CH4 and CO2 Emissions from the Decomposition of Microplastics in the Bottom Sediment—Preliminary Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Preparation and Experimental Conditions
2.3. Instrumental Analysis
2.3.1. Determination of CH4 and CO2
2.3.2. Particle Analysis
2.4. Statistical Analysis
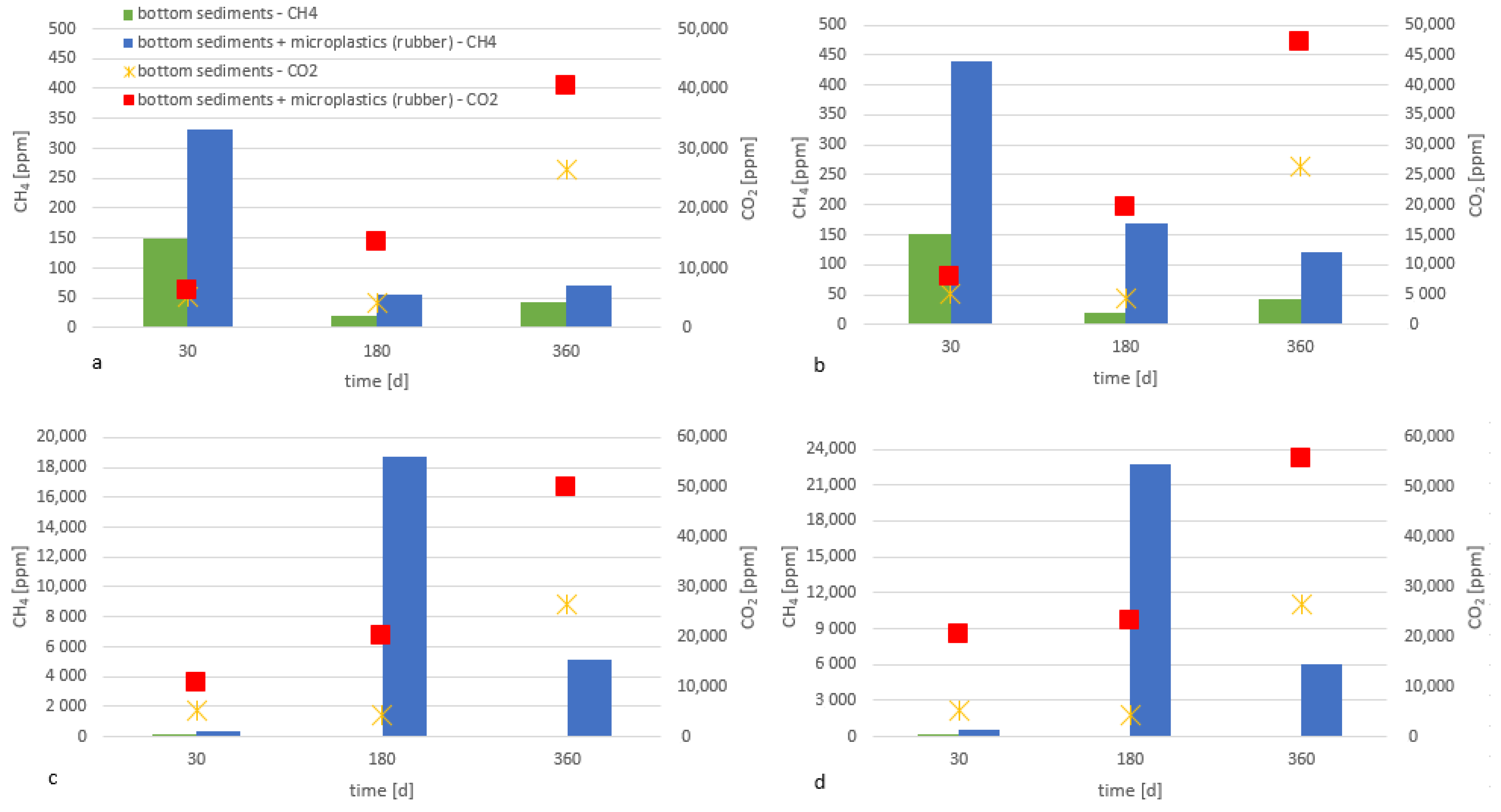
3. Results and Discussion
4. Limitations of the Study
5. Conclusions
- Microplastic accumulated in the bottom sediments in surface waters is degraded even in the absence of UV radiation and under anaerobic conditions.
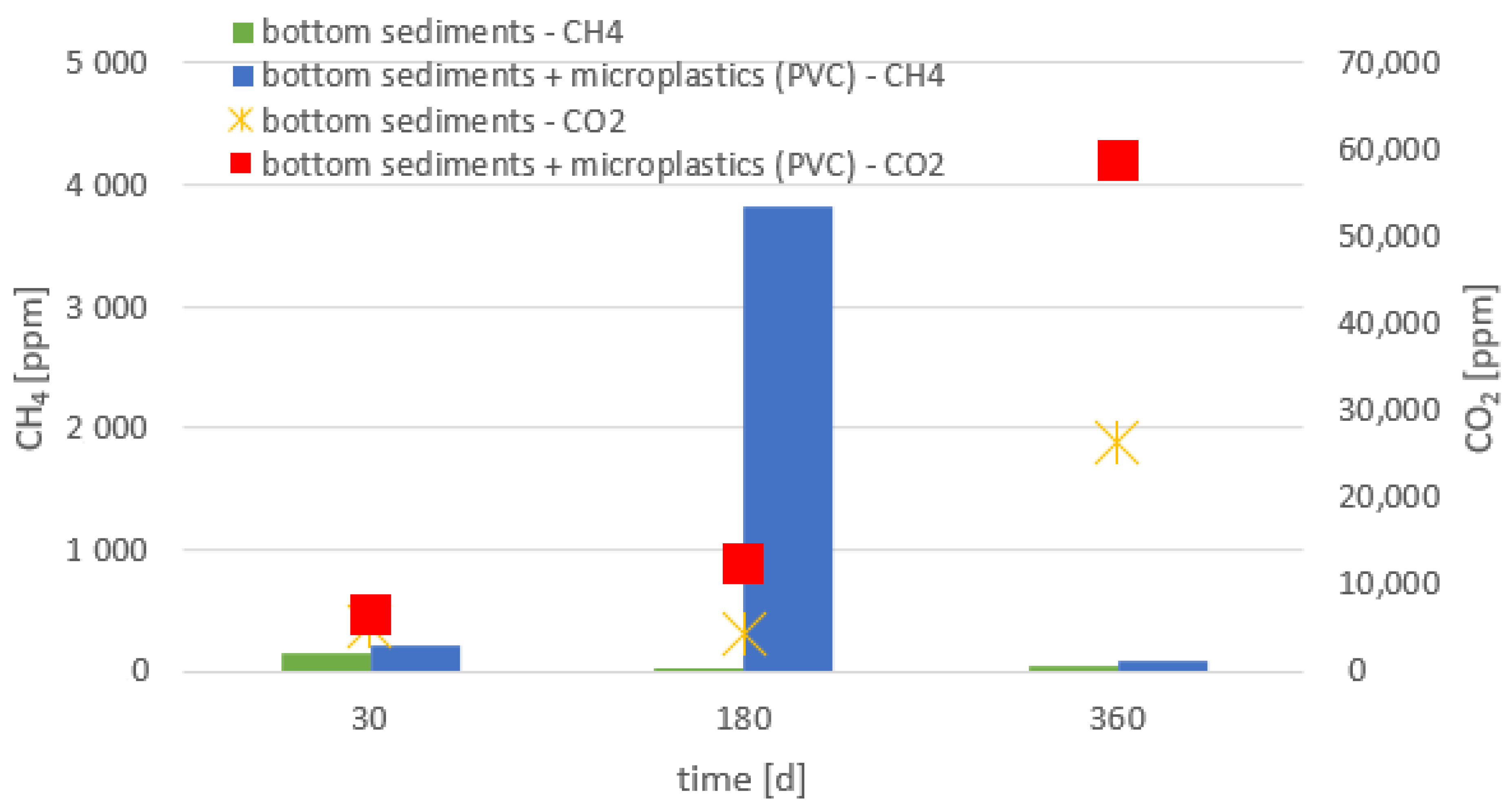
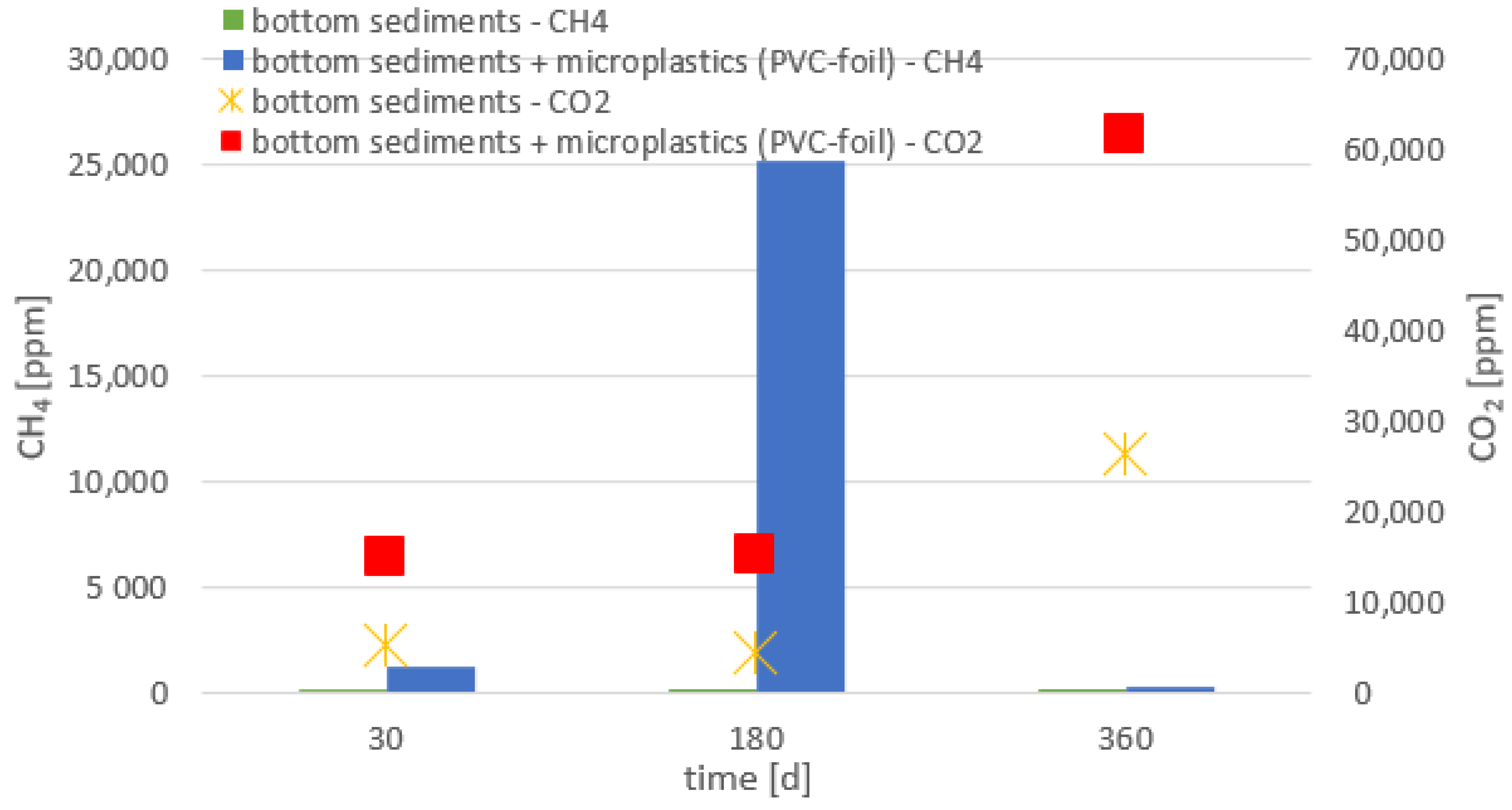
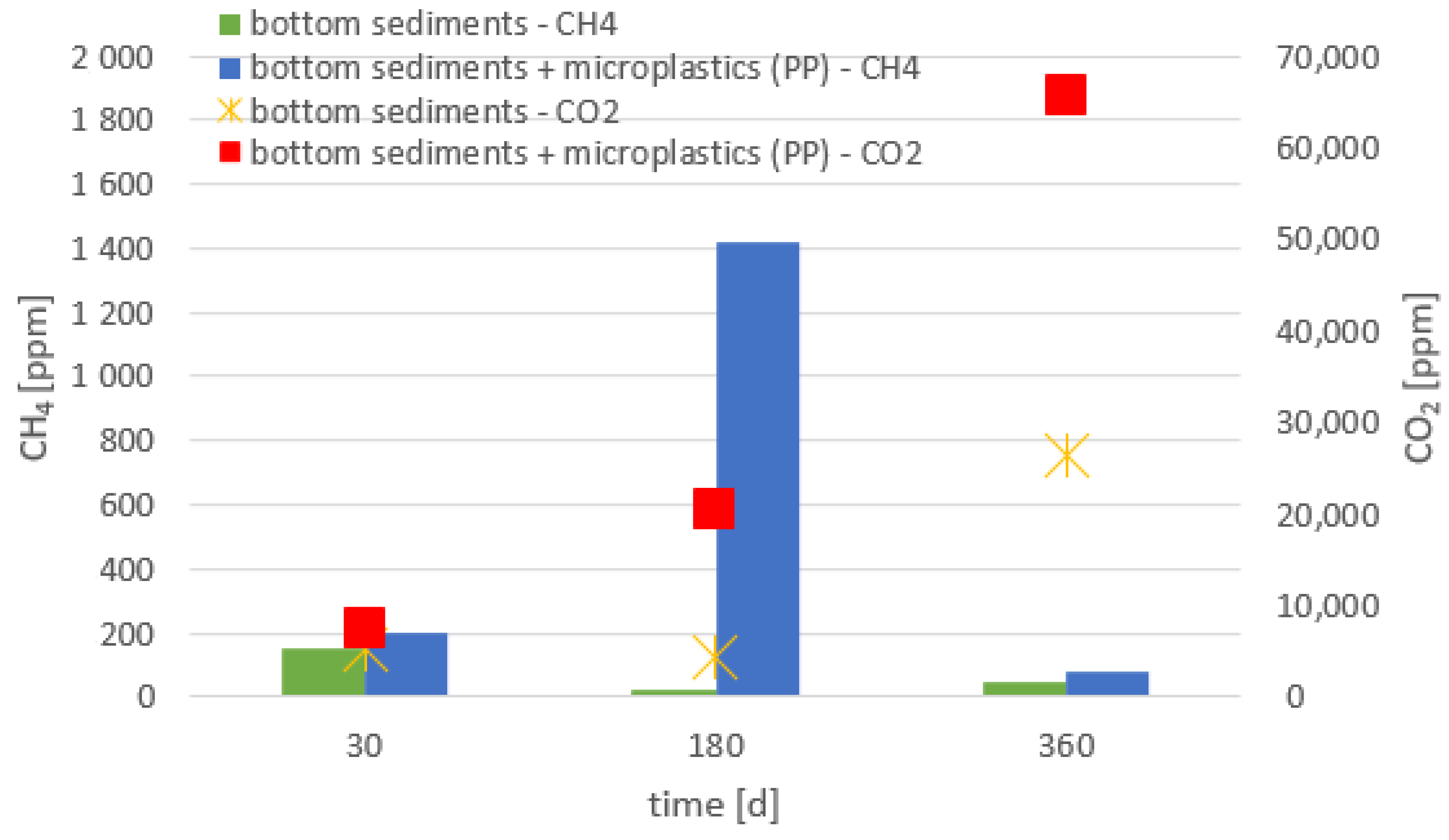
- The presence of all tested materials made of polymers in the bottom sediments resulted in an increased emission of greenhouse gases (methane and carbon dioxide), compared to the bottom sediments without the addition of the analyzed microplastics.
- Higher emissions of carbon dioxide were observed compared to methane; methane can be oxidized to carbon dioxide (i.e., reverse methanogenesis).
- The emission of the gases analyzed depended on the chemical composition of the material and the size of the plastic particles. The highest emission of these gases was recorded for the smallest particles.
- The obtained results confirm the need for further research in this area and the extension of research in other components of the environment.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, H.; Park, B.S. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chen, G.; Xu, K.; Gong, H.; Huang, K.; Wang, J. Microplastics in Surface Waters and Sediments from Guangdong Coastal Areas, South China. Sustainability 2021, 13, 2691. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Stachurek, I. Problems with biodegradation of plastics in the environment. Sci. Pap. Coll. Labor Prot. Manag. Katow. 2012, 1, 74–108. [Google Scholar]
- Vaid, M.; Mehra, K.; Gupta, A. Microplastics as contaminants in Indian environment: A review. Environ. Sci. Pollut. Res. 2021, 28, 1–28. [Google Scholar] [CrossRef]
- Koyilath Nandakumar, V.; Palani, S.G.; Raja Raja Varma, M. Interactions between microplastics and unit processes of wastewater treatment plants: A critical review. Water Sci. Technol. 2022, 85, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Książek, S.; Kida, M.; Koszelnik, P. The occurrence and source of polycyclic aromatic hydrocarbons in bottom sediments of the Wisłok river. Polish J. Natural Sci. 2016, 31, 373–386. [Google Scholar]
- Mourgkogiannis, N.; Kalavrouziotis, I.K.; Karapanagioti, H.K. Questionnaire-based survey to managers of 101 wastewater treatment plants in Greece confirms their potential as plastic marine litter sources. Mar. Pollut. Bull. 2018, 133, 822–827. [Google Scholar] [CrossRef]
- Karapanagioti, H.; Kalavrouziotis, I.K. (Eds.) Microplastics in Water and Wastewater, 2nd ed.; IWA Book: London, UK, 2019; ISBN 13:9781789061680. Available online: https://www.iwapublishing.com/books/9781789061680/microplastics-water-and-wastewater-2nd-edition (accessed on 5 July 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ. 2018, 636, 20–29. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef] [Green Version]
- Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plasticlitter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Norén, F. Small plastic particles in coastal Swedish waters. KIMO Swed. 2007, 11, 1–11. [Google Scholar]
- Tunçer, S.; Artüz, O.B.; Demirkol, M.; Artüz, M.L. First report of occurrence, distribution, and composition of microplastics in surface waters of the Sea of Marmara, Turkey. Mar. Pollut. Bull. 2018, 135, 283–289. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Wang, G.; Yue, W.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2019, 707, 135601. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.; Madden, O. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Hamilton, L.A.; Feit, S.; Muffett, C.; Kelso, M.; Rubright, S.M.; Bernhardt, C.; Labbé Bellas, R. Plastic and Climate: The Hidden Cost of A Plastic Planet. Cent. Int. Environ. Law 2019, 1–96. Available online: https://www.ciel.org/plasticandclimate/ (accessed on 21 March 2022).
- Royer, S.J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef]
- Cerruti, P.; Lavorgna, M.; Carfagna, C.; Nicolais, L. Comparison of photo-oxidative degradation of polyamide 6, 6 films stabilized with HALS and CuCl2+ KI mixtures. Polymer 2005, 46, 4571–4583. [Google Scholar] [CrossRef]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Kida, M.; Koszelnik, P. Investigation of the presence and possible migration from microplastics of phthalic acid esters and polycyclic aromatic hydrocarbons. J. Polym. Environ. 2021, 29, 599–611. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic biodegradation tests of poly (lactic acid) under mesophilic and thermophilic conditions using a new evaluation system for methane fermentation in anaerobic sludge. Int. J. Mol. Sci. 2009, 10, 3824–3835. [Google Scholar] [CrossRef] [Green Version]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Orphan, V.J.; House, C.H.; Hinrichs, K.U.; McKeegan, K.D.; DeLong, E.F. Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc. Natl. Acad. Sci. USA 2002, 99, 7663–7668. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.G. Does size and buoyancy affect the long-distance transport of floating debris? Environ. Res. Lett. 2015, 10, 084019. [Google Scholar] [CrossRef]
- Pochwat, K. Assessment of Rainwater Retention Efficiency in Urban Drainage Systems—Model Studies. Resources 2022, 11, 14. [Google Scholar] [CrossRef]
- Binda, G.; Spanu, D.; Monticelli, D.; Pozzi, A.; Bellasi, A.; Bettinetti, R.; Carnati, S.; Nizzetto, L. Unfolding the interaction between microplastics and (trace) elements in water: A critical review. Water Res. 2021, 204, 117637. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.S.; Sun, J.; Wang, J.Y.; Wu, S.L.; Ni, B.J. Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef]
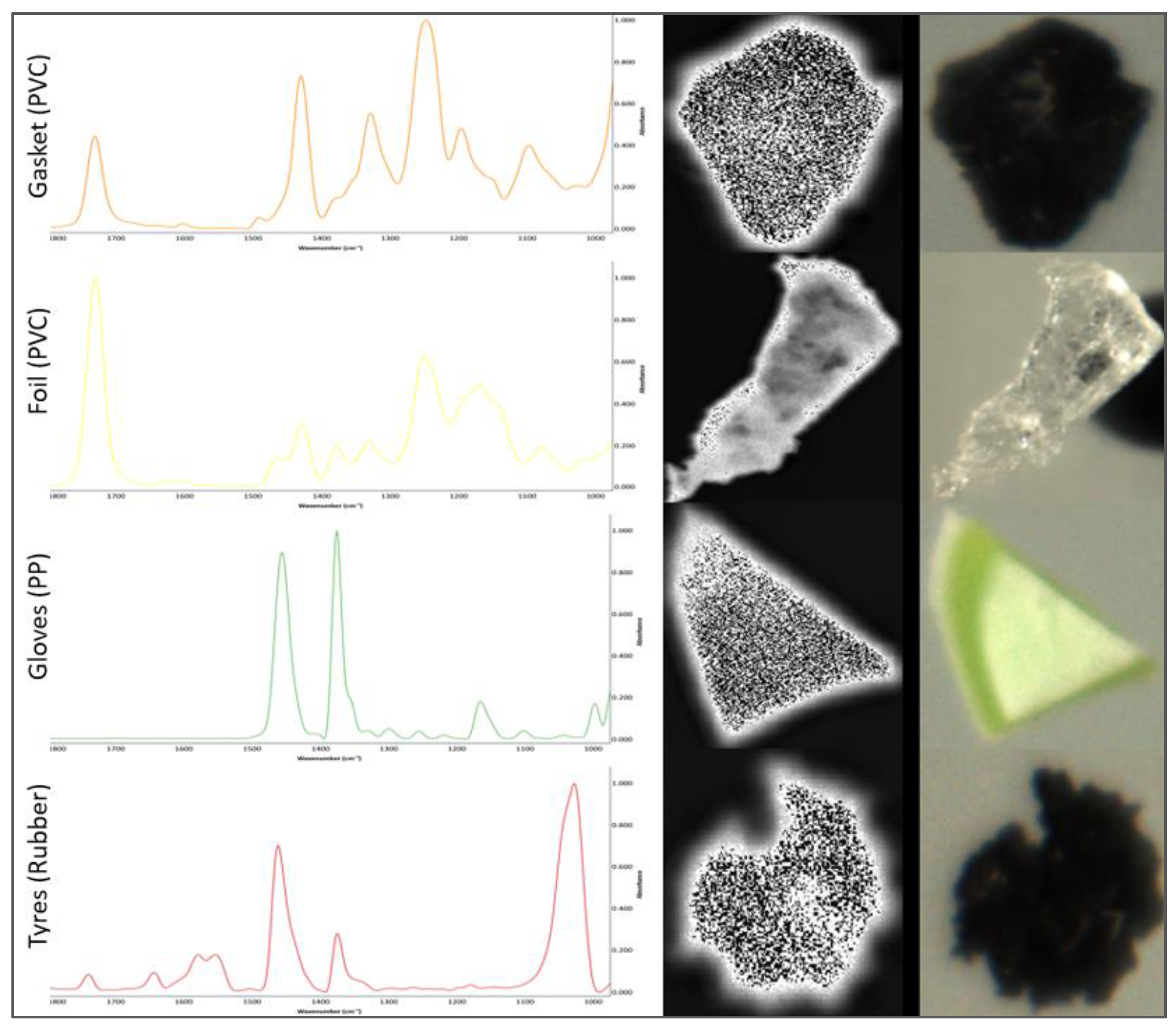
| Matrix | Location | Concentration | Reference |
|---|---|---|---|
| Surface Waters | Ma’an Archipelago | 200 ± 100 to 600 ± 200 pieces/m3 | [19] |
| Marmara Sea | 1263 pieces/m2 | [16] | |
| Yellow Sea | 545 ± 282 pieces/m3 | [12] | |
| West Coast of Sweden | 102,550 pieces/m3 | [15] | |
| Yellow River | 930,000 (dry season) and 497,000 (wet season) pieces/m3 | [18] | |
| Pearl River (China) | 379–7924 pieces/m3 | [19] | |
| Lake Taihu | 6.8 × 106 pieces/km2 | [20] | |
| Bottom Sediments | Ma’an Archipelago | 30 to 80 pieces/kg d.w. | [17] |
| Yellow Sea | 37.1 ± 42.7 pieces/kg d.w. | [12] | |
| Pearl River (China) | 80–9597 pieces/kg d.w. | [19] | |
| Lake Ontario | 2.8 × 104 pieces/kg | [21] | |
| Treated Wastewater | Helsinki | 8600 pieces/m3 | [22] |
| Polymer | Product | Color | Source | |
|---|---|---|---|---|
| polyvinyl chloride | PVC | gasket | black | Jano, Poland |
| polyvinyl chloride | PVC | phthalate foil | transparent | Europak, Poland |
| rubber (caoutchouc) | Rubber (caoutchouc) | tires | black | - |
| polypropylene | PP | gloves | green | W5, Germany |
| Parameter | pH | OM | DOC | CaCO3 | Fe | Ni | Cu | Zn | Pb | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| [–] | [%] | [mg/g d.w.] | [%] | g/kg d.w. | mg/kg d.w. | g/kg d.w. | ||||
| 7.88 | 8.45 | 4.38 | 3.22 | 34.12 | 36.20 | 35.60 | 116.70 | 42.91 | 39.96 | |
| Parameters | |
|---|---|
| Type of column | Shin Carbon ST column (2 m, 1.00 mm ID) |
| Injector temperature | 150 °C |
| Detector temperature | 100 °C |
| Column temperature | 60 °C |
| Carrier gas | helium |
| Carrier gas flow rate | 50.0 mL/min |
| Parameters | Unit | Gasket (PVC) | Foil (PVC) | Gloves (PP) | Tires (Rubber)—600 µm |
|---|---|---|---|---|---|
| Width | µm | 964 | 1470 | 1207 | 734 |
| Height | µm | 898 | 3394 | 1051 | 799 |
| Diameter | µm | 913 | 1932 | 1013 | 744 |
| Area | µm2 | 655,025 | 29,331,00 | 806,875 | 435,875 |
| Circularity | - | 0.67 | 0.35 | 0.58 | 0.62 |
| Solidity | - | 0.96 | 0.82 | 0.96 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, M.; Ziembowicz, S.; Koszelnik, P. CH4 and CO2 Emissions from the Decomposition of Microplastics in the Bottom Sediment—Preliminary Studies. Environments 2022, 9, 91. https://doi.org/10.3390/environments9070091
Kida M, Ziembowicz S, Koszelnik P. CH4 and CO2 Emissions from the Decomposition of Microplastics in the Bottom Sediment—Preliminary Studies. Environments. 2022; 9(7):91. https://doi.org/10.3390/environments9070091
Chicago/Turabian StyleKida, Małgorzata, Sabina Ziembowicz, and Piotr Koszelnik. 2022. "CH4 and CO2 Emissions from the Decomposition of Microplastics in the Bottom Sediment—Preliminary Studies" Environments 9, no. 7: 91. https://doi.org/10.3390/environments9070091
APA StyleKida, M., Ziembowicz, S., & Koszelnik, P. (2022). CH4 and CO2 Emissions from the Decomposition of Microplastics in the Bottom Sediment—Preliminary Studies. Environments, 9(7), 91. https://doi.org/10.3390/environments9070091






