Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression?
Abstract
:1. Introduction
2. Arachidonic Acid Sources
3. Sphingolipids and Sphingomyelinase Role in Depression
4. Mast Cells and Depression
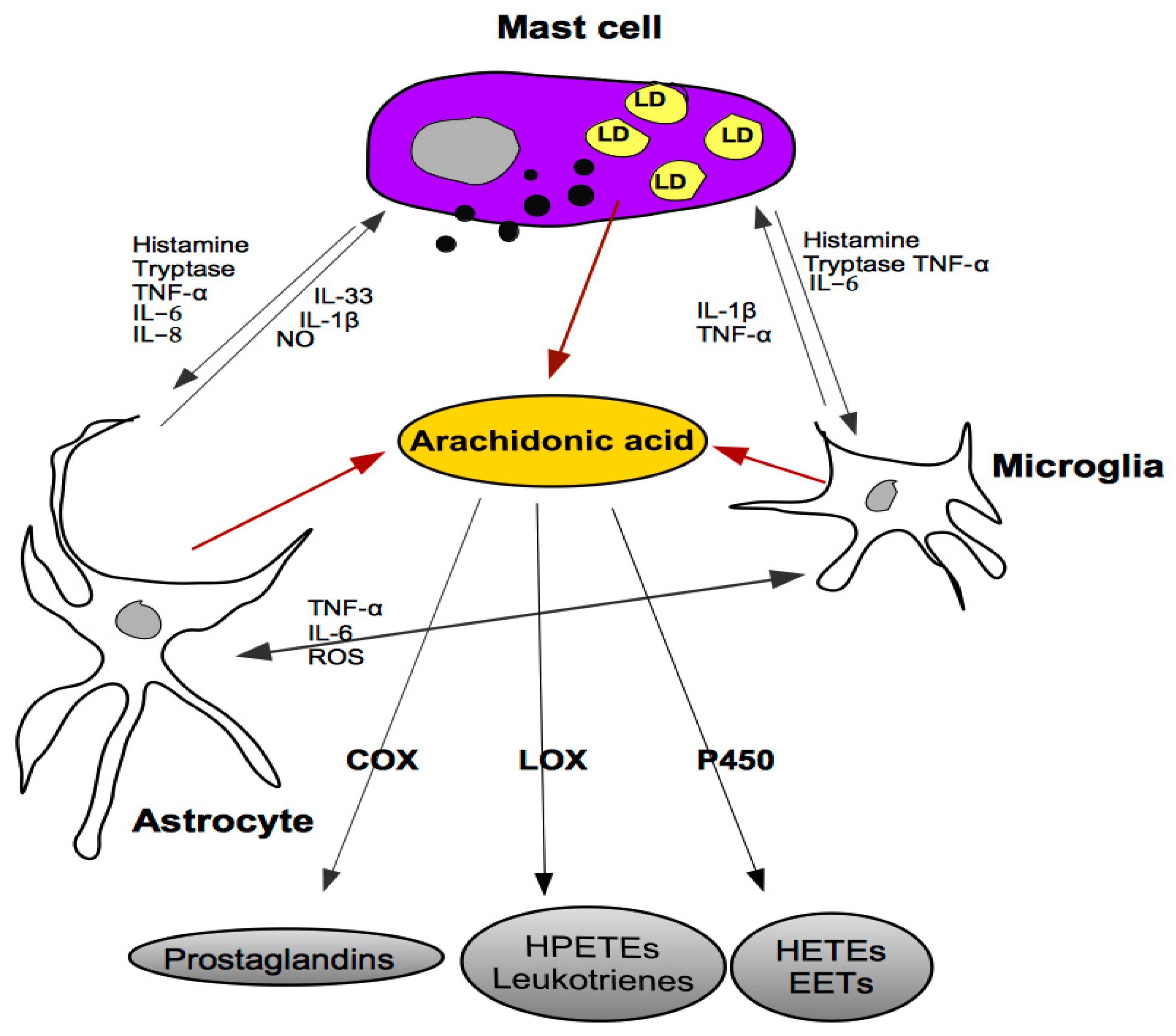
5. Cross-Talk between Mast Cells, Astrocytes and Microglia
6. Serotonin and Depression
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| BD | bipolar disorder |
| CNS | central nervous system |
| COX | cyclo-oxygenase |
| DHA | docosahexaenoic acid |
| EC | endocrine cell |
| 5-HT | 5-hydroxytryptamine, serotonin |
| HETE | hydroxyeicosatetraenoic acid |
| LA | linoleic acid |
| LD | lipid droplet |
| LOX | lipooxygenase |
| MC | mast cell |
| MD | major depression |
| PA | palmitic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PGE | prostaglandin |
| PL | phospholipid |
| PLA2 | phospholipase A2 |
| PUFA | polyunsaturated fatty acid |
| SCFAs | short-chain fatty acids |
| SP | substance |
| TG | triglyceride |
References
- World Health Organization. World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hauser, W.; Janke, K.H.; Klump, B.; Hinz, A. Anxiety and depression in patients with inflammatory bowel disease: Comparison with chronic liver disease patients and the general population. Inflamm. Bowel Dis. 2011, 17, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Tonello, L. Running the hypothesis of a bio molecular approach to psychiatric disorder characterization and fatty acids therapeutical choices. Ann. Gen. Psychiatry 2010, 9 (Suppl. S1). [Google Scholar] [CrossRef] [Green Version]
- Benedetti, S.; Bucciarelli, S.; Canestrari, F.; Catalani, S.; Mandolini, S.; Marconi, V.; Mastrogiacomo, A.; Silvestri, R.; Tagliamonte, M.; Venanzini, R.; et al. Platelet’s Fatty Acids and Differential Diagnosis of Major Depression and Bipolar Disorder through the Use of an Unsupervised Competitive- Learning Network Algorithm (SOM). Open J. Depress. 2014, 3, 52–73. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, M.; Tonello, L.; Rasenick, M. Human depression: A new approach in quantitative psychiatry. Ann. Gen. Psychiatry 2010, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagini, G.; Pich, E.M.; Carani, C.; Marrama, P.; Gustafsson, J.A.; Fuxe, K.; Agnati, L.F. Indole-pyruvic acid, a tryptophan ketoanalogue, antagonizes the endocrine but not the behavioral effects of repeated stress in a model of depression. Biol. Psychiatry 1993, 33, 712–719. [Google Scholar] [CrossRef]
- Agnati, L.F.; Cortelli, P.; Biagini, G.; Bjelke, B.; Fuxe, K. Different classes of volume transmission signals exist in the central nervous system and are affected by metabolic signals, temperature gradients and pressure waves. Neuroreport 1994, 6, 9–12. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Sable, J.E.; Döbereiner, H.-G. Continuous Membrane-Cytoskeleton Adhesion Requires Continuous Accommodation to Lipid and Cytoskeleton Dynamics. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 417–434. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Gabrielli, F. Possible Roles of Cell Membrane & Cytoskeleton in Quantum Aspect of Psychiatry. J. Conscious. Explor. Res. 2012, 3, 1082–1100. [Google Scholar]
- Müller, C.P.; Reichel, M.; Muhle, C.; Rhein, C. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, M.; Tonello, L. Bio molecular considerations in Major Depression and Ischemic Cardiovascular Disease. CNS Agents Med. Chem. 2010, 9, 2–11. [Google Scholar] [CrossRef]
- Goodhand, J.R.; Wahed, M.; Mawdsley, J.E.; Farmer, A.D.; Aziz, Q.; Rampton, D.S. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel Dis. 2012, 18, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Lee, H.-J.; Rapoport, S.I.; Bazinet, R.P. Mode of action of mood stabilizers: Is the arachidonic acid cascate a common target? Mol. Psychiatry 2008, 13, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heron, D.S.; Shinitzky, M.; Hershkowitz, M.; Samuel, D. Lipid Fluidity Markedly Modulates the Binding of Serotonin to Mouse Brain Membranes. Proc. Natl. Acad. Sci. USA 1980, 77, 7463–7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocchi, M.; Gabrielli, F.; Tonello, L.; Pregnolato, M. Interactome hypthesis of Depression. NeuroQuantology 2010, 4, 603–613. [Google Scholar]
- Cocchi, M.; Minuto, C.; Tonello, L.; Gabrielli, F.; Bernroider, G.; Tuszynski, J.A.; Cappello, F.; Rasenick, M. Linoleic acid: Is this the key that unlocks the quantum brain? Insights linking broken symmetries in molecular biology, mood disorders and personalistic emergentism. BMC Neurosci. 2017, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, F.; Nishinaka, T.; Yamashita, T.; Nakamoto, K.; Koyama, Y.; Kasuya, F.; Tokuyama, S. Astrocytes release polyunsatured fatty acids by lipopolysaccharide stimuli. Biol. Pharm. Bull. 2016, 39, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, L.S. Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, and Other Derivatives of Carbon-20 Unsaturated Fatty Acids. J. Neurochem. 1982, 38, 1–14. [Google Scholar] [CrossRef]
- Piomelli, D.; Volterra, A.; Dale, N.; Siegelbaum, S.A.; Kandel, E.R.; Schwartz, J.H.; Belardetti, F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature 1987, 328, 38–43. [Google Scholar] [CrossRef]
- Su, K.-P.; Huang, S.-Y.; Peng, C.-Y.; Lai, H.-C.; Huang, C.-L.; Chen, Y.-C.; Aitchison, K.J.; Pariante, C.M. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsatured fatty acids levels. Biol. Psychiatry 2010, 67, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Manev, R.; Manev, H. 5-Lipoxygenase as a putative link between cardiovascular and psychiatric disorders. Critic Rev. Neurobiol. 2004, 16, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Manev, R.; Mrazovac, D.; Manev, H. Possible role for interactions between 5-lipoxygenase (5-LOX) and AMPA GluR1 receptors in depression and in antidepressant therapy. Med. Hypotheses 2007, 69, 1076–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoport, S.I. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem. Neurosci. 2014, 5, 459–467. [Google Scholar] [CrossRef]
- Matsuo, M.; Hamasaki, Y.; Fujiyama, F.; Miyazaki, S. Eicosanoids are produced by microglia, not by astrocytes, in rat glial cell cultures. Brain Res. 1995, 685, 201–204. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Ong, W.Y.; Horrocks, L.A. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neu- rologic disorders. Pharmacol. Rev. 2006, 58, 591–620. [Google Scholar] [CrossRef] [Green Version]
- Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. [Google Scholar] [CrossRef]
- Faridhosseini, F.; Sadeghi, R.; Farid, L.; Pourgholami, M. Celecoxib: A new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum. Psychopharmacol. 2014, 29, 216–223. [Google Scholar] [CrossRef]
- Eyre, H.A.; Air, T.; Proctor, S.; Rositano, S.; Baune, B.T. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 11–16. [Google Scholar] [CrossRef]
- Johansson, D.; Falk, A.; Marcus, M.M.; Svensson, T.H. Celecoxib enhances the effect of reboxetine and fluoxetine on cortical noradrenaline and serotonin output in the rat. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 39, 143–148. [Google Scholar] [CrossRef]
- Kornhuber, J.; Medlin, A.; Bleich, S.; Jendrossek, V.; Henkel, A.W.; Wiltfang, J.; Gulbins, E. High activity of acid sphingomyelinase in major depression. J. Neural Transm. 2005, 112, 1583–1590. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Baldi, E.; Bucherelli, C.; Ferri, I.; Sidoni, A.; Codini, M.; Conte, C.; Beccari, T.; Traina, G. Vitamin D receptor expression and acid sphingomyelinase activity in prefrontal region of a learning anial model. Arch. Ital. Biol. 2019, 157, 120–128. [Google Scholar] [PubMed]
- Cataldi, S.; Arcuri, C.; Hunot, S.; Légeron, F.P.; Mecca, C.; Garcia-Gil, M.; Lazzarini, A.; Codini, M.; Beccari, T.; Tasegian, A.; et al. Neutral Sphingomyelinase Behaviour in Hippocampus Neuroinflammation of MPTP-Induced Mouse Model of Parkinson’s Disease and in Embryonic Hippocampal Cells. Mediators Inflamm. 2017, 2017, 2470950. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Murayama, T. The role of sphingolipids in arachidonic acid metabolism. J. Pharmacol. Sci. 2014, 124, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, M.P.; Arrondo, J.L.R.; Goni, F.M.; Alonso, A.; Marsh, D. Interaction of cholesterol with sphingomyelin in mixed membrane containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry 2001, 40, 2614–2622. [Google Scholar] [CrossRef]
- Donati, R.J.; Dwivedi, Y.; Roberts, R.C.; Conley, R.R.; Pandey, G.N.; Rasenick, M.M. Postmortem brain tissue of depressed suicides reveals increased Gs localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J. Neurosci. 2008, 28, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Mühle, C.; Wagner, C.J.; Farber, K.; Richter-Schmidinger, T.; Gulbins, E.; Lenz, B.; Kornhuber, J. Secretory sphingomyelinase in the serum of medicated patients predicts the prospective course of depression. J. Clin. Med. 2019, 8, 846. [Google Scholar] [CrossRef] [Green Version]
- Bryan, P.F.; Karla, C.; Edgar Alejandro, M.T.; Sara Elva, E.P.; Gemma, F.; Luz, C. Sphingolipids as Mediators in the Crosstalk between Microbiota and Intestinal Cells: Implications for Inflammatory Bowel Disease [published correction appears in Mediators Inflamm. 2016;2016:7267956]. Mediat. Inflamm. 2016, 2016, 9890141. [Google Scholar]
- An, D.; Na, C.; Bielawski, J.; Hannun, Y.A.; Kasper, D.L. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4666–4671. [Google Scholar] [CrossRef] [Green Version]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the role of the microbiome in major depressive disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar]
- Kim, Y.K.; Shin, C. The microbiota-gut-brain axis in neuropsychiatric disorders: Pathophysiological and novel treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.S.; Sultan, S.; Georgin-Lavialle, S.; Pillet, N.; Montestruc, F.; Gineste, P.; Barete, S.; Damaj, G.; Moussy, A.; Lortholary, O.; et al. Depression in patients with mastocytosis: Prevalence, features and effects of masitinib therapy. PLoS ONE 2011, 6, e26375. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, E.; van Brgeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Traina, G. Mast cells in the brain- Old cells, new target. J. Integr. Neurosci. 2017, 16, S69–S83. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front. Cell. Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Irmak, D.K.; Kilinc, E.; Tore, F. Shared fate of meningeal mast cells and sensory neurons in migraine. Front. Cell Neurosci. 2019, 13, 136. [Google Scholar] [CrossRef] [Green Version]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef]
- Conte, C.; Sichetti, M.; Traina, G. Gut-brain axis: Focus on neurodegeneration and mast cells. Appl. Sci. 2020, 10, 1828. [Google Scholar] [CrossRef] [Green Version]
- Dichlberger, A.; Kovanen, P.T.; Schneider, J. Mast cells: From lipid droplets to lipid mediators. Clin. Sci. 2013, 125, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Dichlberger, D.; Schlager, S.; Kovanen, P.T.; Schneider, W.J. Lipid droplets in activated mast cells—A significant source of triglyceride-derived arachidonic acid for eicosanoid production. Eur. J. Pharmacol. 2016, 785, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; Dvorak, H.F.; Peters, S.P.; Shulman, E.S.; MacGlashan, D.W., Jr.; Pyne, K.; Harvey, V.S.; Galli, S.J.; Lichtenstein, L.M. Lipid bodies: Cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J. Immunol. 1983, 131, 2965–2976. [Google Scholar] [PubMed]
- Bozza, P.T.; Magalhães, K.G.; Weller, P.F. Leukocyte lipid bodies—Biogenesis and functions in inflammation. Biochim. Biophys. Acta 2009, 1791, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukawa, T.; Izawa, K.; Isobe, M.; Takahashi, M.; Maehara, A.; Yamanishi, Y.; Kaitani, A.; Okumura, K.; Teshima, T.; Kitamura, T.; et al. Ceramide-CD300f binding suppresses experimental colitis by inhibiting ATP-mediated mast cell activation. Gut 2016, 65, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.M. Adult rat brain astrocytes support survival of both NGF- dependent and NGF-insensitive neurones. Nature 1979, 282, 80–82. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Q.; Xie, L.; Yang, F.; Wang, L.; Tu, J. Astrocyte, a Promising Target for Mood Disorder Interventions. Front. Mol. Neurosci. 2019, 12, 136. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Eroglu, C. The interplay between neurons and glia in synapse development and plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.A.; Yoder, E.; Murphy, S.; Dutton, G.R.; Spector, A.A. Astrocytes, not neurons, produce docosahexaenoic acid (22:6ω-3) and Arachidonic Acid (20:4ω-6). J. Neurochem. 1991, 56, 518–524. [Google Scholar] [CrossRef]
- Stella, N.; Estelles, A.; Siciliano, J.; Tencé, M.; Desagher, S.; piomelli, D.; Glowinski, J.; Prémont, J. Interleukin-1 enhances the ATP-evoked release of arachidonic acid from mouse astrocytes. J. Neurosci. 1997, 17, 2939–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.C.; Kim, H.W.; Rapoport, S.I.; Rao, J.S. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: Cross-talk between excitotoxicity and neuroinflammation. Neurochem. Res. 2008, 33, 2318–2323. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Reiche, E.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple Immune-Inflammatory and Oxidative and Nitrosative Stress Pathways Explain the Frequent Presence of Depression in Multiple Sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.F.; Fa, F.; Li, X.; Zhang, Q.Q.; Ding, J.; Wang, X. Different behavioral and pathological changes between epilepsy-associated depression and primary depression models. Epilepsy Behav. 2018, 83, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ruhé, H.G.; Mason, N.S.; Schene, A.H. Mood is indeirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef] [Green Version]
- Gabbay, V.; Ely, B.A.; Babb, J.; Liebes, L. The possible role of the kynurenine pathway in anhedonia in adolescents. J. Neural Transm. 2012, 119, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Schiepers, O.J.G.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenter. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Kushnir-Sukhov, N.M.; Gilfillan, A.M.; Coleman, J.W.; Brown, J.M.; Bruening, S.; Toth, M.; Metcalfe, D.D. 5-ydroxytryptamine Induces Mast Cell Adhesion and Migration. J. Immunol. 2006, 177, 6422–6432. [Google Scholar] [CrossRef] [Green Version]
- Waclawiková, B.; El Aidy, S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felder, C.C.; Kanterman, R.Y.; Ma, A.L.; Axelrod, J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc. Natl. Acad. Sci. USA 1990, 87, 2187–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, R.F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem. J. 1982, 204, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarsi, S.; GarciaGil, M.; Traina, G.; Brunelli, M. Seasonal-variation of serotonin content and nonassociative learning of swim induction in the leech Hirudo medicinalis. J. Comp. Physiol. A 1990, 167, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.H.; Chen, M.; Keller, F.; Kandel, E.R. Serotonin-mediated endocytosis of apCAM: An early step of learning-related synaptic growth in Aplysia. Science 1992, 256, 645–649. [Google Scholar] [CrossRef]
- Zaccardi, M.L.; Traina, G.; Cataldo, E.; Brunelli, M. Sensitization and dishabituation of swim induction in the leech Hirudo medicinalis: Role of serotonin and cyclic AMP. Behav. Brain Res. 2004, 153, 317–326. [Google Scholar] [CrossRef]
- Zaccardi, M.L.; Mozzachiodi, R.; Traina, G.; Brunelli, M.; Scuri, R. Molecular mechanisms of short-term habituation in the leech Hirudo medicinalis. Behav. Brain Res. 2012, 229, 235–243. [Google Scholar] [CrossRef]
- Traina, G.; Ristori, C.; Brunelli, M.; Scuri, R. Acetyl-l-carnitine prevents serotonin-induced behavioural sensitization and dishabituation in Hirudo medicinalis. Behav. Brain Res. 2013, 253, 323–328. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity- Links beetween molecular, functional and structural pathphysiology in depression. Neurosci. Behav. Res. 2017, 77, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Ostojic, P.; Zivojinovic, S.; Reza, T.; Milivojevic, D.; Damjanov, N. Symptoms of depression and anxiety in Serbian patients with systemic sclerosis: Impact of disease severity and socioeconomic factors. Mod. Rheumatol. 2010, 20, 353–357. [Google Scholar] [CrossRef]
- Kurina, L.; Goldacre, M.; Yeates, D.; Gill, L. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Comm. Health 2001, 55, 716–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graff, L.A.; Walker, J.R.; Bernstein, C.N. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm. Bowel Dis. 2009, 15, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Tonello, L.; Lercker, G. Fatty acids, membrane viscosity, serotonin and ischemic heart disease. Lipids Health Dis. 2010, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bab, I.A.; Yirmiya, R. Depression and bone mass. Ann. N. Y. Acad. Sci. 2010, 1192, 170–175. [Google Scholar] [CrossRef]
- Bester, J.; Pretorious, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Tonello, L.; Amato, P.; De Lucia, A. Platelet and Brain Fatty acid transfer: Hypothesis on Arachidonic Acid and its relationship to Major Depression. J. Biol. Res. 2009, 82, 47–53. [Google Scholar] [CrossRef]
- Tonello, L.; Cocchi, M. The Cell Membrane: Is it a bridge from psychiatry to quantum consciousness? NeuroQuantology 2010, 1, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Tonello, L.; Cocchi, M.; Gabrielli, F.; Tuszynski, J.A. On the possible quantum role of serotonin in consciousness. J. Integr. Neurosci. 2015, 14, 295. [Google Scholar] [CrossRef]
- Cocchi, M.; Bernroider, G.; Rasenick, M.; Tonello, L.; Gabrielli, F.; Tuszynski, J.A. Document of Trapani on animal consciousness and quantum brain function: A hypothesis. J. Integr. Neurosci. 2017, 16, 1–5. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Gabrielli, F. Linoleic Acid: Fine tuning regulator of mood disorders? In Proceedings of the 13th Conference of Italian Researchers in the World, Dallas, TX, USA, 1 December 2018. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traina, G.; Cocchi, M. Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression? Appl. Sci. 2020, 10, 3455. https://doi.org/10.3390/app10103455
Traina G, Cocchi M. Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression? Applied Sciences. 2020; 10(10):3455. https://doi.org/10.3390/app10103455
Chicago/Turabian StyleTraina, Giovanna, and Massimo Cocchi. 2020. "Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression?" Applied Sciences 10, no. 10: 3455. https://doi.org/10.3390/app10103455
APA StyleTraina, G., & Cocchi, M. (2020). Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression? Applied Sciences, 10(10), 3455. https://doi.org/10.3390/app10103455





