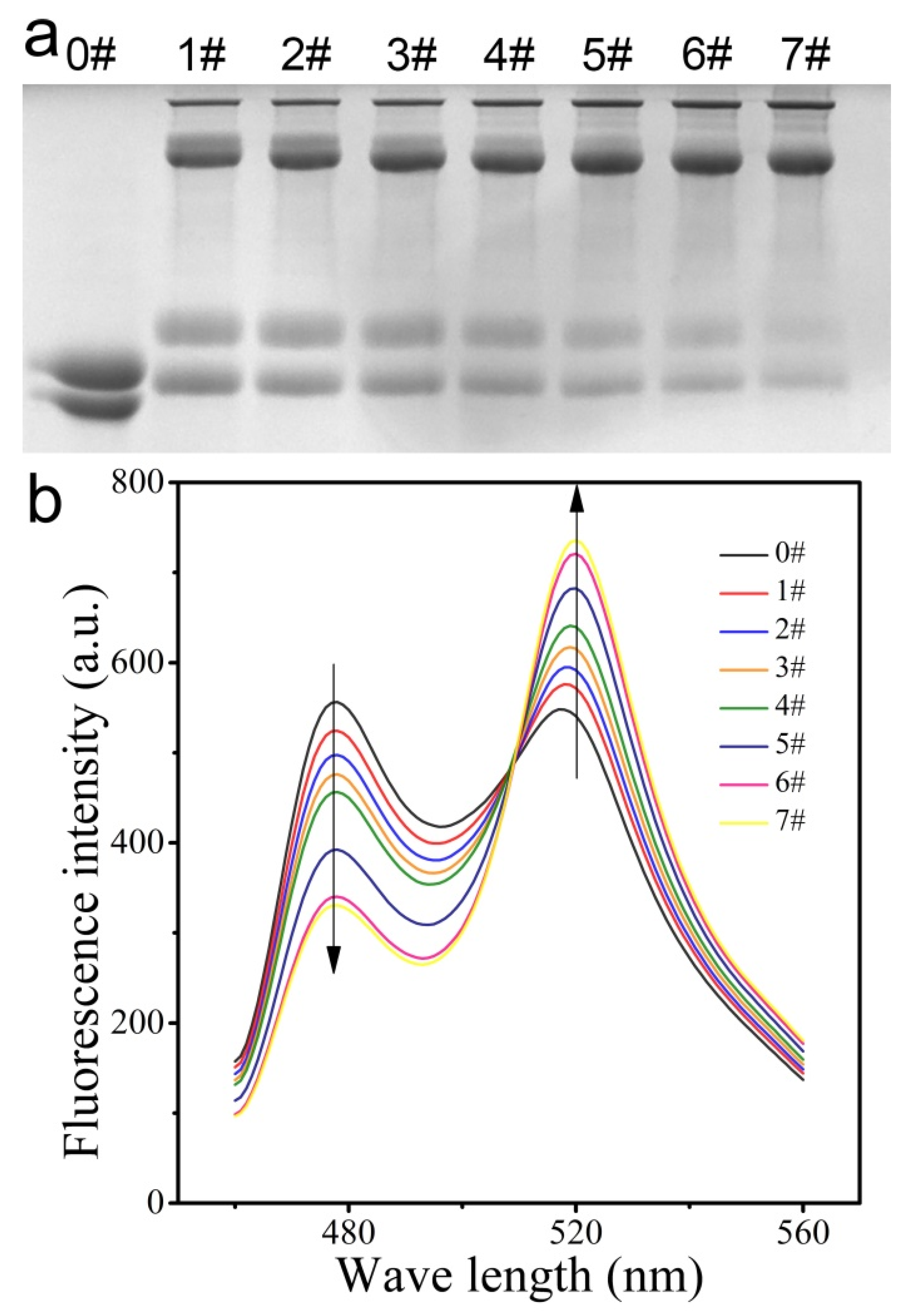
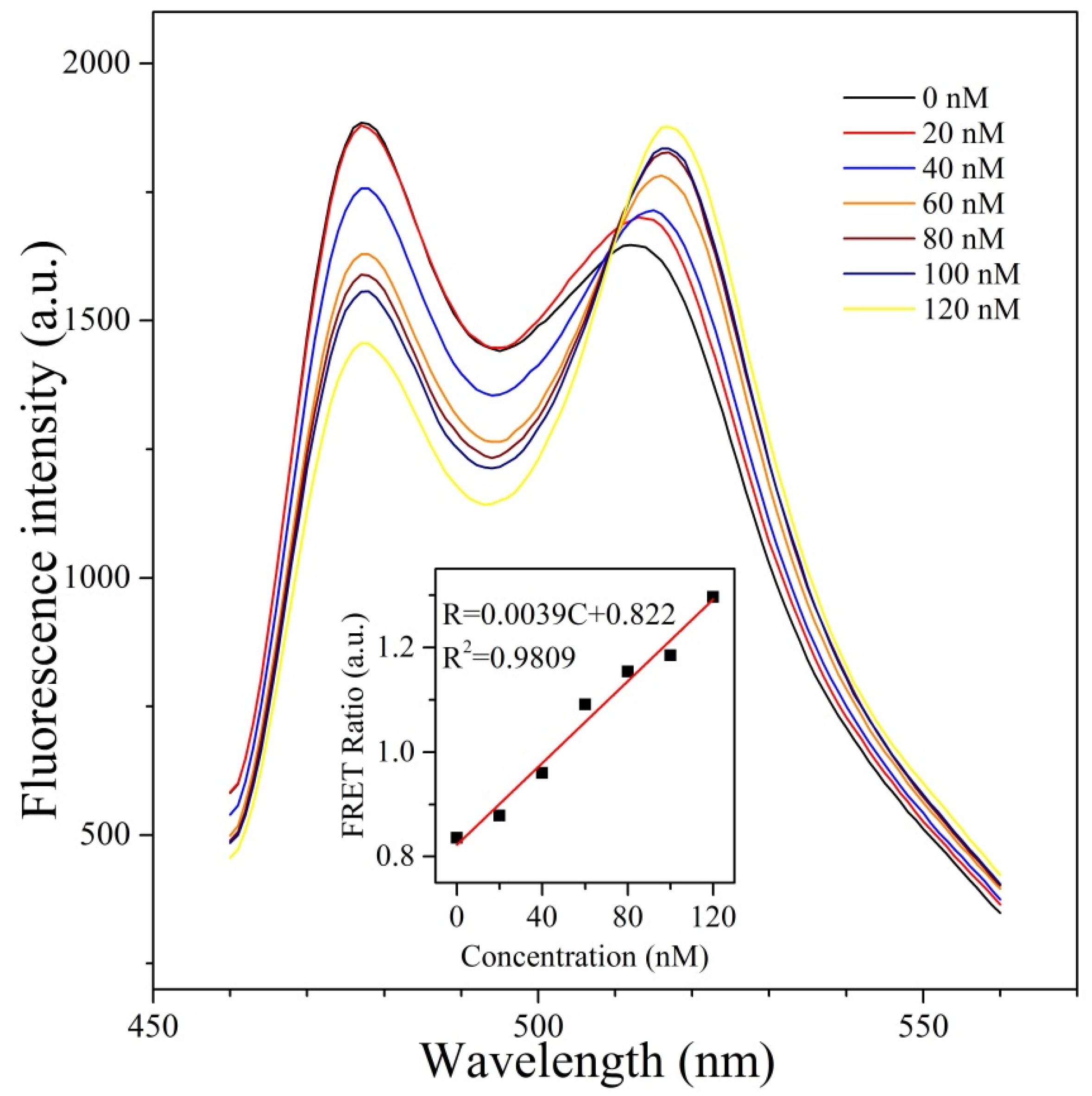
3.3. Ability of CPmTY to Detect H2O2 with Low Limit and Wide Range by Optimal Protein Concentration
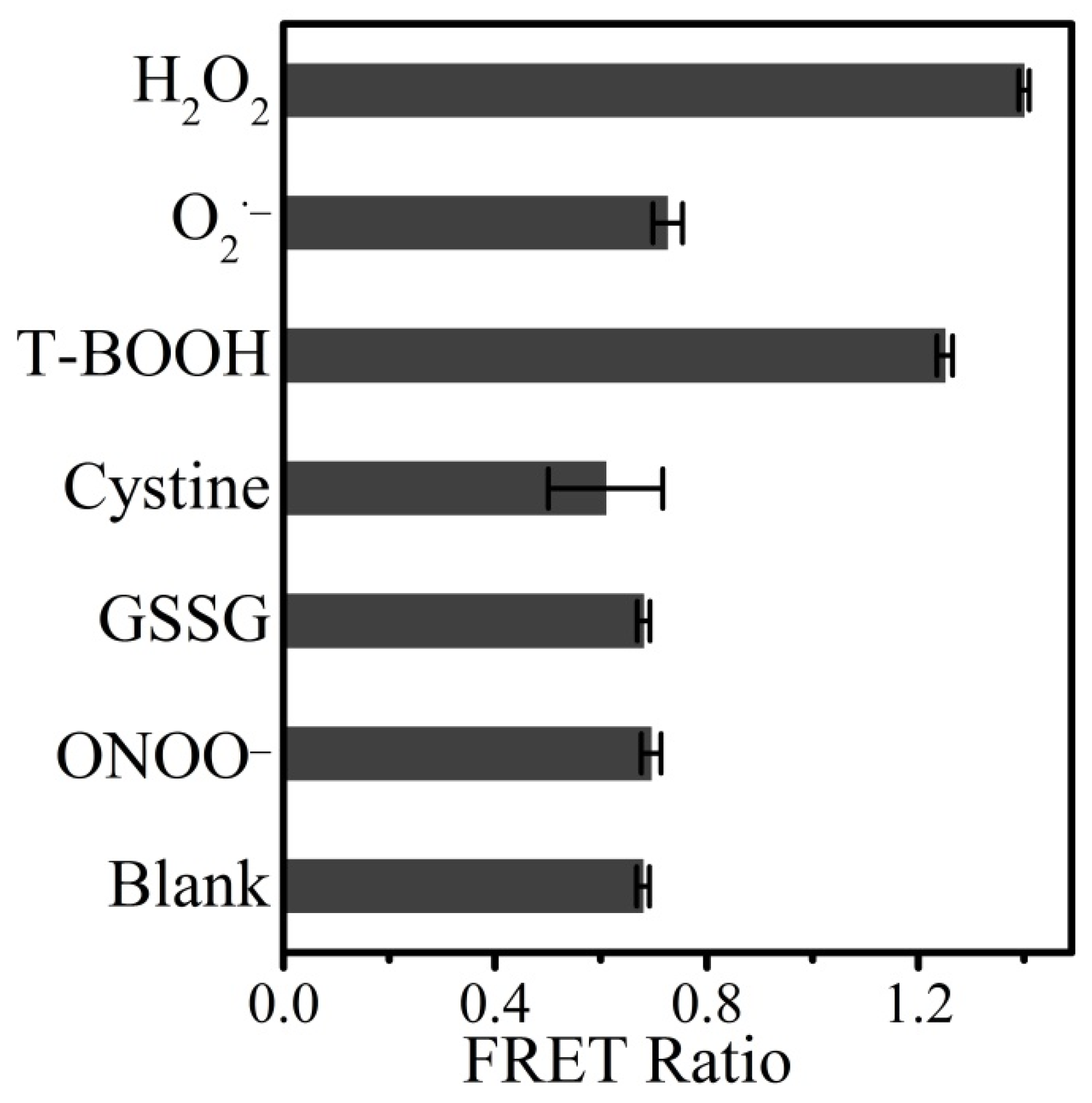
3.4. Detection Selectivity
3.5. Detection of Glucose
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, P.; Ji, B.; Sun, G. Whiteness Improvement of Citric Acid Crosslinked Cotton Fabrics: H2O2 Bleaching under Alkaline Condition. Carbohydr. Polym. 2016, 147, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L.; Crisol, R.; Schuchardt, U. Bleaching of Commercial Pulps with H2O2 Catalyzed by Heteropolyacids. Bioresour. Technol. 1999, 68, 17–21. [Google Scholar] [CrossRef]
- Le Toquin, E.; Faure, S.; Orange, N.; Gas, F. New Biocide Foam Containing Hydrogen Peroxide for the Decontamination of Vertical Surface Contaminated With Bacillus Thuringiensis Spores. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, C.; Hao, Y.; Ye, B.; Xu, M. Oriented Growth of Cross-Linked Metal-Organic Framework Film on Graphene Surface for Non-Enzymatic Electrochemical Sensor of Hydrogen Peroxide in Disinfectant. Talanta 2018, 188, 282–287. [Google Scholar] [CrossRef]
- Pletjushkina, O.Y.; Fetisova, E.K.; Lyamzaev, K.G.; Ivanova, O.Y.; Domnina, L.V.; Vyssokikh, M.Y.; Pustovidko, A.V.; Vasiliev, J.M.; Murphy, M.P.; Chernyak, B.V.; et al. Long-Distance Apoptotic Killing of Cells Is Mediated by Hydrogen Peroxide in a Mitochondrial ROS-Dependent Fashion. Cell Death Differ. 2005, 12, 1442–1444. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Abramov, A.Y. Role of Mitochondrial ROS in the Brain: From Physiology to Neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS Promotes Cancer Cell Survival through Calcium Signaling. Cancer Cell 2018, 33, 949–951. [Google Scholar] [CrossRef]
- Mori, S.; Morihiro, K.; Okuda, T.; Kasahara, Y.; Obika, S. Hydrogen Peroxide-Triggered Gene Silencing in Mammalian Cells through Boronated Antisense Oligonucleotides. Chem. Sci. 2018, 9, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Reczek, C.R.; Chandel, N.S. ROS-Dependent Signal Transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajah, J.R.; Chang, J.; Goettel, J.A.; Verkman, A.S.; Lencer, W.I. Aquaporin-3 Mediates Hydrogen Peroxide-Dependent Responses to Environmental Stress in Colonic Epithelia. Proc. Natl. Acad. Sci. USA 2017, 114, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Allaoui, A.; Botteaux, A.; Dumont, J.E.; Hoste, C.; De Deken, X. Dual Oxidases and Hydrogen Peroxide in a Complex Dialogue between Host Mucosae and Bacteria. Trends Mol. Med. 2009, 15, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Fedurayev, P.V.; Mironov, K.S.; Gabrielyan, D.A.; Bedbenov, V.S.; Zorina, A.A.; Shumskaya, M.; Los, D.A. Hydrogen Peroxide Participates in Perception and Transduction of Cold Stress Signal in Synechocystis. Plant Cell Physiol. 2018, 59, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Velasco-Lozano, S.; Guisán, J.M.; López-Gallego, F. Oxidation of Phenolic Compounds Catalyzed by Immobilized Multi-Enzyme Systems with Integrated Hydrogen Peroxide Production. Green Chem. 2014, 16, 303–311. [Google Scholar] [CrossRef]
- Messner, K.R.; Imlay, J.A. Mechanism of Superoxide and Hydrogen Peroxide Formation by Fumarate Reductase, Succinate Dehydrogenase, and Aspartate Oxidase. J. Biol. Chem. 2002, 277, 42563–42571. [Google Scholar] [CrossRef] [Green Version]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Tzanov, T.; Costa, S.A.; Gübitz, G.M.; Cavaco-Paulo, A. Hydrogen Peroxide Generation with Immobilized Glucose Oxidase for Textile Bleaching. J. Biotechnol. 2002, 93, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of Hydrogen Peroxide (H2O2 ) Using a Colorimetric Sensor Based on Cellulose Nanowhiskers and Silver Nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Su, W.; Gu, B.; Xu, H.; Wu, C.; Yin, P.; Li, H.; Zhang, Y. A Dual-Signal Colorimetric and near-Infrared Fluorescence Probe for the Detection of Exogenous and Endogenous Hydrogen Peroxide in Living Cells. Sens. Actuators B Chem. 2019, 280, 120–128. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Liu, Y.; Wang, Y.; Tang, J. Catalase-Inorganic Hybrid Microflowers Modified Glassy Carbon Electrode for Amperometric Detection of Hydrogen Peroxide. Mater. Lett. 2019, 243, 9–12. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Wu, C.; Zhao, Y. A Doubly-Quenched Fluorescent Probe for Low-Background Detection of Mitochondrial H2O2. Anal. Chem. 2019, 91, 6902–6909. [Google Scholar] [CrossRef]
- Zheng, D.J.; Yang, Y.S.; Zhu, H.L. Recent Progress in the Development of Small-Molecule Fluorescent Probes for the Detection of Hydrogen Peroxide. TrAC Trends Anal. Chem. 2019, 118, 625–651. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, M.; Wang, P.; Gu, Y. FRET-Based Upconversion Nanoprobe Sensitized by Nd3+ for the Ratiometric Detection of Hydrogen Peroxide in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 7441–7449. [Google Scholar] [CrossRef] [PubMed]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bilan, D.S.; Pase, L.; Joosen, L.; Gorokhovatsky, A.Y.; Ermakova, Y.G.; Gadella, T.W.J.; Grabher, C.; Schultz, C.; Lukyanov, S.; Belousov, V.V. HyPer-3: A Genetically Encoded H2O2 Probe with Improved Performance for Ratiometric and Fluorescence Lifetime Imaging. ACS Chem. Biol. 2013, 8, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, Y.G.; Bilan, D.S.; Matlashov, M.E.; Mishina, N.M.; Markvicheva, K.N.; Subach, O.M.; Subach, F.V.; Bogeski, I.; Hoth, M.; Enikolopov, G.; et al. Red Fluorescent Genetically Encoded Indicator for Intracellular Hydrogen Peroxide. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Gutscher, M.; Sobotta, M.C.; Wabnitz, G.H.; Ballikaya, S.; Meyer, A.J.; Samstag, Y.; Dick, T.P. Proximity-Based Protein Thiol Oxidation by H2O2-Scavenging Peroxidases. J. Biol. Chem. 2009, 284, 31532–31540. [Google Scholar] [CrossRef] [Green Version]
- Enyedi, B.; Zana, M.; Donkó, Á.; Geiszt, M. Spatial and Temporal Analysis of NADPH Oxidase-Generated Hydrogen Peroxide Signals by Novel Fluorescent Reporter Proteins. Antioxid. Redox Signal. 2013, 19, 523–534. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [Green Version]
- Peskin, A.V.; Pace, P.E.; Behring, J.B.; Paton, L.N.; Soethoudt, M.; Bachschmid, M.M.; Winterbourn, C.C. Glutathionylation of the Active Site Cysteines of Peroxiredoxin 2 and Recycling by Glutaredoxin. J. Biol. Chem. 2016, 291, 3053–3062. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.; Van Laer, K.; Owusu, T.N.E.; Ezeriņa, D.; Pastor-Flores, D.; Amponsah, P.S.; Tursch, A.; Dick, T.P. Real-Time Monitoring of Basal H2O2 Levels with Peroxiredoxin-Based Probes. Nat. Chem. Biol. 2016, 12, 437–443. [Google Scholar] [CrossRef]
- Fan, Y.; Makar, M.; Wang, M.X.; Ai, H.W. Monitoring Thioredoxin Redox with a Genetically Encoded Red Fluorescent Biosensor. Nat. Chem. Biol. 2017, 13, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, F.M.; Yu, J.; Xiao-Xiao, M.; Yu, X.J.; Chen, Y.; Zhou, C.Z. Structural Snapshots of Yeast Alkyl Hydroperoxide Reductase Ahp1 Peroxiredoxin Reveal a Novel Two-Cysteine Mechanism of Electron Transfer to Eliminate Reactive Oxygen Species. J. Biol. Chem. 2012, 287, 17077–17087. [Google Scholar] [CrossRef] [Green Version]
- Kouwen, T.R.H.M.; Andréll, J.; Schrijver, R.; Dubois, J.Y.F.; Maher, M.J.; Iwata, S.; Carpenter, E.P.; van Dijl, J.M.; Thioredoxin, A. Active-Site Mutants Form Mixed Disulfide Dimers That Resemble Enzyme-Substrate Reaction Intermediates. J. Mol. Biol. 2008, 379, 520–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisabori, T.; Hara, S.; Fujii, T.; Yamazaki, D.; Hosoya-Matsuda, N.; Motohashi, K. Thioredoxin Affinity Chromatography: A Useful Method for Further Understanding the Thioredoxin Network. J. Exp. Bot. 2005, 56, 1463–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhou, Y.; Li, J.; Yu, H.; Takaya, N.; Wang, P.; Zhou, S. Novel Peroxiredoxin-Based Sensor for Sensitive Detection of Hydrogen Peroxide. Biochem. Biophys. Res. Commun. 2019, 517, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, Q.; Wang, Y.; Yuan, C.; Qin, X.; Xu, Y. Colorimetric Detection of Hydrogen Peroxide and Glucose by Exploiting the Peroxidase-like Activity of Papain. RSC Adv. 2019, 9, 16566–16570. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Miao, L.; Yu, L.; Chen, Q.; Qiao, Y.; Zhang, J.F.; Zhou, Y. A Near-Infrared Fluorescent Probe for Detection of Exogenous and Endogenous Hydrogen Peroxide in Vivo. Dye. Pigment. 2019, 168, 160–165. [Google Scholar] [CrossRef]
- Xiong, J.; Xia, L.; Li, L.; Cui, M.; Gu, Y.; Wang, P. An Acetate-Based NIR Fluorescent Probe for Selectively Imaging of Hydrogen Peroxide in Living Cells and in Vivo. Sens. Actuators B Chem. 2019, 288, 127–132. [Google Scholar] [CrossRef]
- Yu, X.; Gong, Y.; Xiong, W.; Li, M.; Zhao, J.; Che, Y. Turn-on Fluorescent Detection of Hydrogen Peroxide and Triacetone Triperoxide via Enhancing Interfacial Interactions of a Blended System. Anal. Chem. 2019, 91, 6967–6970. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Tian, C.; Yuan, M.S.; Li, Z.; Wang, W.; Li, T.; Chen, S.W.; Wang, J. Colorimetric Hydrazine Detection and Fluorescent Hydrogen Peroxide Imaging by Using a Multifunctional Chemical Probe. Anal. Chim. Acta 2019, 1052, 137–144. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Cirone, J.; Chen, A. Fluorescent Fe3O4 Quantum Dots for H2O2 Detection. ACS Appl. Nano Mater. 2019, 2, 2076–2085. [Google Scholar] [CrossRef]
- Ahmed, A.; Hayat, A.; Nawaz, M.H.; John, P.; Nasir, M. Construction of Sponge-like Graphitic Carbon Nitride and Silver Oxide Nanocomposite Probe for Highly Sensitive and Selective Turn-off Fluorometric Detection of Hydrogen Peroxide. J. Colloid Interface Sci. 2019, 558, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, Y.; Du, J. Bifunctional Gold Nanoclusters Enable Ratiometric Fluorescence Nanosensing of Hydrogen Peroxide and Glucose. Talanta 2019, 197, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, R.M.; El-Nagar, G.A.; Abouserie, A.; Roth, C. Silver-Iron Hierarchical Microflowers for Highly Efficient H2O2 Nonenzymatic Amperometric Detection. ACS Sustain. Chem. Eng. 2019, 7, 4335–4342. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Lan, L.; Zhang, C.; Pan, M.; Wang, Y.; Han, B.; Wang, Z.; Jiao, J.; Chen, Q. A Novel Catalase Mimicking Nanocomposite of Mn(II)-Poly-L-Histidine-Carboxylated Multi Walled Carbon Nanotubes and the Application to Hydrogen Peroxide Sensing. Anal. Biochem. 2019, 567, 51–62. [Google Scholar] [CrossRef]
- Riaz, M.A.; Zhai, S.; Wei, L.; Zhou, Z.; Yuan, Z.; Wang, Y.; Huang, Q.; Liao, X.; Chen, Y. Ultralow-Platinum-Loading Nanocarbon Hybrids for Highly Sensitive Hydrogen Peroxide Detection. Sens. Actuators B Chem. 2019, 283, 304–311. [Google Scholar] [CrossRef]
- Mani, V.; Shanthi, S.; Peng, T.K.; Lin, H.Y.; Ikeda, H.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C.; Huang, S.T. Real-Time Quantification of Hydrogen Peroxide Production in Living Cells Using NiCo2S4@CoS2 Heterostructure. Sens. Actuators B Chem. 2019, 287, 124–130. [Google Scholar] [CrossRef]
- Wang, P.; Cao, L.; Chen, Y.; Wu, Y.; Di, J. Photoelectrochemical Biosensor Based on Co3O4 Nanoenzyme Coupled with PbS Quantum Dots for Hydrogen Peroxide Detection. ACS Appl. Nano Mater. 2019, 2, 2204–2211. [Google Scholar] [CrossRef]

| Method | Detection Limit | Linear Range | Reference |
|---|---|---|---|
| Enzymatic colorimetric detection | 2.5 μM | 0.05–0.50 mM | [36] |
| Hybrid microflower enzymatic amperometric detection | 50 μM | 100 μM–100 mM | [19] |
| Small-molecule fluorescence detection | 5.3 μM | [37] | |
| Small-molecule fluorescence detection | 0.07 μM | 0.5–200 μM | [18] |
| Small-molecule fluorescence detection | 21 nM | 3–500 μM | [38] |
| Small-molecule fluorescence detection | 25 nM | 1–60 μM | [39] |
| Small-molecule fluorescence detection | 160 nM | 5–20 μM | [40] |
| Nanocomposite colorimetric detection | 14 nM | 0.01–30 μM | [17] |
| 112 μM | 60–600 μM | ||
| Nanocomposite ratiometric fluorescence Detection | 168 nM | 0.5–10 μM | [22] |
| Nanocomposite nonenzymatic colorimetric detection | 3.87 nM | 10 nM–10 mM | [41] |
| Nanocomposite nonenzymatic fluorescence detection | 22 nM | 30–300 nM | [42] |
| 11 nM | 30–110 nM | ||
| Nanocomposite nonenzymatic ratiometric fluorescence detection | 10 nM | 0.025–5.0 μM | [43] |
| Nanocomposite nonenzymatic amperometric detection | 1.6 μM | Up to 14 mM | [44] |
| 2.2 μM | Up to 15 mM | ||
| 0.1 μM | Up to 20 mM | ||
| Nanocomposite nonenzymatic amperometric detection | 0.5 μM | 0.002–1.0 mM | [45] |
| Nanocomposite nonenzymatic amperometric detection | 1 μM | 10 μM–15 mM | [46] |
| Nanocomposite nonenzymatic amperometric detection | 2 nM | 12.64 nM–2104 μM | [47] |
| Nanocomposite nonenzymatic photoelectrochemical detection | 1.2 μM | 5–250 μM | [48] |
| CFP-Prx/mTrx-YFP ratiometric fluorescence Detection | 4 nM | 10–320 nM | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, H.; Zhou, Y.; Zhou, S.; Wang, P. Ultra-Sensitive Hydrogen Peroxide Sensor Based on Peroxiredoxin and Fluorescence Resonance Energy Transfer. Appl. Sci. 2020, 10, 3508. https://doi.org/10.3390/app10103508
Yu H, Li H, Zhou Y, Zhou S, Wang P. Ultra-Sensitive Hydrogen Peroxide Sensor Based on Peroxiredoxin and Fluorescence Resonance Energy Transfer. Applied Sciences. 2020; 10(10):3508. https://doi.org/10.3390/app10103508
Chicago/Turabian StyleYu, Haijun, Haoxiang Li, Yao Zhou, Shengmin Zhou, and Ping Wang. 2020. "Ultra-Sensitive Hydrogen Peroxide Sensor Based on Peroxiredoxin and Fluorescence Resonance Energy Transfer" Applied Sciences 10, no. 10: 3508. https://doi.org/10.3390/app10103508
APA StyleYu, H., Li, H., Zhou, Y., Zhou, S., & Wang, P. (2020). Ultra-Sensitive Hydrogen Peroxide Sensor Based on Peroxiredoxin and Fluorescence Resonance Energy Transfer. Applied Sciences, 10(10), 3508. https://doi.org/10.3390/app10103508




