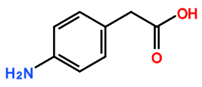
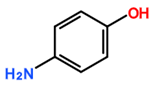
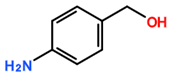
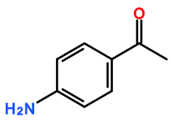
Featured Application
The present work is expected to have potential applications in the field of silicone composites, rubbers, ceramics, and epoxides. Also, it provides a novel way to understand the nature of solvents based on Hansen solubility parameters (HSP) theory.
Abstract
The functionalization of carbon nanotubes (CNTs) plays a key role in their solubilization and compatibility for many applications. Among the many possible ways to functionalize CNTs, the creation of an Si–O–C bond is crucial for the formation of silicone composites. Catalyst-mediated silylesterification is useful in creating Si–O–C bonds because it is cost-effective and uses a hydrosilane precursor of lower reactivity than that of chlorosilane. However, it was previously demonstrated that two important silylesterification catalysts (zinc chloride and Karstedt’s catalyst) exhibit different selectivity for oxidized functional groups that are present on the surface of CNTs after oxidative acid treatment. This report details the selective modification of CNTs with various oxygenated functional groups (aromatic and nonaromatic alcohols, carboxylic acids, ethers, and ketones) using diazonium chemistry. Modified CNTs were used to assess the specifity of zinc chloride and Karstedt’s catalyst for oxygenated functional groups during a silylesterification reaction. Karstedt’s catalyst appeared to be widely applicable, allowing for the silylesterification of all of the aforementioned oxygenated functional groups. However, it showed lower efficacy for ethers and ketones. By contrast, zinc chloride was found to be very specific for nonaromatic carboxylic acids. This study also examined the Hansen solubility parameters of modified CNTs.
1. Introduction
Carbon nanotubes (CNTs) [1] have exceptional electrical, mechanical, and physical properties [2]. However, their major drawbacks are their insolubility in most common solvents, and their tendency to form aggregates due to strong π–π and van der Waals interactions. Therefore, due to the chemical functionalization of CNTs, it is often necessary in order to exfoliate and solubilize them before their practical application [3,4]. Moreover, their use as reinforcement in polymers requires anchoring groups that must be introduced by chemical functionalization.
Many different methods for the chemical functionalization of CNT sidewalls were reported [5]. One approach consists of the direct functionalization of the CNT sidewall using, for example, carbenes, nitrenes, the Diels–Alder reaction, and diazonium [6]. This option has the advantage of preserving the aspect ratio of CNTs, as well as their mechanical and electrical properties [7]. However, this depends on the number of defects created in the outermost aromatic layer. A second approach concerns the derivatization of oxidized functional groups introduced by acidic treatments [8] at the extremities or defective areas of CNTs. Derivatization reactions include amidation [9,10], esterification, and reactions with silicon derivatives such as hydrosilanes and chlorosilanes [11].
Among the various options for the derivatization of oxidized functional groups, silylesterification is a powerful way to modify the CNT surface using silane compounds [12]. This functionalization allows for modification by many silane derivatives bearing various side chains. Hydrosilanes have the advantage of being less reactive than their chlorosilane counterparts, which allows for the use of less stringent experiment conditions. Usually, the coupling of hydrosilanes with oxygen-containing molecules requires the presence of a catalyst. Depending on the catalyst’s nature, hydrosilanes react differently with oxygenated molecules. CNT modification with an Si–O–C bond creates opportunities for the addition of different functional groups for various applications. These include the filling of silicone composites, rubbers [13], ceramics, and epoxides [14].
In a previous study [11], our group reported on the functionalization of oxidized CNTs (using oxidative acid treatment) with hydrosilane as an anchoring molecule. Specifically, we used a fluorine-bearing hydrosilane, diisopropyl(3,3,4,4,5,5,6,6,6-nonafluorohexyl) silane, which is also referred to as Rf(iPr)2SiH. We compared two different catalysts—zinc chloride (ZnCl2) and Karstedt’s catalyst (1,3-divinyl-1,1,3,3-tetramethyldisiloxane platinum (0) complex). CNTs oxidized by concentrated acids, bearing several different oxygenated functional groups, reacted differently with each catalyst. We hypothesized that the zinc chloride catalyst allowed for reactions involving only carboxyl groups, while Karstedt’s catalyst facilitated reactions of both alcohol and carboxyl groups that were introduced during oxidative acid treatment.
The aim of this study was to assess the selectivity of silylesterification reactions on CNTs by using CNTs modified by derivatives of aryl diazonium salts as a starting material. In contrast to products obtained after oxidation using concentrated acid mixtures, diazonium-mediated modification allows for the preparation of CNTs with controlled surface chemistry [15,16,17]. In addition, the advantage of using diazonium modification is that oxidative treatment can affect CNT conductivity. This has been proven in a previous study by measuring the electrical conductivity of buckypaper (a thin sheet of aggregated CNTs) [7] prepared from diazonium-modified CNTs and oxidized CNTs. Using a silylesterification reaction [11], the selectivity of both catalysts for various oxygenated functional groups on aryl diazonium derivatives was investigated (Table 1).

Table 1.
Carbon-nanotube (CNT) samples, aniline precursors used for in situ diazonium-mediated functionalization, and side chain targeted for hydrosilylation.
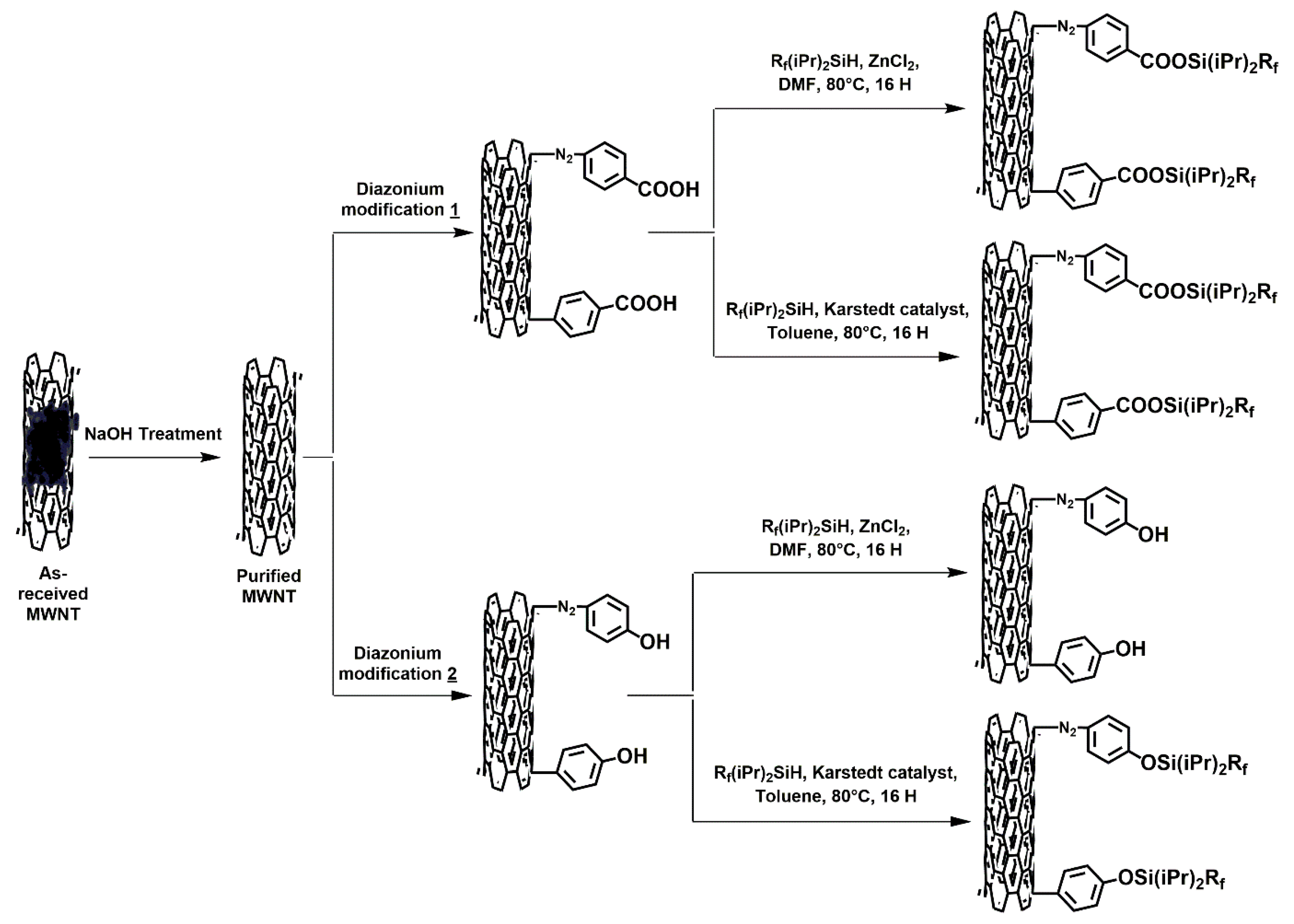
A schematic representation of the approach proposed in this work is shown in Figure 1.
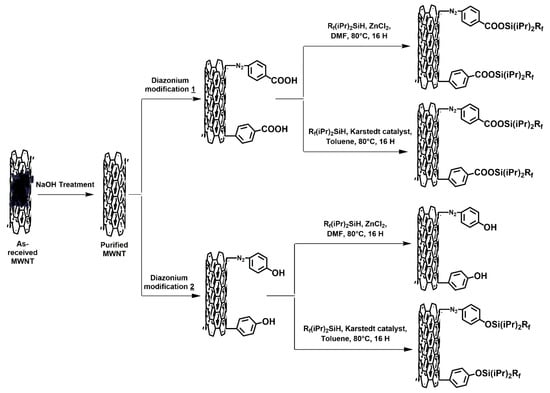
Figure 1.
Schematic representation of this study’s approach. Expected results based on above hypothesis.
CNTs functionalized by diazonium chemistry were characterized by using their Hansen solubility parameters (HSPs).
2. Materials and Methods
2.1. Diazonium-Mediated Modification
Multiwalled carbon nanotubes (MWNTs) were purified prior to modification by diazonium in order to remove alumina (a catalyst support). Purification was achieved by refluxing 4 g of MWNTs into 500 mL sodium hydroxide solution (6 moles) for 12 h. The purified MWNT sample was then thoroughly washed with deionized water [15]. MWNT functionalization by diazonium chemistry was carried out by modifying a previously reported procedure [15]. Briefly, 20 mg of purified CNTs was dispersed in 10 mL of deionised water containing 6.0 × 10−4 moles of the appropriate aniline derivative (depending on the desired functional group, refer to Table 1), along with 6.0 × 10−4 moles of sodium nitrite and 8.0 × 10−4 moles of perchloric acid. This mixture was stirred for 60 min under IR irradiation and then washed with deionized water. It was then washed with acetone and dried at room temperature.
2.2. Silylesterification
All MWNT 6 samples were functionalized with diisopropyl(3,3,4,4,5,5,6,6,6-nonafluorohexyl)silane (Rf(iPr)2SiH) using either ZnCl2 or Karstedt’s catalyst.
When using ZnCl2 as a catalyst, 10 mg of functionalized MWNTs, 40 µl of diisopropyl(3,3,4,4,5,5,6,6,6-nonafluorohexyl)silane, and 1 mg of ZnCl2 were added into 20 mL of dimethylformamide (DMF) and stirred at 80 °C for 16 h at atmospheric pressure. The product was then washed with DMF and dried at 120 °C.
When using Karstedt’s catalyst, 10 mg of functionalized MWNTs, 40 µl of diisopropyl(3,3,4,4,5,5,6,6,6-nonafluorohexyl)silane, and 1 µl of a 0.120 M solution of Karstedt’s catalyst in xylene were added to 20 mL of toluene and stirred at 80 °C for 16 h at atmospheric pressure. The product was then washed with toluene and dried at 120 °C.
2.3. Determination of Hansen Solubility Parameters
Determination of the HSPs of a given solute was described in previous publications [18,19]. Briefly, 1 mg of solute was added to 1 mL of the tested solvent. The sample was sonicated for 1 min using a sonication tip (1 cycle and maximum amplitude) and centrifugated for 30 min at 3500 rpm (PICCOLO, 220 V and 60 W). Solvent quality (i.e., its ability to maintain a high amount of solute in a stable solution) was estimated with a visual comparison to standards of known concentration. Although rough, this protocol was demonstrated to provide valuable results with regard to HSP determination [20]. The solubility of each CNT sample in 40 different solvents was determined. Solvents were classified as “bad,” “good,” or “very good”, and then processed using HSPiP software [19]. This yielded the most reliable solubility sphere with regard to “good” solvents. The center of the sphere corresponded to the HSPs of functionalized CNTs.
3. Results
In the first part of this section, the characterizations of functionalized CNTs are described. In the second part, the solubility of functionalized CNTs in various solvents, the corresponding HSPs, and their correlation with grafted functional groups are described.
3.1. Diazonium-Mediated Modification
MWNT samples functionalized by diazonium (CNT–COOH, CNT–CH2–COOH, CNT–OH, CNT–CH2OH, CNT–C(O)–CH3, and CNT–O–CH3) were characterized using X-ray photoelectron spectroscopy spectra and changes to their HSPs. In the case of characterization using XPS, difficulties were created by the fact that functionalization does not introduce a new element to the modified sample. Therefore, the fit of the C1s peak was used to assess functionalization efficacy. The C1s fit table (Table 2) was established using previously reported values [21,22] and analysis of standard samples such as those containing regraphitized MWNTs. The element composition and components of the C1s peaks for the six samples are summarized in Table 3 and Figure 2, respectively. Figure 2 shows the C1s high-resolution XPS spectra for the six samples and contributions of the various components.

Table 2.
XPS fitting parameters of various C1s peak components.

Table 3.
Quantity of oxygen, carbon, and nitrogen in XPS spectra for six different diazonium-modified multiwalled carbon nanotubes (MWNTs).
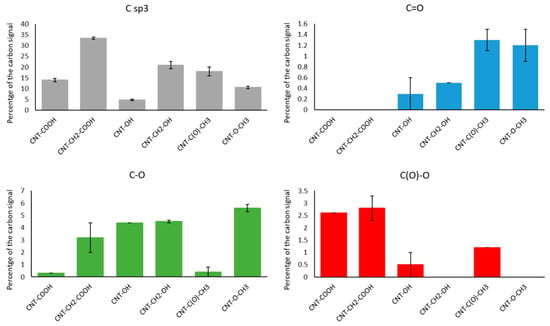
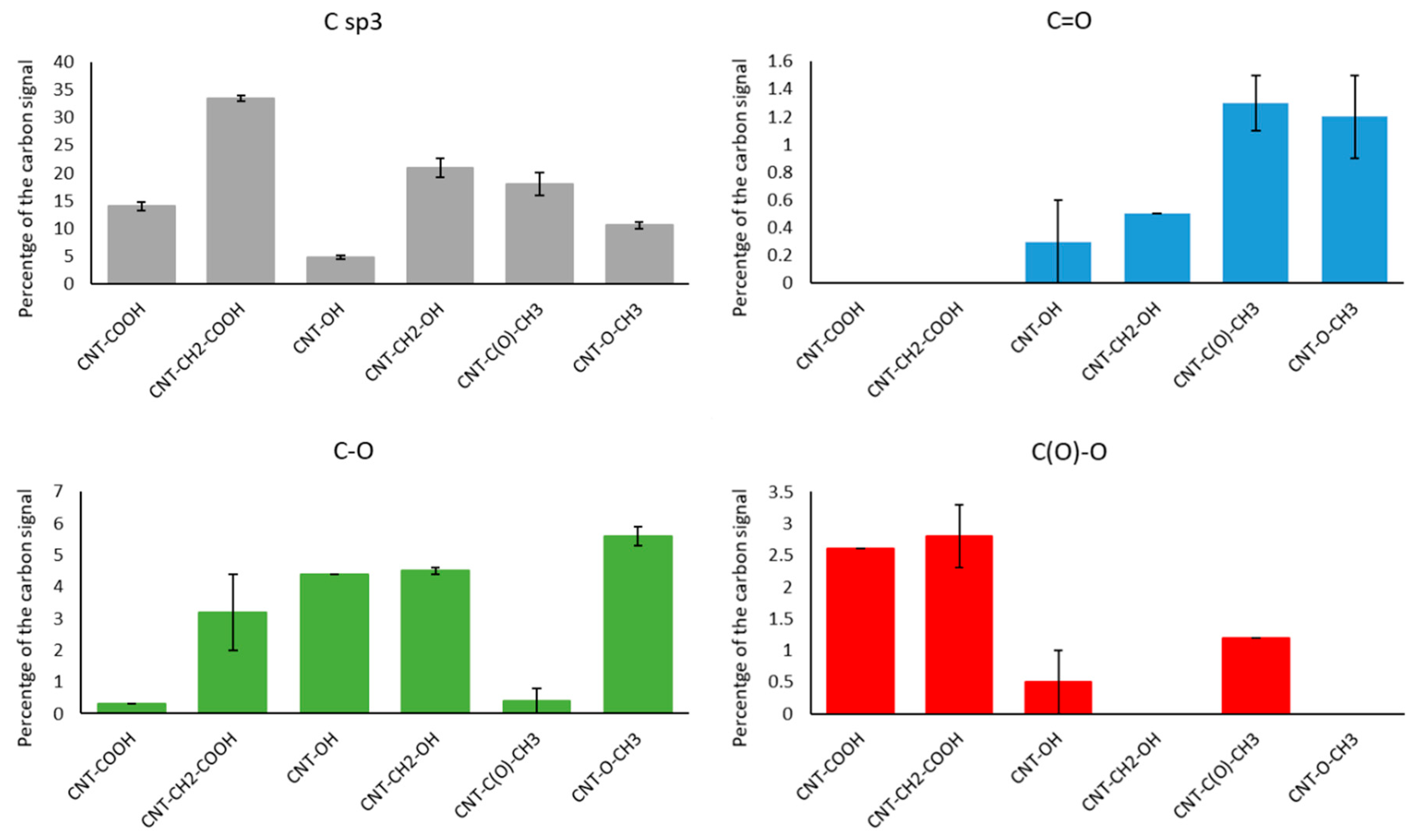
Figure 2.
Quantity of various components of C1s peak for six diazonium-modified carbon nanotube samples.
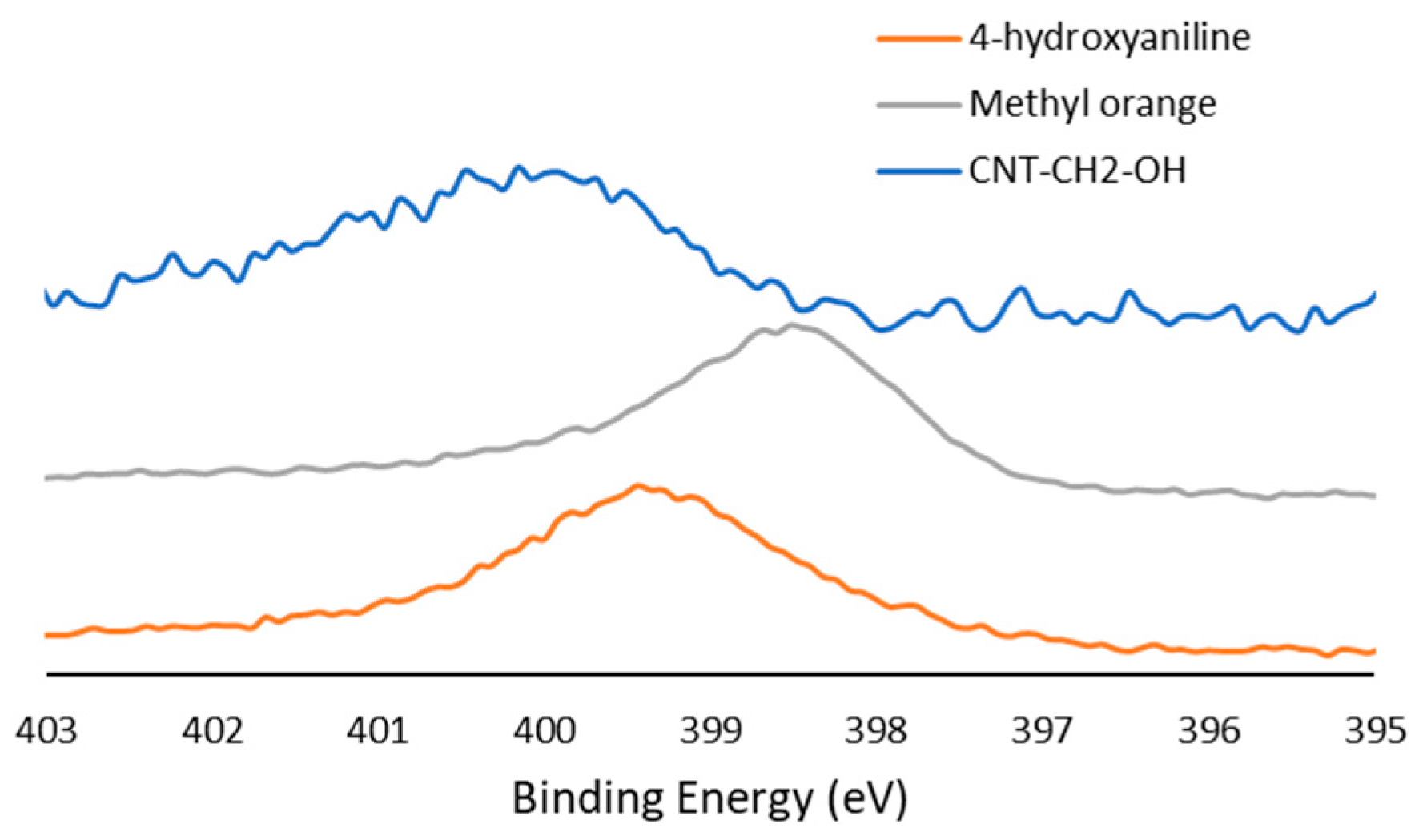
Both the element composition and contributions of various components of the C1s peak were highly reproducible, as demonstrated by small standard deviations. Table 3 shows the amount of increased oxygen in the six samples after functionalization. In samples where the side chain was separated from the aromatic core by a methylene group, a noticeable nitrogen peak was seen. High-resolution spectra of the N1s peak in those samples (CNT–CH2–COOH and CNT–CH2–OH) showed an asymmetric peak at 400.0 eV. Diazonium bridges exhibited a binding energy of 398.5 eV (Figure 3, standardization using methyl orange), which was close to that of amines (399.5 eV, depending on the side chain). Therefore, the conclusion that the N1s peak originated from a diazonium bond was derived from (i) the asymmetry of the peak, which is characteristic of delocalized electrons, and (ii) the absence of a decrease in intensity after further washing, which removes adsorbed molecules.
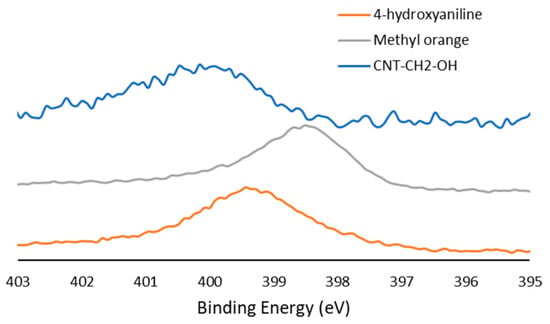
Figure 3.
High-resolution N1s spectra for CNT–CH2–OH sample, pure 4-hydroxyaniline sample, and methyl orange (diazoic compound).
Figure 4 shows an increase in the Csp3 component in all functionalized samples. This increase was consistent with diazonium-mediated functionalization, as this modification transforms sp2 carbons of the CNT sidewall into Csp3 carbons [23].
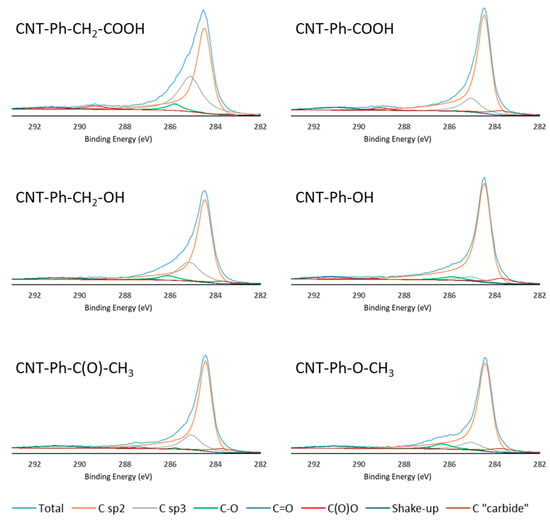
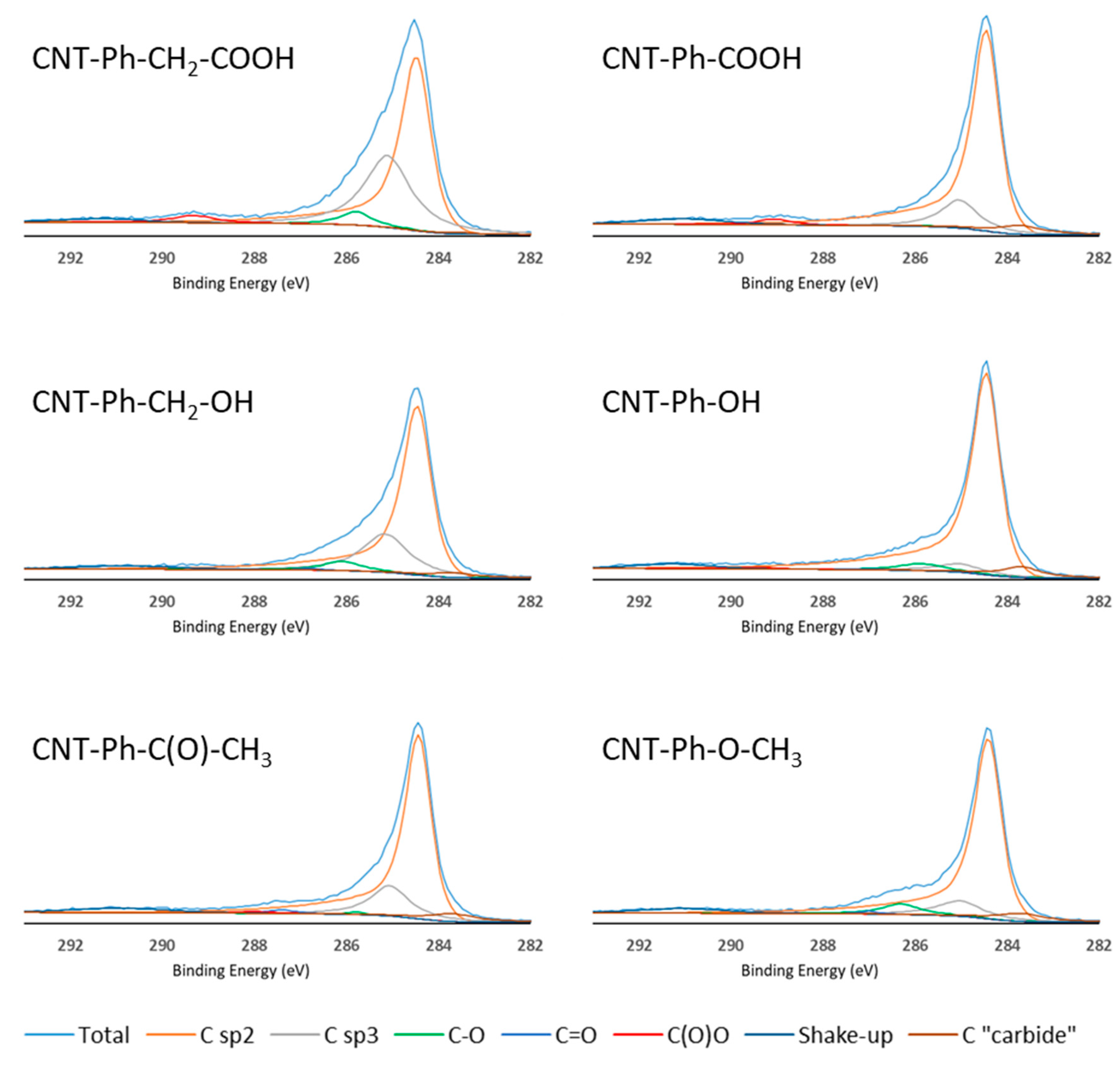
Figure 4.
High-resolution C1s spectra of the six samples of diazonium-modified CNTs with various components described in Table 2.
When assessing functionalization via C1s peak fitting, it was found that MWNT–COOH and MWNT–CH2–COOH samples contained a C(O)–O component (2.6% and 2.8%, respectively). Additionally, the Csp3 component was larger in the CNTs functionalized with –CH2–COOH than in their –C(O)–O counterpart, which was consistent with the presence of an additional methylene group.
For CNTs functionalized with –OH and –CH2–OH, the C–O component (285.8 ± 0.2 eV) increased by 4.4% and 4.5%, respectively, with a larger sp3 component in the case of –CH2–OH functionalization. This confirmed the grafting effectiveness for both side chains.
The MWNT–C(O)–CH3 sample contained larger C=O (1.3%, 286.9 eV) and C(O)–O (1.2%, 289.2 eV) components. This confirmed the presence of a highly oxidized functional group (a ketone) on the MWNT surface. An increase in Csp3 contribution was observed due to the presence of a methyl group on the ketone molecule. CNTs modified with an –O–CH3 side chain displayed high C–O contribution (5.6%, 285.8 eV), which is characteristic of ether.
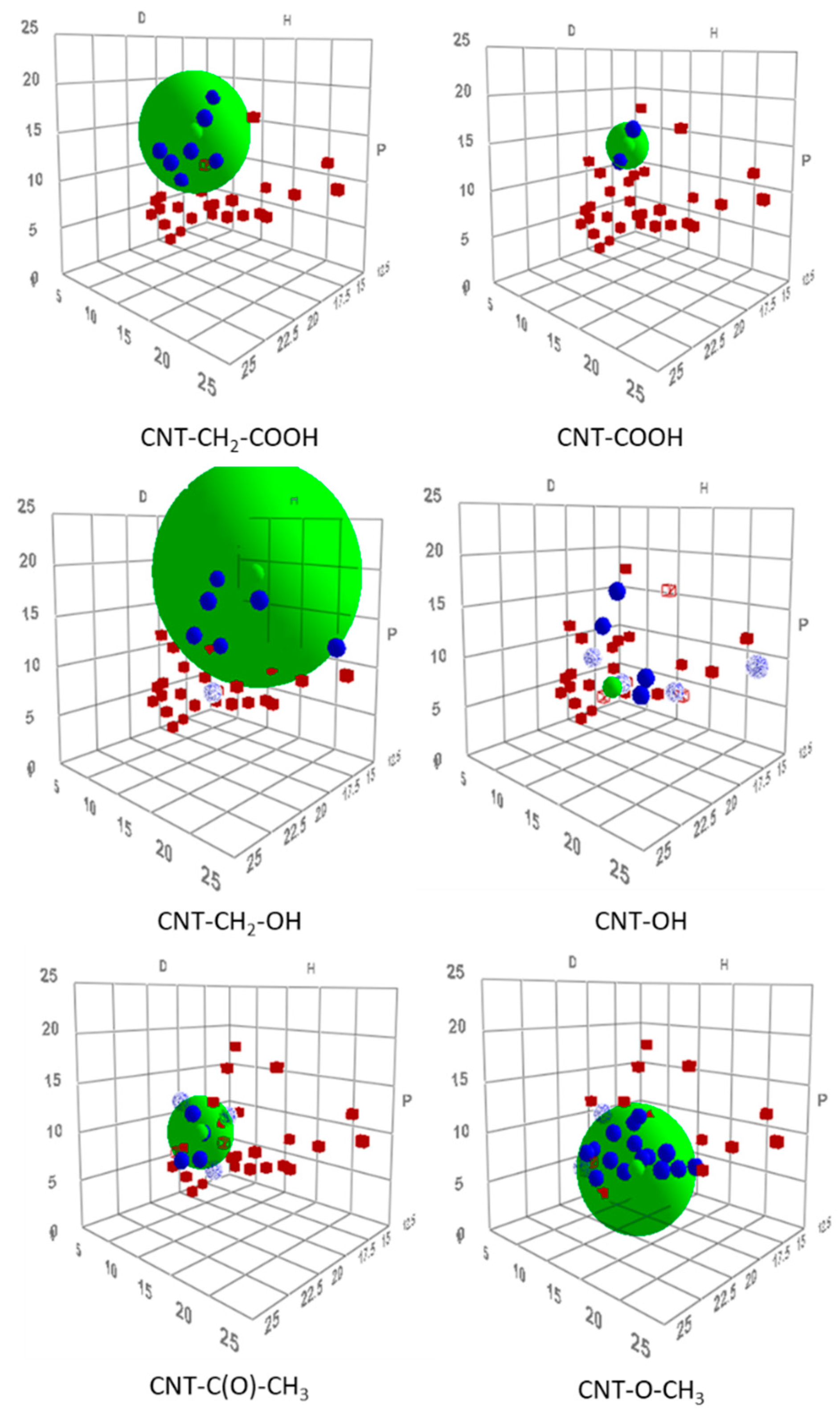
HSPs are a means to describe interactions between a solute and a solvent. Each compound is described by three parameters: A dispersion parameter, accounting for dispersion forces (δd); a polar parameter (δp), accounting for dipole–dipole interactions; and a hydrogen-bond parameter (δh). A solute and solvent that are characterized by close parameters are compatible and thus soluble in each other. By contrast, compounds with largely different parameters are immiscible. Therefore, it is possible to determine HSPs of an unknown compound by testing its solubility in several solvents with known HSPs. Some solvents appear to be “good solvents.” On a tridimensional plot (using the three parameters as axes), “good solvents” are encompassed by a sphere with a center corresponding to the solubility of the compound with unknown HSPs. HSP theory [20] was proven to be applicable in the case of CNTs (crude and functionalized), and could therefore be used to characterize functionalized CNTs. Modified CNTs should be characterized by different HSPs from those of the starting material, and each functionalization produces CNTs with different parameters. HSPs were determined for six modified samples (Table 4), and the corresponding solubility spheres are shown in Figure 5.

Table 4.
Hansen solubility parameters (HSPs) for six samples of diazonium-modified CNTs.
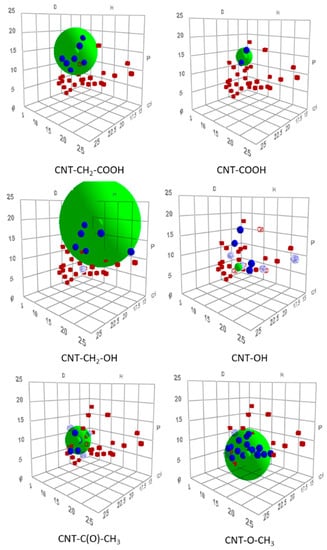
Figure 5.
Tridimensional representation of Hansen solubility spheres for six diazonium-modified CNT samples. Note: good solvents, blue; bad solvents, red. For CNT–OH sample, only sphere center is shown.
The six modified samples exhibited HSPs (Figure 6 and Table 4) that differed from those of nonfunctionalized MWNTs (δd = 19.7 MPa1/2, δp = 6.2 MPa1/2, and δh = 4.2 MPa1/2). COOH- and CH2–COOH-modified CNTs were characterized by similar HSPs. They showed strong polar parameters and moderate hydrogen bond parameters, which was consistent with the grafting of a carboxyl-like functional group. Comparatively, CNTs modified with MWNT–OH and MWNT–CH2–OH displayed HSPs with greater hydrogen-bond contribution but lower (at least for MWNT–OH) polar contribution, which was consistent with an alcohol-like functional group. CNTs modified with ketone and ether side chains were characterized by lower polar and hydrogen-bond parameters compared with samples functionalized with alcohol or carboxyl functional groups. As shown in Table 5, the HSPs of MWNT–C(O)–CH3 and MWNT–O–CH3 were consistent with the tabulated values for ketones and ethers. Ketones are characterized by a greater polar parameter than the hydrogen-bond parameter. The ether showed low polar and hydrogen-bond parameters, with δp being lower than δh [19]. A comparison between Table 4 and Table 5 clearly shows that CNTs modified by MWNT–C(O)–CH3 and MWNT–O–CH3 were characterized by HSPs that were very close to those of aromatic molecules with corresponding functions (acetophenone and anisole, respectively).
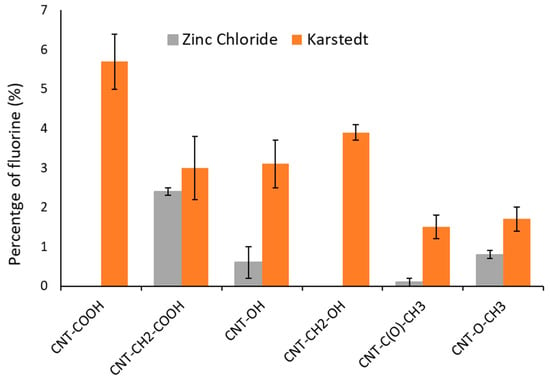
Figure 6.
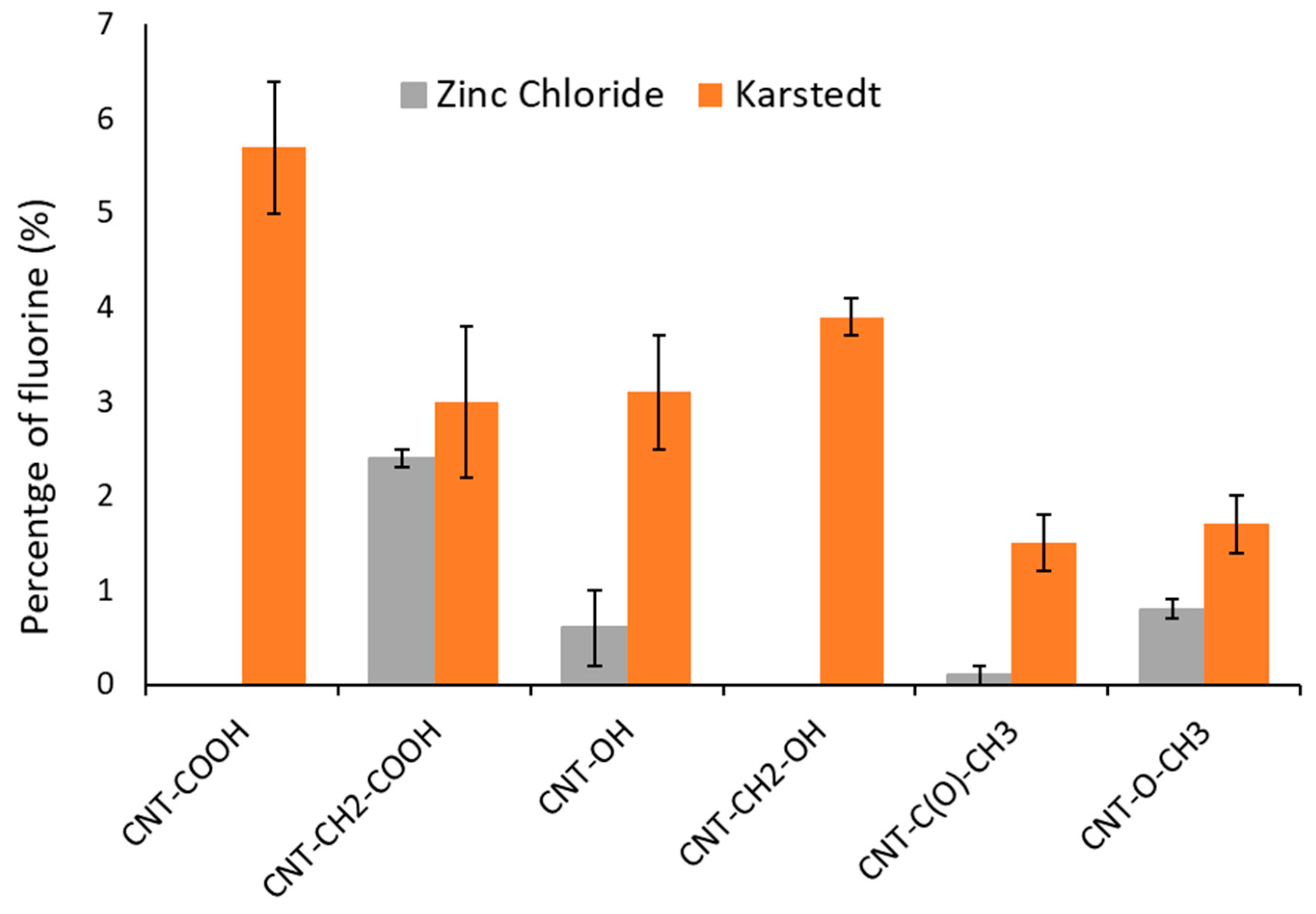
Changes in fluorine quantity as function of oxygenated functional group grafted by diazonium chemistry and catalyst used for silylesterification.

Table 5.
Hansen solubility parameters for molecular characteristics of ethers and ketones. Values compared to those in Table 4.
On the basis of XPS and HSP characterizations, it could be concluded that the six different modifications were successful. Therefore, those samples were used to test the selectivity of silylesterification catalysts.
3.2. Silylesterification
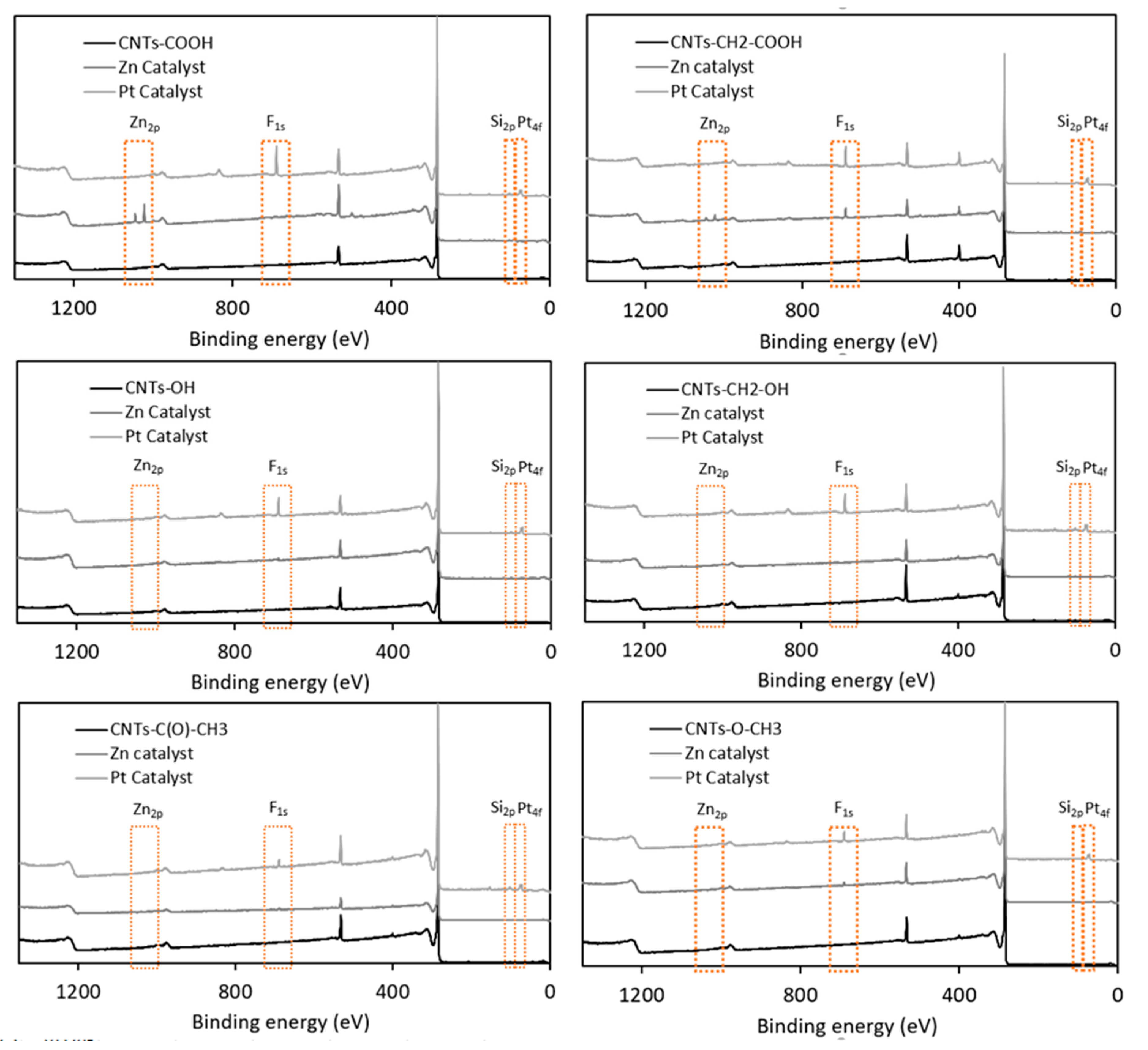
Table 6 and Figure 6 summarize the atomic percentages of various elements in 12 samples produced by the derivatization of six types of diazonium-modified CNTs using either zinc chloride or Karstedt’s catalyst. Figure 7 displays XPS comparisons of the samples’ survey spectra after derivatization with diazonium and silylesterification with either zinc chloride or Karstedt’s catalyst.

Table 6.
Atomic % of various elements in six samples of diazonium-modified CNTs that were silylesterified with either zinc chloride or Karstedt’s catalyst.
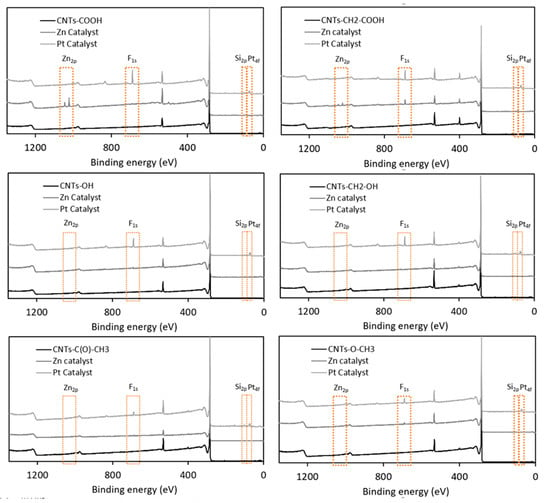
Figure 7.
Comparison of XPS survey spectra for six diazonium-modified samples before and after silylesterification using zinc chloride or Karstedt’s catalyst.
The observed fluorine percentages (in bold) showed two extremely different types of behavior depending on the used catalyst.
Karstedt’s catalyst allowed for the silylesterification of all oxygenated functional groups. The process was more efficient for carboxyls and alcohols, regardless of whether they contained an aromatic ring [24,25].
Zinc chloride catalyzed the silylesterification of only nonaromatic carboxylic acids. This observation complements our previous report that found zinc chloride to be selective for carboxyl groups [11].
4. Conclusions
In this report, we demonstrated the selectivity of two catalysts for several oxygenated functional groups in CNT silylesterification.
To achieve this goal, we used a two-step approach that consisted of (i) the selective functionalization of CNT sidewalls with various oxygenated functional groups (carboxylic acid, alcohol, ketone, and ether) and (ii) a study of the selectivity of silylesterification catalysts using fluorinated hydrosilane as a probe molecule.
Selective CNT sidewall functionalization with six different oxygenated functional groups was assessed using XPS survey spectra, high-resolution spectra of C1s peaks, and their characteristic HSPs. XPS C1s high-resolution spectra showed components that were characteristic of each of the grafted functional groups. HSP solubility characterization also demonstrated behaviors typical of the grafted functional groups.
Subsequent derivatization of oxygenated side chains by silylesterification using either zinc chloride or Karstedt’s catalyst showed differences in their selectivity. Karstedt’s catalyst appeared to be highly efficient in the silylsterification of both alcohol and carboxylic acid, regardless of the presence of an aromatic ring. This catalyst was slightly less effective in the silylesterification of ethers and ketones. As previously reported, zinc chloride appears to be specific to carboxylic acid groups. Moreover, its selectivity is strictly limited to nonaromatic carboxylic acids.
The difference in selectivity described in this report paves the way for the modification of CNTs using two different oxygenated groups.
Author Contributions
Conceptualization, S.D., J.D., and Z.M.; methodology, S.D. and P.N.; software, S.D. and A.K.B.; validation, S.D., A.K.B., P.N., Z.M., and J.D.; resources, Z.M.; writing—original-draft preparation, S.D. and A.K.B.; writing—review and editing, S.D., A.K.B., P.N., Z.M., and J.D.; supervision, ZM. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Arvind K. Bhakta thanks the University of Namur for the CERUNA doctoral fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Suman, L.; Vikas. Dispersibility of carbon nanotubes in organic solvents: Do we really have predictive models? J. Nanoparticle Res. 2017, 19, 211. [Google Scholar] [CrossRef]
- Farghali, A.; Tawab, H.A.A.; Moaty, S.A.A.; Khaled, R. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J. Nanostructure Chem. 2017, 7, 101–111. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Salmi, Z.; Dahoumane, S.A.; Mekki, A.; Carbonnier, B.; Chehimi, M.M. Functionalization of nanomaterials with aryldiazonium salts. Adv. Colloid Interface Sci. 2015, 225, 16–36. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Detriche, S.; Kumari, S.; Hussain, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Multi-wall Carbon Nanotubes Decorated with Bismuth Oxide Nanocrystals Using Infrared Irradiation and Diazonium Chemistry. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1402–1413. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Leija, G.H.M. Functionalization of SWCNTs with amine derivatives and comparative solubilization studies. J. Mater. Sci. 2018, 53, 466–478. [Google Scholar] [CrossRef]
- Musayeva, N.; Orujov, T.; Hasanov, R.; Sultanov, C.; Ferrari, C.; Frigeri, C.; Trevisi, G.; Beretta, S.; Bosi, M.; Verucchi, R.; et al. Growth and functionalization of carbon nanotubes for nitroaromatic explosive detection. Mater. Today Proc. 2019. [Google Scholar] [CrossRef]
- Modugno, G.; Ksar, F.; Battigelli, A.; Russier, J.; Lonchambon, P.; Eleto Da Silva, E.; Ménard-Moyon, C.; Soula, B.; Galibert, A.M.; Pinault, M.; et al. A comparative study on the enzymatic biodegradability of covalently functionalized double- and multi-walled carbon nanotubes. Carbon 2016, 100, 367–374. [Google Scholar] [CrossRef]
- Seffer, J.-F.; Detriche, S.; Nagy, J.B.; Delhalle, J.; Mekhalif, Z. Silylesterification of oxidized multi-wall carbon nanotubes by catalyzed dehydrogenative cross-coupling between carboxylic and hydrosilane functions. Appl. Surf. Sci. 2014, 305, 301–308. [Google Scholar] [CrossRef]
- Karabörk, M.; Fattah, B.S. Synthesis, characterization and application in the water purification of the multi-walled carbon nanotubes hybrid material. Inorg. Nano-Metal Chem. 2019, 48, 312–322. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Ryu, S.H. Influence of aminosilane-functionalized carbon nanotubes on the rheometric, mechanical, electrical and thermal degradation properties of epoxidized natural rubber nanocomposites. Polym. Int. 2013, 62, 1433–1441. [Google Scholar] [CrossRef]
- Vennerberg, D.; Hall, R.; Kessler, M.R. Supercritical carbon dioxide-assisted silanization of multi-walled carbon nanotubes and their effect on the thermo-mechanical properties of epoxy nanocomposites. Polymer (Guildf) 2014, 55, 4156–4163. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Detriche, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Decoration of tricarboxylic and monocarboxylic aryl diazonium functionalized multi-wall carbon nanotubes with iron nanoparticles. J. Mater. Sci. 2017, 52, 9648–9660. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Mascarenhas, R.J.; Martis, P.; Delhalle, J.; Mekhalif, Z. Multi-wall carbon nanotubes decorated with barium oxide nanoparticles. Synth. Catal. Open Access 2018, 3, 1–4. [Google Scholar]
- Chehimi, M.M. (Ed.) Aryl Diazonium Salts: New Coupling Agents in Polymer and Surface Science; Wiley-VCH: Weinheim, Germany, 2012; ISBN 978-3-527-32998-4. [Google Scholar]
- Detriche, S.; Nagy, J.B.; Mekhalif, Z.; Delhalle, J. Surface state of carbon nanotubes and Hansen solubility parameters. J. Nanosci. Nanotechnol. 2009, 9, 6015–6025. [Google Scholar] [CrossRef]
- Charles, M.H. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2000; ISBN 0849315255. [Google Scholar]
- Detriche, S.; Zorzini, G.; Colomer, J.-F.; Fonseca, A.; Nagy, J.B. Application of the Hansen solubility parameters theory to carbon nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 6082–6092. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High resolution monochromated X-ray photoelectron spectroscopy of organic polymers : A comparison between solid state data for organic polymers and gas phase data for small molecules. Mol. Phys. An Int. J. Interface Chem. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Detriche, S. Chimie de surface des nanotubes de carbone, vers une meilleure dispersion et solubilisation, Ph.D. Thesis, University of Namur, Namur, Belgium, 31 August 2010. [Google Scholar]
- Bhakta, A.K.; Kumari, S.; Hussain, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Synthesis and characterization of maghemite nanocrystals decorated multi-wall carbon nanotubes for methylene blue dye removal. J. Mater. Sci. 2019, 54, 200–216. [Google Scholar] [CrossRef]
- Fujita, M.; Hiyama, T. Highly Diastereocontrolled Reduction of Ketones by means of Hydrosilanes. Practical Synthesis of Optically Active 1,2-Diols and 2-Amino Alcohols of Threo or Erythro Configuration. J. Am. Chem. Soc. 1984, 106, 4629–4630. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Enthaler, S.; Junge, K.; Beller, M. Iron-catalyzed enantioselective hydrosilylation of ketones. Angew. Chemie Int. Ed. 2008, 47, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).