Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis
Abstract
1. Introduction
2. Review Method
2.1. Literature Search Strategy
2.2. Study Selection: Inclusion Criteria and Quality Assessment
3. Results
3.1. Common Wearable Sensors Used in Gait, Balance, and RoM Analysis
- obtain objective measures that characterize how and why functional performance of gait and balance are impaired,
- increase the sensitivity of gait and balance measures,
- increase the opportunity for immediate biofeedback provided to patients.
3.1.1. Inertial Measurement Units and Magnetometers
3.1.2. Smart Devices
3.1.3. Electromyography (EMG) Sensors
- technical limitations may be present in cases of obesity or advanced age,
- EMG cannot be used for all muscles for all activities,
- EMG does not give RoM information,
- electrode placement is vital,
- traditional EMG cannot detect passive movements,
- for surface EMG (SEMG), skin must be cleaned and static charges on the skin can alter the signals.
3.1.4. Insole Pressure and Force Sensors
4. Design Issues in Gait, Balance and RoM Wearable Systems
4.1. Obtrusiveness
4.2. Sensor Location
4.3. Sensor-to-Segment Alignment
4.4. Soft Tissue Artifact
4.5. Processing
4.6. Energy Consumption
4.7. Mobility
4.8. Cost
4.9. Noise
4.10. Thresholds
4.11. Magnetic Disturbances
4.12. Sensor Fusion
- data-level fusion: implements de-nosing, feature extraction, data classification, and data compression,
- feature-level fusion: creates a new high-dimension feature set that represents the input for classification or pattern recognition, and
- decision-level fusion: utilizes the abstracted information from either data-level or feature-level fusion to make a decision [103].
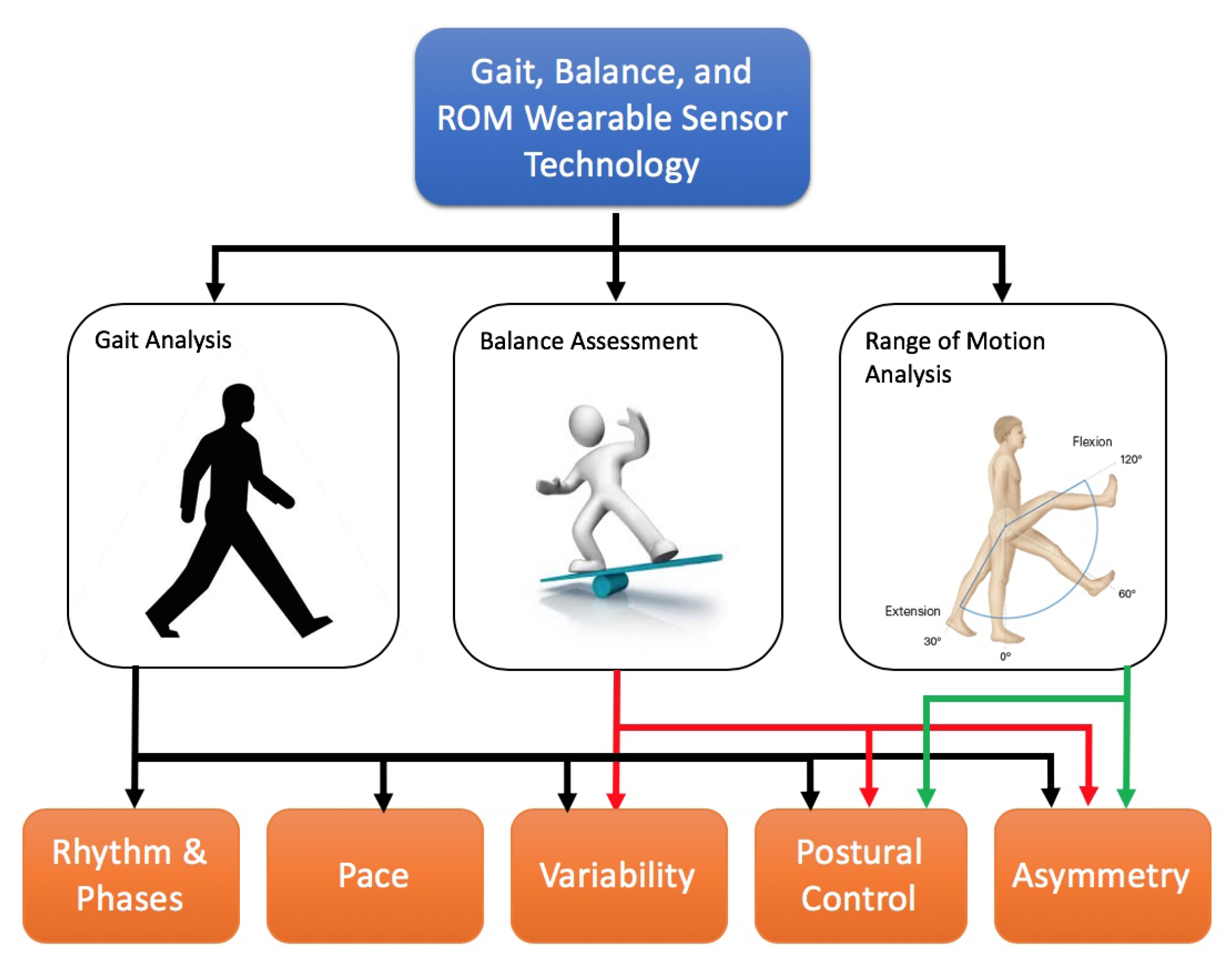
5. A Taxonomy for Gait, Balance, RoM Analysis
5.1. Gait Analysis
5.1.1. Gait Analysis: Rhythm and Phases
5.1.2. Gait Analysis: Pace
5.1.3. Gait Analysis: Variability
5.1.4. Gait Analysis: Postural Control
5.1.5. Gait Analysis: Asymmetry
5.2. Analysis of Postural Control and Balance
- Romberg Test,
- Limits of Stability Test,
- Single Leg Stance Test (SLST),
- 5 Times Sit to Stand (STS) Test,
- Functional Reach Test (FRT),
- Clinical Test of Sensory Interaction and Balance (CTSIB),
- Timed Up and Go (TUG) Test,
- Tinetti Test,
- Berg Balance Scale (BBS), and
- BESTest.
5.2.1. Postural Sway
5.2.2. Postural Sway: Asymmetry and Variability
5.3. Analysis of Joint Range of Motion
- passive RoM: physical therapist is moving the subject’s joint and no active movement is performed by the subject,
- active assistive RoM: the subject can perform movements but cannot complete it because of pain or muscle weakness; assistance of the physical therapist is needed, and
- active RoM: the subject can perform the movement without manual assistance from the therapist.
5.4. Validation against a Gold Standard
6. Discussion
6.1. Revealing Features in Population
6.2. Biofeedback
6.3. Wearable Sensor Technology Validation
6.4. Machine Learning in Gait, Balance, and RoM
6.5. Limitations
7. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AP | Anteroposterior |
| AV | Acceleration Variance |
| BESS | Balance Error Scoring System |
| BBS | Berg Balance Score |
| CoM | Center of Mass |
| CTSIB | Clinical Test of Sensory Interaction and Balance |
| DTW | Dynamic Time Warping |
| EMG | Electromyography |
| FA | Friedreich’s ataxia |
| FGA | Functional Gait Assessment |
| FoG | Freezing of Gait |
| FRT | Functional Reach Test |
| GVI | Gait Variability Index |
| IMU | Inertial Measurement Unit |
| ISB | International Society of Biomechanics |
| KNN | K-Nearest Neighbors |
| LyE | Lyapunov Exponent |
| MAE | Mean Absolute Error |
| ML | Mediolateral |
| MS | Multiple Sclerosis |
| PCC | Pearson Correlation Coefficient |
| PD | Parkinson’s Disease |
| RMSE | Root Mean Square Error |
| RoM | Range of Motion |
| SEM | Standard Error of Measurement |
| SEMG | Surface Electromyography |
| SL | Step length |
| SLST | Single Leg Stance Test |
| STA | Soft Tissue Artifact |
| STD | Standard Deviation |
| STS | Sit to Stand |
| SVM | Support Vector Machine |
| TUG | Time Up and Go |
| WF | Walking Frequency |
References
- Balance Disorder. Available online: https://www.nidcd.nih.gov/health/balance-disorders (accessed on 2 February 2018).
- Alzheimer’s Disease. Available online: https://www.cdc.gov/aging/aginginfo/alzheimers.htm (accessed on 12 January 2018).
- Causes. Available online: http://www.parkinson.org/understanding-parkinsons/causes-and-statistics?gclid=Cj0KCQjw_ODWBRCTARIsAE2_EvXlXEuMZbQRwtf9zVwUoDZxTd_w3TONstVpIBTa7uhbagCOeFuKKgoaArQGEALw_wcB (accessed on 12 January 2018).
- Markowitz, C.E. Multiple sclerosis update. Am. J. Manag. Care 2013, 19, s294–s300. [Google Scholar]
- What Is Ataxia? Available online: https://ataxia.org/what-is-ataxia/ (accessed on 12 January 2018).
- 10 Reasons Why Physical Therapy Is Beneficial. Available online: https://www.burke.org/blog/2015/10/10-reasons-why-physical-therapy-is-beneficial/58 (accessed on 14 October 2017).
- Lara, O.D.; Labrador, M.A. A survey on human activity recognition using wearable sensors. IEEE Commun. Surv. Tutor. 2013, 15, 1192–1209. [Google Scholar] [CrossRef]
- Muro-De-La-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef]
- Delahoz, Y.S.; Labrador, M.A. Survey on fall detection and fall prevention using wearable and external sensors. Sensors 2014, 14, 19806–19842. [Google Scholar] [CrossRef]
- El-Gohary, M.A.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F. Continuous Monitoring of Movement in Patients with Parkinson’s Disease Using Inertial Sensors. In Proceedings of the 33rd International Conference of Biomechanics in Sports, Poitiers, France, 29 June–3 July 2015. [Google Scholar]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Horak, F.; King, L.; Mancini, M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys. Ther. 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices 2016, 13, 455–462. [Google Scholar] [CrossRef]
- Caldas, R.; Mundt, M.; Potthast, W.; de Lima Neto, F.B.; Markert, B. A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait Posture 2017, 57, 204–210. [Google Scholar] [CrossRef]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J. Validity and reliability of wearable sensors for joint angle estimation: A systematic review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Hagströmer, M.; Ainsworth, B.E.; Kwak, L.; Bowles, H.R. A checklist for evaluating the methodological quality of validation studies on self-report instruments for physical activity and sedentary behavior. J. Phys. Act. Health 2012, 9, S29–S36. [Google Scholar]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef]
- Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.K.; Wan, A.H.-P.; Lee, W.C.-C. Balance improvement effects of biofeedback systems with state-of-the-art wearable sensors: A systematic review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Alexiou, K.I.; Roushias, A.; Varitimidis, S.E.; Malizos, K.N. Quality of life and psychological consequences in elderly patients after a hip fracture: A review. Clin. Interv. Aging 2018, 13, 143. [Google Scholar] [CrossRef]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Appl. Bionics Biomech. 2018, 2018, 19. [Google Scholar] [CrossRef]
- van den Noort, J.C.; Scholtes, V.A.; Harlaar, J. Evaluation of clinical spasticity assessment in cerebral palsy using inertial sensors. Gait Posture 2009, 30, 138–143. [Google Scholar] [CrossRef]
- Franco, C.; Fleury, A.; Guméry, P.; Diot, B.; Demongeot, J.; Vuillerme, N. iBalance-ABF: A smartphone-based audio-biofeedback balance system. IEEE Trans. Biomed. Eng. 2012, 60, 211–215. [Google Scholar] [CrossRef]
- Spain, R.; George, R.S.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Martori, A.L. A Wearable Motion Analysis System to Evaluate Gait Deviations. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2013. [Google Scholar]
- Crea, S.; Cipriani, C.; Donati, M.; Carrozza, M.C.; Vitiello, N. Providing time-discrete gait information by wearable feedback apparatus for lower-limb amputees: Usability and functional validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 250–257. [Google Scholar] [CrossRef]
- Dewey, D.C.; Miocinovic, S.; Bernstein, I.; Khemani, P.; Dewey, R.B., III; Querry, R.; Chitnis, S.; Dewey, R.B., Jr. Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J. Neurol. Sci. 2014, 345, 131–138. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chung, P.C.; Wang, W.H.; Pai, M.C.; Wang, C.Y.; Lin, C.W.; Wu, H.L.; Wang, J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014, 18, 1822–1830. [Google Scholar] [CrossRef]
- Patterson, J.A.; Amick, R.Z.; Pandya, P.D.; Hakansson, N.; Jorgensen, M.J. Comparison of a mobile technology application with the balance error scoring system. Int. J. Athl. Ther. Train. 2014, 19, 4–7. [Google Scholar] [CrossRef]
- Tzallas, A.; Tsipouras, M.; Rigas, G.; Tsalikakis, D.; Karvounis, E.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.; et al. PERFORM: A system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef]
- Wentink, E.C.; Schut, V.G.H.; Prinsen, E.C.; Rietman, J.S.; Veltink, P.H. Detection of the onset of gait initiation using kinematic sensors and EMG in transfemoral amputees. Gait Posture 2014, 39, 391–396. [Google Scholar] [CrossRef]
- Alberts, J.L.; Thota, A.; Hirsch, J.; Ozinga, S.; Dey, T.; Schindler, D.D.; Koop, M.M.; Burke, D.; Linder, S.M. Quantification of the balance error scoring system with mobile technology. Med. Sci. Sport. Exerc. 2015, 47, 2233. [Google Scholar] [CrossRef]
- Alberts, J.L.; Hirsch, J.R.; Koop, M.M.; Schindler, D.; Kana, D.E.; Linder, S.M.; Campbell, S.; Thota, A.K. Using accelerometer and gyroscopic measures to quantify postural stability. J. Athl. Train. 2015, 50, 578–588. [Google Scholar] [CrossRef]
- Bauer, C.M.; Rast, F.M.; Ernst, M.J.; Kool, J.; Oetiker, S.; Rissanen, S.M.; Suni, J.H.; Kankaanpää, M. Concurrent validity and reliability of a novel wireless inertial measurement system to assess trunk movement. J. Electromyogr. Kinesiol. 2015, 25, 782–790. [Google Scholar] [CrossRef]
- Zhu, S.; Ellis, R.J.; Schlaug, G.; Ng, Y.S.; Wang, Y. Validating an iOS-based Rhythmic Auditory Cueing Evaluation (iRACE) for Parkinson’s Disease. In Proceedings of the 22nd ACM International Conference on Multimedia, Orlando, FL, USA, 3–7 November 2014; pp. 487–496. [Google Scholar]
- Ellis, R.J.; Ng, Y.S.; Zhu, S.; Tan, D.M.; Anderson, B.; Schlaug, G.; Wang, Y. A validated smartphone-based assessment of gait and gait variability in Parkinson’s disease. PLoS ONE 2015, 10, e0141694. [Google Scholar] [CrossRef]
- Godfrey, A.; Del Din, S.; Barry, G.; Mathers, J.C.; Rochester, L. Instrumenting gait with an accelerometer: A system and algorithm examination. Med Eng. Phys. 2015, 37, 400–407. [Google Scholar] [CrossRef]
- Jaysrichai, T.; Suputtitada, A.; Khovidhungij, W. Mobile sensor application for kinematic detection of the knees. Ann. Rehabil. Med. 2015, 39, 599. [Google Scholar] [CrossRef]
- Kanzler, C.M.; Barth, J.; Rampp, A.; Schlarb, H.; Rott, F.; Klucken, J.; Eskofier, B.M. Inertial sensor based and shoe size independent gait analysis including heel and toe clearance estimation. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5424–5427. [Google Scholar]
- Lee, W.W.; Yen, S.C.; Tay, E.B.A.; Zhao, Z.; Xu, T.M.; Ling, K.K.M.; Ng, Y.S.; Chew, E.; Cheong, A.L.K.; Huat, G.K.C. A smartphone-centric system for the range of motion assessment in stroke patients. IEEE J. Biomed. Health Inform. 2014, 18, 1839–1847. [Google Scholar] [CrossRef]
- Kumar, Y.; Yen, S.C.; Tay, A.; Lee, W.; Gao, F.; Zhao, Z.; Li, J.; Hon, B.; Xu, T.T.; Cheong, A.; et al. Wireless wearable range-of-motion sensor system for upper and lower extremity joints: A validation study. Healthc. Technol. Lett. 2015, 2, 12–17. [Google Scholar] [CrossRef]
- Lin, F.; Wang, A.; Song, C.; Xu, W.; Li, Z.; Li, Q. A comparative study of smart insole on real-world step count. In Proceedings of the 2015 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 12 December 2015; pp. 1–6. [Google Scholar]
- Postolache, O.; Girão, P.S.; Pereira, J.M.D.; Postolache, G. Wearable system for gait assessment during physical rehabilitation process. In Proceedings of the 2015 9th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 7–9 May 2015; pp. 321–326. [Google Scholar]
- Sijobert, B.; Benoussaad, M.; Denys, J.; Pissard-Gibollet, R.; Geny, C.; Coste, C.A. Implementation and Validation of a Stride Length Estimation Algorithm, Using a Single Basic Inertial Sensor on Healthy Subjects and Patients Suffering from Parkinson’s Disease. ElectronicHealthcare 2015, 704–714. [Google Scholar] [CrossRef]
- Nouredanesh, M.; Tung, J. Machine learning based detection of compensatory balance responses to lateral perturbation using wearable sensors. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Atlanta, GA, USA, 22–24 October 2015; pp. 1–4. [Google Scholar]
- Nouredanesh, M.; Kukreja, S.L.; Tung, J. Detection of compensatory balance responses using wearable electromyography sensors for fall-risk assessment. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 1680–1683. [Google Scholar]
- Bertolotti, G.M.; Cristiani, A.M.; Colagiorgio, P.; Romano, F.; Bassani, E.; Caramia, N.; Ramat, S. A wearable and modular inertial unit for measuring limb movements and balance control abilities. IEEE Sens. J. 2016, 16, 790–797. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J. Biomed. Health Inform. 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Horak, F.B.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; Salarian, A. Balance and gait represent independent domains of mobility in Parkinson disease. Phys. Ther. 2016, 96, 1364–1371. [Google Scholar] [CrossRef]
- Lee, C.; Sun, T.; Jiang, B.; Choi, V. Using wearable accelerometers in a community service context to categorize falling behavior. Entropy 2016, 18, 257. [Google Scholar] [CrossRef]
- LeMoyne, R.; Heerinckx, F.; Aranca, T.; De Jager, R.; Zesiewicz, T.; Saal, H.J. Wearable body and wireless inertial sensors for machine learning classification of gait for people with Friedreich’s ataxia. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 147–151. [Google Scholar]
- Li, B.; Gui, Q.; Ali, H.B.; Li, H.; Jin, Z. A wearable sit-to-stand detection system based on angle tracking and lower limb EMG. In Proceedings of the 2016 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 3 December 2016; pp. 1–6. [Google Scholar]
- Storm, F.A.; Buckley, C.J.; Mazzà, C. Gait event detection in laboratory and real life settings: Accuracy of ankle and waist sensor based methods. Gait Posture 2016, 50, 42–46. [Google Scholar] [CrossRef]
- Wang, P. Autocorrelation analysis of lower limb EMG signals for the initial evaluation of hemiparetic gaits. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 974–977. [Google Scholar]
- Andò, B.; Baglio, S.; Marletta, V.; Pistorio, A.; Dibilio, V.; Mostile, G.; Nicoletti, A.; Zappia, M. A multisensor architecture for the assessment of postural sway in elderly and people with neurological disease. In Proceedings of the 2017 IEEE Sensors Applications Symposium (SAS), Glassboro, NJ, USA, 13–15 March 2017; pp. 1–5. [Google Scholar]
- Iijima, M.; Mitoma, H.; Uchiyama, S.; Kitagawa, K. Long-term monitoring gait analysis using a wearable device in daily lives of patients with Parkinson’s disease: The efficacy of selegiline hydrochloride for gait disturbance. Front. Neurol. 2017, 8, 542. [Google Scholar] [CrossRef]
- Lebel, K.; Boissy, P.; Nguyen, H.; Duval, C. Inertial measurement systems for segments and joints kinematics assessment: Towards an understanding of the variations in sensors accuracy. Biomed. Eng. Online 2017, 16, 56. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Validation of inertial measurement units with an optoelectronic system for whole-body motion analysis. Med Biol. Eng. Comput. 2017, 55, 609–619. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef]
- Shahzad, A.; Ko, S.; Lee, S.; Lee, J.; Kim, K. Quantitative Assessment of Balance Impairment for Fall-Risk Estimation Using Wearable Triaxial Accelerometer. IEEE Sens. J. 2017, 17, 6743–6751. [Google Scholar] [CrossRef]
- Aich, S.; Pradhan, P.; Park, J.; Sethi, N.; Vathsa, V.; Kim, H. A validation study of freezing of gait (FoG) detection and machine-learning-based FoG prediction using estimated gait characteristics with a wearable accelerometer. Sensors 2018, 18, 3287. [Google Scholar] [CrossRef]
- Díaz, S.; Disdier, S.; Labrador, M.A. Step Length and Step Width Estimation using Wearable Sensors. In Proceedings of the 2018 9th IEEE Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON), New York, NY, USA, 8–10 November 2018; pp. 997–1001. [Google Scholar]
- Stack, E.; Agarwal, V.; King, R.; Burnett, M.; Tahavori, F.; Janko, B.; Harwin, W.; Ashburn, A.; Kunkel, D. Identifying balance impairments in people with Parkinson’s disease using video and wearable sensors. Gait Posture 2018, 62, 321–326. [Google Scholar] [CrossRef]
- Zhang, W.; Smuck, M.; Legault, C.; Ith, M.A.; Muaremi, A.; Aminian, K. Gait symmetry assessment with a low back 3d accelerometer in post-stroke patients. Sensors 2018, 18, 3322. [Google Scholar] [CrossRef]
- Chomiak, T.; Sidhu, A.S.; Watts, A.; Su, L.; Graham, B.; Wu, J.; Classen, S.; Falter, B.; Hu, B. Development and validation of ambulosono: A wearable sensor for bio-feedback rehabilitation training. Sensors 2019, 19, 686. [Google Scholar] [CrossRef]
- Chomiak, T.; Xian, W.; Pei, Z.; Hu, B. A novel single-sensor-based method for the detection of gait-cycle breakdown and freezing of gait in Parkinson’s disease. J. Neural Transm. 2019, 126, 1029–1036. [Google Scholar] [CrossRef]
- Grinberg, Y.; Berkowitz, S.; Hershkovitz, L.; Malcay, O.; Kalron, A. The ability of the instrumented tandem walking tests to discriminate fully ambulatory people with MS from healthy adults. Gait Posture 2019, 70, 90–94. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Roach, K.L.; Wajda, D.A.; Sosnoff, J.J. Smartphone technology can measure postural stability and discriminate fall risk in older adults. Gait Posture 2019, 67, 160–165. [Google Scholar] [CrossRef]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable sensors system for an improved analysis of freezing of gait in Parkinson’s disease using electromyography and inertial signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef]
- Mikos, V.; Heng, C.-H.; Tay, A.; Yen, S.-C.; Chia, N.S.Y.; Koh, K.M.L.; Tan, D.M.L.; Au, W.L. A Wearable, Patient-Adaptive Freezing of Gait Detection System for Biofeedback Cueing in Parkinson’s Disease. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 503–515. [Google Scholar] [CrossRef]
- Ngueleu, A.M.; Blanchette, A.K.; Bouyer, L.; Maltais, D.; McFadyen, B.J.; Moffet, H.; Batcho, C.S. Design and Accuracy of an Instrumented Insole Using Pressure Sensors for Step Count. Sensors 2019, 13, 984. [Google Scholar] [CrossRef]
- Phan, D.; Nguyen, N.; Pathirana, P.N.; Horne, M.; Power, L.; Szmulewicz, D. Quantitative Assessment of Ataxic Gait using Inertial Sensing at Different Walking Speeds. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4600–4603. [Google Scholar]
- Reeves, J.; Jones, R.; Liu, A.; Bent, L.; Nester, C. The between-day reliability of peroneus longus EMG during walking. J. Biomech. 2019, 86, 243–246. [Google Scholar] [CrossRef]
- Rivolta, M.W.; Aktaruzzaman, M.; Rizzo, G.; Lafortuna, C.L.; Ferrarin, M.; Bovi, G.; Bonardi, D.R.; Caspani, A.; Sassi, R. Evaluation of the Tinetti score and fall risk assessment via accelerometry-based movement analysis. Artif. Intell. Med. 2019, 95, 38–47. [Google Scholar] [CrossRef]
- Tang, W.; Fulk, G.; Zeigler, S.; Zhang, T.; Sazonov, E. Estimating Berg Balance Scale and Mini Balance Evaluation System Test Scores by Using Wearable Shoe Sensors. In Proceedings of the 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar]
- Weiss, A.; Herman, T.; Mirelman, A.; Shiratzky, S.S.; Giladi, N.; Barnes, L.L.; Bennett, D.A.; Buchman, A.S.; Hausdorff, J.M. The transition between turning and sitting in patients with Parkinson’s disease: A wearable device detects an unexpected sequence of events. Gait Posture 2019, 67, 227–229. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Qiu, S.; Wang, J.; Xu, F.; Wang, Z.; Shen, Y. Adaptive gait detection based on foot-mounted inertial sensors and multi-sensor fusion. Inf. Fusion 2019, 52, 157–166. [Google Scholar] [CrossRef]
- Hwang, P.Y. Inertial Measurement Unit with Magnetometer for Detecting Stationarity. U.S. Patent 6,496,779, 17 December 2002. [Google Scholar]
- Khandelwal, S.; Wickström, N. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2018, 59, 278–285. [Google Scholar]
- Baker, R. Measuring Walking: A Handbook of Clinical Gait Analysis; Mac Keith Press: London, UK, 2013. [Google Scholar]
- Day, S.A. The Advantages and Limits of Electromyography. Available online: https://oregon.providence.org/forms-and-information/t/the-advantages-and-limits-of-electromyography/ (accessed on 9 March 2019).
- TarniŢă, D. Wearable sensors used for human gait analysis. Rom J. Morphol. Embryol. 2016, 57, 373–382. [Google Scholar]
- Hackett, L.; Reed, D.; Halaki, M.; Ginn, K.A. Assessing the validity of surface electromyography for recording muscle activation patterns from serratus anterior. J. Electromyogr. Kinesiol. 2014, 24, 221–227. [Google Scholar] [CrossRef]
- Knarr, B.A.; Zeni, J.A., Jr.; Higginson, J.S. Comparison of electromyography and joint moment as indicators of co-contraction. J. Electromyogr. Kinesiol. 2012, 22, 607–611. [Google Scholar] [CrossRef]
- Zimmermann, T.; Taetz, B.; Bleser, G. IMU-to-segment assignment and orientation alignment for the lower body using deep learning. Sensors 2018, 18, 302. [Google Scholar] [CrossRef]
- Miezal, M.; Taetz, B.; Bleser, G. On inertial body tracking in the presence of model calibration errors. Sensors 2016, 16, 1132. [Google Scholar] [CrossRef]
- Bouvier, B.; Duprey, S.; Claudon, L.; Dumas, R.; Savescu, A. Upper limb kinematics using inertial and magnetic sensors: Comparison of sensor-to-segment calibrations. Sensors 2015, 15, 18813–18833. [Google Scholar] [CrossRef]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- De Vries, W.; Veeger, H.; Cutti, A.; Baten, C.; Van Der Helm, F. Functionally interpretable local coordinate systems for the upper extremity using inertial & magnetic measurement systems. J. Biomech. 2010, 43, 1983–1988. [Google Scholar]
- Fiorentino, N.M.; Atkins, P.R.; Kutschke, M.J.; Goebel, J.M.; Foreman, K.; Anderson, A.E. Soft tissue artifact causes significant errors in the calculation of joint angles and range of motion at the hip. Gait Posture 2017, 55, 184–190. [Google Scholar] [CrossRef]
- Frick, E.; Rahmatalla, S. Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation. Sensors 2018, 18, 2563. [Google Scholar] [CrossRef]
- Olsson, F.; Halvorsen, K. Experimental evaluation of joint position estimation using inertial sensors. In Proceedings of the 2017 20th International Conference on Information Fusion (Fusion), Xi’an, China, 10–13 July 2017; pp. 1–8. [Google Scholar]
- Cappozzo, A.; Catani, F.; Della Croce, U.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Cappozzo, A.; Catani, F.; Leardini, A.; Benedetti, M.; Della Croce, U. Position and orientation in space of bones during movement: Experimental artefacts. Clin. Biomech. 1996, 11, 90–100. [Google Scholar] [CrossRef]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry: Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar]
- Chiari, L.; Della Croce, U.; Leardini, A.; Cappozzo, A. Human movement analysis using stereophotogrammetry: Part 2: Instrumental errors. Gait Posture 2005, 21, 197–211. [Google Scholar] [CrossRef]
- Leardini, A.; Chiari, L.; Della Croce, U.; Cappozzo, A. Human movement analysis using stereophotogrammetry: Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–225. [Google Scholar] [CrossRef]
- Laidig, D.; Schauer, T.; Seel, T. Exploiting kinematic constraints to compensate magnetic disturbances when calculating joint angles of approximate hinge joints from orientation estimates of inertial sensors. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 971–976. [Google Scholar]
- Sabatini, A.M. Estimating three-dimensional orientation of human body parts by inertial/magnetic sensing. Sensors 2011, 11, 1489–1525. [Google Scholar] [CrossRef]
- Elmenreich, W. An introduction to sensor fusion. Vienna University Technology Austria 2002, Volume 502. [Google Scholar]
- Murphy, R.R. Biological and cognitive foundations of intelligent sensor fusion. IEEE Trans. Syst. Man Cybern. Part Syst. Hum. 1996, 26, 42–51. [Google Scholar] [CrossRef]
- Yang, G.Z.; Yang, G. Body Sensor Networks; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1. [Google Scholar]
- Gravina, R.; Alinia, P.; Ghasemzadeh, H.; Fortino, G. Multi-sensor fusion in body sensor networks: State-of-the-art and research challenges. Inf. Fusion 2017, 35, 68–80. [Google Scholar] [CrossRef]
- Gouelle, A.; Mégrot, F. Interpreting Spatiotemporal Parameters, Symmetry, and Variability in Clinical Gait Analysis. In Handbook of Human Motion; Springer: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Bhosale, T.; Kudale, H.; Kumthekar, V.; Garude, S.; Dhumal, P. Gait analysis using wearable sensors. In Proceedings of the 2015 International Conference on Energy Systems and Applications, Pune, India, 30 October–1 November 2015; pp. 267–269. [Google Scholar]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait partitioning methods: A systematic review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef]
- Whittle, M.W. Gait analysis: An introduction. Heidi Harrison 1991, 1, 47–100. [Google Scholar]
- Shin, S.H.; Park, C.G. Adaptive step length estimation algorithm using optimal parameters and movement status awareness. Med Eng. Phys. 2011, 33, 1064–1071. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Fritz, S.; Michelle, L. White paper: Walking speed: The Sixth Vital Sign. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Q. Inertial sensor-based methods in walking speed estimation: A systematic review. Sensors 2012, 12, 6102–6116. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Rozumalski, A. The Gait Deviation Index: A new comprehensive index of gait pathology. Gait Posture 2008, 28, 351–357. [Google Scholar] [CrossRef]
- Gouelle, A.; Mégrot, F.; Presedo, A.; Husson, I.; Yelnik, A.; Penneçot, G.F. The gait variability index: A new way to quantify fluctuation magnitude of spatiotemporal parameters during gait. Gait Posture 2013, 38, 461–465. [Google Scholar] [CrossRef]
- Huisinga, J.M.; Mancini, M.; George, R.J.; Horak, F.B. Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Ann. Biomed. Eng. 2013, 41, 1670–1679. [Google Scholar] [CrossRef]
- Perez, A.A.; Labrador, M.A. A Smartphone-Based System for Clinical Gait Assessment. In Proceedings of the 2016 IEEE International Conference on Smart Computing (SMARTCOMP), St. Louis, MO, USA, 18–20 May 2016; pp. 1–8. [Google Scholar]
- Perez, A.A. A Smartphone-Based System for Clinical Gait Assessment. Master’s Thesis, Computer Science-University of South Florida, Tampa, FL, USA, 2016. [Google Scholar]
- Senin, P. Dynamic time warping algorithm review. Inf. Comput. Sci. Dep. Univ. Hawaii Manoa Honolulu USA 2008, 855, 1–23. [Google Scholar]
- Moe-Nilssen, R.; Helbostad, J.L. Estimation of gait cycle characteristics by trunk accelerometry. J. Biomech. 2004, 37, 121–126. [Google Scholar] [CrossRef]
- Black, F.O.; Wall, C., III; Rockette, H.E., Jr.; Kitch, R. Normal subject postural sway during the Romberg test. Am. J. Otolaryngol. 1982, 3, 309–318. [Google Scholar] [CrossRef]
- Clark, S.; Rose, D.J.; Fujimoto, K. Generalizability of the limits of stability test in the evaluation of dynamic balance among older adults. Arch. Phys. Med. Rehabil. 1997, 78, 1078–1084. [Google Scholar] [CrossRef]
- Chomiak, T.; Pereira, F.V.; Hu, B. The single-leg-stance test in Parkinson’s disease. J. Clin. Med. Res. 2015, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Khattar, V.; Hathiram, B. The clinical test for the sensory interaction of balance. Int. Otorhinolaryngol. Clin. 2012, 4, 41–45. [Google Scholar]
- Podsiadlo, D.; Richardson, S. Timed Up and Go (TUG) Test. J. Am. Geriatr. Soc. 1991, 39, 142148. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Mancini, M.; Carlson-Kuhta, P.; Zampieri, C.; Nutt, J.G.; Chiari, L.; Horak, F.B. Postural sway as a marker of progression in Parkinson’s disease: A pilot longitudinal study. Gait Posture 2012, 36, 471–476. [Google Scholar] [CrossRef]
- Martinez-Mendez, R.; Sekine, M.; Tamura, T. Postural sway parameters using a triaxial accelerometer: Comparing elderly and young healthy adults. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, C.F. Using the Ellipse to Fit and Enclose Data Points; Department of Computer Science Cornell University: Ithaca, NY, USA, 2008; p. 54. [Google Scholar]
- Flash, T.; Hogan, N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985, 5, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.B.; Rice, T.; Stoskus, J.L.; Lambert, K.H.; Dannenbaum, E.; Scherer, M.R. Measurement characteristics and clinical utility of the clinical test of sensory interaction on balance (CTSIB) and modified CTSIB in individuals with vestibular dysfunction. Arch. Phys. Med. Rehabil. 2015, 96, 1747–1748. [Google Scholar] [CrossRef] [PubMed]
- Gajdosik, R.L.; Bohannon, R.W. Clinical measurement of range of motion: Review of goniometry emphasizing reliability and validity. Phys. Ther. 1987, 67, 1867–1872. [Google Scholar] [CrossRef]
- Range of Motion—Types of Range of Motion Exercises. Available online: http://www.physicaltherapynotes.com/2010/11/range-of-motion-types-of-range-of.html (accessed on 11 December 2017).
- Pedley, M. Tilt sensing using a three-axis accelerometer. Free. Semicond. Appl. Note 2013, 1, 2012–2013. [Google Scholar]
- Kuipers, J.B. Quaternions and Rotation Sequences; MPrinceton—Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Diebel, J. Representing attitude: Euler angles, unit quaternions, and rotation vectors. Matrix 2006, 58, 1–35. [Google Scholar]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D D’Lima, D.; Cristofolini, L.; Witte, H. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Wu, G.; Van der Helm, F.C.; Veeger, H.D.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]




| Item | Criteria | Validity Type | Outcome |
|---|---|---|---|
| 1 | The purpose of the study is clearly stated | IV | Yes/No |
| 2 | The research question is relevant to the purpose of the study | EV | Yes/No |
| 3 | Inclusion and/or exclusion criteria are described | EV | Yes/No |
| 4 | Data collection clearly described | IV/EV | Yes/No |
| 5 | Same data collection procedure for all subjects | EV | Yes/No |
| 6 | Reliable data processing clearly described | IV/EV | Yes/No |
| 7 | Data loss <20% | EV | Yes/No |
| 8 | Outcomes are relevant to the topic | EV | Yes/No |
| 9 | Outcomes are same for all subjects | IV | Yes/No |
| 10 | Scientific question stated in the aim is answered | IV | Yes/No |
| 11 | Results are clearly presented and discussed | IV | Yes/No |
| 12 | Appropriate statistical analysis techniques used | QV | Yes/No |
| 13 | Statistical test used clearly stated | QV | Yes/No |
| 14 | Analytical software used is clearly stated and referenced | QV | Yes/No |
| 15 | Sufficient number of subjects | QV | Yes/No |
| Reference | Year | Objective |
|---|---|---|
| Van den Noort et al. [22] | 2009 | Evaluate the use of goniometry in estimating the joint angle of the catch simultaneously with inertial sensors. |
| Franco et al. [23] | 2012 | Implement a Kalman filter using a smartphone to estimate 3-D angulation of the trunk. |
| Spain et al. [24] | 2012 | Study if wearable sensors can detect differences in balance and gait between people with MS with normal walking speeds and healthy controls. |
| Martori et al. [25] | 2013 | Develop a wearable motion analysis system to evaluate gait that consists on six IMUs. |
| Crea et al. [26] | 2014 | Describe a wearable pressure-sensitive insole sensor for lower-limb amputees feedbacks. |
| Dewey et al. [27] | 2014 | Assess suitability of instrumented gait and balance measures for PD diagnosis and estimation. |
| Hsu et al. [28] | 2014 | Develop gait and balance analysis algorithms to gather quantitative data considered early indicators of AD. |
| Patterson et al. [29] | 2014 | Compare a mobile technology application with a commonly used subjective balance assessment. |
| Tzallas et al. [30] | 2014 | Describe a system for continuous remote monitoring of patients with PD. |
| Wentick et al. [31] | 2014 | Investigate whether detection of gait initiation in transfemoral amputees can be useful for voluntary control of lower extremity prostheses. |
| Alberts et al. [32,33] | 2015 | Develop a biomechanically based quantification of the BESS using inertial sensors data. Determine whether inertial data provide sufficient resolution of center of gravity movements to quantify postural stability. |
| Bauer et al. [34] | 2015 | Evaluate IMU-system when assessing movement dysfunctions of concurrent validity and reliability. |
| Ellis & Zhu et al. [35,36] | 2015 | Describe a smartphone-based application to quantify gait variability. |
| Godfrey et al. [37] | 2015 | Investigate the use of a wearable sensor compared to laboratory reference. |
| Jaysrichai et al. [38] | 2015 | Measure the knee joint angle using IMUs and reference it with a motion capture system. |
| Kanzler et al. [39] | 2015 | Present a method for calculating continuous heel and toe clearance and foot angle in the sagittal plane without knowing shoe dimensions. |
| Lee & Kumar et al. [40,41] | 2015 | Design and validate a smartphone-based system for motor assessment using IMUs. |
| Lin et al. [42] | 2015 | Present and evaluate the step count performance of a smart insole system. |
| Postolache et al. [43] | 2015 | Develop a system to objectively record ground reaction forces, acceleration and direction of the feet using wearable sensors. |
| Sijobert et al. [44] | 2015 | Present an algorithm to estimate stride length using an accelerometer and a gyroscope. |
| Nouredanesh et al. [45,46] | 2015-16 | Develop a method that automatically distinguishes compensatory balance responses from regular stepping pattern. |
| Bertolotti et al. [47] | 2016 | Assemble an IMU to provide measurements of limb movements and balance abilities. |
| Del Din et al. [48] | 2015 | Quantify a comprehensive range of gait parameters using a single tri-axial accelerometer. Compare gait data of older adults with PD subjects. |
| Horak et al. [49] | 2016 | Study balance and gait to represent independent domains of mobility in PD. |
| Lee et al. [50] | 2016 | Compare Multiscale Entropy (MSE) analysis of acceleration data with other features to observe falling behavior and traditional clinical scales to evaluate falling behavior. |
| LeMoyne et al. [51] | 2016 | Facilitate the acuity of the timed 25 foot walk test with the synthesis of wearable and sensors and machine learning. |
| Li et al. [52] | 2016 | Develop a sit to stand detection system to raise an alarm when a individuals stand up without proper technique or assistance. |
| Storm et al. [53] | 2016 | Evaluate accuracy of two algorithms for detection of gait events and temporal parameters during free-living walking. |
| Wang et al. [54] | 2016 | Improve autocorrelation method for gait analysis using EMG signals collected from six muscle groups of the lower limbs in hemiparetic subjects. |
| Andó et al. [55] | 2017 | Propose a multi-sensor architecture for postural sway assessment in elderly and in people with neurological disorders. |
| Iijima et al. [56] | 2017 | Assess quantitatively the gait disorders in the daily lives ofpatients with PD using with a newly developed portable gait rhythmogram. |
| Lebel et al. [57] | 2017 | Assess attitude and heading reference system at multiple segments and joints. |
| Robert-Lachaine et al. [58] | 2017 | Determine the technological error and biomechanical model differences between IMUs and an optoelectronic system. |
| Schlachetzki et al. [59] | 2017 | Develop a gait analysis system with wearable sensors to assess gait parameters in PD. |
| Shazad et al. [60] | 2017 | Provide an objective, cost-effective method to obtain balance and mobility based fall-risk in older adults. |
| Aich et al. [61] | 2018 | Quantify gait parameters using wearable accelerometers; compare five estimated gait parameters with a 3D motion capture system automatic discrimination of FoG patients from no FoG patients using machine learning. |
| Diaz et al. [62] | 2018 | Propose methods to estimate step length and step width using wearable sensors. |
| Stack et al. [63] | 2018 | Detect instability using wearable sensors. |
| Zhang et al. [64] | 2018 | Propose a new gait symmetry index to quantify gait symmetry using one accelerometer. |
| Chomiak et al. [65] | 2019 | Assess the accuracy and reliability of a wearable sensor system for bio-feedback training. |
| Chomiak et al. [66] | 2019 | Describe a pattern recognition algorithm for the automated detection of gait-cycle breakdown and freezing episodes. |
| Grinberg et al. [67] | 2019 | Investigate different types of 3-meter tandem walking tests in fully ambulatory PwMS. |
| Hsied et al. [68] | 2019 | Determine if a smartphone can measure static postural stability and distinguish elderly with fall risk. |
| Mazzeta et al. [69] | 2019 | Propose a wearable sensor system for auto-continuous analysis of FoG in PD patients. |
| Mikos et al. [70] | 2019 | Demonstrate the integration of an FoG detection system into a single sensor node. |
| Ngueleu et al. [71] | 2019 | Equip an insole with pressure sensors to detect steps. |
| Phan et al. [72] | 2019 | Investigate wearable sensor technology to identify the kinematic features associated with gait abnormalities seen in cerebellar ataxia. |
| Reeves et al. [73] | 2019 | Determine the between-day reliability of peroneus longus EMG in healthy subjects while walking. |
| Rivolta et al. [74] | 2019 | Investigate the use of wearable accelerometer to evaluate the fall risk determined by the Tinetti clinical scale. |
| Tang et al. [75] | 2019 | Propose an objective approach to access functional balance using an insole wearable sensor and an accelerometer. |
| Weiss et al. [76] | 2019 | Evaluate strategies employed by PD patients when transitioning from turning to sitting. |
| Zhao et al. [77] | 2019 | Present an adaptive method for gait detection. |
| Reference | Analysis | Parameters Extracted | Population | Sensor(s) Used | Sensor(s) Location | Obtrusiveness Level |
|---|---|---|---|---|---|---|
| Van den Noort et al. [22] | ROM | Knee Angle Ankle Angle | 1 healthy | IMU | Thigh | Medium |
| Franco et al. [23] | Balance ROM | Trunk angles Sway ranges | 20 healthy | Smart | Lumbar | Low |
| Martori et al. [25] | Gait ROM | Stride length Cadence Knee flexion | 10 healthy | IMU | Sternum Waist Thighs Shanks | High |
| Crea et al. [26] | Gait | Swing time Stance time Cadence | 10 healthy | Pressure | Insole | Low |
| Dewey et al. [27] | Gait Balance | Velocity Cadence Arm swing Sway area Jerk Path length Sway distance | 135 PD | IMU | Ankles Wrists Lumbar Sternum | High |
| Hsu et al. [28] | Gait Balance | Stride time Stride Velocity Stance time Swing time Cadence | 21 AD 50 healthy | IMU | Feet Waist | Medium |
| Patterson et al. [29] | Balance | Postural measure | 21 healthy | Smart | Hold on chest | Low |
| Tzallas et al. [30] | Gait | Not specified | 20 PD short-term 24 PD long-term | IMU | Ankles Wrists Waist | High |
| Wentick et al. [31] | Gait | Gait initiation | 3 transfemoral amputees 3 through the knee amputees | IMU EMG | Upper leg | High |
| Alberts et al. [32,33] | Balance | Path length RMS Equilibrium score | 49 healthy for one study 32 healthy for other study | Smart | Lumbar | Low |
| Bauer et al. [34] | ROM | Flexion Extension Lateral flexion | 22 asymptomatic for validity 24 asymptomatic for reliability | IMU | Right thigh Sacrum L1 back level T1 back level | Medium |
| Ellis & Zhu et al. [35,36] | Gait | Step time Step length Variability | 12 healthy elderly 12 PD | Smart | Abdomen | Low |
| Godfrey et al. [37] | Gait | Step length Step velocity Asymmetry | 40 healthy young 40 healthy old | IMU | Lumbar | Low |
| Jaysrichai et al. [38] | ROM | Knee angle | 10 healthy | IMU | Shanks Thighs | Medium |
| Kanzler et al. [39] | Gait | Heel clearance Toe clearance Foot angle | 20 healthy | IMU | Ankle | Low |
| Lee & Kumar et al. [40,41] | ROM | Joint angles | 19 healthy 20 disable | IMU Smart | Thighs Shanks Ankles | High |
| Lin et al. [42] | Gait | Step count | 10 healthy | Pressure | Insole | Low |
| Postolache et al. [43] | Gait | Step length Stride length Cadence Gait Speed | 6 healthy | IMU Pressure | Shanks Insole | Low |
| Sijobert et al. [44] | Gait | Stride length | 10 healthy 12 PD | IMU | Shanks | Low |
| Nouredanesh et al. [45,46] | Gait Balance | Normal step Side step Crossover step | 5 healthy | IMU EMG | Thighs Shanks Lumbar | Medium |
| Bertolotti et al. [47] | Balance ROM | Trunk inclination Sway path Sway area Sway mean velocity | 10 healthy | IMU | Lumbar | Low |
| Del Din et al. [48] | Gait | Stride time Stance time Swing time Step velocity Step length Variability Diff. Asymmetry | 5 healthy | IMU | Lumbar | Low |
| Horak et al. [49] | Gait Balance | Postural measures Trunk acceleration Gait speed Cadence | 10 healthy 12 PD | IMU | Lumbar Shanks Arms | High |
| Lee et al. [50] | Gait Balance | Jerk Sway range Sit-to-stand time Mean & STD Step length | 65 elderly | IMU | Lumbar | Low |
| LeMoyne et al. [51] | Gait | Stride time Gyroscope statistics | 1 healthy 1 FA | IMU | Ankles | Low |
| Li et al. [52] | Gait Balance | Trunk angle Muscle strength | 6 healthy | EMG Smart | Lumbar Thighs | Medium |
| Storm et al. [53] | Gait | Stride time Step time Stance times | 10 healthy | IMU | Lumbar Ankles | Low |
| Wang et al. [54] | Gait | Autocorrelation | 10 healthy 1 hemipheris | EMG | Legs muscle | High |
| Andó et al. [55] | Balance | Sway range Sway mean velocity Sway mean frequency | 22 healthy | IMU | Waist Sternum | High |
| Iijima et al. [56] | Gait | Gait cycle Cadence Acceleration magnitude | 14 PD | IMU | Waist | Low |
| Lebel et al. [57] | Gait ROM | Multiple ROM angles | 20 asymptomatic | IMU | Left feet Pelvis Back Head Left Calf Left Thigh | High |
| Robert-Lachaine et al. [58] | ROM | Multiple ROM angles | 12 healthy | IMU | Feet Shanks Arms Thighs Pelvis Sternum Head | High |
| Schlachetzki et al. [59] | Gait | Stride length Stride time Velocity Gait phases times Foot clearance Heel-strike Toe-off angles | 63 PD | IMU | Ankle | Low |
| Shazad et al. [60] | Gait Balance | Step count Step frequency Avg. step length Walking speed | 23 elderly | IMU | Waist | Low |
| Aich et al. [61] | Gait | Step time Stride time Step length Stride length Walking speed | 51 PD | IMU | Ankles | Low |
| Diaz et al. [62] | Gait | Step length Step width | 4 healthy | IMU | Lumbar Thighs Shanks | Medium |
| Stack et al. [63] | Gait Balance | TUG Times Turns’ Step Count | 4 healthy | IMU | Wrists Ankle Waist | Medium |
| Zhang et al. [64] | Gait | Symmetry | 16 Post-Stroke 9 healthy | IMU | Feet Lower Back | Low |
| Chomiak et al. [65] | Gait | Walking speed Cadence Step length | 15 healthy | IMU Smart | Knee | Low |
| Chomiak et al. [66] | Gait | Rence quantification analysis | 9 healthy 21 PD | Smart | Thigh | Low |
| Grinberg et al. [67] | Gait | Velocity Cadence Double support Swing phase | 25 MS 25 healthy | IMU | Feet Lower Back | Low |
| Hsied et al. [68] | Balance | RMS AP & ML movements | 30 elderly | Smart | Hold on chest | Low |
| Mazzeta et al. [69] | Gait | Step time Ratio: Max value/sEMG | 7 PD | IMU EMG | IMU-calf EMG-lower leg | High |
| Mikos et al. [70] | Gait | Frequency RMS & STD Range Stride length Stride time | 63 PD | IMU | Ankles | Low |
| Ngueleu et al. [71] | Gait | Step Count | 20 healthy | Pressure | Insole | Low |
| Phan et al. [72] | Gait | PCA generated features | 29 cerebellar ataxia 22 healthy | IMU | Ankles | Low |
| Reeves et al. [73] | Gait | Peroneus longus | 10 healthy | EMG | Right leg (SENIAM guideline) | Medium |
| Rivolta et al. [74] | Gait Balance ROM | Accelerometer features Tilt angle | 79 hospitalized | IMU | Chest | Low |
| Tang et al. [75] | Gait Balance | RMS & STD Entropy Mean absolute deviation Lempel-ziv Dominant frequency | 33 elderly | IMU Pressure | Waist pouch Insole | Low |
| Weiss et al. [76] | Balance | TUG times | 96 PD | IMU | Lumbar | Low |
| Zhao et al. [77] | Gait | Gait cycle phases | 9 healthy | IMU | Feet | Low |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, S.; Stephenson, J.B.; Labrador, M.A. Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis. Appl. Sci. 2020, 10, 234. https://doi.org/10.3390/app10010234
Díaz S, Stephenson JB, Labrador MA. Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis. Applied Sciences. 2020; 10(1):234. https://doi.org/10.3390/app10010234
Chicago/Turabian StyleDíaz, Steven, Jeannie B. Stephenson, and Miguel A. Labrador. 2020. "Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis" Applied Sciences 10, no. 1: 234. https://doi.org/10.3390/app10010234
APA StyleDíaz, S., Stephenson, J. B., & Labrador, M. A. (2020). Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis. Applied Sciences, 10(1), 234. https://doi.org/10.3390/app10010234





