Hydrophobicity/Oleophilicity of Autoclaved Aerated Concrete (AAC) Grains Coated with Oleic and Stearic Acids for Application as Oil/Water Separating Filtration and Adsorbent Materials in Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. AAC Grains and Sands
2.2. Oil
2.3. Hydrophobic Agents and Coating
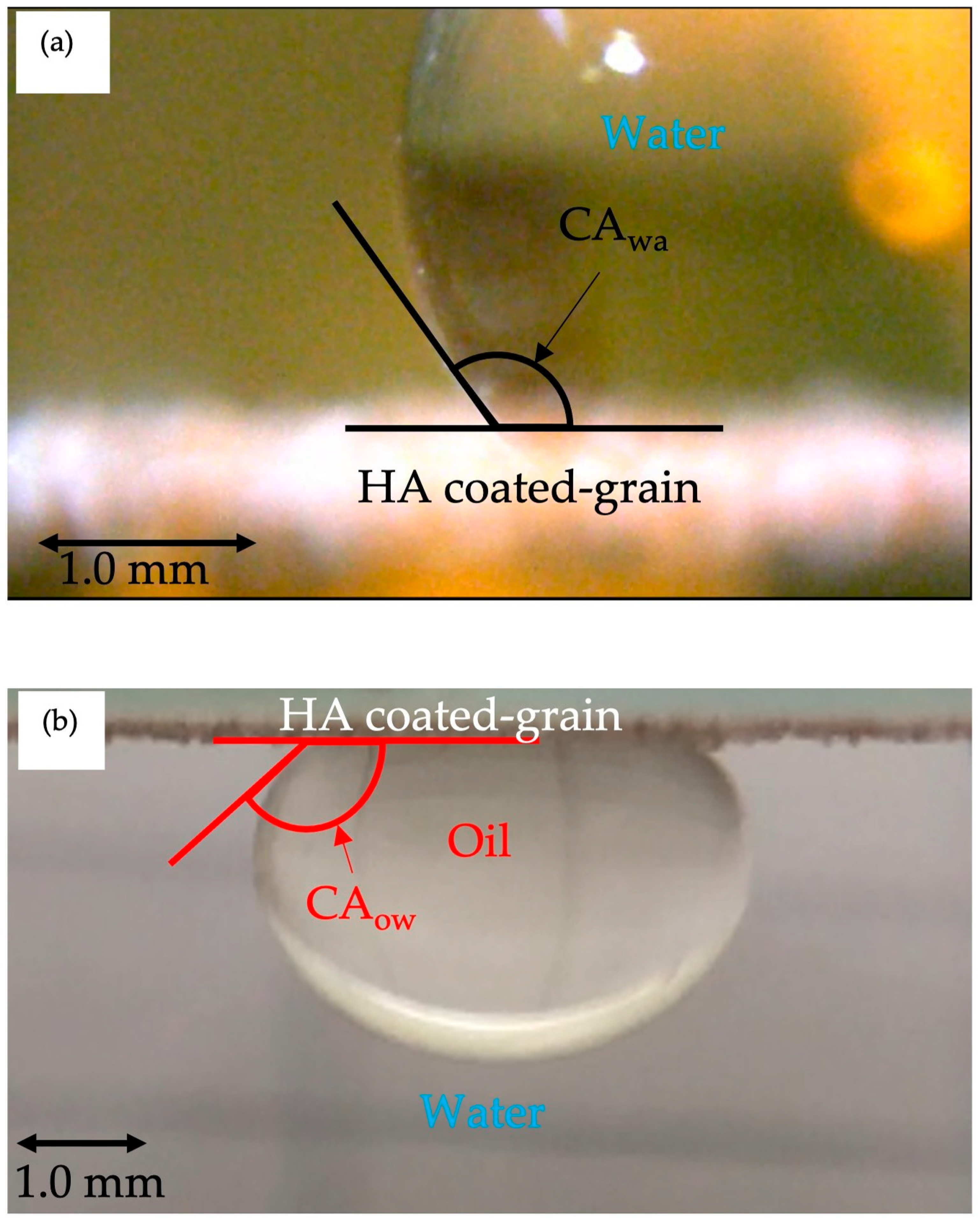
2.4. Measurement of Contact Angles
2.5. Physical and Chemical Properties of Tested Grains and Characterization of Hydrophobicity of HA-Coated Grains
3. Results and Discussion
3.1. Physical and Chemical Characterization
3.2. Contact Angles of Water in Air (CAwa)
3.3. Contact Angles of Oil in Water (CAow)
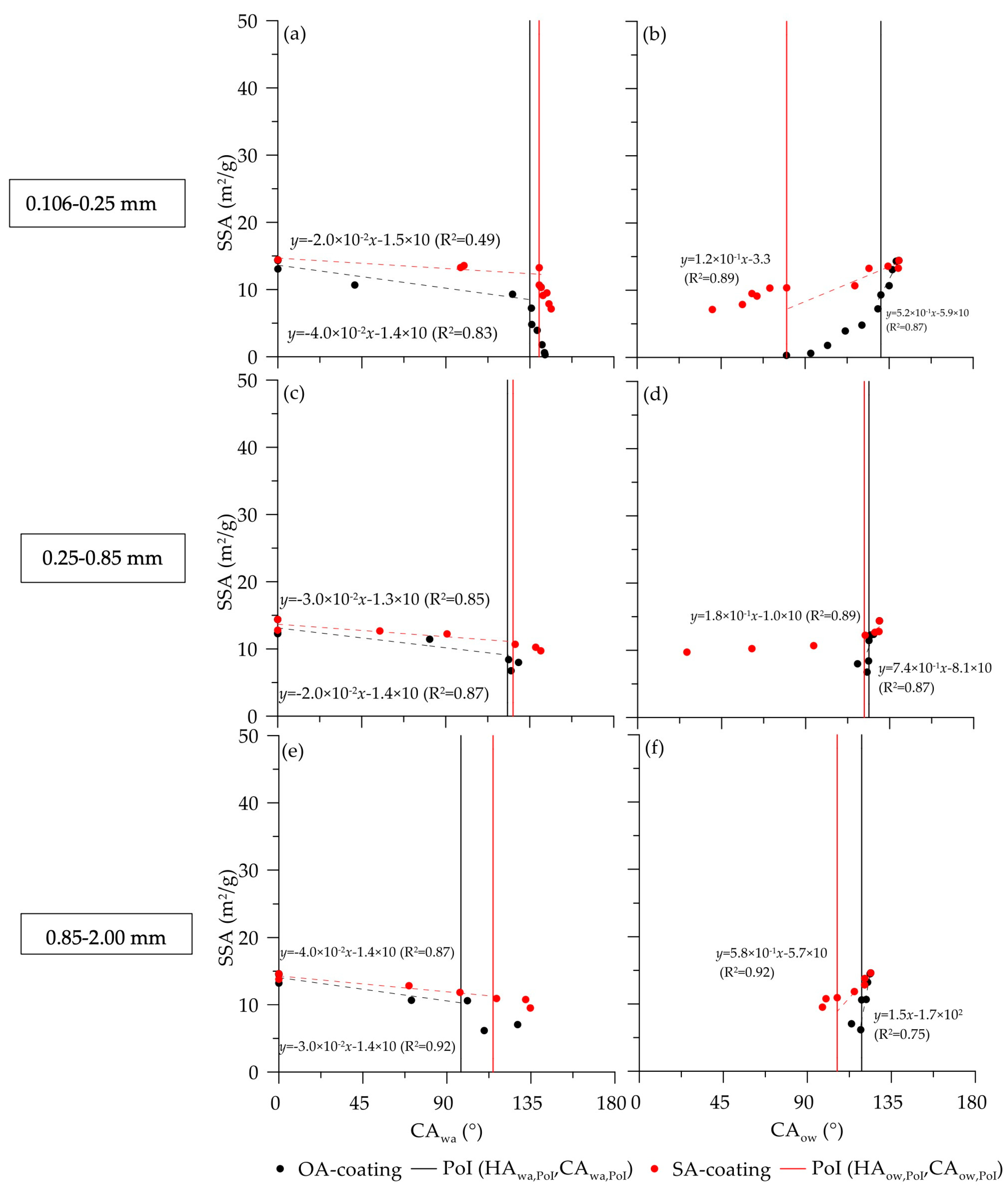
3.4. Relationships between Physicochemical Properties and Measured CAwa and CAow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Autoclaved aerated concrete |
| A/B | The ratio of hydrophobicity to hydrophilicity |
| BET | Brunauer Emmett Teller |
| CA | Contact angles |
| CAow | Contact angles of oil droplets in water |
| CAow,max | Maximum CAow of in the measured range |
| CAow,PoI | CAow at PoI |
| CAwa | Contact angles of water droplets in air |
| CAwa,max | Maximum CAwa of in the measured range |
| CAwa,PoI | CAwa at PoI |
| D10 | Grain size at 10% passing |
| D50 | Grain size at 50% passing (Median diameter) |
| D60 | Grain size at 60% passing |
| EC | Electrical conductivity |
| EDS | Energy dispersive X–ray spectroscope |
| Gs | Specific gravity |
| HA | Hydrophobic agents |
| HAow,PoI | HA concentrations corresponding to the PoI (CAow) |
| HAwa,PoI | HA concentrations corresponding to the PoI (CAwa) |
| OA | Oleic acid |
| OC | Organic carbon |
| pH | Potential hydrogen |
| PoI | Point of intersection |
| PVDF | Polyvinylidene fluoride |
| SA | Stearic acid |
| SDM | Sessile droplet method |
| SEM | Scanning electron microscope |
| SSA | Specific surface area |
| Uc | The Uniformity Coefficient |
| VN | Vietnam |
| VN-AAC | AAC scrap in Vietnam |
| WBP | Waste brick powder |
Appendix A
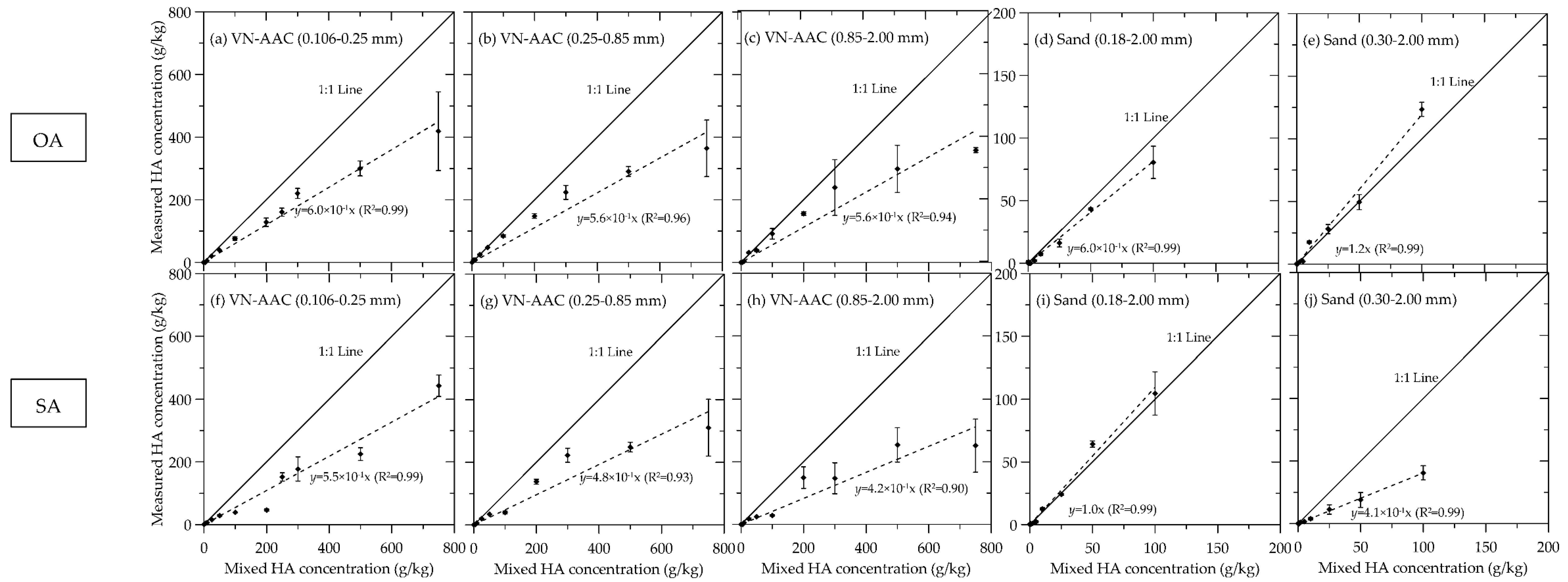
References
- Walker, A.H. Chapter 1—Oil spills and risk perceptions. In Oil Spill Science and Technology, 2nd ed.; Fingas, M., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2017; pp. 1–70. [Google Scholar]
- Huynh, B.Q.; Kwong, L.H.; Kiang, M.V.; Chin, E.T.; Mohareb, A.M.; Jumaan, A.O.; Basu, S.; Geldsetzer, P.; Karaki, F.M.; Rehkopf, D.H. Public health impacts of an imminent Red Sea oil spill. Nat. Sustain. 2021, 4, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Murawski, S.A.; Ainsworth, C.H.; Gilbert, S.; Hollander, D.J.; Paris, C.B.; Schlüter, M.; Wetzel, D.L. Introduction to the volume. In Scenarios and Responses to Future Deep Oil Spills: Fighting the Next War; Murawski, S.A., Ainsworth, C.H., Gilbert, S., Hollander, D.J., Paris, C.B., Schlüter, M., Wetzel, D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 4–15. [Google Scholar]
- Ministry of Natural Resources Environment Vietnam (MoNRE); World Bank; DANIDA. Vietnam Environment Monitor 2003: Water; World Bank: Washington, DC, USA, 2003.
- Asia Pro Eco Programme. The Risks and Consequences of Oil Pollution in Vietnam and Guidelines on Bioremediation of Oil Spills; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Institute for Global Environmental Strategies (IGES). Water resources management in Ho Chi Minh City. In Sustainable Groundwater Management in Asian Cities: A Final Report of Research on Sustainable Water Management Policy; Institute for Global Environmental Strategies: Hayama, Japan, 2006; p. 97. [Google Scholar]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/water separation based on natural materials with super-wettability: Recent advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Ministry of Natural Resources Environment Vietnam (MoNRE); World Bank; DANIDA. Vietnam Environment Monitor 2006: Water Quality in Viet Nam with a Focus on the Cau, Nhue-Day and Dong Nai River Basins; World Bank: Washington, DC, USA, 2006.
- Ministry of Natural Resources Environment Vietnam (MoNRE). National State of Environment 2008: Vietnam Craft Village Environment; Ministry of Natural Resources Environment Vietnam: Cầu Giấy, Vietnam, 2008.
- WWF. WWF REPORT 2018 Textile and Garment Sector in Vietnam: Water Risks and Solutions; WWF: Gland, Switzerland, 2018. [Google Scholar]
- Yong, J.; Chen, F.; Yang, Q.; Bian, H.; Du, G.; Shan, C.; Huo, J.; Fang, Y.; Hou, X. Oil-water separation: A gift from the desert. Adv. Mater. Interfaces 2016, 3, 1500650. [Google Scholar] [CrossRef]
- Kong, L.H.; Chen, X.H.; Yu, L.G.; Wu, Z.S.; Zhang, P.Y. Superhydrophobic cuprous oxide nanostructures on phosphor-copper meshes and their oil-water separation and oil spill cleanup. ACS Appl. Mater. Interfaces 2015, 7, 2616–2625. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Guo, C.; Tian, H.; Zha, F.; Guo, L. Underoil superhydrophilic desert sand layer for efficient gravity-directed water-in-oil emulsions separation with high flux. J. Mater. Chem. A 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Ren, J.; Tao, F.; Liu, L.; Wang, X.; Cui, Y. A novel TiO2@stearic acid/chitosan coating with reversible wettability for controllable oil/water and emulsions separation. Carbohydr. Polym. 2020, 232, 115807. [Google Scholar] [CrossRef]
- Ezazi, M.; Shrestha, B.; Kim, S.I.; Jeong, B.; Gorney, J.; Hutchison, K.; Lee, D.H.; Kwon, G. Selective wettability membrane for continuous oil-water separation and in situ visible light-driven photocatalytic purification of water. Glob. Chall. 2020, 4, 2000009. [Google Scholar] [CrossRef]
- Shrestha, B.; Ezazi, M.; Kwon, G. Engineered nanoparticles with decoupled photocatalysis and wettability for membrane-based desalination and separation of oil-saline water mixtures. Nanomaterials 2021, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Ezazi, M.; Kwon, G. Delamination-free in-air and underwater oil-repellent filters for oil-water separation: Gravity-driven and cross-flow operations. Energies 2021, 14, 7429. [Google Scholar] [CrossRef]
- Liu, P.; Niu, L.; Tao, X.; Li, X.; Zhang, Z.; Yu, L. Preparation of superhydrophobic-oleophilic quartz sand filter and its application in oil-water separation. Appl. Surf. Sci. 2018, 447, 656–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Cao, Q.; Wang, C.; Yang, C.; Li, Y.; Zhou, J. Novel porous oil-water separation material with super-hydrophobicity and super-oleophilicity prepared from beeswax, lignin, and cotton. Sci. Total Environ. 2020, 706, 135807. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Zhang, H.; Wu, F.; Wei, B.; Chang, Q. Superhydrophobic coating on quartz sand filter media for oily wastewater filtration. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 509–514. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Islam, M.A.; Sadrzadeh, M. Industrial waste lignin as an antifouling coating for the treatment of oily wastewater: Creating wealth from waste. J. Clean. Prod. 2020, 256, 120304. [Google Scholar] [CrossRef]
- Shi, G.; Shen, Y.; Mu, P.; Wang, Q.; Yang, Y.; Ma, S.; Li, J. Effective separation of surfactant-stabilized crude oil-in-water emulsions by using waste brick powder-coated membranes under corrosive conditions. Green Chem. 2020, 22, 1345–1352. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Wang, Y.; Yue, X.; Yang, D.; Qiu, F. Waste-to-resource strategy to fabricate functionalized material from waste brick. Sci. Total Environ. 2020, 703, 135032. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Wang, M.; Qiu, F.; Yue, X.; Yang, D. Hierarchical structurized waste brick with opposite wettability for on-demand oil/water separation. Chemosphere 2020, 251, 126348. [Google Scholar] [CrossRef]
- Zhao, B.; Ren, L.; Du, Y.; Wang, J. Eco-friendly separation layers based on waste peanut shell for gravity-driven water-in-oil emulsion separation. J. Clean. Prod. 2020, 255, 120184. [Google Scholar] [CrossRef]
- Matsuno, A.; Junaid, Z.M.; Saito, T.; Dang, H.T.T.; Huyen, P.T.; Nga, T.T.V.; Kawamoto, K. Oil/water separation techniques using hydrophobized/oleophilized grains: A review of recent studies. Int. J. GEOMATE 2021, 20, 28–34. [Google Scholar] [CrossRef]
- Decision No. 567/QD-TTg of Apirl 28, 2010, Approving the Program on Development of Non-Baked Building Materials through 2020. Available online: https://vanbanphapluat.co/decision-no-567-qd-ttg-approving-the-program-on-development-of-non-baked (accessed on 12 July 2022).
- Alexanderson, J. Relations between structure and mechanical properties of autoclaved aerated concrete. Cem. Concr. Res. 1979, 9, 507–514. [Google Scholar] [CrossRef]
- Tada, S. Microstructural approach to properties of moist cellular concrete. In Autoclaved Aerated Concrete-Moisture and Properties; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Wittmann, F.H. Autoclaved Aerated Concrete Moisture and Properties; Elsevier Scientific Publishing, Co.: New York, NY, USA, 1983. [Google Scholar]
- Aroni, S. Autoclaved Aerated Concrete—Properties, Testing and Design; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Nguyen Trong, L.; Asamoto, S.; Matsui, K. Sorption isotherm and length change behavior of autoclaved aerated concrete. Cem. Concr. Compos. 2018, 94, 136–144. [Google Scholar] [CrossRef]
- UNDP. The Role of Systemic Investing in Viet Nam’s Transition to a Circular Economy: A Case Study on Achieving Transformative Change in the Waste Management and Recycling System; UNDP: New York, NY, USA, 2021. [Google Scholar]
- Decision No. 889/QD-TTg on the National Action Program on Sustainable Production and Consumption in the 2021–2030 Period. Available online: https://vietnamcirculareconomy.vn/en/policy-library/decision-no-889-qd-ttg-on-the-national-action-program-on-sustainable-production-and-consumption-in-the-2021-2030-period-2/ (accessed on 12 July 2022).
- Kumara, G.M.P.; Kawamoto, K. Steel slag and autoclaved aerated concrete grains as low-cost adsorbents to remove Cd2+ and Pb2+ in wastewater: Effects of mixing proportions of grains and liquid-to-solid ratio. Sustainability 2021, 13, 10321. [Google Scholar] [CrossRef]
- Subedi, S.; Kawamoto, K.; Jayarathna, L.; Vithanage, M.; Moldrup, P.; de Jonge, L.W.; Komatsu, T. Characterizing time-dependent contact angles for sands hydrophobized with oleic and stearic acids. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Thai, H.N.; Kawamoto, K.; Nguyen, H.G.; Sakaki, T.; Komatsu, T.; Moldrup, P. Measurements and modeling of thermal conductivity of recycled aggregates from concrete, clay brick, and their mixtures with autoclaved aerated concrete grains. Sustainability 2022, 14, 2417. [Google Scholar] [CrossRef]
- Van Boggelen, W.; van Boggelen, J. Sustainable building solutions with new generation autoclaved aerated concrete panel applications. CE/Papers 2018, 2, 513–525. [Google Scholar] [CrossRef]
- Matsuno, A.; Ishizuka, S.; Nguyen, T.L.; Nguyen, T.D.; Nguyen, V.T.; Nguyen, H.G.; Kawamoto, K. Comparison of macropore structure and network of autoclaved aerated concrete blocks using micro-focus X-ray computed tomography. Int. J. GEOMATE 2020, 19, 160–165. [Google Scholar] [CrossRef]
- Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S.-Y.; Ogawa, A.; Matsui, K.; Sato, M. In situ time-resolved X-ray diffraction of tobermorite formation process under autoclave condition. J. Am. Ceram. Soc. 2010, 93, 2667–2674. [Google Scholar] [CrossRef]
- Japanese Standards Association (JSA). JIS Z 8801-1: 2019 Test Sieves—Part 1: Test Sieves of Metal Wire Cloth; Japanese Standards Association (JSA): Tokyo, Japan, 2019. [Google Scholar]
- Japan Water Works Association (JWWA). JWWA A 103-1:2006-2 Filter Sand for Water Supply; Japan Water Works Association (JWWA): Tokyo, Japan, 2006; 18p. [Google Scholar]
- The Chemical Daily. 15710 Chemical Products; The Chemical Daily Co., Ltd.: Tokyo, Japan, 2010. [Google Scholar]
- Wijewardana, N.S.; Kawamoto, K.; Moldrup, P.; Komatsu, T.; Kurukulasuriya, L.C.; Priyankara, N.H. Characterization of water repellency for hydrophobized grains with different geometries and sizes. Environ. Earth Sci. 2015, 74, 5525–5539. [Google Scholar] [CrossRef]
- Leelamanie, D.A.L.; Karube, J.; Yoshida, A. Characterizing water repellency indices: Contact angle and water drop penetration time of hydrophobized sand. Soil Sci. Plant Nutr. 2008, 54, 179–187. [Google Scholar] [CrossRef]
- Karatza, Z.; Buckman, J.; Medero, G.M.; Beckett, C.T.S. Evolution of meniscus structures in hydrophobic granular systems. J. Hydrol. 2021, 603, 126954. [Google Scholar] [CrossRef]
- Zafar, M.J. Assessment of hydrophobicity/oleophilicity for artificially coated autoclave aerated concrete grains and its applicability to treat oily wastewater. Master’s Thesis, Saitama University, Saitama, Japan, 2021. [Google Scholar]
- Bardet, J.P.; Jesmani, M.; Jabbari, N. Permeability and compressibility of wax-coated sands. Géotechnique 2014, 64, 341–350. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, F.; Tao, L.; Liu, N.; Gao, C.; Feng, L.; Wei, Y. Bio-inspired anti-oil-fouling chitosan-coated mesh for oil/water separation suitable for broad pH range and hyper-saline environments. ACS Appl. Mater. Interfaces 2013, 5, 11971–11976. [Google Scholar] [CrossRef]
- Lu, F.; Chen, Y.; Liu, N.; Cao, Y.; Xu, L.; Wei, Y.; Feng, L. A fast and convenient cellulose hydrogel-coated colander for high-efficiency oil–water separation. RSC Adv. 2014, 4, 32544–32548. [Google Scholar] [CrossRef]
- Fan, J.; Duan, J.; Yu, Z.; Wu, D.; Zhu, H. Oleophobicity of chitosan/micron-alumina-coated stainless steel mesh for oil/water separation. Water Air Soil Pollut. 2016, 227, 163. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nishimura, T. Soil Physics Experimental Methods; University of Tokyo Press: Tokyo, Japan, 2011; p. 224. [Google Scholar]
- Japanese Geotechnical Society (JGS). Geotechnical Test: Basics and Guidance, 2nd ed.; Japanese Geotechnical Society (JGS): Tokyo, Japan, 2011; p. 270. [Google Scholar]
- Capriel, P.; Beck, T.; Borchert, H.; Gronholz, J.; Zachmann, G. Hydrophobicity of the organic matter in arable soils. Soil Biol. Biochem. 1995, 27, 1453–1458. [Google Scholar] [CrossRef]
- Celi, L.; Schnitzer, M.I.; NÈgre, M. Analysis of carboxyl groups in soil humic acids by a wet chemical method, Fourier-transform infrared spectrophotometry, and solution-state carbon-13 nuclear magnetic resonance. A comparative study. Soil Sci. 1997, 162, 189–197. [Google Scholar] [CrossRef]
- Tasumi, M. The Spectroscopical Society of Japan. Infrared Spectroscopy Measurement Method: Basics and Latest Methods; S.T. Japan: Köln, Germany, 2012; p. 201. [Google Scholar]
- Ellerbrock, R.H.; Gerke, H.H.; Bachmann, J.; Goebel, M.-O. Composition of organic matter fractions for explaining wettability of three forest soils. Soil Sci. Soc. Am. J. 2005, 69, 57–66. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lambe, T.W.; Whitman, R.V. Soil Mechanics; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- González-Peñaloza, F.A.; Zavala, L.M.; Jordán, A.; Bellinfante, N.; Bárcenas-Moreno, G.; Mataix-Solera, J.; Granged, A.J.P.; Granja-Martins, F.M.; Neto-Paixão, H.M. Water repellency as conditioned by particle size and drying in hydrophobized sand. Geoderma 2013, 209–210, 31–40. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Hydrophobic plasma polymer coated silica particles for petroleum hydrocarbon removal. ACS Appl. Mater. Interfaces 2013, 5, 8563–8571. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Yuan, M.; He, J.; Xue, M.; Ma, X.; Hou, L.; Zhang, T.; Liu, X.; He, J. Substrate-versatile approach to multifunctional superamphiphobic coatings with mechanical durable property from quartz sand. Surf. Coat. Technol. 2018, 352, 191–200. [Google Scholar] [CrossRef]
- Bigui, W.; Cheng, Y.; Jianlin, L.; Gang, W.; Liang, D.; Xiaosan, S.; Fuping, W.; Hua, L.; Qing, C. Fabrication of superhydrophilic and underwater superoleophobic quartz sand filter for oil/water separation. Sep. Purif. Technol. 2019, 229, 115808. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Mohamed, N.H. Novel superhydrophobic sand and polyurethane sponge coated with silica/modified asphaltene nanoparticles for rapid oil spill cleanup. Nanomaterials 2019, 9, 187. [Google Scholar] [CrossRef]
- Bigui, W.; Jianlin, L.; Gang, W.; Qing, C. Filtration of oil from oily wastewater via hydrophobic modified quartz sand filter medium. J. Water Reuse Desalin. 2018, 8, 544–552. [Google Scholar] [CrossRef]
- Mitra, D.; Tai, M.H.; Abdullah, E.B.; Wang, C.-H.; Neoh, K.G. Facile fabrication of porous waste-derived carbon-polyethylene terephthalate composite sorbent for separation of free and emulsified oil from water. Sep. Purif. Technol. 2021, 279, 119664. [Google Scholar] [CrossRef]
- Tarchitzky, J.; Lerner, O.; Shani, U.; Arye, G.; Lowengart-Aycicegi, A.; Brener, A.; Chen, Y. Water distribution pattern in treated wastewater irrigated soils: Hydrophobicity effect. Eur. J. Soil Sci. 2007, 58, 573–588. [Google Scholar] [CrossRef]
- Bachmann, J.; Guggenberger, G.; Baumgartl, T.; Ellerbrock, R.H.; Urbanek, E.; Goebel, M.O.; Kaiser, K.; Horn, R.; Fischer, W.R. Physical carbon-sequestration mechanisms under special consideration of soil wettability. J. Plant Nutr. Soil Sci. 2008, 171, 14–26. [Google Scholar] [CrossRef]
- Voelkner, A.; Holthusen, D.; Ellerbrock, R.H.; Horn, R. Quantity of hydrophobic functional CH-groups—Decisive for soil water repellency caused by digestate amendment. Int. Agrophys. 2015, 29, 247–255. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Loewy, R.M. Characterization of soil organic matter by FT-IR spectroscopy and its relationship with chlorpyrifos sorption. J. Environ. Manag. 2017, 196, 316–322. [Google Scholar] [CrossRef] [PubMed]

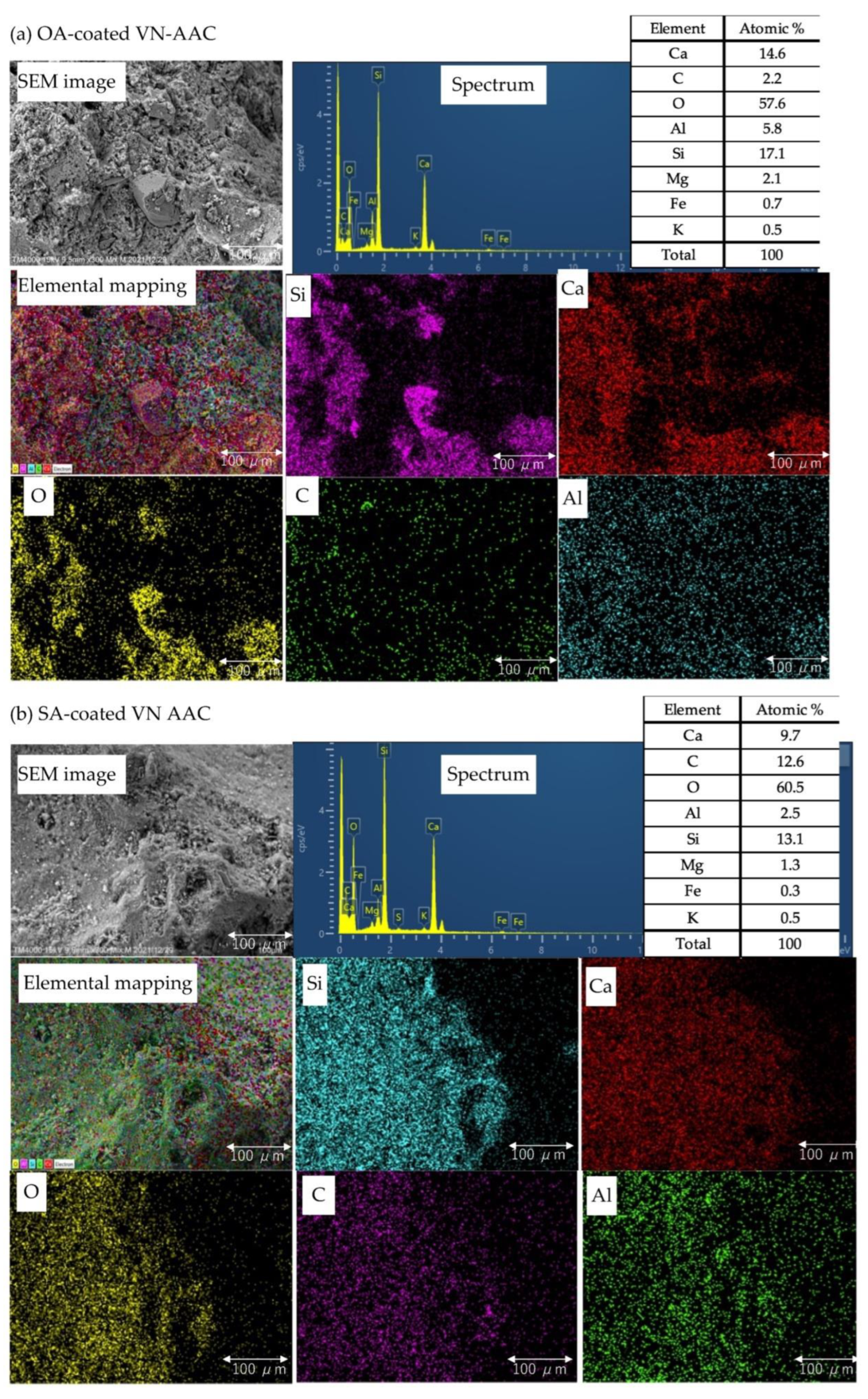

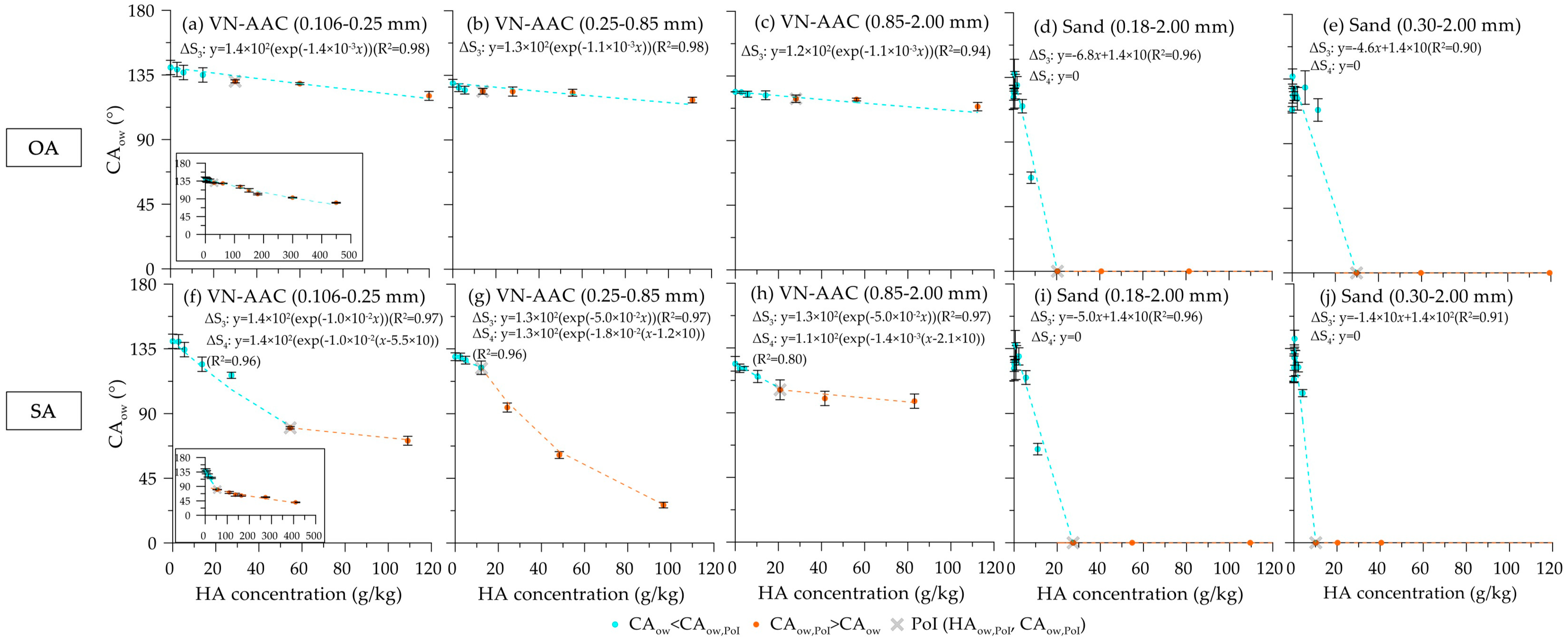
| Sample | Grain Size(mm) | D10 (mm) | D50 (mm) | D60 (mm) | Uc (D50/D10) | SSA (m2/g) | VT (cm3/g) | LOI (%) | wAD (%) | Gs (g/cm3) | EC(mS/cm) | pH (H2O) | pH (1 mol KCl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VN-AAC | 0.106–0.25 | 0.15 | 0.20 | 0.21 | 1.44 | 15.3 | 4.5 × 10−2 | 9.2 | 1.44 | 2.57 | 1.04 | 8.90 | 7.27 |
| VN-AAC | 0.25–0.85 | 0.34 | 0.51 | 0.56 | 1.64 | 16.9 | 4.9 × 10−2 | 9.5 | 2.03 | 2.55 | 0.94 | 8.57 | 7.20 |
| VN-AAC | 0.85–2.00 | 0.90 | 1.20 | 1.30 | 0.83 | 17.1 | 4.8 × 10−2 | 9.4 | 2.01 | 2.46 | 0.83 | 8.97 | 7.33 |
| Slow sand | 0.18–2.00 | 0.31 | 0.48 | 0.55 | 1.54 | 3.0 | 6.0 × 10−3 | 0.6 | 0.44 | 2.66 | 0.01 | 7.83 | 6.13 |
| Rapid sand | 0.30–2.00 | 0.62 | 0.79 | 0.83 | 1.28 | 3.5 | 7.0 × 10−3 | 0.5 | 0.40 | 2.63 | 0.02 | 7.27 | 6.00 |
| Sample | Grain Size (mm) | Coating | CAwa | CAow | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAwa,max (°) | CAwa,PoI (°) | HAwa,PoI (g/kg) | ΔS1 (Slope) | ΔS2 (Slope) | CAow,max (°) | CAow,PoI (°) | HAow,PoI (g/kg) | ||||
| VN-AAC | 0.106–0.25 | OA | 143 | 135 | 31.5 | 5.28 | 0.03 | 140 | 131 | 30.0 | This Study |
| VN-AAC | 0.25–0.85 | 129 | 123 | 18.2 | 9.77 | 0.06 | 130 | 124 | 13.9 | ||
| VN-AAC | 0.85–2.00 | 128 | 98 | 17.2 | 8.42 | 0.32 | 124 | 119 | 28.1 | ||
| Slow sand | 0.18–2.00 | 82 | 82 | 2.5 | 32.36 | −0.42 | 138 | 0 | 20.3 | ||
| Rapid sand | 0.30–2.00 | 73 | 70 | 2.4 | 28.77 | −0.25 | 137 | 0 | 29.9 | ||
| VN-AAC | 0.106–0.25 | SA | 146 | 140 | 7.7 | 18.29 | 0.01 | 138 | 80 | 54.5 | |
| VN-AAC | 0.25–0.85 | 141 | 126 | 17.5 | 8.37 | 0.17 | 130 | 121 | 12.2 | ||
| VN-AAC | 0.85–2.00 | 135 | 115 | 13.6 | 9.99 | 0.26 | 124 | 106 | 20.8 | ||
| Slow sand | 0.18–2.00 | 131 | 131 | 3.9 | 33.49 | −0.06 | 138 | 0 | 27.4 | ||
| Rapid sand | 0.30–2.00 | 130 | 130 | 1.1 | 115.14 | −0.16 | 142 | 0 | 10.1 | ||
| Glass bead | 0.075–0.25 | OA | 106 | 106 | 0.3 | 172.00 | 22.5 | NA | NA | NA | Wijewardana et al., 2015 [47] |
| Accusand | 0.105–0.25 | 76 | 76 | 1.0 | 58.00 | 15.1 | NA | NA | NA | ||
| Toyoura sand | 0.105–0.25 | 97 | 97 | 0.3 | 388.00 | 0.25 | NA | NA | NA | ||
| Narita sand | 0.105–0.25 | 94 | 94 | 1.3 | 86.00 | 0.25 | NA | NA | NA | ||
| Narita sand | 0.25–0.425 | 93 | 93 | 1.0 | 93.00 | 0.1 | NA | NA | NA | ||
| Narita sand | 0.425–0.84 | 79 | 79 | 1.3 | 89.00 | 0.11 | NA | NA | NA | ||
| Toyoura sand | 0.105–0.25 | OA | 101 | 101 | 0.3 | 329.00 | 0.25 | NA | NA | NA | Subedi et al., 2012 [39] |
| Quartz sand | 0.05–0.25 | SA | 99 | 99 | 0.3 | 329.00 | 0.25 | NA | NA | NA | González-Peñaloza et al., 2013 [64] |
| Quartz sand | 0.25–0.5 | 100 | 100 | 1.0 | 36.00 | 0.06 | NA | NA | NA | ||
| Quartz sand | 0.5–2.0 | 101 | 101 | 2.0 | 28.00 | 0.01 | NA | NA | NA | ||
| Sample | Coating | CAwa (°) | CAow (°) | SSA (m2/g) | OC (%) | A/B | No. of Samples | |
|---|---|---|---|---|---|---|---|---|
| VN-AAC | OA | CAwa (°) | 1.00 | −0.61 | −0.88 | 0.72 | 0.72 | 25 |
| CAow (°) | 1.00 | 0.82 | −0.92 | −0.49 | ||||
| SSA (m2/g) | 1.00 | −0.91 | −0.69 | |||||
| OC (%) | 1.00 | 0.61 | ||||||
| A/B | 1.00 | |||||||
| SA | CAwa (°) | 1.00 | −0.66 | −0.80 | 0.50 | 0.59 | 25 | |
| CAow (°) | 1.00 | 0.86 | −0.75 | −0.75 | ||||
| SSA (m2/g) | 1.00 | −0.81 | −0.74 | |||||
| OC (%) | 1.00 | 0.50 | ||||||
| A/B | 1.00 | |||||||
| Sand | OA | CAwa (°) | 1.00 | −0.15 | −0.70 | −0.01 | 0.59 | 23 |
| CAow (°) | 1.00 | 0.75 | −0.78 | −0.61 | ||||
| SSA (m2/g) | 1.00 | −0.54 | −0.82 | |||||
| OC (%) | 1.00 | 0.69 | ||||||
| A/B | 1.00 | |||||||
| SA | CAwa (°) | 1.00 | −0.55 | −0.91 | 0.41 | 0.58 | 24 | |
| CAow (°) | 1.00 | 0.66 | −0.75 | −0.31 | ||||
| SSA (m2/g) | 1.00 | −0.43 | −0.85 | |||||
| OC (%) | 1.00 | 0.29 | ||||||
| A/B | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuno, A.; Kawamoto, K. Hydrophobicity/Oleophilicity of Autoclaved Aerated Concrete (AAC) Grains Coated with Oleic and Stearic Acids for Application as Oil/Water Separating Filtration and Adsorbent Materials in Vietnam. Environments 2022, 9, 101. https://doi.org/10.3390/environments9080101
Matsuno A, Kawamoto K. Hydrophobicity/Oleophilicity of Autoclaved Aerated Concrete (AAC) Grains Coated with Oleic and Stearic Acids for Application as Oil/Water Separating Filtration and Adsorbent Materials in Vietnam. Environments. 2022; 9(8):101. https://doi.org/10.3390/environments9080101
Chicago/Turabian StyleMatsuno, Akihiro, and Ken Kawamoto. 2022. "Hydrophobicity/Oleophilicity of Autoclaved Aerated Concrete (AAC) Grains Coated with Oleic and Stearic Acids for Application as Oil/Water Separating Filtration and Adsorbent Materials in Vietnam" Environments 9, no. 8: 101. https://doi.org/10.3390/environments9080101
APA StyleMatsuno, A., & Kawamoto, K. (2022). Hydrophobicity/Oleophilicity of Autoclaved Aerated Concrete (AAC) Grains Coated with Oleic and Stearic Acids for Application as Oil/Water Separating Filtration and Adsorbent Materials in Vietnam. Environments, 9(8), 101. https://doi.org/10.3390/environments9080101







