From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems
Abstract
:1. Introduction
2. Pharmaceuticals and Personal Care Products (PPCPs) in the Ecosystems
2.1. Microbial Remediation in Culture
2.1.1. Bioremediation or Bacterial Remediation
2.1.2. Mycoremediation
2.1.3. Phycoremediation
2.1.4. Mixed Culture
2.2. Phytoremediation
2.3. Combined Chemical Engineering-Biological Methods
2.3.1. Bioreactors
- Conventional activated sludge
- Membrane bioreactors
- Photobioreactors
2.3.2. Constructed Wetlands
3. Ecotoxicology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| PPCPs | pharmaceuticals and personal care products |
| SDG | sustainable development goal |
| WWTP | Wastewater treatment plants |
| ROS | reactive oxygen species |
| UV | ultraviolet |
| CSCM | Circular supply chain management |
| DFC | diclofenac |
| EST | estradiol |
| CBZ | carbamazepine |
| IBU | ibuprofen |
| NPX | naproxen |
| CDN | codeine |
| SMX | sulfamethoxazole |
| CFN | caffeine |
| GFZ | gemfibrozil |
| TCS | triclosan |
| NP | nonylphenol |
| ATN | artemisine |
| FLU | flumequine |
| E2α | 17α-ethinylestradiol |
| MBR | membrane bioreactors |
| HRT | hydraulic retention time |
| HRAP | high rate algal pond |
| CWs | constructed wetlands |
| WRF | White rot fungi |
| AChE | Acetylcholinesterase |
| EROD | Ethoxyresorufin-O-deethylase |
References
- Hanski, I. Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 1999, 87, 209. [Google Scholar] [CrossRef]
- Quereda Sala, J.; Gil Olcina, A.; Perez Cuevas, A.; Olcina Cantos, J.; Rico Amoros, A.; Montón Chiva, E. Climatic warming in the Spanish Mediterranean: Natural trend or urban effect. Clim. Chang. 2000, 46, 473–483. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Schmeller, D.S.; Loyau, A.; Bao, K.; Brack, W.; Chatzinotas, A.; De Vleeschouwer, F.; Friesen, J.; Gandois, L.; Hansson, S.V.; Haver, M.; et al. People, pollution and pathogens – Global change impacts in mountain freshwater ecosystems. Sci. Total Environ. 2018, 622, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Leston, S.; Nunes, M.; Viegas, I.; Nebot, C.; Cepeda, A.; Pardal, M.Â.; Ramos, F. The influence of sulfathiazole on the macroalgae ulva lactuca. Chemosphere 2014, 100, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Roy, K. Risk assessment for ecotoxicity of pharmaceuticals an emerging issue. Expert Opin. Drug Saf. 2012, 11, 235–274. [Google Scholar] [CrossRef]
- Zwiener, C. Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal. Bioanal. Chem. 2007, 387, 1159–1162. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Esteban, S.; Moreno-Merino, L.; Matellanes, R.; Catalá, M.; Gorga, M.; Petrovic, M.; López de Alda, M.; Barceló, D.; Silva, A.; Durán, J.J.; et al. Presence of endocrine disruptors in freshwater in the northern Antarctic Peninsula region. Environ. Res. 2016, 147, 179–192. [Google Scholar] [CrossRef]
- González-Alonso, S.; Merino, L.M.; Esteban, S.; López de Alda, M.; Barceló, D.; Durán, J.J.; López-Martínez, J.; Aceña, J.; Pérez, S.; Mastroianni, N.; et al. Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environ. Pollut. 2017, 229, 241–254. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Wheeler, A.P.; James, A.R.; Roland, S.; Summer, W.R.; Kenneth, P.S.; Fulkerson, W.J.; Patrick, E.W.; Christman, B.; William, D.D.; et al. The Effects of Ibuprofen on the Physiology and Survival of Patients with Sepsis. N. Engl. J. Med. 1997, 152, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Musmarra, D.; Prisciandaro, M.; Capocelli, M.; Karatza, D.; Iovino, P.; Canzano, S.; Lancia, A. Degradation of ibuprofen by hydrodynamic cavitation: Reaction pathways and effect of operational parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar] [CrossRef]
- García-Cambero, J.P.; García-Cortés, H.; Valcárcel, Y.; Catalá, M. Environmental concentrations of the cocaine metabolite benzoylecgonine induced sublethal toxicity in the development of plants but not in a zebrafish embryo-larval model. J. Hazard. Mater. 2015, 300, 866–872. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Kincaid, D.W.; Bechtold, H.A.; Royer, T.V.; Rojas, M.; Kelly, J.J. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol. Appl. 2013, 23, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Paspalof, A.M.; Snow, D.D.; Richmond, E.K.; Rosi-Marshall, E.J.; Kelly, J.J. Occurrence and potential biological effects of amphetamine on stream communities. Environ. Sci. Technol. 2016, 50, 9727–9735. [Google Scholar] [CrossRef]
- Catalá, M.; Gasulla, F.; Pradas Del Real, A.E.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. The organic air pollutant cumene hydroperoxide interferes with NO antioxidant role in rehydrating lichen. Environ. Pollut. 2013, 179, 277–284. [Google Scholar] [CrossRef]
- Domínguez-Morueco, N.; Moreno, H.; Barreno, E.; Catalá, M. Preliminary assessment of terrestrial microalgae isolated from lichens as testing species for environmental monitoring: lichen phycobionts present high sensitivity to environmental micropollutants. Ecotoxicol. Environ. Saf. 2014, 99, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, B.; Jiang, X.; Yang, Y.; Wells, G.F.; Zhang, T.; Li, X. Antibiotic resistome in a large-scale healthy human gut microbiota deciphered. Environ. Microbiol. 2013, 15, 3119–3120. [Google Scholar]
- Franklin, A.M.; Williams, C.F.; Andrews, D.M.; Woodward, E.E.; Watson, J.E. Uptake of Three Antibiotics and an Antiepileptic Drug by Wheat Crops Spray Irrigated with Wastewater Treatment Plant Effluent. J. Environ. Qual. 2015, 45, 546. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Garcia, A.; Riquelme, A.; Otero, W.; Camargo, C.A.; Hernandez-Garcia, T.; Candia, R.; Bruce, M.G.; Rabkin, C.S. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am. J. Gastroenterol. 2014, 109, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Y.; Xia, Z.; Wang, Y.; Wu, Y.; Gong, Z. Rapid determination of phytosterols by NIRS and chemometric methods. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2019, 211, 336–341. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Q.; Sun, F.; Zhang, L. Ecotoxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.). J. Hazard. Mater. 2009, 169, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gil, J.L.; San Sebastián Sauto, J.; González-Alonso, S.; Sánchez Sanchez, P.; Valcarcel, Y.; Catalá, M. Development of cost-effective strategies for environmental monitoring of irrigated areas in Mediterranean regions: Traditional and new approaches in a changing world. Agric. Ecosyst. Environ. 2013, 181, 41–49. [Google Scholar] [CrossRef]
- Feito, R.; Valcárcel, Y.; Catalá, M. Biomarker assessment of toxicity with miniaturised bioassays: Diclofenac as a case study. Ecotoxicology 2012, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Feito, R.; Valcárcel, Y.; Catalá, M. Preliminary data suggest that venlafaxine environmental concentrations could be toxic to plants. Chemosphere 2013, 90, 2065–2069. [Google Scholar] [CrossRef]
- Esteban, S.; Llamas, P.M.M.; García-Cortés, H.; Catalá, M. The endocrine disruptor nonylphenol induces sublethal toxicity in vascular plant development at environmental concentrations: A risk for riparian plants and irrigated crops? Environ. Pollut. 2016, 216, 480–486. [Google Scholar] [CrossRef]
- Naidoo, V.; Wolter, K.; Cuthbert, R.; Duncan, N. Veterinary diclofenac threatens Africa’s endangered vulture species. Regul. Toxicol. Pharmacol. 2009, 53, 205–208. [Google Scholar] [CrossRef]
- Ashraf, S.; Naveed, M.; Zahir, Z.A.; Afzal, M.; Rehman, K. Plant-endophyte synergism in constructed wetlands enhances the remediation of tannery effluent. Water Sci. Technol. 2018, 77, 1262–1270. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Jamshed, M.; Chaudhry, I.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Velevski, M.; Nikolov, S.C.; Hallmann, B.; Dobrev, V.; Sidiropoulos, L.; Saravia, V.; Tsiakiris, R.; Arkumarev, V.; Galanaki, A.; Kominos, T.; et al. Population decline and range contraction of the Egyptian Vulture Neophron percnopterus in the Balkan Peninsula. Bird Conserv. Int. 2015, 25, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, K.; Deng, Y.; Zhao, Y.; Wu, B.; Xu, K.; Ren, H. Evaluation of the toxic effects of municipal wastewater effluent on mice using omic approaches. Environ. Sci. Technol. 2013, 47, 9470–9477. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Ortiz de García, S.A.; Pinto Pinto, G.; García-Encina, P.A.; Irusta-Mata, R. Ecotoxicity and environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants. Ecotoxicology 2014, 23, 1517–1533. [Google Scholar] [CrossRef]
- Ortiz de García, S.; García-Encina, P.A.; Irusta-Mata, R. Dose–response behavior of the bacterium Vibrio fischeri exposed to pharmaceuticals and personal care products. Ecotoxicology 2016, 25, 141–162. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Wang, S.; Zhu, Z.; Zhao, F.; Liu, F. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ. Pollut. 2018, 246, 509–517. [Google Scholar]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Molina, M.C.; González, N.; Bautista, L.F.; Sanz, R.; Simarro, R.; Sánchez, I.; Sanz, J.L. Isolation and genetic identification of PAH degrading bacteria from a microbial consortium. Biodegradation 2009, 20, 789–800. [Google Scholar] [CrossRef]
- Marugán, J.; Bru, D.; Pablos, C.; Catalá, M. Comparative evaluation of acute toxicity by Vibrio fischeri and fern spore based bioassays in the follow-up of toxic chemicals degradation by photocatalysis. J. Hazard. Mater. 2012, 213–214, 117–122. [Google Scholar]
- Khunjar, W.O.; Mackintosh, S.A.; Baik, S.; Aga, D.S.; Love, N.G. Elucidating the Relative Roles of Ammonia Oxidizing and Heterotrophic Bacteria during the Biotransformation of 17alpha-Ethinylestradiol and Trimethoprim. Environ. Sci. Technol. 2011, 45, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Domaradzka, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Cometabolic Degradation of Naproxen by Planococcus sp. Strain S5. Water Air Soil Pollut. 2015, 226, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, P.M.; Jaramillo, J.; López-Piñero, F.; Plucinski, P.K. Preparation and characterization of magnetic TiO2 nanoparticles and their utilization for the degradation of emerging pollutants in water. Appl. Catal. B Environ. 2010, 100, 338–345. [Google Scholar] [CrossRef]
- Grenni, P.; Patrolecco, L.; Ademollo, N.; Di Lenola, M.; Barra Caracciolo, A. Capability of the natural microbial community in a river water ecosystem to degrade the drug naproxen. Environ. Sci. Pollut. Res. 2014, 21, 13470–13479. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.; Durán, N.; Rubilar, O.; Parada, M.; Diez, M.C. Are white-rot fungi a real biotechnological option for the improvement of environmental health? Crit. Rev. Biotechnol. 2015, 35, 165–172. [Google Scholar] [CrossRef]
- Farooque, M.; Zhang, A.; Thürer, M.; Qu, T.; Huisingh, D. Circular supply chain management: A definition and structured literature review. J. Clean. Prod. 2019, 228, 882–900. [Google Scholar] [CrossRef]
- Glavan, M.; Ojstršek Zorčič, P.; Pintar, M. A tool for the selection and implementation of eco-remediation mitigation measures. Ecol. Eng. 2019, 130, 53–66. [Google Scholar] [CrossRef]
- Lahiri, S.; Ghosh, D.; Bhakta, J.N. Role of Microbes in Eco-Remediation of Perturbed Aquatic Ecosystem. Oceanogr. Coast. Informatics 2018, 25–61. [Google Scholar] [CrossRef]
- Svirčev, Z.; Krstić, S.; Vazić, T. The phylosophy and applicability of ecoremediations for the protection of water ecosystems. Acta Geogr. Slov. 2014, 54, 179–188. [Google Scholar] [CrossRef] [Green Version]
- El Bakouri, H.; Morillo, J.; Usero, J.; Ouassini, A. Potential use of organic waste substances as an ecological technique to reduce pesticide ground water contamination. J. Hydrol. 2008, 353, 335–342. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xiao, E.Y.; Rengel, Z. Phytoremediation facilitates removal of nitrogen and phosphorus from eutrophicated water and release from sediment. Environ. Monit. Assess. 2009, 157, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Du, L.N.; Zou, Y.; Li, Y.H. Eco-remediation of branch river in plain river-net at estuary area. Procedia Environ. Sci. 2011, 10, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Du, S.; Fan, L.; Lin, X.; Wang, H.; Zhang, Y. Microbial activity facilitates phosphorus adsorption to shallow lake sediment. J. Soils Sediments 2011, 11, 185–193. [Google Scholar] [CrossRef]
- Sun, J.H.; Li, Y.; Yang, Y.J.; Cui, P.; Cheng, Z.Y.; Qiao, X.T.; Xu, Y.J. Developing a way to select plants for eutrophication eco-remediation by their nutrient uptake and growth kinetics characteristics. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 022070. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yanagi, T.; Yamada, M.; Suzuki, M. A challenge of water purification in Dokai Bay, Japan. Mar. Pollut. Bull. 1999, 38, 1063–1069. [Google Scholar] [CrossRef]
- Hong, J.; Yu, Z.; Fu, X.; Hong, J. Life cycle environmental and economic assessment of coal seam gas-based electricity generation. Int. J. Life Cycle Assess. 2019, 24, 1828–1839. [Google Scholar] [CrossRef]
- Cottin, N.; Merlin, G. Fate of chlorinated benzenes in laboratory peat and pozzolana filters. Water Air Soil Pollut. 2010, 213, 425–435. [Google Scholar] [CrossRef]
- Bozic, M.; Nikolic, G.; Rudic, Z.; Raicevic, V.; Lalevic, B. Constructed wetlands as an alternative restoration measure for shallow lakes. Water Sci. Technol. 2013, 68, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Huang, Y.; Pan, R.; Wang, F.; Wang, H. Effect of eco-remediation using planted floating bed system on nutrients and heavy metals in urban river water and sediment: A field study in China. Sci. Total Environ. 2014, 485–486, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Sasmal, K.; Sinha, S.S.; Singh, M. Analysis of cytotoxicity and genotoxicity on E. coli, human blood cells and Allium cepa suggests a greater toxic potential of hair dye. Ecotoxicol. Environ. Saf. 2016, 124, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Martinez Gomez, D.A.; Baca, S.; Walsh, E.J. Lethal and sublethal effects of selected PPCPs on the freshwater rotifer, Plationus patulus. Environ. Toxicol. Chem. 2015, 34, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Maszkowska, J.; Białk-Bielińska, A.; Steudte, S.; Kumirska, J.; Stepnowski, P.; Stolte, S. Aquatic toxicity of four veterinary drugs commonly applied in fish farming and animal husbandry. Chemosphere 2013, 92, 1253–1259. [Google Scholar] [CrossRef]
- Mu, L.; Zeng, X.; Liu, W.; Chen, H.; Bi, R.; Xie, L.; Bouchez, A.; Li, P.; Li, D.; Tang, J.; et al. Sensitivities of seven algal species to triclosan, fluoxetine and their mixtures. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Quinn, B.; Schmidt, W.; O’Rourke, K.; Hernan, R. Effects of the pharmaceuticals gemfibrozil and diclofenac on biomarker expression in the zebra mussel (Dreissena polymorpha) and their comparison with standardised toxicity tests. Chemosphere 2011, 84, 657–663. [Google Scholar] [CrossRef]
- Seoane, M.; Rioboo, C.; Herrero, C.; Cid, Á. Toxicity induced by three antibiotics commonly used in aquaculture on the marine microalga Tetraselmis suecica (Kylin) Butch. Mar. Environ. Res. 2014, 101, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gagné, F.; Blaise, C.; Fournier, M.; Hansen, P.D. Effects of selected pharmaceutical products on phagocytic activity in Elliptio complanata mussels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 179–186. [Google Scholar] [CrossRef]
- de Solla, S.R.; Campbell, S.D.; McInnis, R.; Gillis, P.L.; Gilroy, È.A.M.; Klinck, J.S. Toxicity and bioconcentration of the pharmaceuticals moxifloxacin, rosuvastatin, and drospirenone to the unionid mussel Lampsilis siliquoidea. Sci. Total Environ. 2014, 487, 537–544. [Google Scholar]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Osadchuk, O.Y.; Farkas, A.; Vehovszky, A.; Carpenter, D.O.; Gyori, J.; Stoliar, O.B. Diversity of the molecular responses to separate wastewater effluents in freshwater mussels. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 164, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2012, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.M.; Pascoe, D.; Carroll, K. Chronic exposure to 17α-ethinylestradiol and bisphenol A-effects on development and reproduction in the freshwater invertebrate Chironomus riparius (Diptera: Chironomidae). Aquat. Toxicol. 2001, 55, 113–124. [Google Scholar] [CrossRef]
- Watts, M.M.; Pascoe, D.; Carroll, K. Exposure to 17α-ethinylestradiol and bisphenol A - Effects on larval moulting and mouthpart structure of Chironomus riparius. Ecotoxicol. Environ. Saf. 2003, 54, 207–215. [Google Scholar] [CrossRef]
- Luna, T.O.; Plautz, S.C.; Salice, C.J. Chronic effects of 17α-ethinylestradiol, fluoxetine, and the mixture on individual and population-level end points in Daphnia magna. Arch. Environ. Contam. Toxicol. 2015, 68, 603–611. [Google Scholar] [CrossRef]
- Richmond, E.K.; Rosi-Marshall, E.J.; Lee, S.S.; Thompson, R.M.; Grace, M.R. Antidepressants in stream ecosystems: Influence of selective serotonin reuptake inhibitors (SSRIs) on algal production and insect emergence. Freshw. Sci. 2016, 35, 845–855. [Google Scholar] [CrossRef] [Green Version]
- Bundschuh, M.; Hahn, T.; Gessner, M.O.; Schulz, R. Antibiotic mixture effects on growth of the leaf-shredding stream detritivore Gammarus fossarum. Ecotoxicology 2017, 26, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Canela, C.; Miller, T.H.; Bury, N.R.; Tauler, R.; Barron, L.P. Targeted metabolomics of Gammarus pulex following controlled exposures to selected pharmaceuticals in water. Sci. Total Environ. 2016, 562, 777–788. [Google Scholar] [CrossRef]
- Garcia, R.N.; Chung, K.W.; Delorenzo, M.E.; Curran, M.C. Individual and mixture effects of caffeine and sulfamethoxazole on the daggerblade grass shrimp Palaemonetes pugio following maternal exposure. Environ. Toxicol. Chem. 2014, 33, 2120–2125. [Google Scholar] [CrossRef]
- Huang, S.S.Y.; Benskin, J.P.; Veldhoen, N.; Chandramouli, B.; Butler, H.; Helbing, C.C.; Cosgrove, J.R. A multi-omic approach to elucidate low-dose effects of xenobiotics in zebrafish (Danio rerio) larvae. Aquat. Toxicol. 2017, 182, 102–112. [Google Scholar] [CrossRef]
- Galus, M.; Jeyaranjaan, J.; Smith, E.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat. Toxicol. 2013, 132–133, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.B.D.; Trudeau, V.L.; Marlatt, V.L.; Moon, T.W.; Sherry, J.P.; Metcalfe, C.D. Interaction of stilbene compounds with human and rainbow trout estrogen receptors. Environ. Toxicol. Chem. 2008, 27, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Fedorova, G.; Burkina, V.; Steinbach, C.; Schmidt-Posthaus, H.; Zlabek, V.; Kocour Kroupova, H.; Grabic, R.; Randak, T. Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol. Environ. Saf. 2013, 96, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Carbonell, G.; Babín, M. Effects of individual and a mixture of pharmaceuticals and personal-care products on cytotoxicity, EROD activity and ROS production in a rainbow trout gonadal cell line (RTG-2). J. Appl. Toxicol. 2013, 33, 1203–1212. [Google Scholar] [CrossRef]
- Yokota, H.; Eguchi, S.; Hasegawa, S.; Okada, K.; Yamamoto, F.; Sunagawa, A.; Tanaka, M.; Yamamoto, R.; Nakano, E. Assessment of in vitro antiovulatory activities of nonsteroidal anti-inflammatory drugs and comparison with in vivo reproductive toxicities of medaka (Oryzias latipes). Environ. Toxicol. 2015, 24, 296–303. [Google Scholar] [CrossRef]
- Nassef, M.; Matsumoto, S.; Seki, M.; Khalil, F.; Kang, I.J.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Acute effects of triclosan, diclofenac and carbamazepine on feeding performance of Japanese medaka fish (Oryzias latipes). Chemosphere 2010, 80, 1095–1100. [Google Scholar] [CrossRef]
- Zenobio, J.E.; Sanchez, B.C.; Archuleta, L.C.; Sepulveda, M.S. Effects of triclocarban, N,N-diethyl-meta-toluamide, and a mixture of pharmaceuticals and personal care products on fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2014, 33, 910–919. [Google Scholar] [CrossRef]
- Yan, L.; Mu, L.; Chen, H.X.; Guo, Z.B.; Luo, Y.J.; Xie, L.T. Combined effects of fluoxetine and triclosan on Pseudorasbora parva. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2018, 29, 3058–3066. [Google Scholar]
- Northcott, G.; Graham, L.; Emnet, P.; Storey, B.; Gaw, S. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2014, 136, 331–342. [Google Scholar]
- Hu, C.; Hermann, G.; Pen-Mouratov, S.; Shore, L.; Steinberger, Y. Mammalian steroid hormones can reduce abundance and affect the sex ratio in a soil nematode community. Agric. Ecosyst. Environ. 2011, 142, 275–279. [Google Scholar] [CrossRef]
- Beltrán Rodríguez, M.E.; Carbonell, G.; Escuer, M.; Fernández, C.; Gutiérrez, C.; Rodríguez Martín, J.A.; Campos-Herrera, R. Effect of soil properties, heavy metals and emerging contaminants in the soil nematodes diversity. Environ. Pollut. 2016, 213, 184–194. [Google Scholar]
- Chevillot, F.; Guyot, M.; Desrosiers, M.; Cadoret, N.; Veilleux, É.; Cabana, H.; Bellenger, J.P. Accumulation and sublethal effects of triclosan and its transformation product methyl-triclosan in the earthworm Eisenia andrei exposed to environmental concentrations in an artificial soil. Environ. Toxicol. Chem. 2018, 37, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.J.; Rivas, N.G.; Prager, S.M.; Walton, W.E.; Trumble, J.T. Pharmaceuticals and personal care products alter the holobiome and development of a medically important mosquito. Environ. Pollut. 2015, 203, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larcher, S.; Yargeau, V. The effect of ozone on the biodegradation of 17α-ethinylestradiol and sulfamethoxazole by mixed bacterial cultures. Appl. Microbiol. Biotechnol. 2013, 97, 2201–2210. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Yan, P.; Gong, H.; Fang, F. Sorption-desorption behavior of sulfamethoxazole, carbamazepine, bisphenol A and 17A-ethinylestradiol in sewage sludge. J. Hazard. Mater. 2019, 739–745. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Halling-Sørensen, B. Biodegradation of selected emerging organic contaminants in the environment-an overview. In Leading-Edge Environmental Biogradation Research; Pawley, L.E., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2007; ISBN 1600219039. [Google Scholar]
- Combalbert, S.; Hernandez-Raquet, G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Appl. Microbiol. Biotechnol. 2010, 86, 1671–1692. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Rousseau, D.P.L.; Lens, P.N.L. Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems. Environ. Sci. Pollut. Res. 2010, 17, 824–833. [Google Scholar] [CrossRef]
- Al-Ansari, A.M.; Saleem, A.; Kimpe, L.E.; Sherry, J.P.; McMaster, M.E.; Trudeau, V.L.; Blais, J.M. Bioaccumulation of the pharmaceutical 17α-ethinylestradiol in shorthead redhorse suckers (Moxostoma macrolepidotum) from the St. Clair River, Canada. Environ. Pollut. 2010, 158, 2566–2571. [Google Scholar] [CrossRef]
- Eldridge, H.C.; Milliken, A.; Farmer, C.; Hampton, A.S.; Wendland, N.; Coward, L.; Gregory, D.J.; Johnson, C.M. Efficient remediation of 17 α-ethinylestradiol by Lentinula edodes (shiitake) laccase. Biocatal. Agric. Biotechnol. 2017, 10, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Otto, B.; Beuchel, C.; Liers, C.; Reisser, W.; Harms, H.; Schlosser, D. Laccase-like enzyme activities from chlorophycean green algae with potential for bioconversion of phenolic pollutants. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikesková, H.; Novotný, C.; Svobodová, K. Interspecific interactions in mixed microbial cultures in a biodegradation perspective. Appl. Microbiol. Biotechnol. 2012. [CrossRef] [PubMed]
- Guo, Y.P.; Hu, Y.Y.; Lin, H.; Ou, X.L. Sorption and desorption of 17α-ethinylestradiol onto sediments affected by rhamnolipidic biosurfactants. J. Hazard. Mater. 2018, 344, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lai, C.; Dai, H.; Mu, K.; Xu, Z.; Gu, L.; Pan, X. Microbially reduced humic acid promotes the anaerobic photodegradation of 17α-ethinylestradiol. Ecotoxicol. Environ. Saf. 2019, 171, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540. [Google Scholar] [CrossRef]
- Furgal, K.M.; Meyer, R.L.; Bester, K. Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels. Chemosphere 2014, 136, 321–326. [Google Scholar] [CrossRef]
- Tran, T.N.; Kim, D.G.; Ko, S.O. Synergistic effects of biogenic manganese oxide and Mn(II)-oxidizing bacterium Pseudomonas putida strain MnB1 on the degradation of 17 A-ethinylestradiol. J. Hazard. Mater. 2018, 344, 350–359. [Google Scholar] [CrossRef]
- Rovani, S.; Censi, M.T.; Pedrotti, S.L.; Lima, É.C.; Cataluña, R.; Fernandes, A.N. Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. J. Hazard. Mater. 2014, 344, 350–359. [Google Scholar] [CrossRef]
- Roh, H.; Subramanya, N.; Zhao, F.; Yu, C.P.; Sandt, J.; Chu, K.H. Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 2009, 77, 1084–1089. [Google Scholar] [CrossRef]
- Barth, S.; Fischer, M.; Schmid, R.D.; Pleiss, J. Sequence and structure of epoxide hydrolases: A systematic analysis. Proteins Struct. Funct. Genet. 2004, 55, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, P.; Oh, T.J.; Liou, K.; Sohng, J.K. Cytochrome P450 (CYP105F2) from Streptomyces peucetius and its activity with oleandomycin. Appl. Microbiol. Biotechnol. 2008, 79, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Favier, L.; Dinica, R.; Semrany, S.; Djelal, H.; Amrane, A.; Bahrim, G. Potential of newly isolated wild Streptomyces strains as agents for the biodegradation of a recalcitrant pharmaceutical, carbamazepine. Environ. Technol. (U. K.) 2014, 35, 3082–3091. [Google Scholar]
- Woo, H.L.; Hazen, T.C.; Simmons, B.A.; DeAngelis, K.M. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst. Appl. Microbiol. 2014, 37, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhang, Y.; Zhang, Y.; Fang, D.; Schauer, J.J. Optimization of the measurement of particle-bound reactive oxygen species with 2′,7′-dichlorofluorescin (DCFH). Water Air Soil Pollut. 2016, 227, 1–10. [Google Scholar] [CrossRef]
- Quandt, E.M.; Summers, R.M.; Subramanian, M.V.; Barrick, J.E. Draft genome sequence of the bacterium Pseudomonas putida CBB5, which can utilize caffeine as a sole carbon and nitrogen source. Genome Announc. 2015, 3, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Thelusmond, J.R.; Strathmann, T.J.; Cupples, A.M. Carbamazepine, triclocarban and triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci. Total Environ. 2019, 657, 1138–1149. [Google Scholar] [CrossRef]
- Devatha, C.P.; Pavithra, N. Isolation and identification of Pseudomonas from wastewater, its immobilization in cellulose biopolymer and performance in degrading Triclosan. J. Environ. Manag. 2019, 232, 584–591. [Google Scholar] [CrossRef]
- Kumari, R.; Ghosh Sachan, S. Bioconversion of toxic micropollutant triclosan to 2,4-dichlorophenol using a wastewater isolate Pseudomonas aeruginosa KS2002. Int. J. Environ. Sci. Technol. 2019, 16, 7663–7672. [Google Scholar] [CrossRef]
- Mahmoud, I.S.; Altaif, K.I.; Sini, M.K.A.; Daoud, S.; Aqel, N.N. Determination of antimicrobial drug resistance among bacterial isolates in two hospitals of Baghdad. Jordan J. Pharm. Sci. 2020, 13, 1–9. [Google Scholar]
- González-Benítez, N.; Molina, M.C.; Arrayás, M. Empirical evidence and mathematical modelling of carbamazepine degradative kinetics by a wood-rotting microbial consortium. Waste Biomass Valori. 2020. [Google Scholar] [CrossRef]
- Li, H.; Sumarah, M.W.; Topp, E. Persistence of the tricyclic antidepressant drugs amitriptyline and nortriptyline in agriculture soils. Environ. Toxicol. Chem. 2013, 32, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ruan, Z.; Liu, J.; Liu, C.; Zhang, F.; Linhardt, R.J.; Li, L. Complete degradation of bisphenol A and nonylphenol by a composite of biogenic manganese oxides and Escherichia coli cells with surface-displayed multicopper oxidase CotA. Chem. Eng. J. 2019, 897–908. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, D.; Peng, W.; Wang, Y.; Wang, X.; Xiong, W.; Liang, R. Characterization of 17β-hydroxysteroid dehydrogenase and regulators involved in estrogen degradation in Pseudomonas putida SJTE-1. Appl. Microbiol. Biotechnol. 2019, 103, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Kjeldal, H.; Zhou, N.A.; Wissenbach, D.K.; Von Bergen, M.; Gough, H.L.; Nielsen, J.L. Genomic, proteomic, and metabolite characterization of gemfibrozil-degrading organism Bacillus sp. GeD10. Environ. Sci. Technol. 2016, 50, 744–755. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1(2015b) is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil Pollut. 2016, 227, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, M.; Liu, X.; Zhang, Q.; Liu, R.; Wang, Z.; Shi, X.; Quan, J.; Shen, X.; Zhang, F. Removal of sulfamethoxazole and trimethoprim from reclaimed water and the biodegradation mechanism. Front. Environ. Sci. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, T.; Niu, M.; Chen, X.; Liu, C.; Wang, Y.; Chen, T. Biodegradation of nonylphenol during aerobic composting of sewage sludge under two intermittent aeration treatments in a full-scale plant. Environ. Pollut. 2018, 238, 783–791. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Wang, J. Biodegradation of typical pharmaceutical compounds by a novel strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. A new pathway for naproxen utilisation by Bacillus thuringiensis B1(2015b) and its decomposition in the presence of organic and inorganic contaminants. J. Environ. Manag. 2019, 239, 1–7. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology 2013, 159, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Thelusmond, J.R.; Strathmann, T.J.; Cupples, A.M. The identification of carbamazepine biodegrading phylotypes and phylotypes sensitive to carbamazepine exposure in two soil microbial communities. Sci. Total Environ. 2016, 571, 1241–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.A.; Lutovsky, A.C.; Andaker, G.L.; Ferguson, J.F.; Gough, H.L. Kinetics modeling predicts bioaugmentation with Sphingomonad cultures as a viable technology for enhanced pharmaceutical and personal care products removal during wastewater treatment. Bioresour. Technol. 2014, 166, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Murugesan, K.; Schmidt, S.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Chang, Y.S. Triclosan susceptibility and co-metabolism - A comparison for three aerobic pollutant-degrading bacteria. Bioresour. Technol. 2011, 102, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Wang, S.; Sun, P.; Abuduaini, R.; Zhu, X.; Zhao, Y. Degradation of nonylphenol polyethoxylates by functionalized Fe3O4 nanoparticle-immobilized Sphingomonas sp. Y2. Sci. Total Environ. 2018, 615, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Cirja, M.; Hommes, G.; Ivashechkin, P.; Prell, J.; Schäffer, A.; Corvini, P.F.X.; Lenz, M. Impact of bio-augmentation with Sphingomonas sp. strain TTNP3 in membrane bioreactors degrading nonylphenol. Appl. Microbiol. Biotechnol. 2009, 84, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, K.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Kim, J.H.; Chang, Y.S. Effect of Fe-Pd bimetallic nanoparticles on Sphingomonas sp. PH-07 and a nano-bio hybrid process for triclosan degradation. Bioresour. Technol. 2011, 102, 6019–6025. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Y.; Zhao, Q.; Li, N.; Zhou, Q.; Xie, S. Nonylphenol biodegradation, functional gene abundance and bacterial community in bioaugmented sediment: effect of external carbon source. Environ. Sci. Pollut. Res. 2015, 22, 12083–12091. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Bacterial degradation of naproxen - Undisclosed pollutant in the environment. J. Environ. Manag. 2014, 145, 157–161. [Google Scholar] [CrossRef]
- Pauwels, B.; Wille, K.; Noppe, H.; De Brabander, H.; Van De Wiele, T.; Verstraete, W.; Boon, N. 17 α-ethinylestradiol cometabolism by bacteria degrading estrone, 17β-estradiol and estriol. Biodegradation 2008, 19, 683–693. [Google Scholar] [CrossRef]
- Reis, P.J.M.; Reis, A.C.; Ricken, B.; Kolvenbach, B.A.; Manaia, C.M.; Corvini, P.F.X.; Nunes, O.C. Biodegradation of sulfamethoxazole and other sulfonamides by Achromobacter denitrificans PR1. J. Hazard. Mater. 2014, 280, 741–749. [Google Scholar] [CrossRef]
- Reis, A.C.; Čvančarová, M.; Liu, Y.; Lenz, M.; Hettich, T.; Kolvenbach, B.A.; Corvini, P.F.X.; Nunes, O.C. Biodegradation of sulfamethoxazole by a bacterial consortium of Achromobacter denitrificans PR1 and Leucobacter sp. GP. Appl. Microbiol. Biotechnol. 2018, 102, 10299–10314. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Mao, Y.; Li, B.; Yang, C.; Zhang, T. Aerobic degradation of sulfadiazine by arthrobacter spp.: Kinetics, pathways, and genomic characterization. Environ. Sci. Technol. 2016, 50, 9566–9575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Y.; Silva, A.F.; Reis, A.C.; Nunes, O.C.; Rodrigues, A.M.; Rodrigues, J.E.; Cardoso, V.V.; Benoliel, M.J.; Reis, M.A.M.; Oehmen, A.; et al. Bioaugmentation of membrane bioreactor with Achromobacter denitrificans strain PR1 for enhanced sulfamethoxazole removal in wastewater. Sci. Total Environ. 2019, 648, 44–55. [Google Scholar] [CrossRef]
- Navrozidou, E.; Melidis, P.; Ntougias, S. Biodegradation aspects of ibuprofen and identification of ibuprofen-degrading microbiota in an immobilized cell bioreactor. Environ. Sci. Pollut. Res. 2019, 26, 14238–14249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Li, Y.; Wang, P.; Wang, C. Background nutrients and bacterial community evolution determine 13 C-17β-estradiol mineralization in lake sediment microcosms. Sci. Total Environ. 2019, 651, 2304–2311. [Google Scholar] [CrossRef]
- Ootsuka, M.; Nishizawa, T.; Hasegawa, M.; Kurusu, Y.; Ohta, H. Comparative analysis of the genetic basis of branched nonylphenol degradation by Sphingobium amiense DSM 16289 T and Sphingobium cloacae JCM 10874 T. Microbes Environ. 2018, 33, 450–454. [Google Scholar] [CrossRef]
- Watahiki, S.; Kimura, N. Draft genome sequence of a caffeine-utilizing bacterium, Cupriavidus sp. strain D384. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Mahanty, B.; Yoon, S.; Kim, C.G. Degradation of the long-resistant pharmaceutical compounds carbamazepine and diatrizoate using mixed microbial culture. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2016, 51, 467–471. [Google Scholar] [CrossRef]
- Lee, D.G.; Chu, K.H. Effects of growth substrate on triclosan biodegradation potential of oxygenase-expressing bacteria. Chemosphere 2013, 93, 1904–1911. [Google Scholar] [CrossRef]
- Villemur, R.; Cunha dos Santos, S.C.; Ouellette, J.; Juteau, P.; Lépine, F.; Déziel, E. Biodegradation of endocrine disruptors in solid-liquid two-phase partitioning systems by enrichment cultures. Appl. Environ. Microbiol. 2013, 79, 4701–4711. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sui, Q.; Lu, S.G.; Zhao, W.T.; Qiu, Z.F.; Miao, Z.W.; Yu, G. Occurrence and removal of six pharmaceuticals and personal care products in a wastewater treatment plant employing anaerobic/anoxic/aerobic and UV processes in Shanghai, China. Environ. Sci. Pollut. Res. 2014, 21, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- de las Heras, I.; Padrino, B.; Molina, R.; Segura, Y.; Melero, J.A.; Mohedano, A.F.; Martinez, F.; Puyol, D. Efficient treatment of synthetic wastewater contaminated with emerging pollutants by anaerobic purple phototrophic bacteria. Front. Wastewater Treat. Model. 2017, 1, 324–330. [Google Scholar]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J.O. Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Front. Microbiol. 2017, 7, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madukasi, E.I.; Dai, X.; He, C.; Zhou, J. Potentials of phototrophic bacteria in treating pharmaceutical wastewater. Int. J. Environ. Sci. Technol. 2010, 7, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Merugu, R.; Prashanthi, Y.; Sarojini, T.; Badgu, N. Bioremediation of waste waters by the anoxygenic photosynthetic bacterium Rhodobacter sphaeroides SMR 009. Int. J. Res. Environ. Sci. Technol. 2014, 4, 16–19. [Google Scholar]
- Yang, Z.; Shi, Y.; Zhang, Y.; Cheng, Q.; Li, X.; Zhao, C.; Zhang, D. Different pathways for 4-n-nonylphenol biodegradation by two Aspergillus strains derived from estuary sediment: Evidence from metabolites determination and key-gene identification. J. Hazard. Mater. 2018, 359, 203–212. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Molina, R.; Pariente, M.I.; Christoforidis, K.C.; Martinez, F.; Melero, J.A. Understanding the role of mediators in the efficiency of advanced oxidation processes using white-rot fungi. Chem. Eng. J. 2019, 359, 1427–1435. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Sánchez-Vázquez, R.; Molina, R.; Martínez, F.; Melero, J.A.; Bautista, L.F.; Iglesias, J.; Morales, G. Biological removal of pharmaceutical compounds using white-rot fungi with concomitant FAME production of the residual biomass. J. Environ. Manag. 2016, 180, 228–237. [Google Scholar] [CrossRef]
- Yang, S.; Hai, F.I.; Nghiem, L.D.; Price, W.E.; Roddick, F.; Moreira, M.T.; Magram, S.F. Understanding the factors controlling the removal of trace organic contaminants by white-rot fungi and their lignin modifying enzymes: A critical review. Bioresour. Technol. 2013, 359, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Asif, M.B.; Hai, F.I.; Singh, L.; Price, W.E.; Nghiem, L.D. Degradation of pharmaceuticals and personal care products by White-Rot Fungi—A critical review. Curr. Pollut. Rep. 2017, 3, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds. Front. Microbiol. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Castellet-Rovira, F.; Villagrasa, M.; Badia-Fabregat, M.; Barceló, D.; Vicent, T.; Caminal, G.; Sarrà, M.; Rodríguez-Mozaz, S. The role of sorption processes in the removal of pharmaceuticals by fungal treatment of wastewater. Sci. Total Environ. 2018, 610–611, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Morales, R.L.; Gerardo-Gerardo, J.L.; Guillén Navarro, K.; Sánchez, J.E. Ligninolytic enzyme production by white rot fungi during paraquat (herbicide) degradation. Rev. Argent. Microbiol. 2017, 49, 189–196. [Google Scholar] [PubMed]
- Singh, H. Mycoremediation: Fungal Bioremediation; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- González, T.; Terrón, M.C.; Yagüe, S.; Junca, H.; Carbajo, J.M.; Zapico, E.J.; Silva, R.; Arana-Cuenca, A.; Téllez, A.; González, A.E. Melanoidin-containing wastewaters induce selective laccase gene expression in the white-rot fungus Trametes sp. I-62. Res. Microbiol. 2008, 59, 103–109. [Google Scholar] [CrossRef]
- Cruz-Morató, C.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Marco-Urrea, E.; Vicent, T.; Sarrà, M. Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res. 2013, 47, 5200–5210. [Google Scholar] [CrossRef]
- Margot, J.; Bennati-Granier, C.; Maillard, J.; Blánquez, P.; Barry, D.A.; Holliger, C. Bacterial versus fungal laccase: Potential for micropollutant degradation. AMB Express 2013, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzyme Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef]
- Ardao, I.; Magnin, D.; Agathos, S.N. Bioinspired production of magnetic laccase-biotitania particles for the removal of endocrine disrupting chemicals. Biotechnol. Bioeng. 2015, 112, 1986–1996. [Google Scholar] [CrossRef]
- Castellet-Rovira, F.; Lucas, D.; Villagrasa, M.; Rodríguez-Mozaz, S.; Barceló, D.; Sarrà, M. Stropharia rugosoannulata and Gymnopilus luteofolius: Promising fungal species for pharmaceutical biodegradation in contaminated water. J. Environ. Manag. 2018, 207, 396–404. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef]
- Liao, C.-S.; Yuan, S.-Y.; Hung, B.-H.; Chang, B. Removal of organic toxic chemicals using the spent mushroom compost of Ganoderma lucidum. J. Environ. Monit. 2012, 14, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Čvančarová, M.; Moeder, M.; Filipová, A.; Reemtsma, T.; Cajthaml, T. Biotransformation of the antibiotic agent flumequine by ligninolytic fungi and residual antibacterial activity of the transformation mixtures. Environ. Sci. Technol. 2013, 47, 14128–14136. [Google Scholar] [CrossRef] [PubMed]
- Přenosilová, L.; Křesinová, Z.; Amemori, A.S.; Cajthaml, T.; Svobodová, K. Transcriptional response of lignin-degrading enzymes to 17 α-ethinyloestradiol in two white rots. Microb. Biotechnol. 2013, 6, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, E.; Castellana, G.; Taskin, E. A Two-step approach to eliminate pesticides and estrogens from a wastewater and reduce its phytotoxicity: Adsorption onto plant-derived materials and fungal degradation. Water Air Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Cajthaml, T.; Křesinová, Z.; Svobodová, K.; Möder, M. Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef]
- Moon, D.S.; Song, H.G. Degradation of alkylphenols by white rot fungus Irpex lacteus and its manganese peroxidase. Appl. Biochem. Biotechnol. 2012, 168, 542–549. [Google Scholar] [CrossRef]
- Parra Guardado, A.L.; Belleville, M.P.; de Jesús Rostro Alanis, M.; Parra Saldivar, R.; Sanchez-Marcano, J. Effect of redox mediators in pharmaceuticals degradation by laccase: A comparative study. Process. Biochem. 2019, 78, 123–131. [Google Scholar] [CrossRef]
- Golveia, J.C.S.; Santiago, M.F.; Sales, P.T.F.; Sartoratto, A.; Ponezi, A.N.; Thomaz, D.V.; de Gil, E.S.; Maria, M.T. Cupuaçu (Theobroma grandiflorum) residue and its potential application in the bioremediation of 17-A-ethinylestradiol as a Pycnoporus sanguineus laccase inducer. Prep. Biochem. Biotechnol. 2018, 48, 541–548. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Q.; Huang, Q. Removal of 17 β-estradiol from poultry litter via solid state cultivation of lignolytic fungi. J. Clean. Prod. 2016, 139, 1400–1407. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of endocrine-disrupting compounds in groundwater: Bisphenol A, nonylphenol, ethynylestradiol and triclosan by a laccase cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226, 251. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. (Special Issue: Biotechnology for the bio and green economy.). N. Biotechnol. 2013, 30, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duarte, C.; Viana, M.T.; Vazquez-Duhalt, R. Laccase-mediated transformations of endocrine disrupting chemicals abolish binding affinities to estrogen receptors and their estrogenic activity in zebrafish. Appl. Biochem. Biotechnol. 2012, 168, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuang, J.; Bester, K. Degradation of triclosan by environmental microbial consortia and by axenic cultures of microorganisms with concerns to wastewater treatment. Appl. Microbiol. Biotechnol. 2018, 102, 5403–5417. [Google Scholar] [CrossRef] [PubMed]
- Bronikowski, A.; Hagedoorn, P.L.; Koschorreck, K.; Urlacher, V.B. Expression of a new laccase from Moniliophthora roreri at high levels in Pichia pastoris and its potential application in micropollutant degradation. AMB Express 2017, 7, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, H.; Yargeau, V.; Cooper, D.G. Biodegradation of pharmaceuticals by Rhodococcus rhodochrous and Aspergillus niger by co-metabolism. Sci. Total Environ. 2010, 408, 1701–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Ma, C.; Wang, H.; Xia, T. Biodegradation of caffeine by whole cells of tea-derived fungi Aspergillus sydowii, Aspergillus niger and optimization for caffeine degradation. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of diclofenac with fungal strains. Arch. Environ. Prot. 2018, 44, 55–62. [Google Scholar]
- Ertit Taştan, B.; Dönmez, G. Biodegradation of pesticide triclosan by A. versicolor in simulated wastewater and semi-synthetic media. Pestic. Biochem. Physiol. 2015, 118, 33–37. [Google Scholar] [CrossRef]
- Pai, P.V.; Pai, A.; Pai, S.; Devadiga, S.Y.; Nayak, V.; Rao, C. V Effect of glucose and nitrogen source on caffeine degradation by four filamentous fungi. Indian J. Biotechnol. 2013, 12, 432–434. [Google Scholar]
- Hussain, J.; Muhammad, Z.; Ullah, R.; Jamila, N.; Ahmad, S.; Khan, N.; Ayaz, S.; Haider, S. Biotransformation of ß-estradiol isolated from Sonchus eruca. African J. Biotechnol. 2011, 10, 5529–5533. [Google Scholar]
- Rodríguez, E.; Ruiz-Dueñas, F.J.; Kooistra, R.; Ram, A.; Martínez, Á.T.; Martínez, M.J. Isolation of two laccase genes from the white-rot fungus Pleurotus eryngii and heterologous expression of the pel3 encoded protein. J. Biotechnol. 2008, 134, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; van de Merwe, J.P.; Hai, F.I.; Leusch, F.D.L.; Kang, J.; Price, W.E.; Roddick, F.; Magram, S.F.; Nghiem, L.D. Laccase-syringaldehyde-mediated degradation of trace organic contaminants in an enzymatic membrane reactor: Removal efficiency and effluent toxicity. Bioresour. Technol. 2016, 200, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olicón-Hernández, D.R.; Camacho-Morales, R.L.; Pozo, C.; González-López, J.; Aranda, E. Evaluation of diclofenac biodegradation by the ascomycete fungus Penicillium oxalicum at flask and bench bioreactor scales. Sci. Total Environ. 2019, 662, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ma, Y.J.; Li, W.Y.; Wang, J.W. Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ. Sci. Pollut. Res. 2018, 25, 8963–8975. [Google Scholar] [CrossRef]
- Kuzikova, I.; Safronova, V.; Zaytseva, T.; Medvedeva, N. Fate and effects of nonylphenol in the filamentous fungus Penicillium expansum isolated from the bottom sediments of the Gulf of Finland. J. Mar. Syst. 2017, 171, 111–119. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, J.; Lee, Z.M.P.; Gersberg, R.M.; Liu, Y.; Tan, S.K.; Ng, W.J. Characterization of microbial communities in wetland mesocosms receiving caffeine-enriched wastewater. Environ. Sci. Pollut. Res. 2016, 23, 14526–14539. [Google Scholar] [CrossRef] [Green Version]
- Tastan, B.E.; Özdemir, C.; Tekinay, T. Effects of different culture media on biodegradation of triclosan by Rhodotorula mucilaginosa and Penicillium sp. Water Sci. Technol. 2016, 74, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Li, Y.; Chen, Y.; Yin, M.; Huang, J.; Zhang, Z.; Shi, X.; Liu, H. Microbial hydroxylation of 17β-estradiol by Penicillium brevicompactum. Biocatal. Biotransform. 2016, 34, 137–143. [Google Scholar] [CrossRef]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites. Zeitschrift fur Naturforsch. 2017, 72, 173–179. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Mycoremediation of 17β-estradiol using Trichoderma citrinoviride strain AJAC3 along with enzyme studies. Environ. Prog. Sustain. Energy 2019, 8, 13142. [Google Scholar] [CrossRef]
- Buchicchio, A.; Bianco, G.; Sofo, A.; Masi, S.; Caniani, D. Biodegradation of carbamazepine and clarithromycin by Trichoderma harzianum and Pleurotus ostreatus investigated by liquid chromatography - high-resolution tandem mass spectrometry (FTICR MS-IRMPD). Sci. Total Environ. 2016, 557, 733–739. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of estrogens by laccase from Myceliophthora thermophila in fed-batch and enzymatic membrane reactors. J. Hazard. Mater. 2012, 213, 175–183. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- García-Delgado, C.; Eymar, E.; Camacho-Arévalo, R.; Petruccioli, M.; Crognale, S.; D’Annibale, A. Degradation of tetracyclines and sulfonamides by stevensite- and biochar-immobilized laccase systems and impact on residual antibiotic activity. J. Chem. Technol. Biotechnol. 2018, 93, 3394–3409. [Google Scholar] [CrossRef]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; Sánchez-Carbente, M.D.R.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First demonstration that ascomycetous halophilic fungi (Aspergillus sydowii and Aspergillus destruens) are useful in xenobiotic mycoremediation under high salinity conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar]
- Parshikov, I.A.; Muraleedharan, K.M.; Avery, M.A.; Williamson, J.S. Transformation of artemisinin by Cunninghamella elegans. Appl. Microbiol. Biotechnol. 2004, 64, 782–786. [Google Scholar] [CrossRef]
- Kang, S.I.; Kang, S.Y.; Kanaly, R.A.; Lee, E.; Lim, Y.; Hur, H.G. Rapid oxidation of ring methyl groups is the primary mechanism of biotransformation of gemfibrozil by the fungus Cunninghamella elegans. Arch. Microbiol. 2009, 191, 509–517. [Google Scholar] [CrossRef]
- Zhong, D.F.; Sun, L.; Liu, L.; Huang, H.H. Microbial transformation of naproxen by Cunninghamella species. Acta Pharmacol. Sin. 2003, 24, 442–447. [Google Scholar]
- Williams, A.J.; Deck, J.; Freeman, J.P.; Paul Chiarelli, M.; Adjei, M.D.; Heinze, T.M.; Sutherland, J.B. Biotransformation of flumequine by the fungus Cunninghamella elegans. Chemosphere 2007, 67, 240–243. [Google Scholar] [CrossRef]
- Goto, M.; Noda, S.; Kamiya, N.; Nakashio, F. Enzymatic resolution of racemic ibuprofen by surfactant-coated lipases in organic media. Biotechnol. Lett. 1996, 18, 839–844. [Google Scholar] [CrossRef]
- Djelal, H.; Amrane, A. Biodegradation by bioaugmentation of dairy wastewater by fungal consortium on a bioreactor lab-scale and on a pilot-scale. J. Environ. Sci. 2013, 25, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.H.; Suzuki, Y.; Lee, B.D.; Nakai, S.; Hosomi, M. Isolation and characterization of the ethynylestradiol-biodegrading microorganism Fusarium proliferatum strain HNS-1. Water Sci. Technol. 2002, 45, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Dubroca, J.; Brault, A.; Kollmann, A.; Touton, I.; Jolivalt, C.; Kerhoas, L.; Mougin, C. Biotransformation of nonylphenol surfactants in soils amended with contaminated sewage sludges. In Environmental Chemistry: Green Chemistry and Pollutants in Ecosystems; Springer-Verlag: Berlin, Germany, 2005; ISBN 3540228608. [Google Scholar]
- Janicki, T.; Długoński, J.; Krupiński, M. Detoxification and simultaneous removal of phenolic xenobiotics and heavy metals with endocrine-disrupting activity by the non-ligninolytic fungus Umbelopsis isabellina. J. Hazard. Mater. 2018, 360, 661–669. [Google Scholar] [CrossRef]
- Janicki, T.; Krupiński, M.; Długoński, J. Degradation and toxicity reduction of the endocrine disruptors nonylphenol, 4-tert-octylphenol and 4-cumylphenol by the non-ligninolytic fungus Umbelopsis isabellina. Bioresour. Technol. 2016, 200, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Czarny, K.; Szczukocki, D.; Krawczyk, B.; Skrzypek, S.; Zieliński, M.; Gadzała-Kopciuch, R. Toxic effects of single animal hormones and their mixtures on the growth of Chlorella vulgaris and Scenedesmus armatus. Chemosphere 2019, 224, 93–102. [Google Scholar] [CrossRef]
- Gosset, A.; Durrieu, C.; Barbe, P.; Bazin, C.; Bayard, R. Microalgal whole-cell biomarkers as sensitive tools for fast toxicity and pollution monitoring of urban wet weather discharges. Chemosphere 2019, 217, 522–533. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Lindberg, R.H.; Tysklind, M.; Funk, C. Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicol. Environ. Saf. 2019, 170, 644–656. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Ding, T.; Yang, M.; Zhang, J.; Yang, B.; Lin, K.; Li, J.; Gan, J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard. Mater. 2017, 330, 127–134. [Google Scholar] [CrossRef]
- Wu, N.; Moreira, C.M.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.J.; Svoronos, S.A.; Pullammanappallil, P.C. Techno-Economic Analysis of Biogas Production from Microalgae through Anaerobic Digestion. In Anaerobic Digestion; IntechOpen Ltd.: London, UK, 2019; ISBN 9781838818500. [Google Scholar]
- Shomal, R.; Hisham, H.; Mlhem, A.; Hassan, R.; Al-Zuhair, S. Simultaneous extraction–reaction process for biodiesel production from microalgae. Energy Rep. 2019, 5, 37–40. [Google Scholar] [CrossRef]
- Lozano-Garcia, D.F.; Cuellar-Bermudez, S.P.; del Rio-Hinojosa, E.; Betancourt, F.; Aleman-Nava, G.S.; Parra-Saldivar, R. Potential land microalgae cultivation in Mexico: From food production to biofuels. Algal Res. 2019, 39, 101459. [Google Scholar] [CrossRef]
- de Souza, M.H.B.; Calijuri, M.L.; Assemany, P.P.; de Siqueira Castro, J.; de Oliveira, A.C.M. Soil application of microalgae for nitrogen recovery: A life-cycle approach. J. Clean. Prod. 2019, 211, 342–349. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Wang, S.; Poon, K.; Cai, Z. Removal and metabolism of triclosan by three different microalgal species in aquatic environment. J. Hazard. Mater. 2018, 342, 643–650. [Google Scholar] [CrossRef]
- Gao, Q.T.; Wong, Y.S.; Tam, N.F.Y. Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. J. Appl. Phycol. 2017, 29, 1317–1329. [Google Scholar] [CrossRef]
- de Siqueira Castro, J.; Calijuri, M.L.; Mattiello, E.M.; Ribeiro, V.J.; Assemany, P.P. Algal biomass from wastewater: Soil phosphorus bioavailability and plants productivity. Sci. Total Environ. 2020, 711, 135088. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Mohamed, W.A.; El-Metwally, A.E. Spirulina platensis attenuates furan reprotoxicity by regulating oxidative stress, inflammation, and apoptosis in testis of rats. Ecotoxicol. Environ. Saf. 2018, 161, 25–33. [Google Scholar] [CrossRef]
- Hamjinda, N.S.; Chiemchaisri, W.; Watanabe, T.; Honda, R.; Chiemchaisri, C. Toxicological assessment of hospital wastewater in different treatment processes. Environ. Sci. Pollut. Res. 2018, 25, 7271–7279. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Paracetamol and salicylic acid removal from contaminated water by microalgae. J. Environ. Manag. 2017, 203, 799–806. [Google Scholar] [CrossRef]
- López-Serna, R.; Posadas, E.; García-Encina, P.A.; Muñoz, R. Removal of contaminants of emerging concern from urban wastewater in novel algal-bacterial photobioreactors. Sci. Total Environ. 2019, 662, 32–40. [Google Scholar] [CrossRef] [Green Version]
- de Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kim, S.J.; Kurade, M.B.; Govindwar, S.; Abou-Shanab, R.A.I.; Kim, J.R.; Roh, H.S.; Khan, M.A.; Jeon, B.H. Combined effects of sulfamethazine and sulfamethoxazole on a freshwater microalga, Scenedesmus obliquus: Toxicity, biodegradation, and metabolic fate. J. Hazard. Mater. 2019, 370, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Govindwar, S.; Kurade, M.B.; Paeng, K.J.; Roh, H.S.; Khan, M.A.; Jeon, B.H. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere 2019, 218, 551–558. [Google Scholar] [CrossRef]
- Maes, H.M.; Maletz, S.X.; Ratte, H.T.; Hollender, J.; Schaeffer, A. Uptake, elimination, and biotransformation of 17 α-ethinylestradiol by the freshwater alga Desmodesmus subspicatus. Environ. Sci. Technol. 2014, 48, 12354–12361. [Google Scholar] [CrossRef]
- Escapa, C.; Torres, T.; Neuparth, T.; Coimbra, R.N.; García, A.I.; Santos, M.M.; Otero, M. Zebrafish embryo bioassays for a comprehensive evaluation of microalgae efficiency in the removal of diclofenac from water. Sci. Total Environ. 2018, 1024–1033. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Vázquez, N.C.; Noriega-Hevia, G.; Otero, M. Utilization of non-living microalgae biomass from two different strains for the adsorptive removal of diclofenac from water. Water 2018, 10, 1401. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.E.M.; Abd El-Aty, A.M.; Badawy, M.I.; Ali, R.K. Removal of pharmaceutical pollutants from synthetic wastewater using chemically modified biomass of green alga Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2018, 151, 144–152. [Google Scholar] [CrossRef]
- Gao, T.; Shi, X. Preparation of a synthetic seed for the common reed harboring an endophytic bacterium promoting seedling growth under cadmium stress. Environ. Sci. Pollut. Res. 2018, 25, 8871–8879. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Improving the bioremoval of sulfamethoxazole and alleviating cytotoxicity of its biotransformation by laccase producing system under coculture of Pycnoporus sanguineus and Alcaligenes faecalis. Bioresour. Technol. 2016, 220, 333–340. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Elimination of endocrine disrupting chemicals using white rot fungi and their lignin modifying enzymes: A review. Eng. Life Sci. 2007, 7, 429–456. [Google Scholar] [CrossRef]
- Hai, F.I.; Modin, O.; Yamamoto, K.; Fukushi, K.; Nakajima, F.; Nghiem, L.D. Pesticide removal by a mixed culture of bacteria and white-rot fungi. J. Taiwan Inst. Chem. Eng. 2012, 43, 459–462. [Google Scholar] [CrossRef] [Green Version]
- Bodin, H.; Daneshvar, A.; Gros, M.; Hultberg, M. Effects of biopellets composed of microalgae and fungi on pharmaceuticals present at environmentally relevant levels in water. Ecol. Eng. 2016, 91, 169–172. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Jaén-Gil, A.; Bello-Laserna, I.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Performance of a microalgal photobioreactor treating toilet wastewater: Pharmaceutically active compound removal and biomass harvesting. Sci. Total Environ. 2017, 91, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Treesubsuntorn, C.; Thiravetyan, P. Botanical biofilter for indoor toluene removal and reduction of carbon dioxide emission under low light intensity by using mixed C3 and CAM plants. J. Clean. Prod. 2018, 194, 94–100. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Dolphen, R.; Dhurakit, P.; Siswanto, D.; Thiravetyan, P. Green Technology Innovation in a Developing Country. 2017. Available online: https://aip.scitation.org/doi/abs/10.1063/1.5012704 (accessed on 29 November 2017).
- Bhatnagar, N.; Bhatnagar, N. Phytoremediation: A Promising Technology of Environmental Pollution Control. Glob. J. Microbiol. Biotechnol. 2016, 4, 39–42. [Google Scholar]
- Kouki, S.; Saidi, N.; M’hiri, F.; Hafiane, A.; Hassen, A. Co-Composting of Macrophyte Biomass and Sludge as an Alternative for Sustainable Management of Constructed Wetland By-Products. Clean Soil Air Water 2016, 44, 694–702. [Google Scholar] [CrossRef]
- Sánchez-Galván, G.; Bolaños-Santiago, Y. Phytofiltration of anaerobically digested sugarcane ethanol stillage using a macrophyte with high potential for biofuel production. Int. J. Phytoremediation 2018, 20, 805–812. [Google Scholar] [CrossRef]
- da Luz, J.M.R.; Paes, S.A.; Torres, D.P.; Nunes, M.D.; da Silva, J.S.; Mantovani, H.C.; Kasuya, M.C.M. Production of edible mushroom and degradation of antinutritional factors in jatropha biodiesel residues. LWT Food Sci. Technol. 2013, 50, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, M.; Prade, T.; Bodin, H.; Vidakovic, A.; Asp, H. Adding benefit to wetlands – Valorization of harvested common reed through mushroom production. Sci. Total Environ. 2018, 637, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Bessa, V.S.; Mesquita, R.B.R.; Brix, H.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Macci, C.; Peruzzi, E.; Doni, S.; Iannelli, R.; Masciandaro, G. Ornamental plants for micropollutant removal in wetland systems. Environ. Sci. Pollut. Res. 2015, 22, 2406–2415. [Google Scholar] [CrossRef]
- Salamanca, E.J.P.; Madera-Parra, C.A.; Avila-Williams, C.A.; Rengifo-Gallego, A.L.; Ríos, D.A. Phytoremediation using terrestrial plants. Phytoremediation 2015, 305–319. [Google Scholar]
- Sopajarn, A.; Sangwichien, C. Optimization of enzymatic saccharification of alkali pretreated Typha angustifolia for Glucose Production. Int. J. Chem. Eng. Appl. 2015, 6, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Emhofer, L.; Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. High-performance liquid chromatography – mass spectrometry analysis of the parent drugs and their metabolites in extracts from cress (Lepidium sativum) grown hydroponically in water containing four non-steroidal anti-inflammatory drugs. J. Chromatogr. A 2017, 1491, 137–144. [Google Scholar] [CrossRef]
- Emhofer, L.; Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Insights into the uptake, metabolization, and translocation of four non-steroidal anti-inflammatory drugs in cress (Lepidium sativum) by HPLC-MS2. Electrophoresis 2018, 39, 1294–1300. [Google Scholar] [CrossRef]
- Klampfl, C.W. Metabolization of pharmaceuticals by plants after uptake from water and soil: A review. TrAC Trends Anal. Chem. 2019, 111, 13–26. [Google Scholar] [CrossRef]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant-microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 7, 416. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; Van Der Lelie, D. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 17, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.X.; Yu, J.; Zhao, H.M.; Cheng, Y.T.; Mo, C.H.; Cai, Q.Y.; Li, Y.W.; Li, H.; Wong, M.H. Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci. Total Environ. 2017, 583, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Dolphen, R.; Thiravetyan, P. Reducing arsenic in rice grains by leonardite and arsenic–resistant endophytic bacteria. Chemosphere 2019, 223, 448–454. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 753. [Google Scholar] [CrossRef]
- Molina, M.C.; Bautista, L.F.; Belda, I.; Carmona, M.; Díaz, E.; Durante-Rodríguez, G.; García-Salgado, S.; López, J.; Martínez-Hidalgo, P.; Quijano, M.A.; et al. Bioremediation of Soil Contaminated with Arsenic; Ashok, K., Swati, S., Eds.; Springer: Singapore, 2019; pp. 321–351. [Google Scholar]
- González, P.S.; Ontañon, O.M.; Armendariz, A.L.; Talano, M.A.; Paisio, C.E.; Agostini, E. Brassica napus hairy roots and rhizobacteria for phenolic compounds removal. Environ. Sci. Pollut. Res. 2013, 20, 1310–1317. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Politi, M.; Weyens, N.; Venieri, D.; Vangronsveld, J.; Kalogerakis, N. Bisphenol-A removal by the halophyte Juncus acutus in a phytoremediation pilot: Characterization and potential role of the endophytic community. J. Hazard. Mater. 2017, 323, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto)remediation purposes? N. Biotechnol. 2017, 38, 43–55. [Google Scholar] [CrossRef]
- Toyama, T.; Murashita, M.; Kobayashi, K.; Kikuchi, S.; Sei, K.; Tanaka, Y.; Ike, M.; Mori, K. Acceleration of nonylphenol and 4-tert-octylphenol degradation in sediment by phragmites australis and associated rhizosphere bacteria. Environ. Sci. Technol. 2011, 45, 6524–6530. [Google Scholar] [CrossRef]
- Toyama, T.; Ojima, T.; Tanaka, Y.; Mori, K.; Morikawa, M. Sustainable biodegradation of phenolic endocrine-disrupting chemicals by Phragmites australis-rhizosphere bacteria association. Water Sci. Technol. 2013, 68, 522–529. [Google Scholar] [CrossRef]
- Gonda, S.; Kiss-Szikszai, A.; Szucs, Z.; Balla, B.; Vasas, G. Efficient biotransformation of non-steroid anti-inflammatory drugs by endophytic and epiphytic fungi from dried leaves of a medicinal plant, Plantago lanceolata L. Int. Biodeterior. Biodegrad. 2016, 108, 115–121. [Google Scholar] [CrossRef]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Cañameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sauvêtre, A.; May, R.; Harpaintner, R.; Poschenrieder, C.; Schröder, P. Metabolism of carbamazepine in plant roots and endophytic rhizobacteria isolated from Phragmites australis. J. Hazard. Mater. 2018, 342, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvêtre, A.; Schröder, P. Uptake of carbamazepine by rhizomes and endophytic bacteria of Phragmites australis. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by an MBR comprising a mixed culture of bacteria and white-rot fungi. Bioresour. Technol. 2013, 148, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Peña-Herrera, J.M.; Rodelas, B.; Osorio, F.; Pozo, C. Removal of anti-inflammatory/analgesic pharmaceuticals from urban wastewater in a pilot-scale A 2 O system: Linking performance and microbial population dynamics to operating variables. Sci. Total Environ. 2018, 643, 1481–1492. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Mao, Y.; Zhang, T. Partnership of Arthrobacter and Pimelobacter in aerobic degradation of sulfadiazine revealed by metagenomics analysis and isolation. Environ. Sci. Technol. 2018, 52, 2963–2972. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijosa-Valsero, M.; Sidrach-Cardona, R.; Pedescoll, A.; Sánchez, O.; Bécares, E. Role of Bacterial Diversity on PPCPs Removal in Constructed Wetlands. In Constructed Wetlands for Industrial Wastewater Treatment; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 405–426. ISBN 9781119268345. [Google Scholar]
- Boonnorat, J.; Kanyatrakul, A.; Prakhongsak, A.; Honda, R.; Panichnumsin, P.; Boonapatcharoen, N. Effect of hydraulic retention time on micropollutant biodegradation in activated sludge system augmented with acclimatized sludge treating low-micropollutants wastewater. Chemosphere 2019, 230, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Hoinkis, J.; Deowan, S.A.; Panten, V.; Figoli, A.; Huang, R.R.; Drioli, E. Membrane bioreactor (MBR) technology - A promising approach for industrial water reuse. Procedia Eng. 2012, 33, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cabassud, C.; Guigui, C. Evaluation of membrane bioreactor on removal of pharmaceutical micropollutants: A review. Desalin. Water Treat. 2015, 55, 845–858. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Sillanpää, M. Removal and fate of emerging organic micropollutants (EOMs) in municipal wastewater by a pilot-scale membrane bioreactor (MBR) treatment under varying solid retention times. Sci. Total Environ. 2019, 667, 671–680. [Google Scholar] [CrossRef]
- Maeng, S.K.; Choi, B.G.; Lee, K.T.; Song, K.G. Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res. 2013, 47, 3151–3162. [Google Scholar] [CrossRef]
- de Melo, R.G.; de Andrade, A.F.; Bezerra, R.P.; Correia, D.S.; de Souza, V.C.; Brasileiro-Vidal, A.C.; de Araújo Viana Marques, D.; Porto, A.L.F. Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro-industrial by-products. Chemosphere 2018, 204, 344–350. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—a review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.M.S.; Carvalho, L.; Leal, C.D.; Dias, M.F.; Martins, K.L.; Garcia, G.B.; Mancuelo, I.D.; Hipólito, T.; Macconell, E.F.A.; Okada, D.; et al. Impact of inocula and operating conditions on the microbial community structure of two anammox reactors. Environ. Technol. 2014, 35, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Khadamkar, H.P.; Mathpati, C.S.; Pandit, R.; Lali, A.M. Computational and experimental studies of high depth algal raceway pond photo-bioreactor. Renew. Energy 2018, 118, 152–159. [Google Scholar] [CrossRef]
- Gentili, F.G.; Fick, J. Algal cultivation in urban wastewater: An efficient way to reduce pharmaceutical pollutants. J. Appl. Phycol. 2017, 29, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment – A review. Sci. Total Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Borde, X.; Guieysse, B.; Delgado, O.; Muoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Synergistic relationships in algal-bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol. 2003, 86, 293–300. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, V. Constructed wetland microcosms as sustainable technology for domestic kwastewater treatment: An overview. Environ. Sci. Pollut. Res. Int. 2019, 26, 11662–11673. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of full-scale natural systems for the removal of PPCPs from wastewater in small communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the veterinary antibiotics enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581–582, 359–368. [Google Scholar] [CrossRef]
- Harrabi, M.; Alexandrino, D.A.M.; Aloulou, F.; Elleuch, B.; Liu, B.; Jia, Z.; Almeida, C.M.R.; Mucha, A.P.; Carvalho, M.F. Biodegradation of oxytetracycline and enrofloxacin by autochthonous microbial communities from estuarine sediments. Sci. Total Environ. 2019, 648, 962–972. [Google Scholar] [CrossRef] [PubMed]
- VanKempen-Fryling, R.J.; Stein, O.R.; Camper, A.K. Presence and persistence of wastewater pathogen Escherichia coli O157:H7 in hydroponic reactors of treatment wetland species. Water Sci. Technol. 2015, 72, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, D.Q.; Dong, J.W.; Tan, S.K. Constructed wetlands for wastewater treatment in cold climate—A review. J. Environ. Sci. 2017, 57, 293–311. [Google Scholar] [CrossRef]
- Maiga, Y.; von Sperling, M.R.; Mihelcic, J. Constructed Wetlands. 2017. Available online: http://www.waterpathogens.org/book/constructed-wetlands (accessed on 22 March 2017).
- Salomo, S.; Münch, C.; Röske, I. Evaluation of the metabolic diversity of microbial communities in four different filter layers of a constructed wetland with vertical flow by BiologTM analysis. Water Res. 2009, 43, 4569–4578. [Google Scholar] [CrossRef]
- Olson, M.R.; Axler, R.P.; Hicks, R.E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods 2004, 122, 147–152. [Google Scholar] [CrossRef]
- Shao, Y.; Yan Pei, H.; Rong Hu, W. Nitrogen removal by bioaugmentation in constructed wetlands for rural domestic wastewater in autumn. Desalin. Water Treat. 2013, 51, 147–152. [Google Scholar] [CrossRef]
- Pei, H.; Shao, Y.; Chanway, C.P.; Hu, W.; Meng, P.; Li, Z.; Chen, Y.; Ma, G. Bioaugmentation in a pilot-scale constructed wetland to treat domestic wastewater in summer and autumn. Environ. Sci. Pollut. Res. 2016, 23, 7776–7785. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, F.; Yao, S.; Liu, Y.; Chen, J. Application of Pseudomonas flava WD-3 for sewage treatment in constructed wetland in winter. Environ. Technol. 2015, 36, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Haberl, R.; Grego, S.; Langergraber, G.; Kadlec, R.H.; Cicalini, A.R.; Dias, S.M.; Novais, J.M.; Aubert, S.; Gerth, A.; Thomas, H.; et al. Constructed wetlands for the treatment of organic pollutants. J. Soils Sediments 2003, 3, 109–124. [Google Scholar] [CrossRef]
- Silvan, N.; Vasander, H.; Laine, J. Vegetation is the main factor in nutrient retention in a constructed wetland buffer. Plant Soil 2004, 258, 179–187. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Christofilopoulos, S.; Syranidou, E.; Gkavrou, G.; Manousaki, E.; Kalogerakis, N. The role of halophyte Juncus acutus L. in the remediation of mixed contamination in a hydroponic greenhouse experiment. J. Chem. Technol. Biotechnol. 2016, 91, 1665–1674. [Google Scholar] [CrossRef]
- Madera-Parra, C.A.; Peña-Salamanca, E.J.; Peña, M.R.; Rousseau, D.P.L.; Lens, P.N.L. Phytoremediation of Landfill Leachate with Colocasia esculenta, Gynerum sagittatum and Heliconia psittacorum in Constructed Wetlands. Int. J. Phytoremediation 2015, 7, 16–24. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, Y.; Yu, Y.; Zhu, Z.W.; Feng, G. Effects of pharmaceuticals on microbial communities and activity of soil enzymes in mesocosm-scale constructed wetlands. Chemosphere 2018, 212, 245–253. [Google Scholar] [CrossRef]
- Tong, X.; Wang, X.; He, X.; Xu, K.; Mao, F. Effects of ofloxacin on nitrogen removal and microbial community structure in constructed wetland. Sci. Total Environ. 2019, 656, 503–511. [Google Scholar] [CrossRef]
- Weber, K.P.; Mitzel, M.R.; Slawson, R.M.; Legge, R.L. Effect of ciprofloxacin on microbiological development in wetland mesocosms. Water Res. 2011, 45, 3185–3196. [Google Scholar] [CrossRef]
- Shao, Y.; Pei, H.; Hu, W.; Chanway, C.P.; Meng, P.; Ji, Y.; Li, Z. Bioaugmentation in lab scale constructed wetland microcosms for treating polluted river water and domestic wastewater in northern China. Int. Biodeterior. Biodegrad. 2014, 95, 151–159. [Google Scholar] [CrossRef]
- Lingua, G.; Copetta, A.; Musso, D.; Aimo, S.; Ranzenigo, A.; Buico, A.; Gianotti, V.; Osella, D.; Berta, G. Effect of arbuscular mycorrhizal and bacterial inocula on nitrate concentration in mesocosms simulating a wastewater treatment system relying on phytodepuration. Environ. Sci. Pollut. Res. 2015, 22, 18616–18625. [Google Scholar] [CrossRef] [PubMed]
- Pat-Espadas, A.M.; Portales, R.L.; Amabilis-Sosa, L.E.; Gómez, G.; Vidal, G. Review of constructed wetlands for acid mine drainage treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef] [Green Version]
- Prum, C.; Dolphen, R.; Thiravetyan, P. Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius−endophytic Arthrobacter creatinolyticus interactions. J. Environ. Manag. 2018, 213, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Treatment of the textile industry effluent in a pilot-scale vertical flow constructed wetland system augmented with bacterial endophytes. Sci. Total Environ. 2018, 645, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, M.; Ben Said, M.; Bousselmi, L.; Ghrabi, A. Application of bioinoculation to enhance rhizocompetence of horizontal subsurface flow constructed wetland system. Desalin. Water Treat. 2016, 57, 22133–22139. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Bai, S.; Ma, F.; Wang, L. Microbial population dynamics in response to bioaugmentation in a constructed wetland system under 10°C. Bioresour. Technol. 2016, 205, 166–173. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.L.; Li, H.; Song, H.L.; Wang, R.C.; Dai, Z.Q. Degradation of sulfamethoxazole in bioelectrochemical system with power supplied by constructed wetland-coupled microbial fuel cells. Bioresour. Technol. 2017, 244, 345–352. [Google Scholar] [CrossRef]
- Paredes, D.; Kuschk, P.; Köser, H. Influence of plants and organic matter on the nitrogen removal in laboratory-scale model subsurface flow constructed wetlands inoculated with anaerobic ammonium oxidizing bacteria. Eng. Life Sci. 2007, 7, 565–576. [Google Scholar] [CrossRef]
- Nowrotek, M.; Kotlarska, E.; Łuczkiewicz, A.; Felis, E.; Sochacki, A.; Miksch, K. The treatment of wastewater containing pharmaceuticals in microcosm constructed wetlands: The occurrence of integrons (int1–2) and associated resistance genes (sul1–3, qacEΔ1). Environ. Sci. Pollut. Res. 2017, 24, 15055–15066. [Google Scholar] [CrossRef] [Green Version]
- Węgrzyn, A.; Felis, E. Isolation of bacterial endophytes from Phalaris arundinacea and their potential in diclofenac and sulfamethoxazole degradation. Polish J. Microbiol. 2018, 67, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iasur-Kruh, L.; Hadar, Y.; Minz, D. Isolation and bioaugmentation of an estradiol-degrading bacterium and its integration into a mature biofilm. Appl. Environ. Microbiol. 2011, 77, 3734–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. N. Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef]
- Gravel, V.; Dorais, M.; Gruyer, N.; Ménard, C. Constructed wetlands implanted with iris versicolor, Juncus sp. and Phragmites australis as a potential treatment for greenhouse effluents. Acta Hortic. 2011, 1139–1146. [Google Scholar] [CrossRef]
- Schäfer, M.L.; Lundström, J.O.; Pfeffer, M.; Lundkvist, E.; Landin, J. Biological diversity versus risk for mosquito nuisance and disease transmission in constructed wetlands in southern Sweden. Med. Vet. Entomol. 2004, 18, 256–267. [Google Scholar] [CrossRef]
- Boucher, J.G.; Boudreau, A.; Ahmed, S.; Atlas, E. Response to “Comment on ‘In Vitro Effects of Bisphenol A β-D-Glucuronide (BPA-G) on Adipogenesis in Human and Murine Preadipocytes. Env. Heal. Perspect 2015, 123, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrella, A.; Rubino, M.; Isidori, M.; Nardelli, A.; Previtera, L.; Lavorgna, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar]
- González, N.; Simarro, R.; Molina, M.C.; Bautista, L.F.; Delgado, L.; Villa, J.A. Effect of surfactants on PAH biodegradation by a bacterial consortium and on the dynamics of the bacterial community during the process. Bioresour. Technol. 2011, 102, 9438–9446. [Google Scholar] [CrossRef]
- Molina, M.D.C.; González Benítez, N.; Simarro, R.; Bautista, L.F.; Vargas, C.; García Cambero, J.P.; Díaz, E.M.; Arrayás, M.; Quijano, M.A. Bioremediation techniques for naproxen and carbamazepine elimination. Toxicity evaluation test. Chim. Oggi/Chemistry Today 2016, 34, 52–55. [Google Scholar]
- Anderson, J.C.; Beyger, L.; Guchardi, J.; Holdway, D. Chronic effects of hydroxypropyl-β-cyclodextrin on reproduction in the American flagfish (Jordanella floridae) over one complete life cycle. Environ. Toxicol. Chem. 2016, 35, 1358–1363. [Google Scholar] [CrossRef]
- Rubasinghege, G.; Gurung, R.; Rijal, H.; Maldonado-Torres, S.; Chan, A.; Acharya, S.; Rogelj, S.; Piyasena, M. Abiotic degradation and environmental toxicity of ibuprofen: Roles of mineral particles and solar radiation. Water Res. 2018, 131, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kase, R.; Korkaric, M.; Werner, I.; Ågerstrand, M. Criteria for Reporting and Evaluating ecotoxicity Data (CRED): Comparison and perception of the Klimisch and CRED methods for evaluating reliability and relevance of ecotoxicity studies. Environ. Sci. Eur. 2016, 28, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, A.L.; Bernot, M.J.; Bernot, R.J. Relationships between the psychiatric drug carbamazepine and freshwater macroinvertebrate community structure. Sci. Total Environ. 2014, 496, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.A. Use of LC-MS and GC-MS Methods to measure emerging contaminants pharmaceutical and personal care products (PPCPs) in fish. J. Chromatogr. Sep. Tech. 2015, 06. [Google Scholar] [CrossRef]
- Dahms, H.U.; Hagiwara, A.; Lee, J.S. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat. Toxicol. 2011, 101, 1–12. [Google Scholar] [CrossRef]
- Prado, R.; Rioboo, C.; Herrero, C.; Cid, A. The herbicide paraquat induces alterations in the elemental and biochemical composition of non-target microalgal species. Chemosphere 2009, 76, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- McCormick, P.V.; Cairns, J. Algae as indicators of environmental change. J. Appl. Phycol. 1994, 6, 509–526. [Google Scholar] [CrossRef]
- Hoppe, P.D.; Rosi-Marshall, E.J.; Bechtold, H.A. The antihistamine cimetidine alters invertebrate growth and population dynamics in artificial streams. Freshw. Sci. 2012, 31, 379–388. [Google Scholar] [CrossRef]
- Stancová, V.; Ziková, A.; Svobodová, Z.; Kloas, W. Effects of the non-steroidal anti-inflammatory drug (NSAID) naproxen on gene expression of antioxidant enzymes in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2015, 40, 343–348. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar]
- Karlsson, M.V.; Marshall, S.; Gouin, T.; Boxall, A.B.A. Routes of uptake of diclofenac, fluoxetine, and triclosan into sediment-dwelling worms. Environ. Toxicol. Chem. 2016, 35, 836–842. [Google Scholar] [CrossRef] [PubMed]
- García-Santiago, X.; Franco-Uría, A.; Omil, F.; Lema, J.M. Risk assessment of persistent pharmaceuticals in biosolids: Dealing with uncertainty. J. Hazard. Mater. 2016, 302, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ngo, H.H.; Guo, W. A critical review on sustainability assessment of recycled water schemes. Sci. Total Environ. 2012, 426, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals, (REACH) establishing a European Chemicals Agency, amending Directive 1999/45/EC, repealing Council Regulation (EEC) No 793/93, Commission Regulation (EC) No 1488/94, Council Directive 76/769/EEC, Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC, 2000/21/EC. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en:PDF (accessed on 29 May 2007).
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Wong, M.H. Integrated wetlands for food production. Environ. Res. 2016, 426, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.M.; Afzal, M.; Ullah, I.; Shahid, N.; Baqar, M.; Arslan, M. Removal of pharmaceuticals and personal care products using constructed wetlands: Effective plant-bacteria synergism may enhance degradation efficiency. Environ. Sci. Pollut. Res. 2019, 26, 21109–21126. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Chen, Z.; Wang, X.C.; Chen, R.; Zhang, X. Microalgae for saline wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1224–1265. [Google Scholar] [CrossRef]
- Homrich, A.S.; Galvão, G.; Abadia, L.G.; Carvalho, M.M. The circular economy umbrella: Trends and gaps on integrating pathways. J. Clean. Prod. 2018, 175, 525–543. [Google Scholar] [CrossRef]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Clean. Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.S.; Am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key green engineering research areas for sustainable manufacturing: A perspective from pharmaceutical and fine chemicals manufacturers. Org. Process Res. Dev. 2011, 51, 900–911. [Google Scholar] [CrossRef]
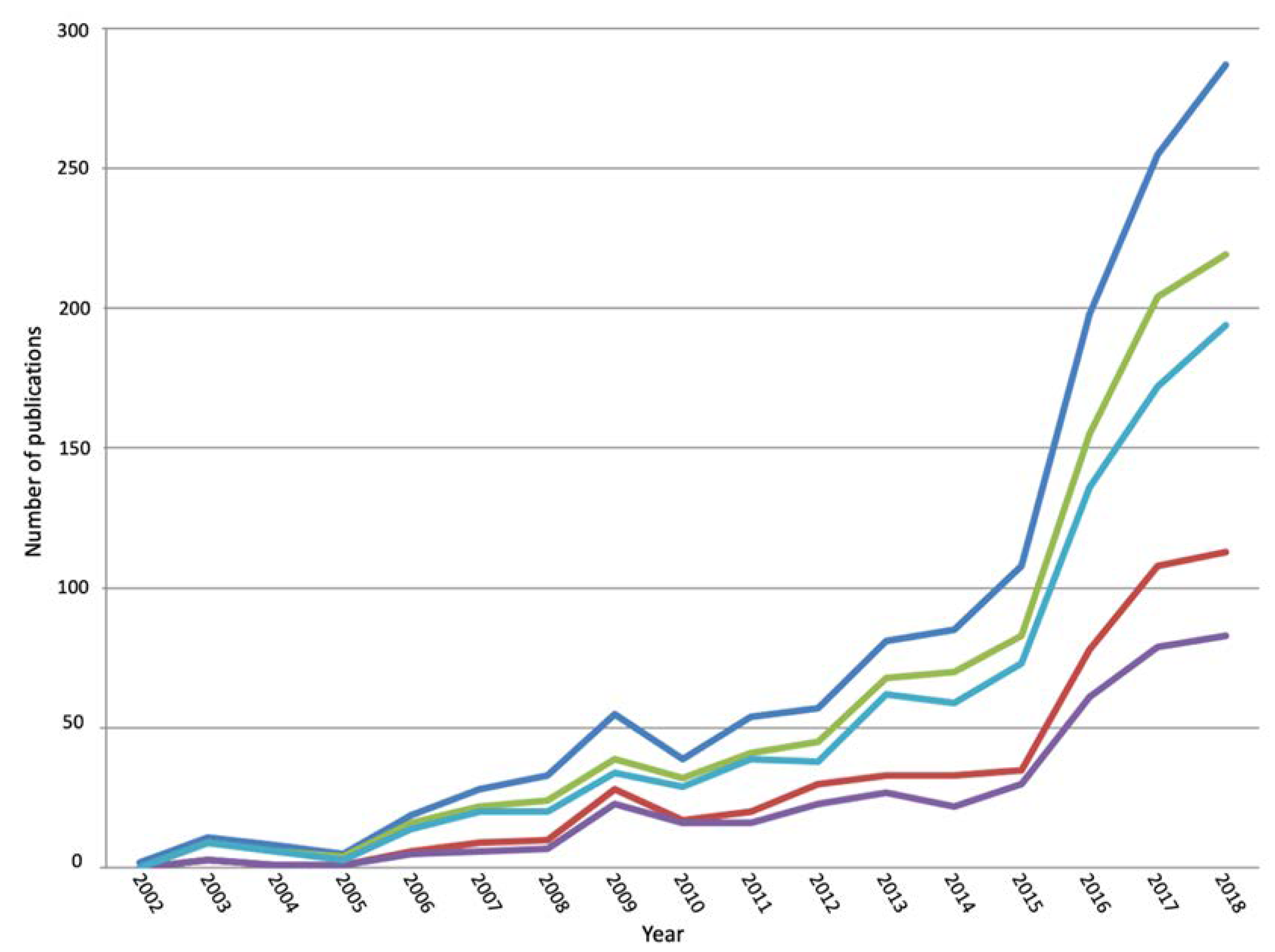
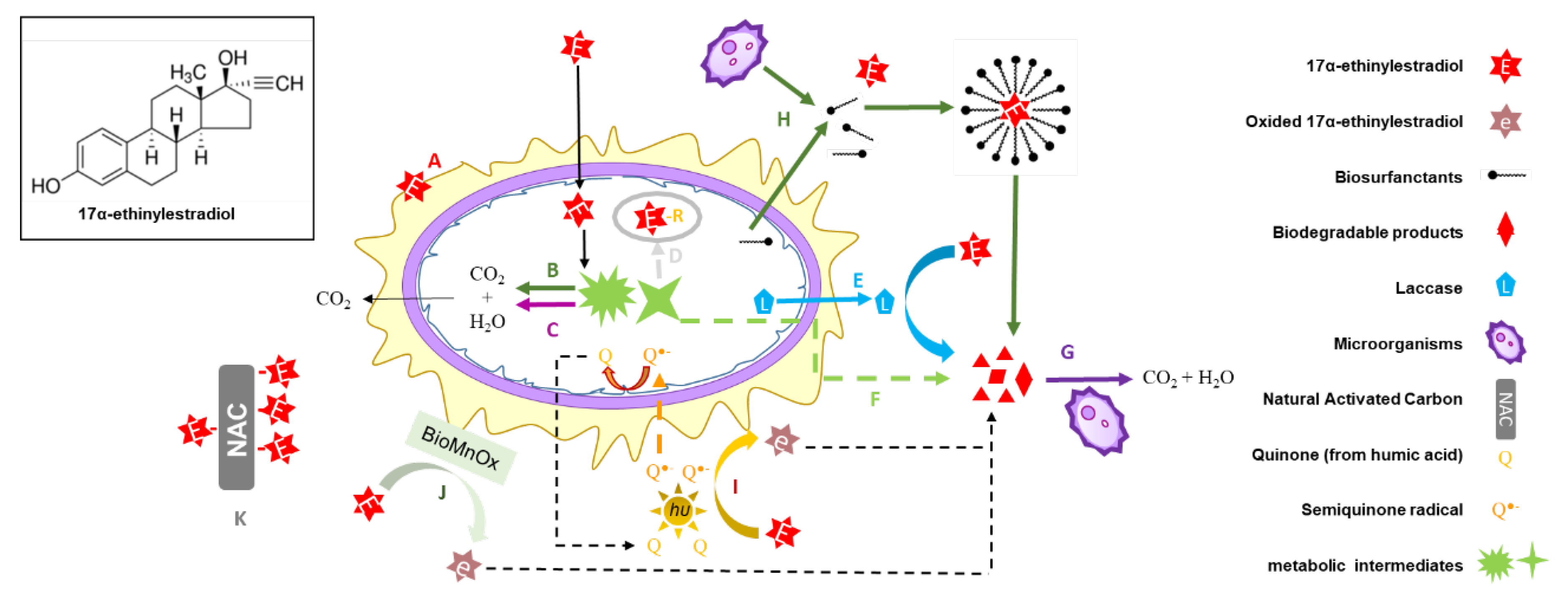
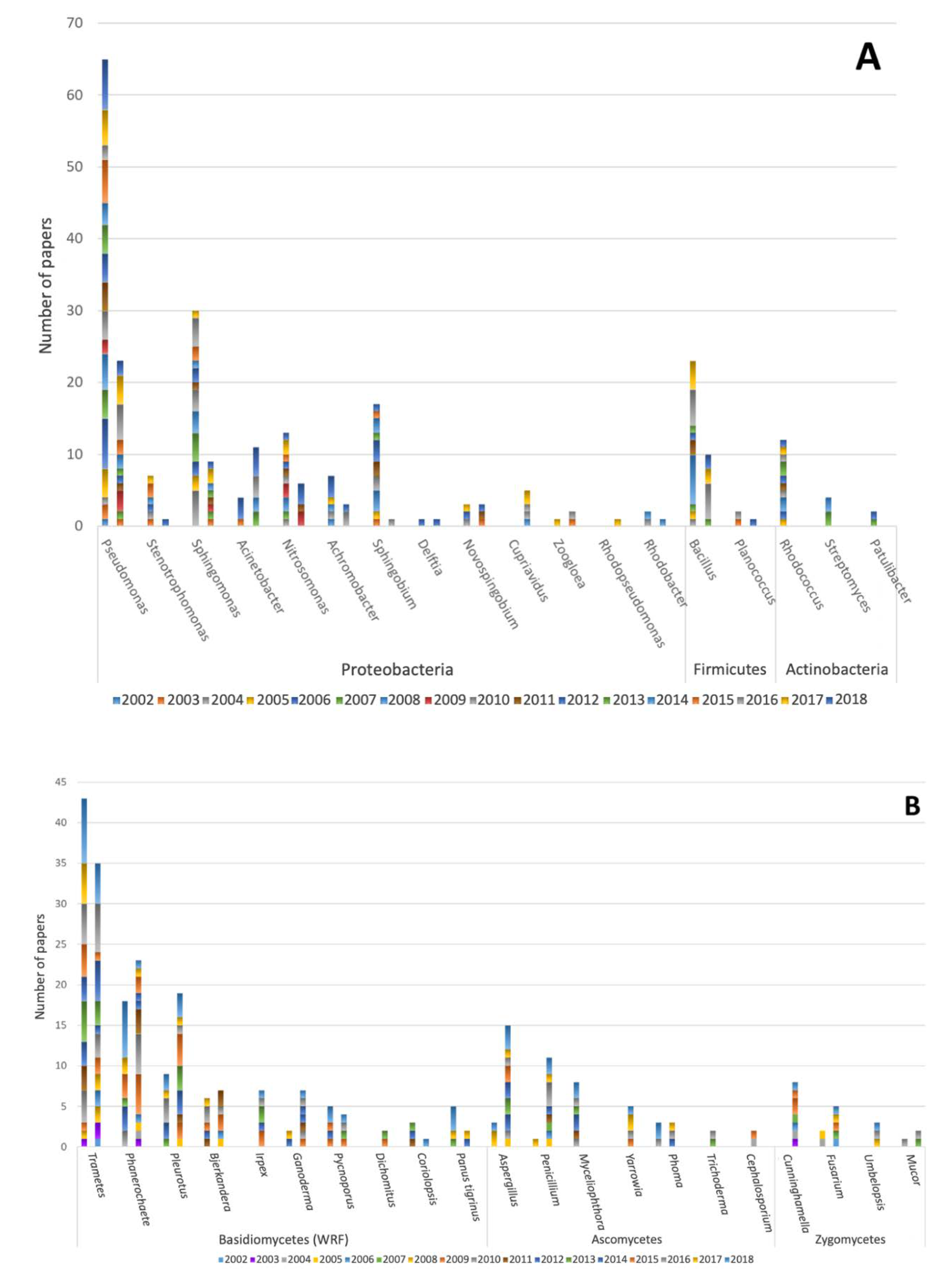
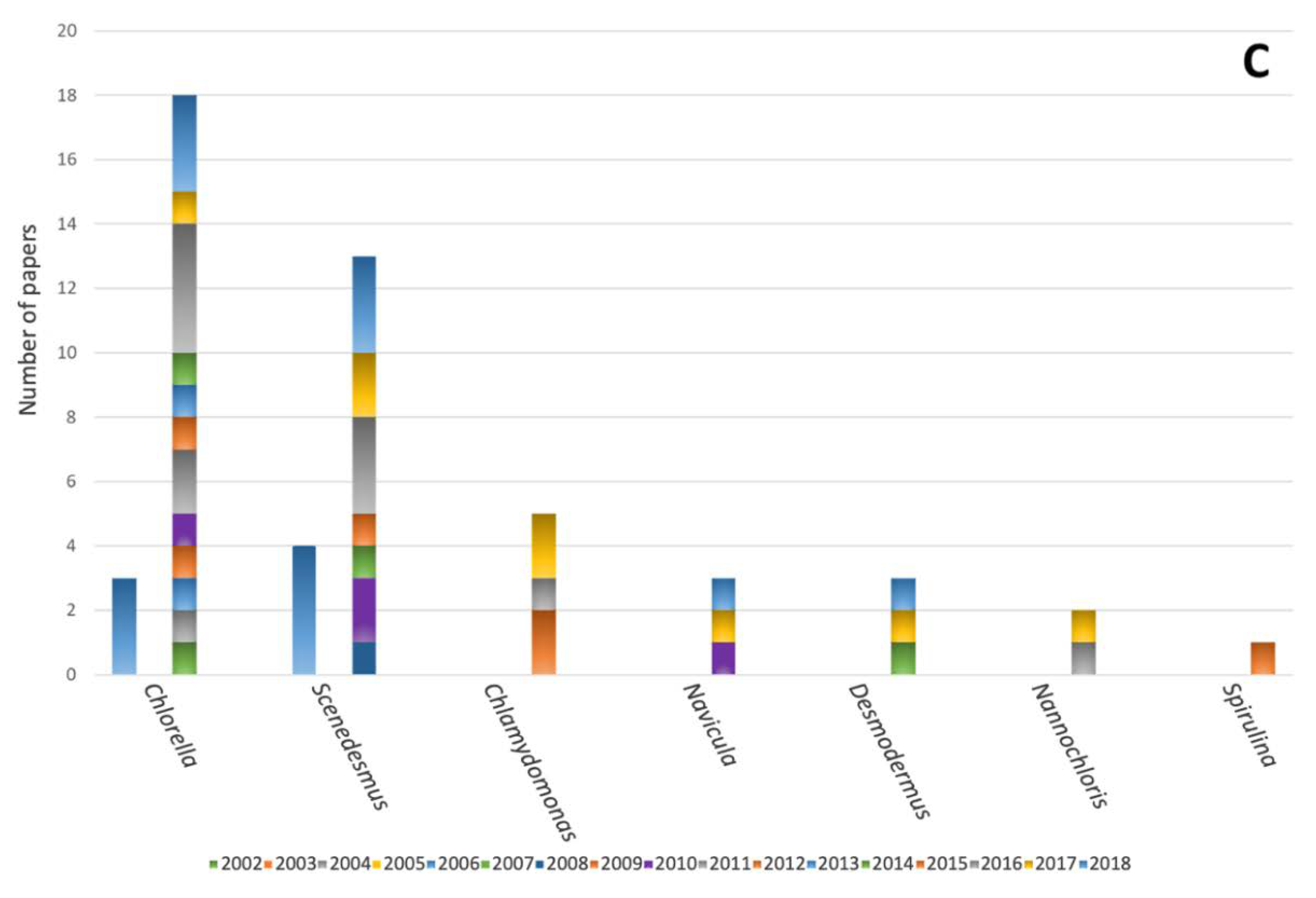
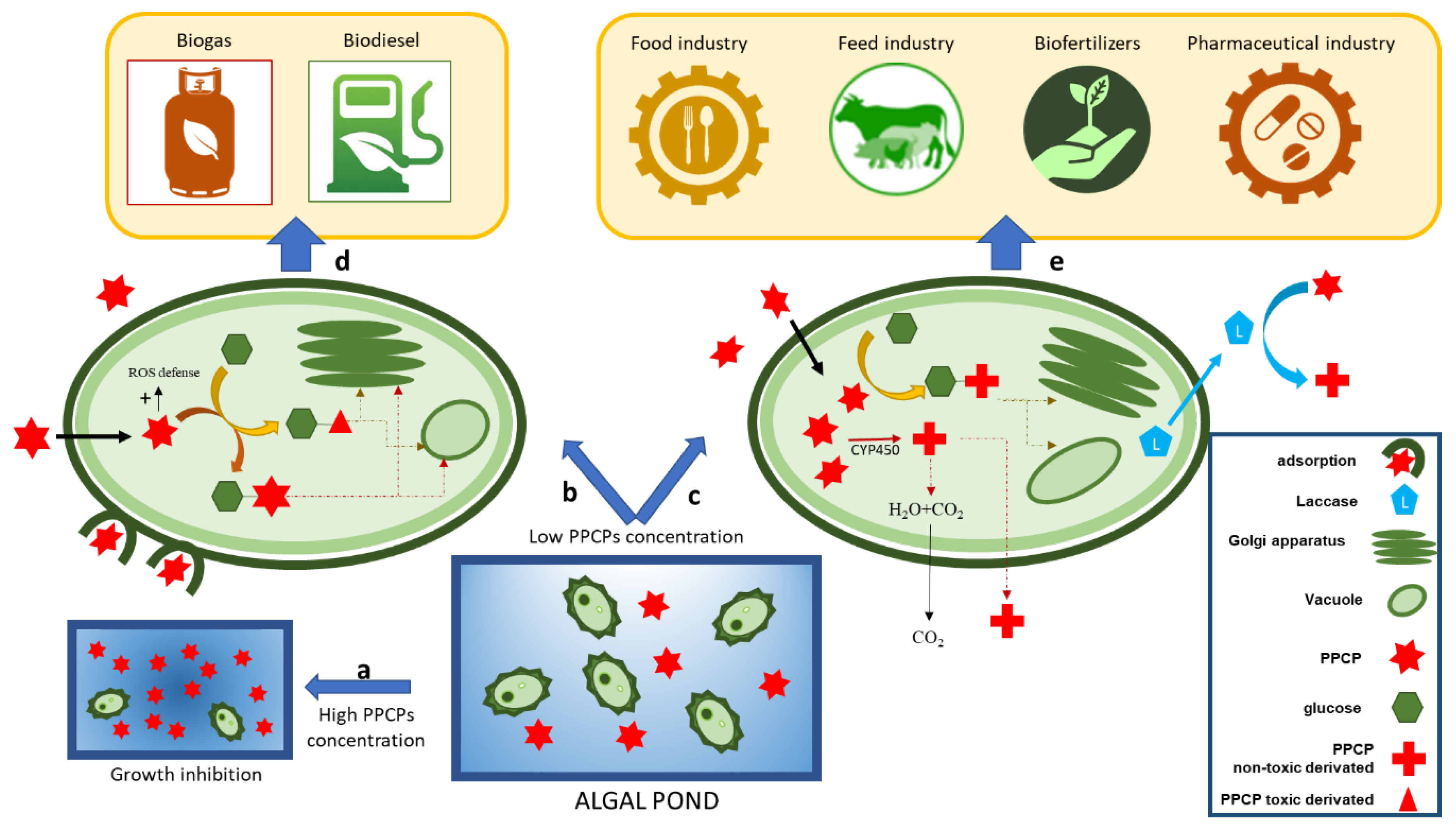
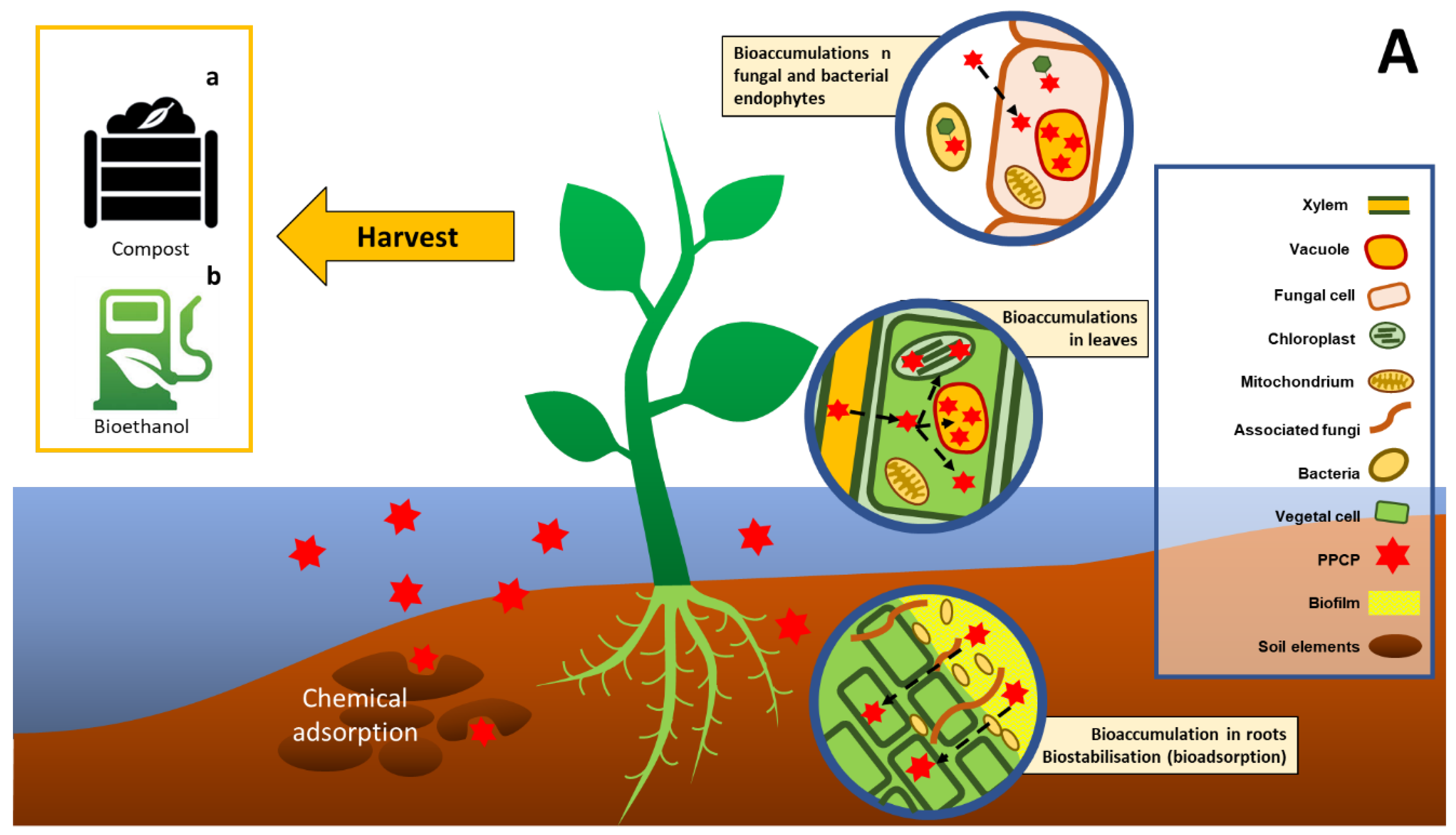
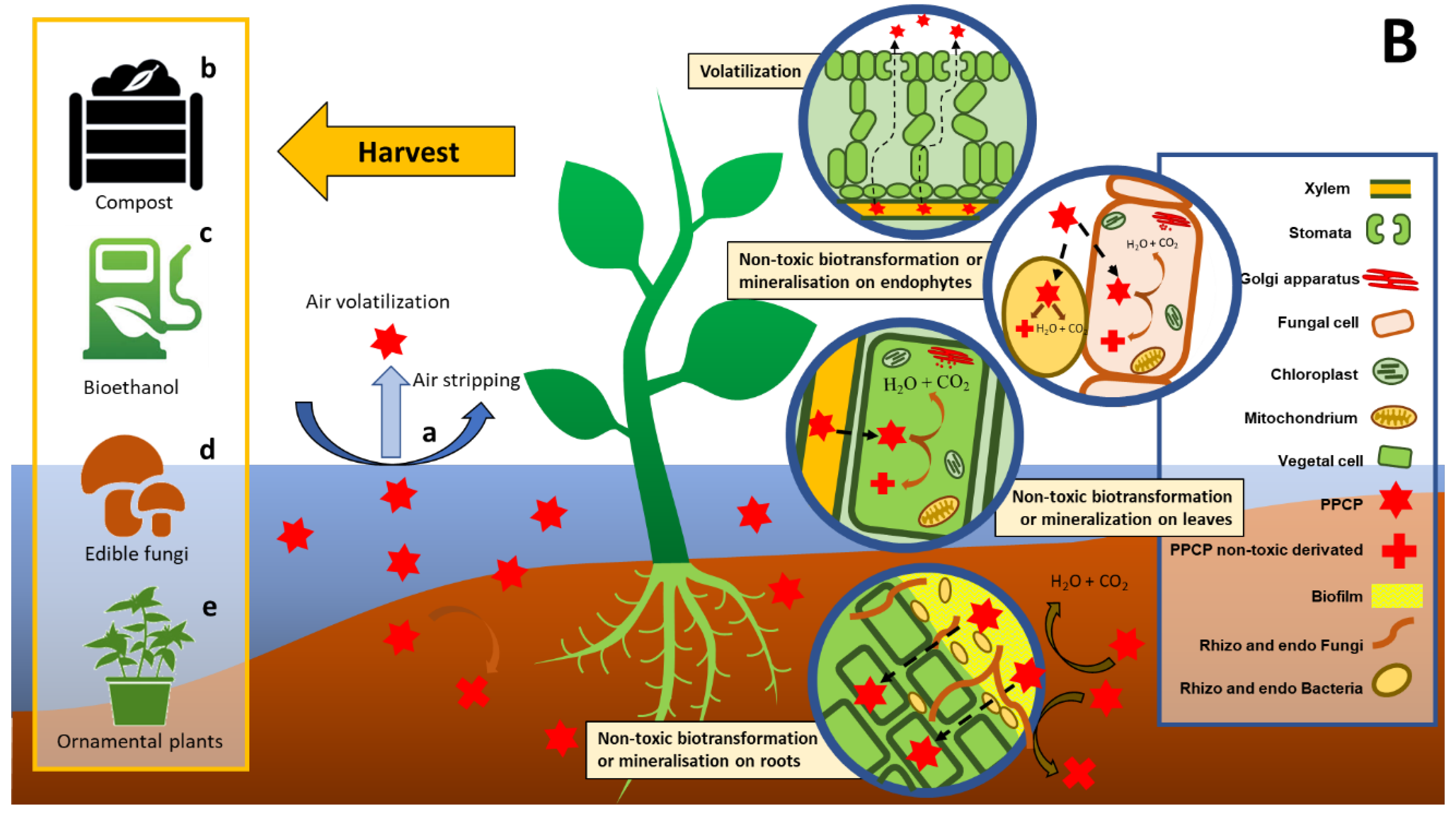
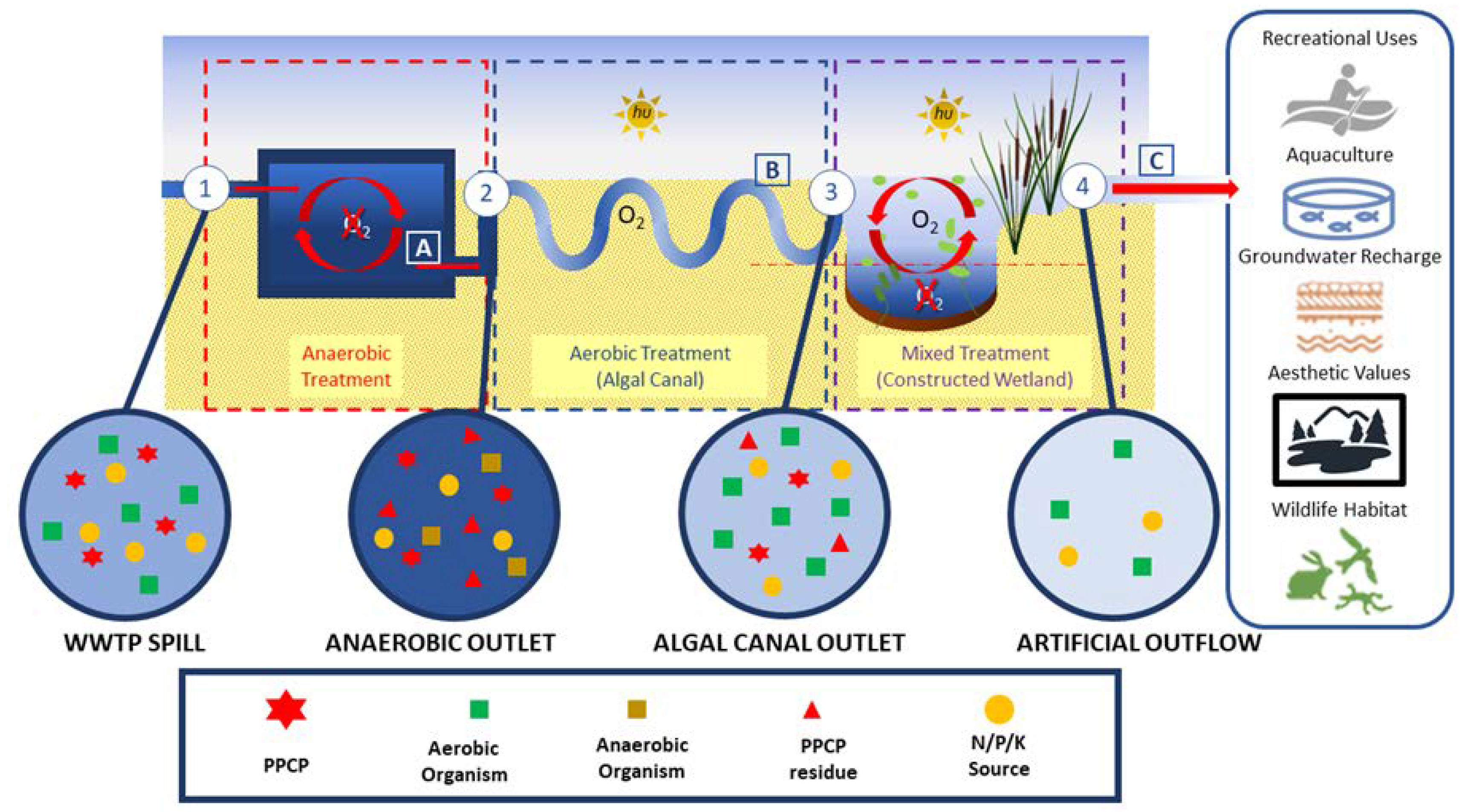
| Phylum | Species | Mixture Type | Endpoint/Biomarker Effect | Reference |
|---|---|---|---|---|
| Bacteroidetes | Flavobacterium sp. | Psychostimulant + Antihistamine + Antibiotic + Antidiabetic, cholesterol reducer | Relative decreases on bacterial community composition | [16] |
| Pseudomonas sp. | Psychostimulant + Antihistamine + Antibiotic + Antidiabetic, cholesterol reducer | Relative increases on bacterial community composition | [16] | |
| Firmicutes | Paracoccus yeei | Analgesic and antipyretic drug + Nonsteroidal anti-inflammatory drug | Susceptibility | [13] |
| Staphylococcus aureus | Analgesic and antipyretic drug + Nonsteroidal anti-inflammatory drug | Susceptibility | [13] | |
| Proteobacteria | Enterobacter aerogenes | Analgesic and antipyretic drug + Nonsteroidal anti-inflammatory drug | Resistance | [13] |
| Enterobacter cloacae | Analgesic and antipyretic drug + Nonsteroidal anti-inflammatory drug | Resistance | [13] | |
| Escherichia coli | Hair dyes | Cytotoxicity and mutagenic effect | [64] | |
| Vibrio fischeri | Antibiotics + Disinfectant + H2 blocker + Lipid regulators + Nonsteroidal anti-inflammatory drug + Preservatives | Narcosis | [37] | |
| Rotifera | Plationus patulus | Antidepressant + Psychostimulant + Disinfectants + Nonsteroidal anti-inflammatory drugs | Egg reduction and detachment | [65] |
| Phanerogams Magnoliophyte | Lemna minor | Antibiotics | Growth reduction | [66] |
| Chlorophyta | Chlamydomonas microsphaera | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] |
| Chlorella pyrenoidosa | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Chlorella ellipsoidea | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Dunaliella salina | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Dunaliella parva | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Pseudokirchneriella subcapitata | Lipid regulators + Nonsteroidal anti-inflammatory drugs | Increased the level of lipid peroxidation, glutathione transferase and metallothioneins. DNA damage. Destabilization of the lysosomal membrane | [68] | |
| Scendesmus obliquus | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Scendesmus quadricauda | Antidepressant + Disinfectants | Inhibitory effect on algal growth | [67] | |
| Scenedesmus vacuolatus | Antibiotics | Growth redution | [66] | |
| Tetraselmis suecica | Antibiotics | Inhibited growth. Decrease in esterase activity and alteration of chlorophyll a, cellular content and autofluorescence | [69] | |
| Mollusca | Elliptio complanata | Analgesic + Antibiotics + Antidepressant + Anticonvulsants + Lipid regulators + Nonsteroidal anti-inflammatory drugs | Adverse effects on the immune system | [70] |
| Dreissena polymorpha | Lipid regulators + Nonsteroidal anti-inflammatory drugs | High levels of lipid peroxidation | [68] | |
| Lampsilis siliquoidea | Antibiotics + Hormones + Lipid regulators | Reduction in filter-feeding | [71] | |
| Unio tumidus | Hormones + Disinfectants + Nonsteroidal anti-inflammatory drugs | Elevated levels of lactate/pyruvate ratio, lipofuscin, DNA fragmentation and caspase-3 activity | [72] | |
| Arthropoda | Chironomus riparius | Hormones | Deformities in the mouth, decreased fertility | [73,74,75] |
| Daphnia magna | Antidepressant + Hormones | Population growth rate | [76] | |
| Antibiotics | Viability reduction | [66] | ||
| Antocha | Antidepressants | Metamorphosis occurred earlier and more frequently | [77] | |
| Corydalus | Antidepressants | Metamorphosis occurred earlier and more frequently | [77] | |
| Ectopria | Antidepressants | Metamorphosis occurred earlier and more frequently | [77] | |
| Psephenus | Antidepressants | Metamorphosis occurred earlier and more frequently | [77] | |
| Gammarus fossarum | Antibiotics | Increase in body mass | [78] | |
| Gammarus pulex | Beta blockers + Disinfectants + Nonsteroidal anti-inflammatory drugs | Alterations in metabolite concentrations | [79] | |
| Palaemonetes pugio | Antibiotics + Psychostimulant | Negative effects on offspring survival and development | [80] | |
| Chordata | Danio rerio | Antidepressant + Anticonvulsant + Antihistamine + Nonsteroidal anti-inflammatory drug | Perturbations in both the metabolome and transcriptome | [81] |
| Antidepressant + Anticonvulsant + Lipid regulators + Nonsteroidal anti-inflammatory drugs | Decreased embryo production | [82] | ||
| Oncorhynchus mykiss | Hormones | Inhibitor of estrogenicity | [83] | |
| Sunscreen agents | Increased activity of certain P450 cytochromes | [84] | ||
| Antidepressant + Anticonvulsants + Beta blockers + Psychostimulant + Hormones + Nonsteroidal anti-inflammatory drugs + Thiazide diuretic + Fragrances | ROS production and the ß-gal inhibition (RTG-2 cell line) | [85] | ||
| Oryzias latipes | Nonsteroidal anti-inflammatory drugs | Antiovulatory activity | [86] | |
| Anticonvulsant + Disinfectant + Nonsteroidal anti-inflammatory drug | Effects on feeding behavior and swimming speed | [87] | ||
| Pimephales promelas | Antihistamine + Beta blocker + Psychostimulant + Disinfectants + Hormone + Lipid regulators + Nonsteroidal anti-inflammatory drugs | Molecular estrogenic effects | [88] | |
| Pseudorasbora parva | Antidepressant + Disinfectants | AChE and EROD activities inhibitionLipid peroxidation | [89] | |
| Trematomus bernachii | Hormones + Component in plastic and epoxy resins + Disinfectants + Precursors to the non-ionic surfactants + Preservatives | Preferential accumulation in fish correlating negatively with fillet size | [90] |
| Phylum | Species | Mixture Type | Endpoint/Biomarker Effect | Reference |
|---|---|---|---|---|
| Nematoda | Soil nematode community | Hormones | Number of nematodes was decreased and change the sex ratio in a free-living nematode community | [91] |
| Soil nematode community | Antibiotics + Anticonvulsants + Antipsychotic, antiemetic + Beta blockers + Psychostimulant + Component in plastic and epoxy resins + Diuretic + Fragrances + Hormones + Lipid regulators + Nonsteroidal anti-inflammatory drugs + Preservatives | The diversity and structure of the soil nematode community vastly altered | [92] | |
| Annelida | Eisenia andrei | Disinfectants | Affect the growth and reproductive performance | [93] |
| Arthropoda | Culex quinquefasciatus | Antibiotics + Psychostimulant + Hormones + Nonsteroidal anti-inflammatory drugs | Increase developmental time of larvae. Altered the mosquito bacterial microbiome | [94] |
| Magnoliophyte | Allium cepa | Hair dyes | Cytotoxic and mutagenic | [64] |
| Triticum aestivum L. | Analgesic and antipyretic drug | Wheat shoot and root elongation decreased. The antioxidative defensive system in roots was damaged | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, M.C.; Bautista, L.F.; Catalá, M.; de las Heras, M.R.; Martínez-Hidalgo, P.; San-Sebastián, J.; González-Benítez, N. From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems. Appl. Sci. 2020, 10, 3391. https://doi.org/10.3390/app10103391
Molina MC, Bautista LF, Catalá M, de las Heras MR, Martínez-Hidalgo P, San-Sebastián J, González-Benítez N. From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems. Applied Sciences. 2020; 10(10):3391. https://doi.org/10.3390/app10103391
Chicago/Turabian StyleMolina, María Carmen, Luis Fernando Bautista, Myriam Catalá, María Rosa de las Heras, Pilar Martínez-Hidalgo, Jon San-Sebastián, and Natalia González-Benítez. 2020. "From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems" Applied Sciences 10, no. 10: 3391. https://doi.org/10.3390/app10103391
APA StyleMolina, M. C., Bautista, L. F., Catalá, M., de las Heras, M. R., Martínez-Hidalgo, P., San-Sebastián, J., & González-Benítez, N. (2020). From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems. Applied Sciences, 10(10), 3391. https://doi.org/10.3390/app10103391







