Influence of Pairing Startling Acoustic Stimuli with Postural Responses Induced by Light Touch Displacement
Abstract
1. Introduction
2. Materials and Methods
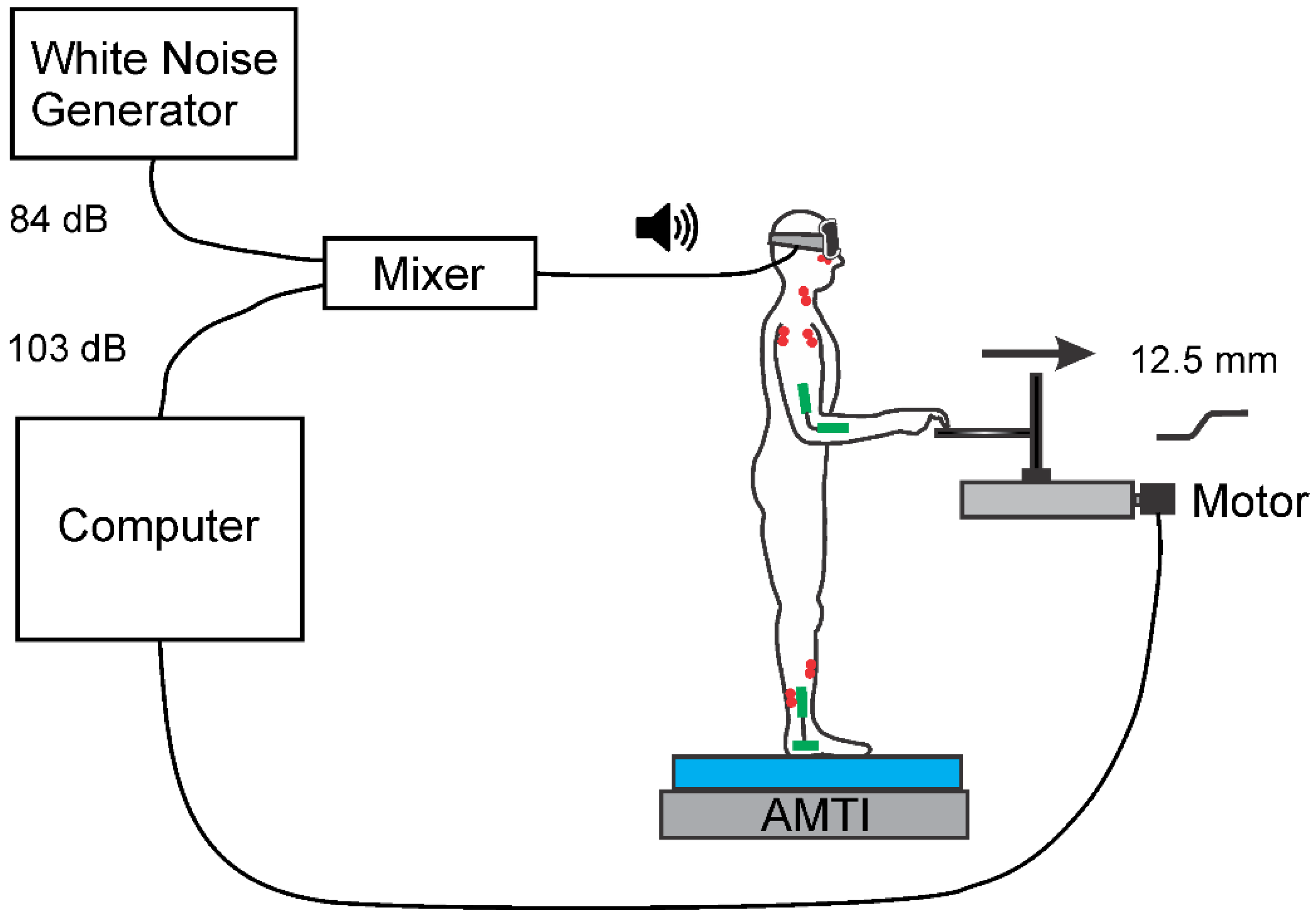
2.1. Set-Up and Apparatus
2.2. Protocol
2.3. Recording and Data Acquisition
2.4. Data Analysis
2.5. Statistics
3. Results
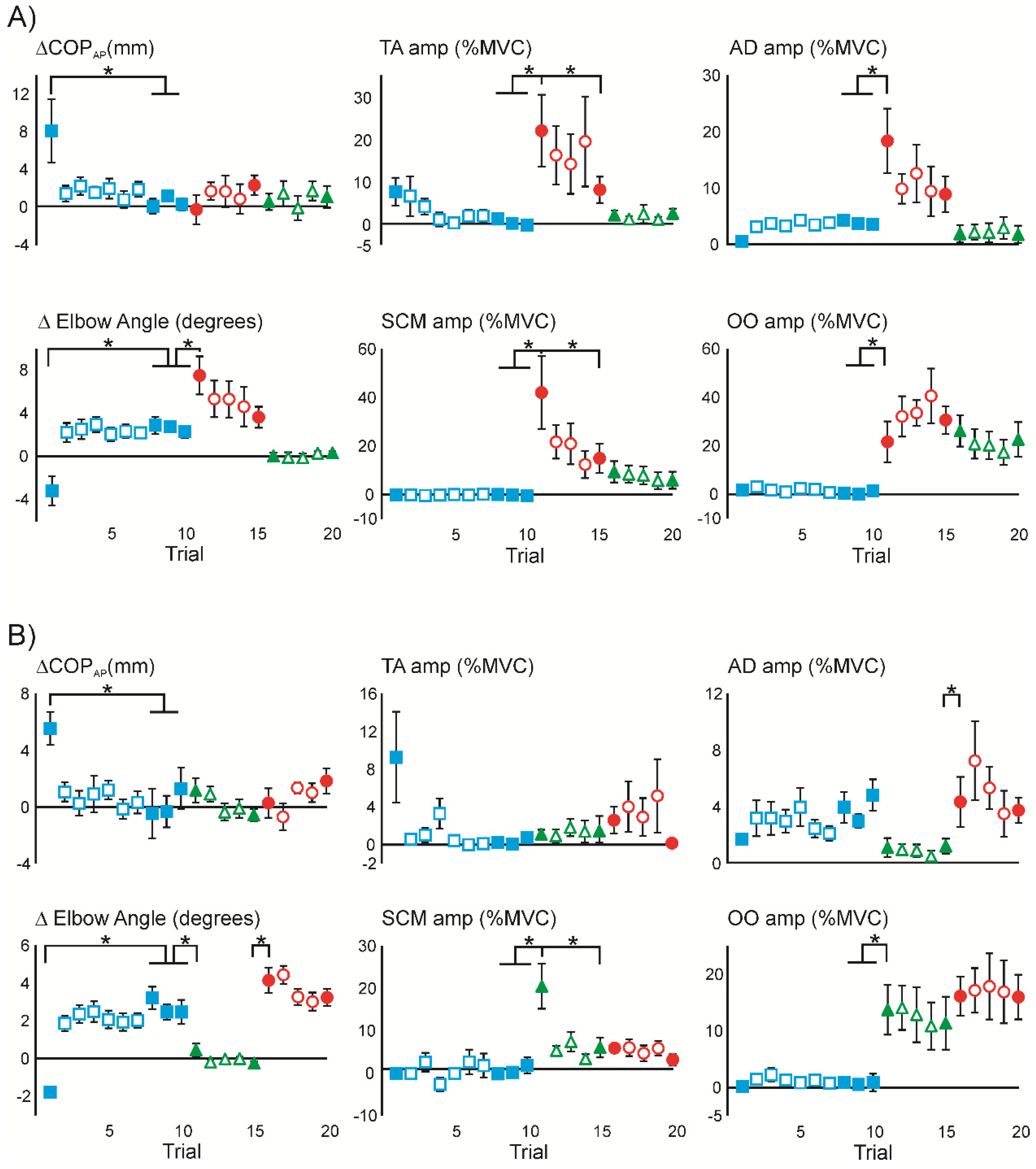
3.1. Superimposition of Acoustic Startle on Habituated Touch Displacement Responses
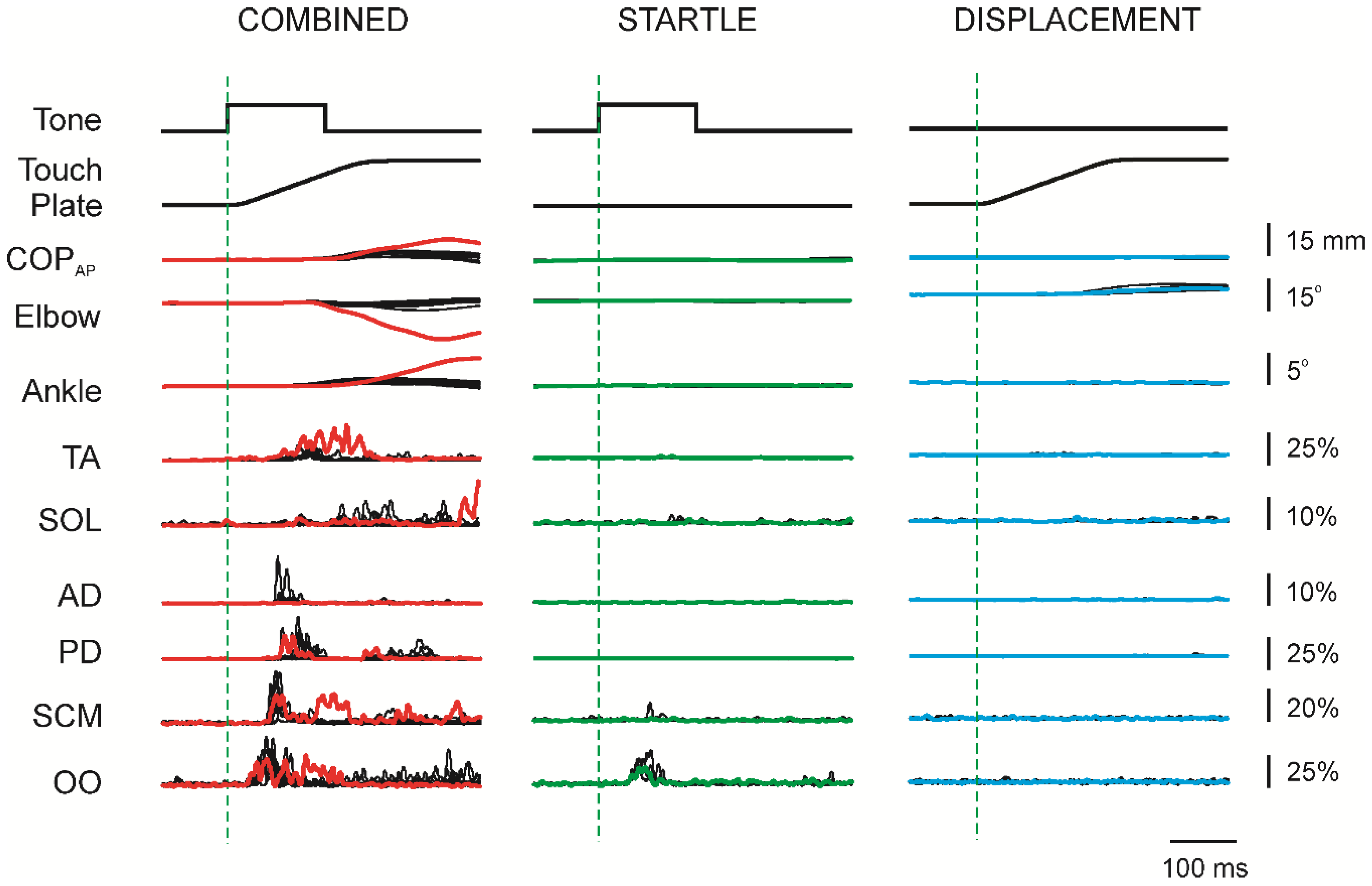
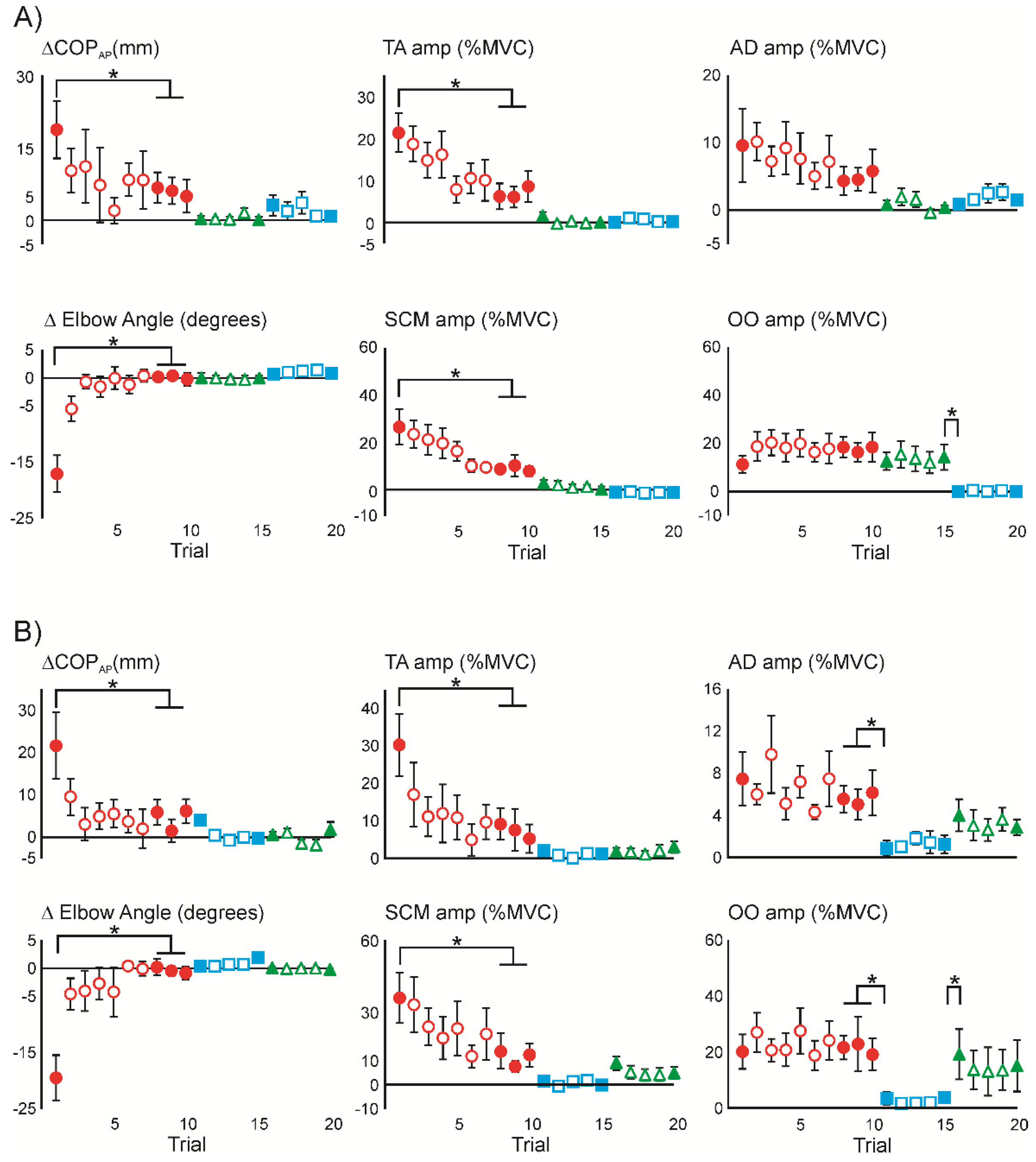
3.2. Initial Superimposition of Acoustic Startle and Touch Displacement
3.3. Comparing Initial COMBINED with Initial DISPLACEMENT
3.4. Superimposition of Touch Displacement on Habituated Acoustic Startle Responses
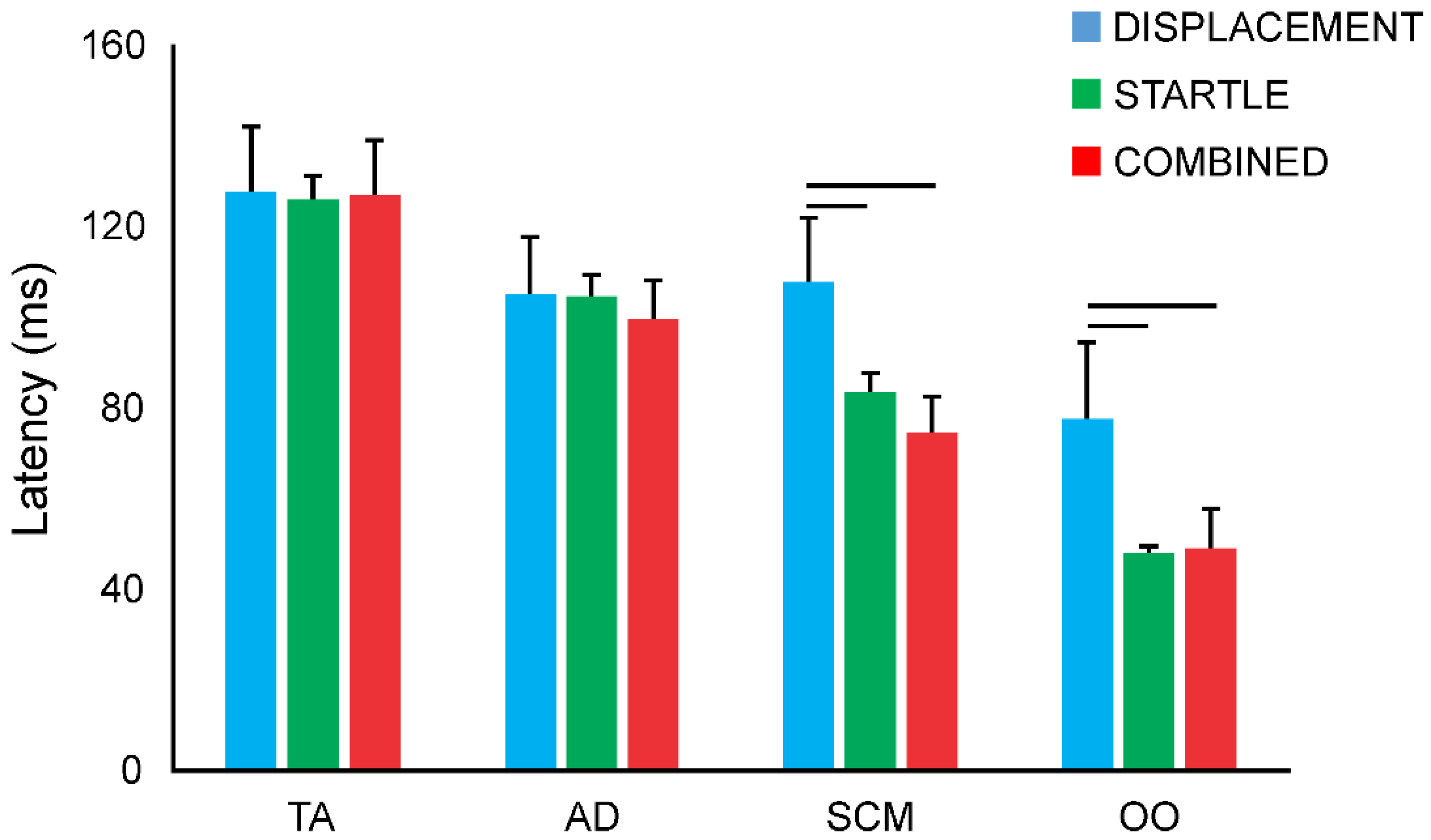
3.5. Response Latencies
4. Discussion
4.1. Interaction between Acoustic Startle and Habituated Touch Displacement Responses
4.2. Interaction of Acoustic Startle and Novel Touch Displacement Responses
4.3. Technical Considerations
4.4. Functional Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marigold, D.S.; Misiaszek, J.M. Whole-body responses: Neural control and implications for rehabilitation and fall prevention. Neuroscientist 2009, 15, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Misiaszek, J.E.; Forero, J.; Hiob, E.; Urbanczyk, T. Automatic postural responses following rapid displacement of a light touch contact during standing. Neuroscience 2016, 316, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Misiaszek, J.E.; Vander Meulen, J. Balance reactions to light touch displacements when standing on foam. Neurosci. Lett. 2017, 639, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.S.; Li, L.; Scott, B.W.; Frankland, P.W. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci. Biobehav. Rev. 2002, 26, 1–11. [Google Scholar] [CrossRef]
- Allum, J.H.J.; Tang, K.S.; Carpenter, M.G.; Oude Nijhuis, L.B.; Bloem, B.R. Review of first trial responses in balance control: Influence of vestibular loss and Parkinson’s disease. Hum. Mov. Sci. 2011, 30, 279–295. [Google Scholar] [CrossRef]
- Campbell, A.D.; Squair, J.W.; Chua, R.; Inglis, J.T.; Carpenter, M.G. First trial and StartReact effects induced by balance perturbations to upright stance. J. Neurophysiol. 2013, 110, 2236–2245. [Google Scholar] [CrossRef][Green Version]
- Oude Nijhuis, L.B.; Allum, J.H.; Borm, G.F.; Honegger, F.; Overeem, S.; Bloem, B.R. Directional sensitivity of “first trial” reactions in human balance control. J. Neurophysiol. 2009, 101, 2802–2814. [Google Scholar] [CrossRef]
- Oude Nijhuis, L.B.; Allum, J.H.J.; Valls-Solé, J.; Overeem, S.; Bloem, B.R. First trial postural reactions to unexpected balance disturbances: A comparison with the acoustic startle reaction. J. Neurophysiol. 2010, 104, 2704–2712. [Google Scholar] [CrossRef][Green Version]
- Sanders, O.P.; Savin, D.N.; Creath, R.A.; Rogers, M.W. Protective balance and startle responses to sudden freefall in standing humans. Neurosci. Lett. 2015, 586, 8–12. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Yeomans, J.S. Summation between acoustic and trigeminal stimuli evoking startle. Neuroscience 1999, 90, 139–152. [Google Scholar] [CrossRef]
- Li, L.; Steidl, S.; Yeomans, J.S. Contributions of the vestibular nucleus and vestibulospinal tract to the startle reflex. Neuroscience 2001, 106, 811–821. [Google Scholar] [CrossRef]
- Blouin, J.S.; Siegmund, G.P.; Inglis, J.T. Interaction between acoustic startle and habituated neck postural responses in seated subjects. J. App. Phsysiol. 2007, 102, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Zaiontz, C. Real Statistics Using Excel. Available online: http://www.real-statistics.com/ (accessed on 22 March 2018).
- Carlsen, A.N.; Maslovat, D.; Lam, M.Y.; Chua, R.; Franks, I.M. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci. Biobehav. Rev. 2011, 35, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Valls-Solé, J.; Rothwell, J.C.; Goulart, F.; Cossu, G.; Muñoz, E. Patterned ballistic movements triggered by a startle in healthy humans. J. Physiol. 1999, 516, 931–938. [Google Scholar] [CrossRef]
- Carlsen, A.N.; Chua, R.; Inglis, J.T.; Sanderson, D.J.; Franks, I.M. Prepared movements are elicited early by startle. J. Mot. Behav. 2004, 36, 253–264. [Google Scholar] [CrossRef]
- Queralt, A.; Weerdesteyn, V.; van Duijnhoven, H.J.; Castellote, J.M.; Valls-Solé, J.; Duysens, J. The effects of an auditory startle on obstacle avoidance during walking. J. Physiol. 2008, 586, 4453–4463. [Google Scholar] [CrossRef]
- Cisek, P. Cortical mechanisms of action selection: The affordance competition hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1585–1599. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Logan, L.; Wolpert, D.M.; Flanagan, J.R. Parallel specification of competing sensorimotor control policies for alternative action options. Nat. Neurosci. 2016, 19, 320–326. [Google Scholar] [CrossRef]
- Brown, P.; Rothwell, J.C.; Thompson, P.D.; Britton, T.C.; Day, B.L.; Marsden, C.D. New observations on the normal auditory startle reflex in man. Brain 1991, 114, 1891–1902. [Google Scholar] [CrossRef]
- Bisdorff, A.R.; Bronstein, A.M.; Gresty, M.A. Responses in neck and facial muscles to sudden free fall and a startling auditory stimulus. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 409–416. [Google Scholar] [CrossRef]
- Wilkins, D.E.; Hallett, M.; Wess, M.M. Audiogenic startle reflex of man and its relationship to startle syndromes. A review. Brain 1986, 109, 561–573. [Google Scholar] [CrossRef]
- Nonnekes, J.; Scotti, A.; Oude Nijhuis, L.B.; Smulders, K.; Queralt, A.; Geurts, A.C.H.; Bloem, B.R.; Weerdesteyn, V. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience 2013, 245, 109–120. [Google Scholar] [CrossRef]
- Mackinnon, C.D.; Bissig, D.; Chiusano, J.; Miller, E.; Rudnick, L.; Jager, C.; Zhang, Y.; Mille, M.L.; Rogers, M.W. Preparation of anticipatory postural adjustments prior to stepping. J. Neurophysiol. 2007, 97, 4368–4379. [Google Scholar] [CrossRef]
- Reynolds, R.F.; Day, B.L. Fast visuomotor processing made faster by sound. J. Physiol. 2007, 583, 1107–1115. [Google Scholar] [CrossRef]
- Van de Kamp, C.; Gawthrop, P.J.; Gollee, H.; Loram, I.D. Refractoriness in sustained visuo-motor control: Is the refractory duration intrinsic or does it depend on external system properties? PLoS Comput. Biol. 2013, 9, e1002843. [Google Scholar] [CrossRef]
- Nonnekes, J.; Carpenter, M.G.; Inglis, J.T.; Duysens, J.; Weerdesteyn, V. What startles tell us about control of posture and gait. Neurosci. Biobehav. Rev. 2015, 53, 131–138. [Google Scholar] [CrossRef]
- Prochazka, A. The fuzzy logic of visuomotor control. Can J. Physiol. Pharm. 1996, 74, 456–462. [Google Scholar] [CrossRef]
- Misiaszek, J.E. Neural control of walking balance: IF falling THEN react ELSE continue. Exerc. Sport Sci. Rev. 2006, 34, 128–134. [Google Scholar] [CrossRef]

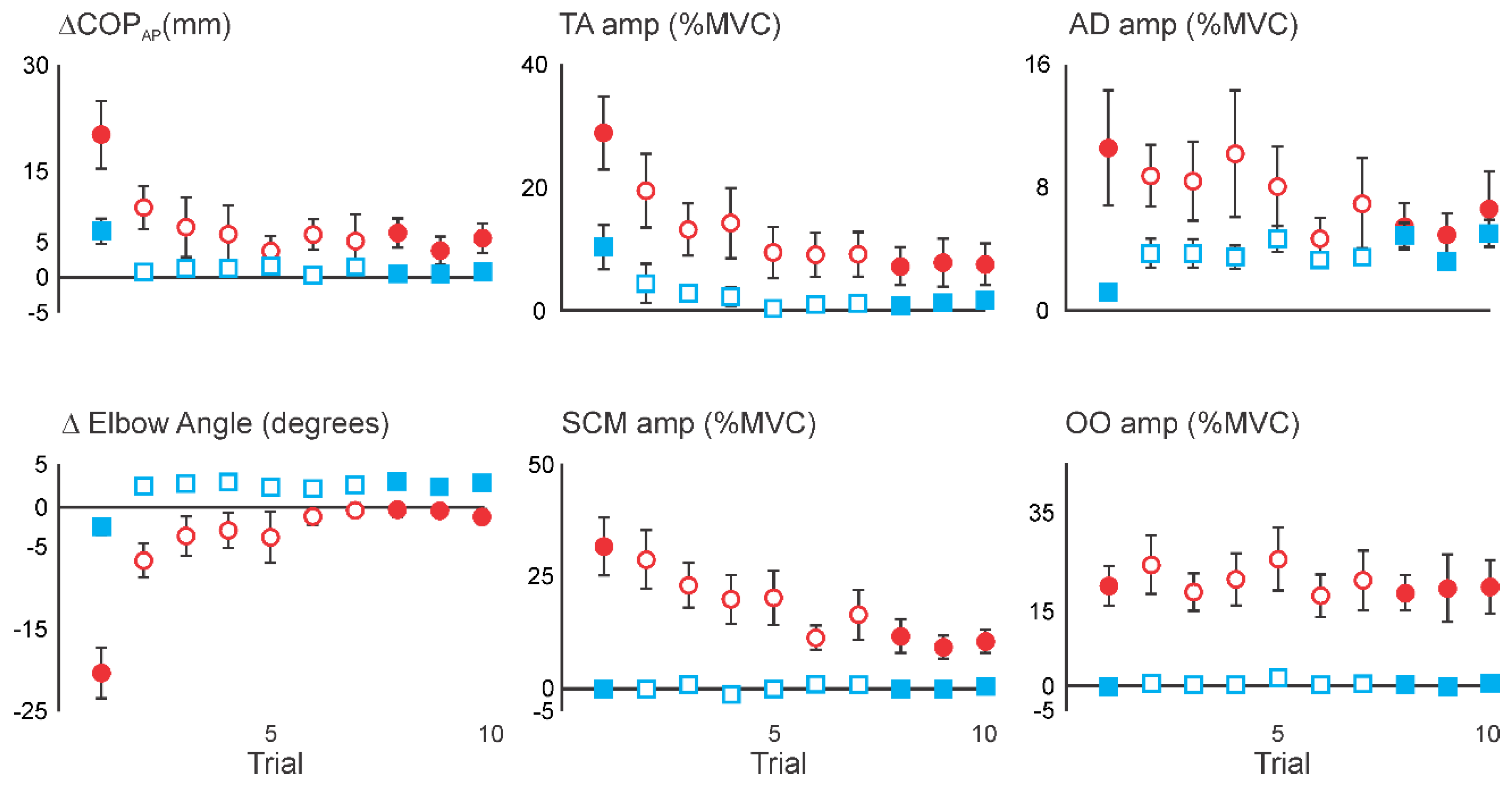

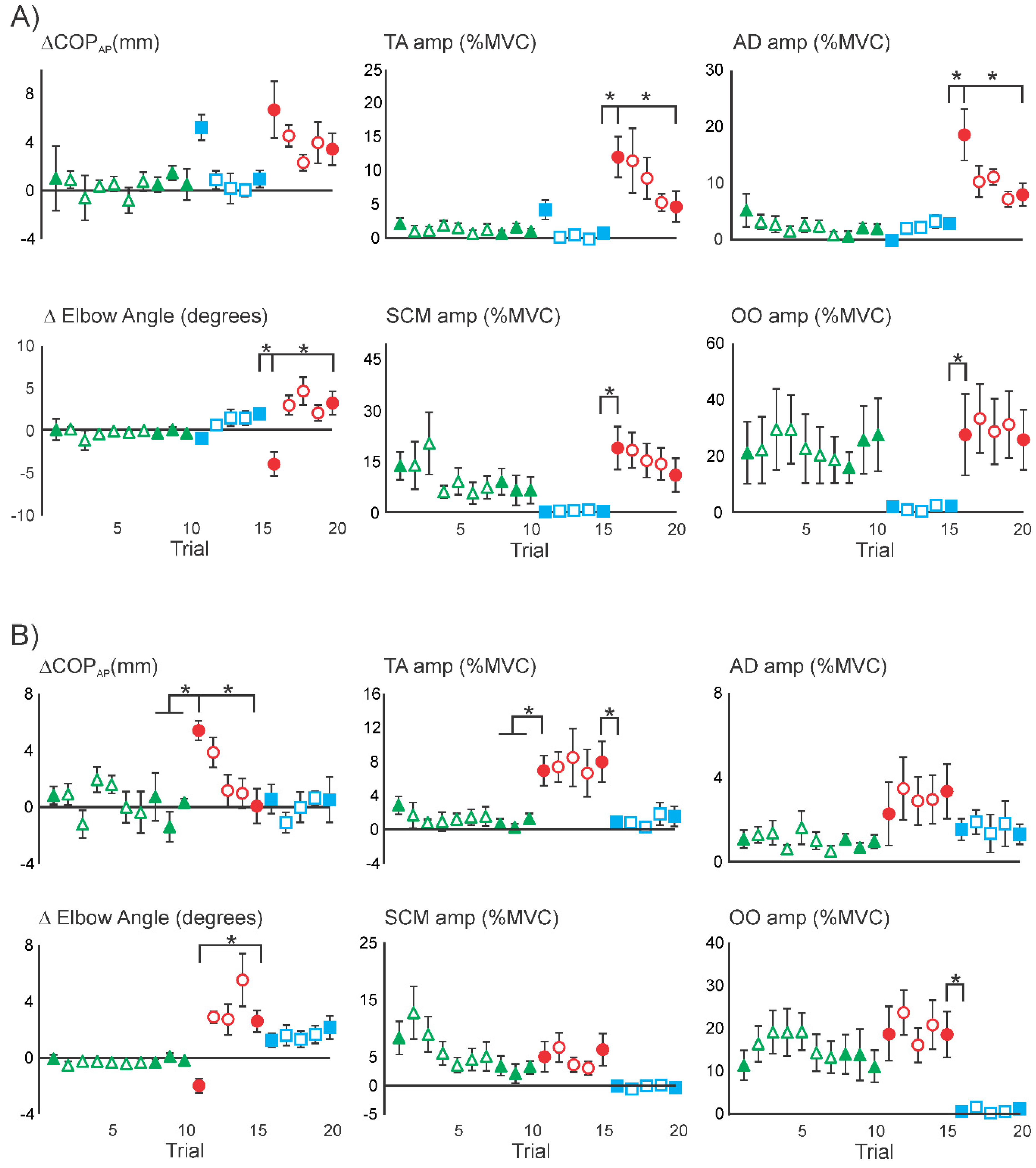
| Stimulus | Statistical Results | ||||||||
| A | D1 | D8–10 | C1 | C5 | S1 | S5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 8.1 (3.4) | 0.5 (0.3) | −0.2 (1.6) | 2.3 (1.1) | 0.6 (0.9) | 1.1 (1.2) | 8 | 3.38 (p = 0.014) | D1 > D8–10 |
| ΔElbow (deg) | −3.2 (1.4) | 2.6 (0.6) | 7.5 (1.9) | 3.6 (1.0) | −0.3 (0.3) | 0.0 (0.2) | 7 | 11.6 (p = 0.000) | D1 < D8–10, D8–10 < C1 |
| EMG (%MVC) | |||||||||
| TA | 7.6 (3.3) | 0.2 (0.2) | 21.9 (8.5) | 8.0 (3.1) | 2.0 (1.1) | 2.4 (1.1) | 8 | 6.31 (p = 0.000) | D8–10 < C1, C1 > C5 |
| AD | 0.7 (0.3) | 3.9 (0.7) | 18.4 (5.7) | 8.9 (3.2) | 1.9 (1.5) | 1.8 (1.6) | 8 | 7.24 (p = 0.000) | D8–10 < C1 |
| SCM | −0.2 (0.2) | −0.2 (0.1) | 42.1 (15.0) | 15.0 (6.0) | 9.4 (4.4) | 6.0 (3.5) | 8 | 6.74 (p = 0.000) | D8–10 < C1, C1 > C5 |
| OO | 1.8 (1.5) | 0.7 (0.4) | 21.7 (8.4) | 30.7 (5.7) | 26.2 (6.4) | 22.7 (7.2) | 8 | 9.71 (p = 0.000) | D8–10 < C1 |
| Stimulus | Statistical Results | ||||||||
| B | D1 | D8–10 | S1 | S5 | C1 | C5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 5.5 (1.2) | 0.2 (1.0) | 2.8 (0.9) | −0.6 (0.4) | 0.3 (1.0) | 1.8 (0.9) | 8 | 7.79 (p = 0.000) | D1 > D8–10 |
| ΔElbow (deg) | −1.8 (0.3) | 2.7 (0.3) | 0.5 (0.3) | −0.2 (0.1) | 4.2 (0.7) | 3.2 (0.4) | 5 | 29.4 (p = 0.000) | D1 < D8–10, D8–10 > S1, S5 < C1 |
| EMG (%MVC) | |||||||||
| TA | 9.3 (4.8) | 0.4 (0.3) | 1.1 (0.5) | 1.6 (1.4) | 2.6 (1.4) | 0.2 (0.2) | 8 | 2.50 (p = 0.049) | |
| AD | 1.7 (0.4) | 4.0 (0.7) | 1.1 (0.7) | 1.2 (0.5) | 4.4 (1.8) | 3.8 (0.9) | 8 | 4.12 (p = 0.005) | S5 < C1 |
| SCM | −0.1 (0.2) | 0.6 (0.8) | 20.5 (6.1) | 6.1 (2.7) | 5.9 (0.6) | 3.2 (1.6) | 6 | 6.95 (p = 0.000) | D8–10 < S1, S1 > S5 |
| OO | 0.1 (0.3) | 0.8 (0.7) | 13.7 (4.4) | 11.4 (4.7) | 16.2 (3.4) | 16.0 (3.9) | 8 | 12.1 (p = 0.000) | D8–10 < S1 |
| Stimulus | Statistical Results | ||||||||
| A | C1 | C8–10 | S1 | S5 | D1 | D5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 18.8 (6.0) | 5.9 (2.7) | 0.3 (1.0) | 0.0 (0.7) | 3.0 (2.1) | 0.8 (1.0) | 8 | 6.98 (p = 0.000) | C1 > C8–10 |
| ΔElbow (deg) | −17.1 (3.3) | −0.8 (0.5) | 0.0 (0.1) | 0.0 (0.1) | 0.7 (0.4) | 1.0 (0.3) | 8 | 27.1 (p = 0.000) | C1 > C8–10 |
| EMG (%MVC) | |||||||||
| TA | 21.3 (4.6) | 7.0 (2.5) | 1.7 (0.9) | 0.1 (0.1) | 0.1 (0.2) | 0.2 (0.1) | 8 | 17.7 (p = 0.000) | C1 > C8–10 |
| AD | 9.6 (5.5) | 4.9 (2.1) | 0.8 (0.7) | 0.4 (0.3) | 0.8 (0.6) | 1.5 (0.8) | 8 | 2.88 (p = 0.028) | |
| SCM | 27.2 (7.8) | 9.8 (2.0) | 3.7 (1.5) | 1.1 (1.4) | 0.0 (0.1) | −0.1 (0.4) | 7 | 11.5 (p = 0.000) | C1 > C8–10 |
| OO | 11.3 (3.5) | 17.8 (4.4) | 12.6 (3.6) | 14.3 (5.3) | 0.0 (0.5) | 0.0 (0.3) | 8 | 9.09 (p = 0.000) | S5 > D1 |
| Stimulus | Statistical Results | ||||||||
| B | C1 | C8–10 | D1 | D5 | S1 | S5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 21.6 (7.9) | 4.5 (2.4) | 4.1 (1.3) | -0.2 (0.5) | 0.6 (0.7) | 1.8 (1.8) | 8 | 5.99 (p = 0.000) | C1 > C8–10 |
| ΔElbow (deg) | −19.5 (4.0) | −0.3(0.8) | 0.4 (0.2) | 1.9 (0.7) | 0.0 (0.3) | -0.2 (0.2) | 8 | 24.1 (p = 0.000) | C1 > C8–10 |
| EMG (%MVC) | |||||||||
| TA | 30.3 (8.3) | 7.3 (4.4) | 2.1 (1.4) | 1.3 (0.9) | 1.8 (1.0) | 3.1 (1.5) | 8 | 10.1 (p = 0.000) | C1 > C8–10 |
| AD | 7.4 (2.6) | 5.6 (1.3) | 0.9 (0.7) | 1.2 (0.9) | 4.0 (1.5) | 2.9 (0.8) | 8 | 4.32 (p = 0.004) | C8–10 > D1 |
| SCM | 36.0 (11.3) | 11.2 (3.7) | 1.3 (1.2) | −0.3 (0.2) | 8.8 (3.1) | 4.7 (2.6) | 7 | 7.58 (p = 0.000) | C1 > C8–10 |
| OO | 20.2 (6.1) | 21.3 (6.2) | 3.7 (2.3) | 4.0 (2.0) | 19.3 (9.0) | 15.2 (9.2) | 8 | 4.10 (p = 0.005) | C8–10 > D1, D5 < S1 |
| Stimulus | Statistical Results | ||||||||
| A | S1 | S8–10 | D1 | D5 | C1 | C5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 1.0 (2.7) | 0.8 (0.6) | 5.2 (1.1) | 1.0 (0.7) | 6.7 (2.4) | 3.4 (1.3) | 8 | 2.07 (p = 0.093) | |
| ΔElbow (deg) | 0.1 (1.2) | −0.2 (0.3) | −0.9 (0.3) | 2.0 (0.4) | −3.9 (1.4) | 3.3 (1.4) | 5 | 5.65 (p = 0.002) | D5 > C1, C1 < C5 |
| EMG (%MVC) | |||||||||
| TA | 2.1 (0.8) | 1.1 (0.4) | 4.2 (1.5) | 0.7 (0.5) | 12.0 (3.0) | 4.6 (2.2) | 8 | 8.03 (p = 0.000) | D5 < C1, C1 > C5 |
| AD | 5.1 (3.1) | 1.5 (0.6) | −0.2 (0.3) | 2.7 (0.7) | 18.5 (4.9) | 7.9 (2.1) | 7 | 8.78 (p = 0.000) | D5 < C1, C1 > C5 |
| SCM | 13.9 (4.4) | 7.5 (3.6) | −0.3 (0.5) | 0.5 (0.6) | 19.1 (6.8) | 11.1 (5.3) | 7 | 4.87 (p = 0.002) | D5 < C1 |
| OO | 21.1 (11.0) | 23.0 (9.8) | 2.1 (1.0) | 2.3 (1.1) | 27.6 (14.4) | 25.8 (10.6) | 8 | 3.29 (p = 0.015) | D5 < C1 |
| Stimulus | Statistical Results | ||||||||
| B | S1 | S8–10 | C1 | C5 | D1 | D5 | n | Main Effect | Tukey’s HSD (p < 0.05) |
| Mechanical | |||||||||
| ΔCOPAP (mm) | 0.8 (0.6) | −0.1 (0.8) | 5.4 (0.7) | 0.1 (1.2) | 0.6 (1.0) | 0.5 (1.6) | 8 | 5.64 (p = 0.001) | S8–10 < C1, C1 > C5 |
| ΔElbow (deg) | −0.1 (0.4) | −0.2 (0.2) | -2.0 (0.6) | 2.6 (1.0) | 1.2 (0.6) | 2.2 (1.0) | 5 | 6.57 (p = 0.000) | C1 < C5 |
| EMG (%MVC) | |||||||||
| TA | 2.8 (1.2) | 0.7 (0.4) | 6.9 (1.9) | 8.0 (2.6) | 0.9 (0.7) | 1.5 (1.3) | 7 | 5.27 (p = 0.001) | S8–10 < C1, C5 > D1 |
| AD | 1.1 (0.4) | 0.9 (0.2) | 2.3 (1.6) | 3.3 (1.4) | 1.5 (0.5) | 1.3 (0.5) | 7 | 1.12 (p = 0.373) | |
| SCM | 8.4 (3.1) | 2.9 (1.5) | 5.0 (2.8) | 6.3 (3.0) | −0.1 (0.3) | −0.4 (0.3) | 7 | 2.74 (p = 0.034) | |
| OO | 11.3 (3.7) | 12.9 (5.0) | 18.7 (6.8) | 18.5 (5.7) | 0.5 (0.4) | 1.1 (0.8) | 7 | 5.61 (p = 0.001) | C5 > D1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiaszek, J.E.; Chodan, S.D.C.; McMahon, A.J.; Fenrich, K.K. Influence of Pairing Startling Acoustic Stimuli with Postural Responses Induced by Light Touch Displacement. Appl. Sci. 2020, 10, 382. https://doi.org/10.3390/app10010382
Misiaszek JE, Chodan SDC, McMahon AJ, Fenrich KK. Influence of Pairing Startling Acoustic Stimuli with Postural Responses Induced by Light Touch Displacement. Applied Sciences. 2020; 10(1):382. https://doi.org/10.3390/app10010382
Chicago/Turabian StyleMisiaszek, John E., Sydney D. C. Chodan, Arden J. McMahon, and Keith K. Fenrich. 2020. "Influence of Pairing Startling Acoustic Stimuli with Postural Responses Induced by Light Touch Displacement" Applied Sciences 10, no. 1: 382. https://doi.org/10.3390/app10010382
APA StyleMisiaszek, J. E., Chodan, S. D. C., McMahon, A. J., & Fenrich, K. K. (2020). Influence of Pairing Startling Acoustic Stimuli with Postural Responses Induced by Light Touch Displacement. Applied Sciences, 10(1), 382. https://doi.org/10.3390/app10010382





