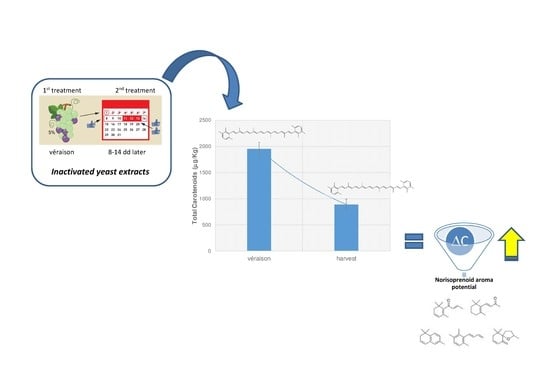
How Pre-Harvest Inactivated Yeast Treatment May Influence the Norisoprenoid Aroma Potential in Wine Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Maturation Indexes of Grapes
2.4. Extraction of Carotenoids from Grapes
2.5. HPLC-DAD-MS Analyses of Carotenoids
2.6. Statistical Analysis
3. Results and Discussion
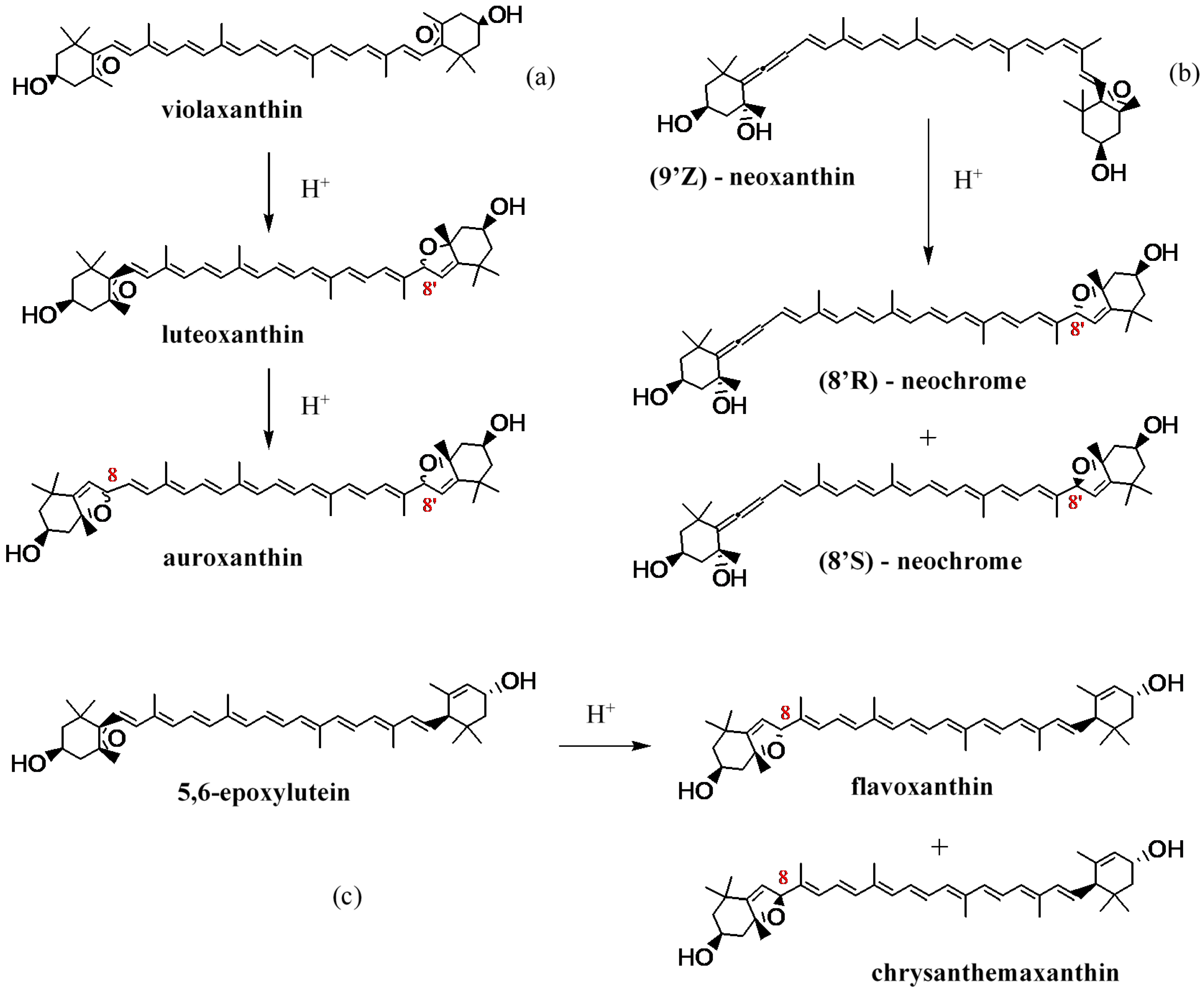
3.1. Carotenoid Composition of the Wine Grape Varieties
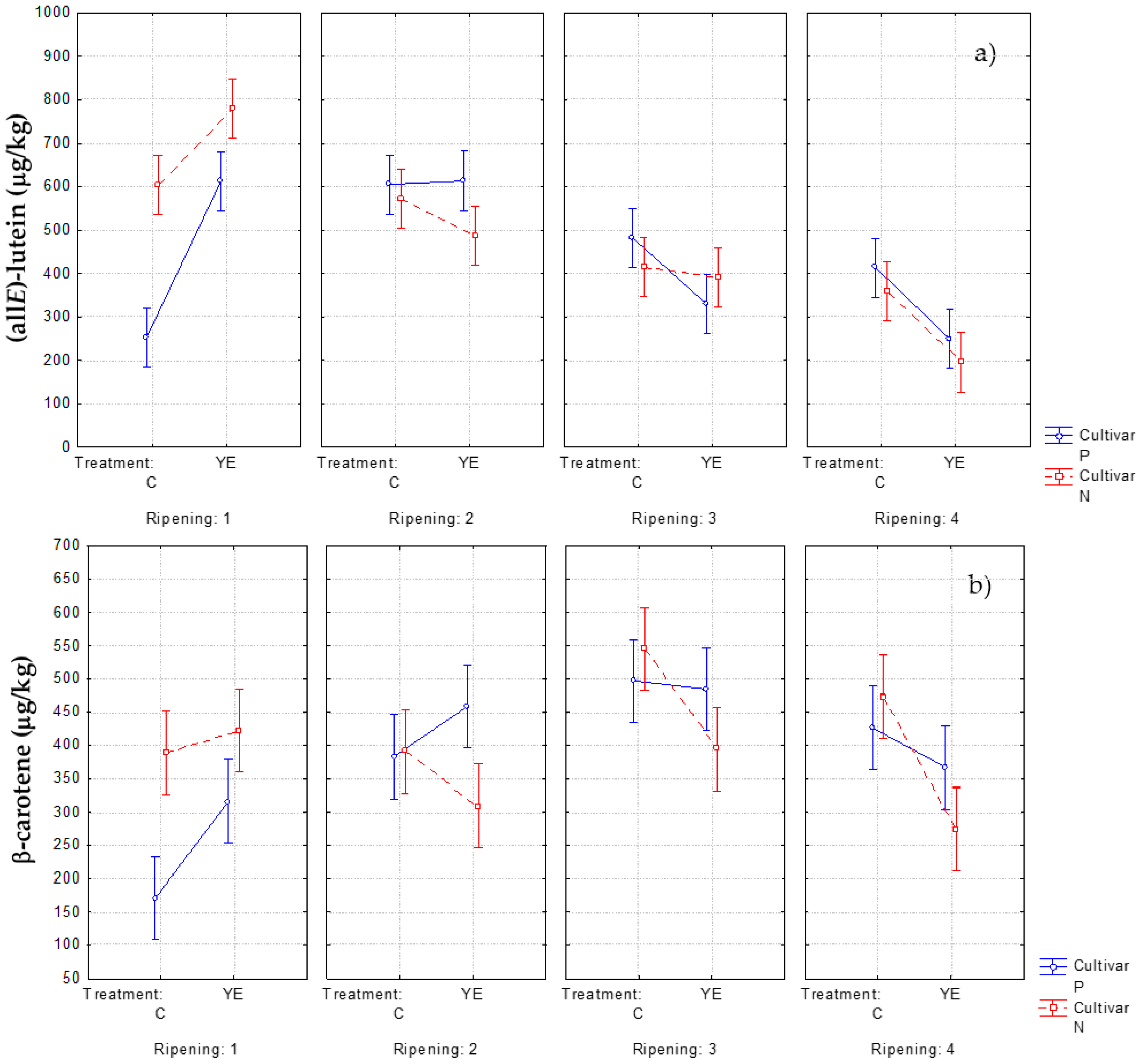
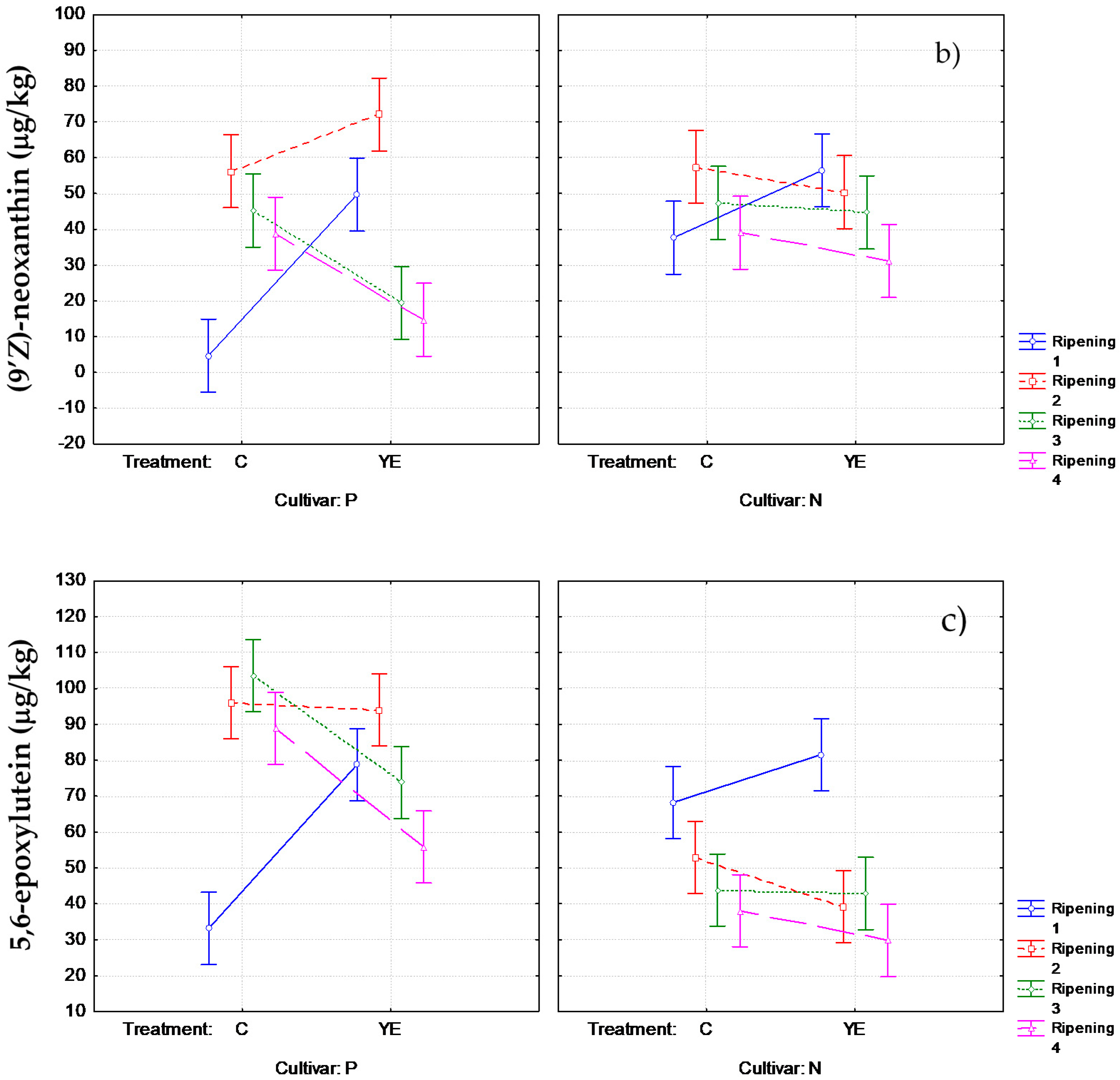
3.2. Effect of Pre-Harvest YE Application on Carotenoid Content
3.3. Influence of YE Treatment on the Norisoprenoid Aroma Potential (ΔC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razungles, A.; Babic, I.; Sapis, J.C.; Bayonove, C.L. Particolar behavior of epoxy xanthophylls during veraison and maturation of grape. J. Agric. Food Chem. 1996, 44, 3821–3825. [Google Scholar] [CrossRef]
- Razungles, A.; Bayonove, C.L.; Cordonnier, R.E.; Baumes, R.L. Etude des Carotenoides du Raisin à Maturitè. Vitis 1987, 26, 183–191. [Google Scholar]
- Razungles, A.; Bayonove, C.L.; Cordonnier, R.E.; Sapis, J.C. Grape carotenoides: Changes during the maturation period and localization in mature berries. Am. J. Enol. Vitic. 1988, 39, 44–48. [Google Scholar]
- Crupi, P.; Milella, R.A.; Antonacci, D. Simultaneous HPLC-DAD-MS (ESI+) determination of structural and geometrical isomers of carotenoids in mature grapes. J. Mass Spectrom. 2010, 45, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Silva-Ferreira, A.C.; Caris-Veyrat, C.; Guedes de Pinho, P. Carotenoid, chlorophyll, and chlorophyll-derived compounds in grapes and port wines. J. Agric. Food Chem. 2005, 53, 10034–10041. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ferreira, A.C.; Monteiro, J.; Oliveira, C.; Guedes de Pinho, P. Study of major aromatic compounds in port wines from carotenoid degradation. Food Chem. 2008, 110, 83–87. [Google Scholar] [CrossRef]
- Crupi, P.; Coletta, A.; Milella, R.A.; Palmisano, G.; Baiano, A.; La Notte, E.; Antonacci, D. Carotenoid and Chlorophyll derived compounds in some wine grapes grown in Apulian region. J. Food Sci. 2010, 75, S191–S198. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Confirmation of the identity of the carotenoids of tropical fruits by HPLC-DAD and HPLC-MS. J. Food Compos. Anal. 2004, 17, 385–396. [Google Scholar] [CrossRef]
- Crupi, P.; Alba, V.; Masi, G.; Caputo, A.R.; Tarricone, L. Effect of two exogenous plant growth regulators on the color and quality parameters of seedless table grape berries. Food Res. Int. 2019, 126, 108667. [Google Scholar] [CrossRef]
- Baumes, R. Wine aroma precursors. In Wine chemistry and biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]
- Crupi, P.; Coletta, A.; Antonacci, D. Analysis of carotenoids in grapes to predict norisoprenoid varietal aroma of wines from Apulia. J. Agric. Food Chem. 2010, 58, 9647–9656. [Google Scholar] [CrossRef]
- Baumes, R.; Wirth, J.; Bureau, S.; Gunata, Y.; Razungles, A. Biogeneration of C13-norisoprenoid compounds: Experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim Acta 2002, 458, 3–14. [Google Scholar] [CrossRef]
- Oliveira, C.; Silva Ferreira, A.C.; Mendes Pinto, M.; Hogg, T.; Alves, F. Carotenoid compounds in grapes and their relationship to plant water status. J. Agric. Food Chem. 2003, 51, 5967–5971. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B. Liquid chromatography/mass spectrometry of carotenoids. Pure App. Chem. 1997, 69, 2061–2066. [Google Scholar] [CrossRef] [Green Version]
- Emenhiser, C.; Simunovic, N.; Sander, L.C.; Schwartz, S.J. Separation of geometrical carotenoid isomers in biological extracts using a polymeric C30 column in reversed-phase liquid chromatography. J. Agric. Food Chem. 1996, 44, 3887–3893. [Google Scholar] [CrossRef]
- Ferrari, S. Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutic. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Landes Bioscience and Springer Science + Business Media LLC: New York, NY, USA, 2010; pp. 152–166. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Šuklje, K.; Antalick, G.; Buica, A.; Coetzee, Z.A.; Brand, J.; Schmidtke, L.M.; Vivier, M.A. Inactive dry yeast application on grapes modify Sauvignon Blanc wine aroma. Food Chem. 2016, 197, 1073–1084. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Marín-San Román, S.; Jofré, V.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Effects on chlorophyll and carotenoid contents in different grape varieties (Vitis vinifera L.) after nitrogen and elicitor foliar applications to the vineyard. Food Chem. 2018, 269, 380–386. [Google Scholar] [CrossRef]
- Betz, M.; Schindler, C.; Schwender, J.; Lichtenthaler, H.K. Jasmonic acid induced changes in carotenoid levels and zeaxanthin cycle performance. In Plant Lipid Metabolism; Kader, J.C., Mazliak, P., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 353–355. [Google Scholar]
- Lu, Y.; Jiang, P.; Liu, S.; Gan, Q.; Cui, H.; Quin, S. Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of β-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour. Technol. 2010, 101, 6468–6474. [Google Scholar] [CrossRef]
- Office International de la Vigne et du Vin (OIV). Recueil des Methodes Internationales d’Analyse des Vins et des Mouts; Office International de la Vigne et du Vin: Paris, France, 1990. [Google Scholar]
- Melendez-Martinez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F.Weber exWiggers). Phytochemistry 2006, 67, 771–777. [Google Scholar] [CrossRef]
- Martì, M.P.; Busto, O.; Guash, J. Application of a headspace mass spectrometry system to the differentiation and classification of wines according to their origin, variety and ageing. J. Chromatogr. A 2004, 1057, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Palou, A. Chromatographic determination of carotenoids in foods. J. Chromatogr. A 2000, 881, 543–555. [Google Scholar] [CrossRef]
- Bureau, S.M.; Razungles, A.J.; Baumes, R.L.; Bayonove, C.L. Effect of vine or bunch shading on the carotenoid composition in Vitis Vinifera L. berries. I. Syrah grapes. Vitic. Enol. Sci. 1998, 53, 64–71. [Google Scholar]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Bernalte, M.J.; Ayuso, M.C.; Rodríguez, A.B. Sweet cherry phytochemicals: Identification and characterization by HPLC-DAD/ESI-MS in six sweet cherry cultivars grown in Valle del Jerte (Spain). J. Food Compos. Anal. 2010, 23, 533–539. [Google Scholar] [CrossRef]
- Oliveira, C.; Barbosa, A.; Silva Ferreira, A.C.; Guerra, J.; Guedes De Pinho, P. Carotenoid profile in grapes related to aromatic compounds in wines from Douro region. J. Food Sci. 2006, 71, S1–S7. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry 2018, 156, 1–8. [Google Scholar] [CrossRef]
- Kogkou, C.; Chorti, E.; Kyraleou, M.; Kallithraka, S.; Koundouras, S.; Logan, G.; Kanakis, I.; Kotseridis, Y. Effects of foliar application of inactivated yeast on the phenolic composition of Vitis vinifera L. cv. Agiorgitiko grapes under different irrigation level. Int. J. Wine Res. 2017, 9, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Influence of the use of elicitors over the composition of cell wall grapes. In Proceedings of the In Vino Analytica Scientia 2017, Salamanca, Spain, 17–20 July 2017. [Google Scholar]
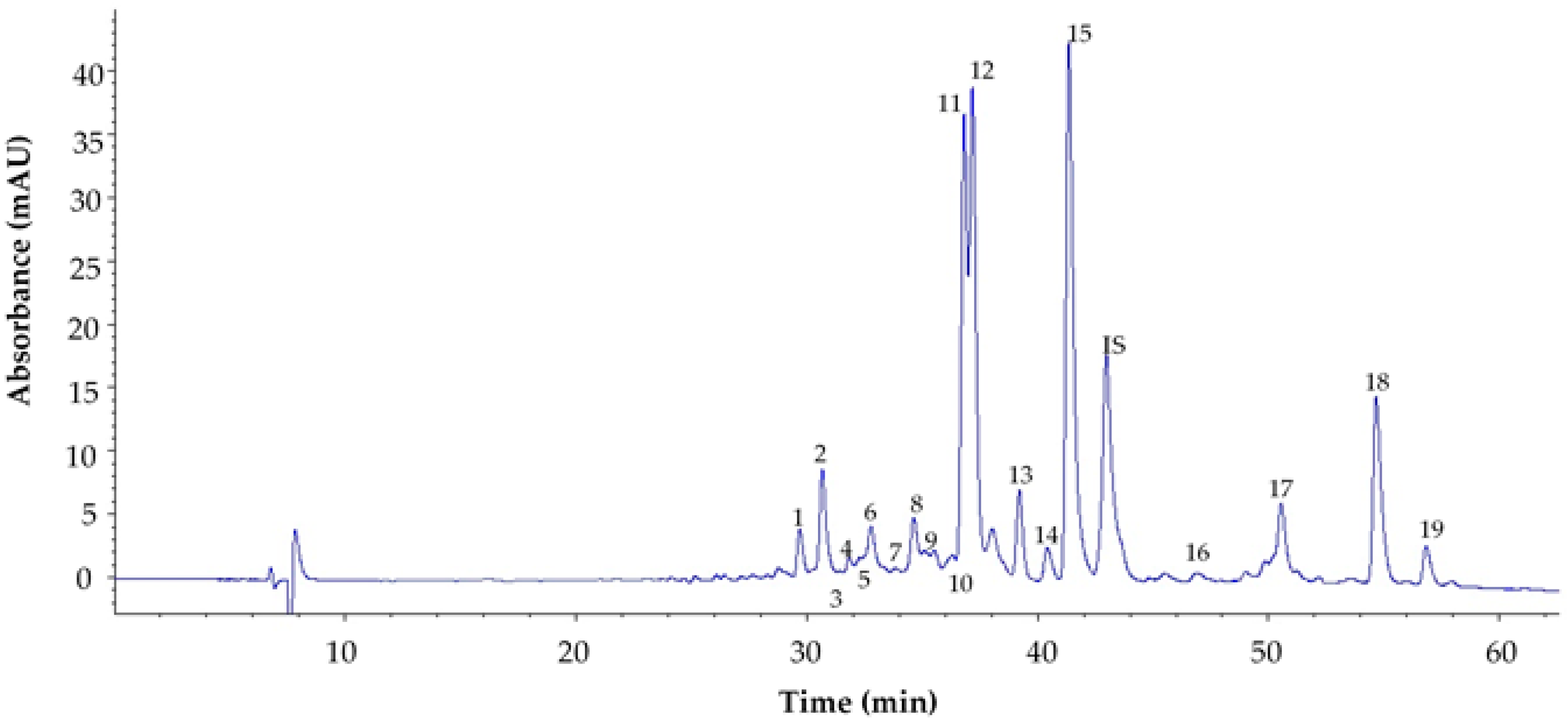




| Peak | Compound | k’ | λmax (nm) | % (III/II)a | DB/DIIb | [M+H]+(m/z) | [M]·+(m/z) | MS2 Product Ions m/z |
|---|---|---|---|---|---|---|---|---|
| 1 | violaxanthin like structure | 2.78 | 418;440;470 | 84 | 601.5 | 600.1 | 583.5, 565.5, 509.5, 491.5, 221.1 | |
| 2 | violaxanthin | 2.92 | 416; 440; 468 | 86 | 601.5 | 600.1 | 583.5, 565.5, 509.5, 491.5, 221.1 | |
| 3 | (8′R)-neochrome | 3.01 | 400; 422; 450 | 88 | 601.5 | 600.1 | 583.2, 565.3, 509.5, 221.1 | |
| 4 | (9′Z)-neoxanthin | 3.06 | 414; 436; 464 | 86 | 601.5 | 600.1 | 583.2, 565.3, 509.5, 221.1 | |
| 5 | (8′S)-neochrome | 3.12 | 400; 422; 450 | 88 | 601.5 | 600.1 | 583.2, 565.3, 509.5, 221.1 | |
| 6 | 5,6-epoxylutein | 3.19 | 416; 440; 468 | 90 | 585.4 | 584.2 | 567.1, 493.1, 221.1 | |
| 7 | luteoxanthin | 3.33 | 399; 422; 448 | 94 | 601.5 | 600.1 | 583.2, 221.1 | |
| 8 | lutein like structure | 3.42 | (425); 446; 474 | 568.9 | 567.9 | 550.9, 532.9, 476.4, 429.4 | ||
| 9 | Z lutein like structure | 3.47 | 328; (412); 436; 464 | 568.9 | 567.9 | 550.9, 532.9, 476.4, 429.4 | ||
| 10 | (8′S)-auroxanthin | 3.64 | 380; 402; 426 | 98 | 601.5 | 600.1 | 583.5, 565.5, 509.5, 491.5, 221.1 | |
| 11 | chlorophyll b | 3.70 | 258; 314; 342; 466; 600; 650 | |||||
| 12 | (allE)-lutein | 3.75 | (422); 446; 472 | 40 | 568.9 | 567.9 | 550.9, 532.9, 476.4, 429.4 | |
| 13 | zeaxanthin | 4.00 | (425); 452; 476 | 22 | 568.9 | 567.9 | 550.9, 532.9, 476.4, 429.4 | |
| 14 | (9Z) or (9’Z)-lutein | 4.16 | 330; (422); 440; 468 | 50 | 0.075 | 568.9 | 567.9 | 550.9, 532.9, 476.4, 429.4 |
| 15 | chlorophyll a | 4.31 | 336; (385); (417); 432; 618; 665 | |||||
| IS | β-apo-8′-carotenal | 4.50 | 460 | |||||
| 16 | pheophytin b | 5.46 | (417); 436; 527; 600; 654 | 885 | ||||
| 17 | pheophytin a | 5.59 | 410; 506; 536; 666 | 871 | ||||
| 18 | β-carotene | 5.98 | (430); 452; 478 | 25 | 536.9 | 535.9 | 444.2, 430.3, 399.3 | |
| 19 | (9Z)- β-carotene | 6.25 | 342; (424); 446; 474 | 17 | 0.03 | 536.9 | 535.9 | 444.2, 430.3, 399.3 |
| Sampling Date | 08/13/19* | 08/26/19 | 09/02/19 | 09/17/19 | ΔCa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | YE | C | YE | C | YE | C | YE | C | YE | ||
| TSSb | 153.0c ± 1.7d | 142.3 ± 1.2 | 165 ± 2 | 163 ± 2 | 171.4 ± 1.4 | 189 ± 1.0 | 212 ± 3 | 220 ± 3 | |||
| pH | 3.11 ± 0.01 | 3.17 ± 0.01 | 3.10 ± 0.02 | 3.26 ± 0.01 | 3.31 ± 0.06 | 3.38 ± 0.04 | 3.36 ± 0.04 | 3.43 ± 0.06 | |||
| TAe | 8.47 ± 0.15 | 7.78 ± 0.16 | 6.25 ± 0.18 | 5.82 ± 0.10 | 5.63 ± 0.07 | 6.6 ± 0.4 | 5.8 ± 0.3 | 5.4 ± 0.4 | |||
| TSS/TAf | 18.1 ± 0.4 | 18.3 ± 0.5 | 26.4 ± 1.1 | 27.9 ± 0.4 | 30.4 ± 0.2 | 29 ± 3 | 37 ± 2 | 40 ± 3 | |||
| Compounds | |||||||||||
| violaxanthin like structureg | 30 ± 5ch | 64 ± 15a | 49 ± 5ab | 34 ± 5bc | 39 ± 6bc | 42 ± 7bc | 34 ± 5bc | 29 ± 4c | |||
| violaxanthing | 96 ± 10b | 126 ± 14a | 80 ± 12bc | 67 ± 10c | 91 ± 14bc | 97 ± 15b | 79 ± 12bc | 68 ± 9c | |||
| (8′R)-neochromei | 8.2 ± 0.6a | 9.5 ± 0.3a | 8.5 ± 0.8a | 4.8 ± 1.0b | 3.2 ± 0.6c | 4.2 ± 0.4bc | 2.8 ± 0.5c | 2.95 ± 0.17c | |||
| (9′Z)-neoxanthini | 38 ± 10ab | 56 ± 10a | 58 ± 12a | 50 ± 8ab | 47 ± 13ab | 45 ± 6ab | 39 ± 7ab | 31 ± 3b | |||
| (8′S)-neochromei | 6.0 ± 0.9b | 9 ± 2a | 7.3 ± 1.3ab | 6.2 ± 1.1bc | 4.6 ± 0.7c | 5.5 ± 0.8bc | 4.0 ± 0.6c | 3.8 ± 0.5c | |||
| 5,6-epoxy-luteinl | 68 ± 5a | 82 ± 8a | 53 ± 9b | 39 ± 6bc | 44 ± 7bc | 43 ± 6bc | 38 ± 6bc | 30 ± 3c | |||
| luteoxanthing | 8.8 ± 1.2b | 14 ± 2a | 8.2 ± 1.0bc | 7.6 ± 1.5bc | 9.0 ± 1.0b | 5.8 ± 0.8c | 7.8 ± 0.9bc | tr | |||
| lutein like structure m | 44 ± 7b | 62 ± 5a | 34 ± 5bc | 32 ± 5bcd | 29 ± 6cd | 29 ± 7cd | 25 ± 6cd | 20 ± 4d | |||
| Z lutein like structure m | 9.8 ± 0.8b | 14.9 ± 1.8a | tr | tr | 5.9 ± 1.2c | 4.1 ± 1.8cd | 5.1 ± 1.1cd | 2.8 ± 1.2d | |||
| (8′S)-auroxanthing | 54 ± 7a | 68 ± 15a | 29 ± 5b | 10 ± 3c | 19 ± 8bc | 18 ± 3bc | 15 ± 5bc | 12 ± 2bc | |||
| (all-E)-luteinm | 600 ± 90b | 780 ± 60a | 570 ± 90bc | 490 ± 90bcd | 410 ± 60cd | 390 ± 70d | 360 ± 60de | 200 ± 20e | |||
| zeaxanthinn | 88 ± 4 | 101 ± 5 | 65 ± 9 | 65 ± 15 | 30 ± 5 | 28 ± 4 | 26 ± 5 | 20 ± 2 | |||
| (9Z)-luteinm | 17 ± 5c | 33 ± 5a | 30 ± 6ab | 20 ± 5bc | 10 ± 3d | 11 ± 3cd | 9 ± 2d | 7 ± 2d | |||
| β-caroteneo | 390 ± 60bc | 420 ± 30abc | 390 ± 60bc | 310 ± 60c | 550 ± 90a | 390 ± 60bc | 470 ± 80ab | 270 ± 40c | |||
| (9Z)-β-caroteneo | 130 ± 50 | 110 ± 20 | 180 ± 50 | 150 ± 40 | 230 ± 20 | 167 ± 19 | 200 ± 20 | 116 ± 8 | |||
| Totalp | 1600 ± 170ab | 1950 ± 90a | 1600 ± 200ab | 1280 ± 180b | 1500 ± 200b | 1280 ± 180b | 1320 ± 170b | 820 ± 30c | 270 ± 300 | 1130 ± 120 | |
| Sampling Date | 08/13/19 | 08/26/19* | 09/04/19 | 09/19/19 | ΔCa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | YE | C | YE | C | YE | C | YE | C | YE | ||
| TSSb | 175c ± 1.0d | 170 ± 2 | 187 ± 1.5 | 200 ± 2 | 226 ± 4 | 227 ± 14 | 236 ± 5 | 239 ± 15 | |||
| pH | 2.98 ± 0.02 | 2.97 ± 0.03 | 3.19 ± 0.02 | 3.22 ± 0.02 | 3.40 ± 0.01 | 3.35 ± 0.08 | 3.45 ± 0.01 | 3.40 ± 0.07 | |||
| TAe | 11.2 ± 0.4 | 13.1 ± 0.6 | 6.8 ± 0.3 | 7.03 ± 0.15 | 6.2 ± 0.4 | 6.7 ± 0.3 | 5.7 ± 0.4 | 6.1 ± 0.4 | |||
| TSS/TAf | 15.7 ± 0.5 | 12.8 ± 0.4 | 27.8 ± 1.3 | 28. 8 ± 0.7 | 36.3 ± 1.9 | 34 ± 3 | 41 ± 2 | 39 ± 3 | |||
| Compounds | |||||||||||
| violaxanthin like structureg | 5 ± 2dh | 21 ± 6bc | 39 ± 9a | 25 ± 7abc | 35 ± 7ab | 22 ± 6bc | 30 ± 6abc | 17 ± 5cd | |||
| violaxanthing | 31 ± 19d | 144 ± 6ab | 160 ± 30a | 129 ± 9ab | 154 ± 18ab | 115 ± 17bc | 132 ± 15ab | 87 ± 12c | |||
| (8′R)-neochromei | tr | tr | tr | 3.2 ± 0.5 | tr | tr | tr | tr | |||
| (9′Z)-neoxanthini | 4.7 ± 1.4d | 50 ± 15ab | 56 ± 15ab | 72 ± 17a | 45 ± 11b | 19 ± 5cd | 39 ± 9bc | 15 ± 4cd | |||
| (8′S)-neochromei | 10 ± 3a | 3.4 ± 1.6b | 2.3 ± 0.6b | 5.3 ± 1.3b | 3.5 ± 1.2b | 3.3 ± 0.7b | 3.0 ± 1.0b | 2.5 ± 0.5b | |||
| 5,6-epoxy-luteinl | 33 ± 13d | 79 ± 9abc | 100 ± 20ab | 94 ± 14ab | 104 ± 10a | 74 ± 7bc | 89 ± 8ab | 56 ± 5cd | |||
| luteoxanthing | 6 ± 2 | tr | tr | tr | tr | tr | tr | tr | |||
| lutein like structure m | 18 ± 12d | 86 ± 12a | 74 ± 13ab | 69 ± 8ab | 61 ± 10b | 36 ± 4cd | 52 ± 9bc | 28 ± 3d | |||
| Z lutein like structure m | tr | 19 ± 2ab | 25 ± 2a | 18 ± 4ab | 24 ± 5a | 18 ± 4ab | 21 ± 4ab | 14 ± 3b | |||
| (8′S)-auroxanthing | 16 ± 3a | 11 ± 2abc | 10 ± 5abc | 15 ± 5ab | 12.3 ± 1.3abc | 8.1 ± 1.5bc | 10.5 ± 1.1abc | 6.1 ± 1.1c | |||
| (all-E)-luteinm | 250 ± 110d | 610 ± 90a | 610 ± 80a | 610 ± 70a | 480 ± 40ab | 329 ± 19cd | 410 ± 30bc | 249 ± 14d | |||
| zeaxanthinn | 30 ± 20c | 110 ± 20a | 68 ± 16b | 64 ± 6b | 39 ± 12bc | 18 ± 3c | 33 ± 10c | 13 ± 2c | |||
| (9Z)-luteinm | 9 ± 7bc | 27 ± 4a | 26 ± 6a | 20 ± 3ab | 15 ± 6bc | 7 ± 5c | 13 ± 5bc | 5 ± 4c | |||
| β-caroteneo | 170 ± 110c | 320 ± 40b | 380 ± 80ab | 460 ± 70ab | 497 ± 19a | 480 ± 40ab | 426 ± 17ab | 370 ± 30ab | |||
| (9Z)-β-caroteneo | 69 ± 4 | 51 ± 6 | 57 ± 10 | 52 ± 11 | 48 ± 6 | 51 ± 11 | 41 ± 5 | 39 ± 9 | |||
| Totalp | 700 ± 300d | 1500 ± 180ab | 1600 ± 200a | 1640 ± 120a | 1520 ± 80ab | 1190 ± 80bc | 1310 ± 70ab | 900 ± 60cd | 290 ± 300 | 740 ± 180 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crupi, P.; Santamaria, M.; Vallejo, F.; Tomás-Barberán, F.A.; Masi, G.; Caputo, A.R.; Battista, F.; Tarricone, L. How Pre-Harvest Inactivated Yeast Treatment May Influence the Norisoprenoid Aroma Potential in Wine Grapes. Appl. Sci. 2020, 10, 3369. https://doi.org/10.3390/app10103369
Crupi P, Santamaria M, Vallejo F, Tomás-Barberán FA, Masi G, Caputo AR, Battista F, Tarricone L. How Pre-Harvest Inactivated Yeast Treatment May Influence the Norisoprenoid Aroma Potential in Wine Grapes. Applied Sciences. 2020; 10(10):3369. https://doi.org/10.3390/app10103369
Chicago/Turabian StyleCrupi, Pasquale, Marika Santamaria, Fernando Vallejo, Francisco A. Tomás-Barberán, Gianvito Masi, Angelo Raffaele Caputo, Fabrizio Battista, and Luigi Tarricone. 2020. "How Pre-Harvest Inactivated Yeast Treatment May Influence the Norisoprenoid Aroma Potential in Wine Grapes" Applied Sciences 10, no. 10: 3369. https://doi.org/10.3390/app10103369
APA StyleCrupi, P., Santamaria, M., Vallejo, F., Tomás-Barberán, F. A., Masi, G., Caputo, A. R., Battista, F., & Tarricone, L. (2020). How Pre-Harvest Inactivated Yeast Treatment May Influence the Norisoprenoid Aroma Potential in Wine Grapes. Applied Sciences, 10(10), 3369. https://doi.org/10.3390/app10103369