Abstract
The abuse of artificial food dyes and the evidence that they harm human health recently prompted a significant effort to introduce vegan substitutes prepared from fruits and vegetables. Not much information, however, has been collected on their possible effects on aquatic and terrestrial ecosystems once released as waste in surface waters. For this purpose, we analyzed the effects of a vegan red (VEG) preparation (concentration 1.2 g/L) on three rapidly proliferating models for terrestrial and aquatic ecosystem contamination. In particular, in vitro cells cultures (exposure for 24 h), Artemia salina nauplii and Cucumis sativus seedlings (exposure 5 days). A comparison was made with the effects exerted by the two dyes that vegan red is intended to replace: an animal dye, cochineal E120 and an artificial dye E124. The analyses of conventional endpoints, indicative of cell proliferation, differentiation, and growth rate, demonstrate that the three dyes affect development and that the vegan substitute is as unsafe as the E124 and E120. Vegan red in fact impairs cell growth in in vitro cells, delays naupliar hatching and early growth in Artemia, and reduces shoot/root biomass in Cucumis. Marked hyperplasia and hypertrophy of mesophyll are also observed in Cucumis leaves. Substitution in food and beverages, therefore, should be carefully reconsidered to avoid unnecessary environmental contamination.
1. Introduction
The potential toxicity of artificial food dyes, including azo dyes, has increased consumers’ attention, and international agencies have introduced daily intake limits (see, for example, regulation EC No 1333/2008 and EC No 257/2010) to mitigate possible adverse effects. Much less attention has been dedicated to the putative threat they represent to the environment. About two-thirds of the azo dyes found in the environment are released by textile industries [1] but, in the remaining one-third, food dyes are a relevant part. The food color market is rapidly growing and with an estimated budget of over two billion USD [2], it significantly contributes to water pollution. Food dyes are released in the superficial waters during production, food manipulation, and consumption, via sewages; eventually, they may contaminate the soils by watering crops. Proof of the concern is the numerous investigations carried out in the last four decades to set methods for evaluating [3,4] and eliminating [5] food dyes in superficial waters.
Red is one of the colors most often used in food preparation to increase perceived flavors and attractiveness. The azo dye Ponceau red E124, in particular, has been widely used until marked side effects, such as attention deficit hyperactivity disorder (ADHD), were demonstrated in children. [6]. Since then, evidence of E124 toxicity has been produced in both plants [7,8] and animal models [8], in which interference with the oxidative state was reported [9]. This evidence led to its progressive replacement with a natural pigment, the cochineal red, also known as carmine or E120. However, E120 also has serious drawbacks caused by the non-vegan origin (the dye is extracted from the insect Dactylopius coccus) and the occasional presence of microbiological and metal impurities. Allergic reactions reported in humans exposed by contact [10], ingestion [11] or inhalation [12] have prompted an additional safer replacement. In the last two decades, the toxicity of the two red dyes has been re-evaluated [13,14], and vegan pigments have been suggested for substitution. Extracted from fruit and vegetables, they are cheap and perceived as safe by consumers because their antioxidant and anti-inflammatory effects add functional properties to the food [15,16].
Contrasting evidence exists so far on the environmental safety of the newly introduced vegan pigments. Curcumin, for example, a yellow dye substituting toxic tartrazine E102, induces favorable effects if added to the diet of cultured fish [17], but it is also reported to induce DNA damage in in vitro hamster cells [18], indicating potential toxicity on unicellular aquatic organisms, bacteria, and protozoa [19,20]. Red pigments are rich in polyphenols and anthocyanins; the latter exert marked antiproliferative effects [21] that may be beneficial in antitumoral therapies but not in developing systems like embryos or fast-growing juveniles. The antioxidant properties of vegan pigments, therefore, may be appreciable in human diets, often lacking essential principles. Still, the same does not necessarily apply to the aquatic organisms, and severe interferences at various levels on plants and animals’ growth may be postulated.
In the present study, we compared the effects of a commercial vegan red dye with those exerted by the two red dyes it is intended to replace on market shelves: E124 and E120. Establishing the environmental toxicity of a product destined to invade the market in the next future is essential. In particular, the interference of the new vegan product was tested on three fast-proliferating models. In vitro cultured cells were chosen as models mimicking single-celled organisms, while Artemia salina nauplii and Cucumis sativus seedlings were chosen to determine the effects on multicellular animal and plant species. The importance of the effects on plants should not be neglected, as contaminated waste waters are used in water gardens and cultures. The concentration of dyes was chosen according to previous papers [8,9], two-fold higher than those reported for other azo dyes in water [1], so facilitating the identification of the potential targets of toxicity.
The selected endpoints vary according to the model system. In cultured cells, the proliferation rate was determined by an MTT viability assay. In A. salina and C. sativus, the rate of development was taken as indicative of the interference of dyes on cell proliferation and differentiation. In particular, in A. salina nauplii, hatching and growth rate were determined together with mortality, while in the plant model C. sativus seedlings’ growth and organization of leaves were examined.
2. Materials and Methods
2.1. Preparation of Red Food Dyes Solutions
The three food dyes used in this study are common shelf products, used to prepare domestic foods. The vegan red, sold in powder, contains extracts of radish, black currant, and apple in undefined quantities. Ponceau red E124 and cochineal red E120 are sold in 3 mL vials, containing water and 20% dye. No attempt was made to determine the exact composition of the vegan preparation. There are no legal restrictions on these natural products and manufacturers have full discretion regarding dye type and content. To reduce variability to the minimum, care was taken to use the same product brand and lot in the experiments.
Dyes were diluted at a final concentration of 1.2 g/L [8]. The solutions were prepared with mineral water (considered pure from impurities) and immediately used to water the C. sativus seeds; for A. salina, water salinity was adjusted (Instant Ocean, salinity 36‰) according to Krishnaraju et al. [22]. For cells, the dyes were directly dissolved in the culture medium (see below).
2.2. Cell’s Maintenance, Growth, and Viability Test
PNT1A cells (human adult prostate epithelial cell line; ECACC Salisbury, UK) were grown in red phenol-free RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and antibiotics (100 U/mL penicillin/streptomycin, 10 mg/mL gentamicin) in a humidified incubator at 37 °C and 5% CO2. The medium was changed every 2 days. At confluence, cells were enzymatically detached with trypsin-EDTA and seeded in a new cell culture flask.
To test viability, cells were seeded in 96-well plates at the density of 5 × 104 cells/well, starved (1% FBS) for 24 h, and treated with vegan red, E120, or E124 for 24 h. The day after, 10 µL of 3-[4,5-dimethylthiazol-2-yl]-3,5 diphenyltetrazolium bromide (MTT) were added to each well for 4 h at 37 °C and 5% CO2. Then, the medium was gently aspirated and substituted with a solution of isopropanol and DMSO (1:1) [23]) to dissolve the formazan crystals produced in each well. The absorbance of the solution was measured at 570 nm using a microplate reader. Each assay was performed in triplicate.
2.3. Artemia salina Maintenance and Evaluation of Hatching and Growth Rate
Artemia salina L. diapause cysts (250 mg) were placed in 500 mL of artificial seawater or artificial seawater contaminated with one of the three food dyes. Incubation was carried out for 10 days, at 22 °C, under constant aeration with a 12 h light photoperiod. If necessary, a few drops of distilled water were added to compensate for evaporation. Nauplii were fed daily with yeasts and algae [24]. The experiments were carried out in quadruplicate, using different batches of cysts; mortality in controls never exceeded the 10% at 2 days [25].
Samples of cysts/nauplii suspension (1 mL) were collected 24 and 48 h after the beginning of the experiment, to determine the hatching percentage, and 5 and 10 days post-hatching, to determine the percent of nauplii at the different larval stages. Samples were fixed in 2% formaldehyde for 20 min, thoroughly washed in ethanol 75% [26], and examined under a dissecting microscope. The naupliar staging was conducted according to Copf et al. [27]. In brief, nauplii in stage L0 have just emerged from the cyst and are often still inside the naupliar membranes. At the L2 and L3 stages, the nauplii elongate the abdomen, developing the first evidence of segmentation during the L3 stage. At stage L4, the anteriormost abdominal segments show the first rudiments of thoracopods which elongate and differentiate during stages L5 to L8. Stage L5 is also characterized by the appearance of the first pigmented cells of the paired compound eyes [8].
2.4. Cucumis sativus Maintenance, Seedling Growth, and Analysis of Leaf Anatomical Structure
Cucumis sativus L. seeds were surface-sterilized [8] and arranged randomly in groups of 13, in 90 mm Petri dishes containing tissue paper wetted with 8 mL of mineral water or water added with the three food dyes, vegan red, E120, and E124. The Petri dishes, set in triplicate, were incubated in the dark in a climatic chamber at a temperature of 25 ± 2 °C and relative humidity of 50 ± 5%. The seed germination and seedling growth were examined daily. The germination experiment was repeated three times, for a total of 117 observations (13 seeds × 3 Petri dishes × 3 replicates).
The germination percentage and the days to 50% emergence (E50), which indicates the rapidity in terms of days to obtain 50% germination, were calculated as reported in Noman et al. [28] using the following formulas:
where N = final number of germinated seeds; ni = number of seeds emerged at time ti when ni < N/2;
G% = (Number of germinated seeds/Total number of seeds) × 100
E50 = ti + (N/2 − ni)(tj − ti)/(nj − ni)
After germination, sprouts were exposed to a Photosynthetic Photon Flux Density (PPFD) of 190 ± 10 μmol photons m2 s−1 provided by white light-emitting diodes, at a photoperiod of 12 h. After 10 days from germination (DAG), sprouts were transferred in 100 mL vials added with water or the dye solutions until 15 DAG. During the seedling growth, distilled water was added to the pots to reintegrate the water lost by evapotranspiration. As a proxy for seedling growth, the following endpoints have been evaluated: plant total length, shoot/root ratio length; total leaf area; total plant biomass, and shoot/root ratio. Total plant length was monitored (n = 20) every 5 days up to 15 DAG; total leaf area and biomass and shoot/root ratio (n = 20) were determined on seedlings at 15 DAG. Measurements were performed by a digital camera on randomly chosen control and treated seedlings and analyzed using the software Image J 1.45. The experiments were repeated in triplicate.
For light microscopy, the leaves of the control plants treated with vegan red, E120, and E124 pigment were fixed according to Motta et al. [8]. They were dehydrated in a graded series of ethanol and embedded in Epon resin. Semi-thin sections were prepared and observed under a light microscope after staining with 1% toluidine blue in 1% sodium tetraborate buffer to show general morphology. The thickness of spongy and palisade mesophyll was determined in serial sections with the aid of software associated with a ZEISS Axiocam Microscope Camera applied to a Zeiss Axioskop microscope.
2.5. Statistical Analysis
Statistically significant differences among treatments were assessed by Graph Pad Prism 7 (GraphPad Software, La Jolla, CA, USA, www.graphpad.com accessed on 4 April 2016), using one-way ANOVA followed by Bonferroni’s multiple comparison test. Data were expressed as mean ± SEM and were considered to be statistically significant if p < 0.05.
3. Results
3.1. Effects of the Red Food Dyes on Cell Viability
MTT assay (Figure 1) indicated that after 24 h of exposure, vegan red induced a significant increase and E124 a significant decrease in cell viability. No effect was noticed after exposure to E120 compared to the control.
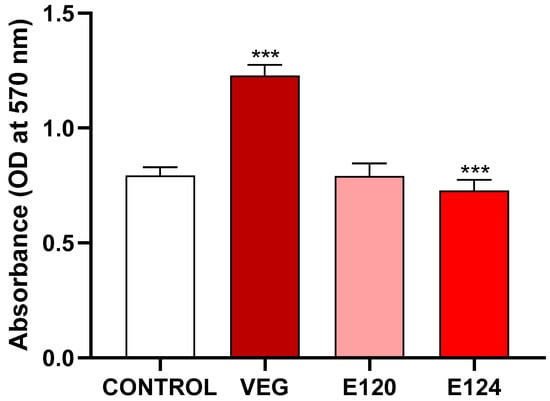
Figure 1.
Effect of vegan red VEG, E120, or E124 on PNT1A cell viability. Notice the significant decrease induced by E124 and the significant increase induced by vegan red. (Data are mean ± SEM; *** p < 0.001).
3.2. Effects of Red Food Dyes on the Early Development of Artemia salina Nauplii
The hatching percentage (Figure 2) was significantly decreased in cysts exposed to vegan red (p < 0.05) or E120 (p < 0.01), at both 24 and 48 h. In cysts exposed to E124, no significant effect was observed at 24 h, while at 48 h a dramatic increase (p < 0.001) occurred with the hatching percentage rising to 66.7 ± 6.4 compared to the 17.4 ± 5.0 of control cysts.
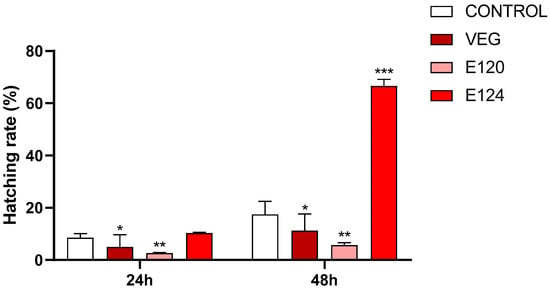
Figure 2.
Hatching rate (%) at 24 and 48 h in Artemia salina cysts exposed to vegan red VEG, E120 or E124. Notice the significant decrease induced by VEG and E120 at both 24 and 48 h and the increase induced by E124 at 48 h. (Data are mean ± SEM; * p < 0.05; ** p < 0.01; *** p < 0.001).
To better understand the dynamics of naupliar populations, mortality was assessed by daily sampling (Figure 3). Results indicated that mortality remained below 5% until day 4. On day 5, a raise was observed in all samples (range 8–12%), with a significant peak in E124 (16.1% vs. 8.6% of control). On day 6, values increased further, with E124 remaining the most toxic dyes (18.6% vs. 9.7% of control). On day 7, an increase in mortality was observed also in samples exposed to E120 (18.6% vs. 14.3% of control). For E124, the mortality was 21.5%, and for vegan red 15.1%.
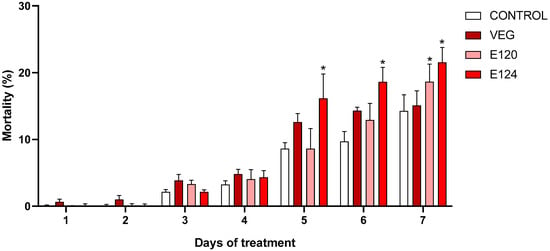
Figure 3.
Mortality percentage in Artemia salina nauplii exposed to vegan red VEG, E120, or E124. Notice the significant increase registered for E124 since day 5 and for E120 on day 7. (Data are mean ± SEM; * p < 0.05).
Analyses of naupliar gross morphology revealed that nauplii were normally developed with no significant evidence of deformities (data not shown).
The effect of dyes on developmental timing was checked by comparing the percent of each stage after 5 and 10 days from hatching. Profound effects were found.
Five days after hatching (Figure 4, left panel), about 30% of control nauplii were at stage L3 and 57% at stage L4. Occasional nauplii were at stage L5. In samples exposed to vegan red, the vast majority of nauplii was at stage L3 (87%) and none at stage L5, suggesting a delay in development. After exposure to E120 or E124, about 10% of nauplii were at stage L5, clearly indicating acceleration of development.
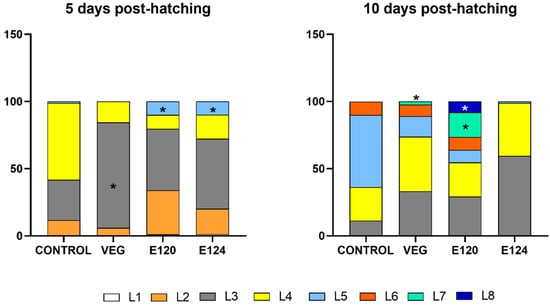
Figure 4.
Naupliar stages (%) in Artemia salina samples exposed to vegan red VEG, E120, or E124. Five days from hatching. VEG delays (increased percentage of L3 nauplii; *) and E124 and E120 accelerate (appearance of L5 nauplii; *) development. Ten days from hatching. Moderate and significant acceleration of development in VEG and E120 (presence of L7 and L7 and L8 nauplii respectively; *). In E124, are present only L3 and L4 nauplii.
Ten days after hatching (Figure 4 right panel), 50% of control nauplii were at stage L5 and about 10% were already at stage L6. The remaining 35% were distributed between stages L3 and L4. In samples exposed to vegan red, a moderate delay of development was observed being the nauplii essentially distributed between stages L3 and L4. Nauplii at the L7 stage were also present but their percentage, 2.5%, was too low to indicate an acceleration of development. In contrast, a marked acceleration was observed in samples exposed to E120: in this case, about 30% of nauplii were at stages L6 and L7 and about 8% were already at stage L8. After exposure to E124, a completely different situation was observed. The occasional presence of nauplii in stage L5 and the complete absence of nauplii in L6–L8 stages were justified by the high mortality rate registered (Figure 3). The survived nauplii were distributed between stages L3 (60%) and L4 (39%).
3.3. Effects of Red Food Dyes on the Early Development of Cucumis sativus Seedlings
3.3.1. Germination Percentage
On 4 DAG the germination percentage reached almost 90% in control and treated seeds, and no significant difference was observed in the time to get 50% germination (T50) between different treatments (Figure 5).
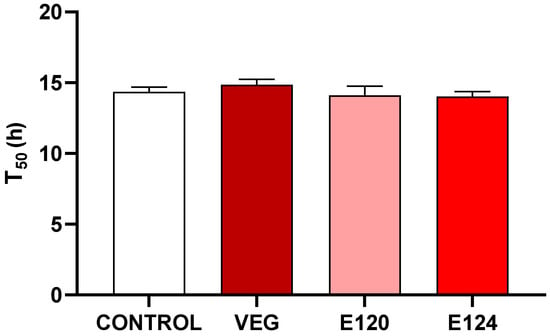
Figure 5.
Effect of vegan red VEG, E120, or E124 on the time (h) to obtain a 50% of Cucumis sativus seeds germinated (T50). Data are mean ± SEM (n = 20). No significant differences are evident among treatments.
3.3.2. Seedling Growth
No significant difference was observed in the seedling elongation up to 15 days after germination (Figure 6A), while seedlings exposed to E120 and E124 showed a higher (p < 0.01) shoot/root length ratio (Figure 6B). Vegan red dye induced a significant reduction of total fresh biomass (p < 0.05) compared to other treatments, due to the decrease of both hypogeal and epigeal portions (Figure 6C). The analysis of biomass distribution between epigeal and hypogeal parts evidenced that E120 and E124 determined a significant increase (p < 0.01 and p < 0.05 respectively) in the hypogeal biomass compared to control and vegan red (Figure 6C). A significant decline (p < 0.05) in the shoot/root mass ratio was also found in all the treatments compared to control (Figure 6D). In addition, seedlings treated with food dyes exhibited a significant reduction (p < 0.01) of the total leaf area (Figure 6E–G).
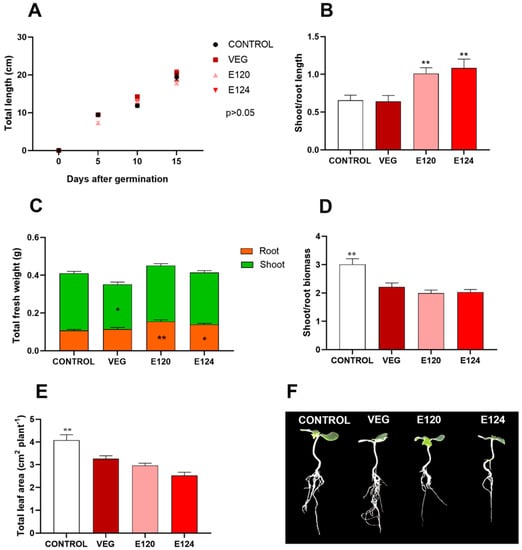
Figure 6.
Effect of vegan red VEG, E120 or E124 on Cucumis sativus seedlings elongation (up to 15 days) (A), and on shoot/root ratio in terms of length (B), total fresh biomass and distribution between shoot and root (C), shoot/root ratio in terms of fresh biomass (D), total leaf area (E) of Cucumis sativus seedlings at 15 days after germination. Individual seedlings at 15 days are compared in (F). (Data are mean ± SE; n = 20; * p < 0.05; ** p < 0.01).
3.3.3. Cotyledonary Leaf Anatomical Structures
A significant thickening accompanied the reduced leaf expansion (Figure 7M, p < 0.001) due to cellular hyperplasia and hypertrophy of the spongy and palisade mesophyll (Figure 7A–D). In seedlings developed in the presence of E124, leaves were more severely altered, with palisade cells tightly packed and crammed (Figure 7D). Amyloplasts were particularly numerous in leaves treated with vegan red (Figure 7B,J) and, to a lesser extent, after treatment with E124 (Figure 7L). No variation was observed in chloroplasts number and/or distribution.
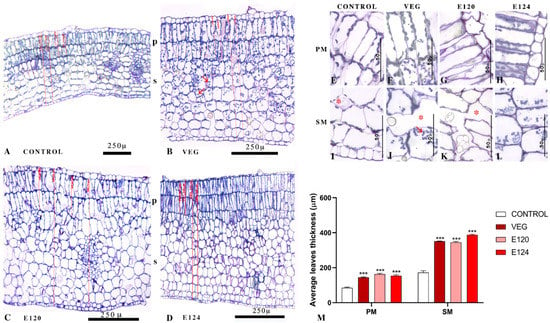
Figure 7.
Effect of vegan red VEG, E120, or E124 on cotyledonary leaves. (A): normal morphology of palisade (p) and spongy (s) mesophyll. (B–D): hyperplasia and hypertrophy of mesophyll. Arrows indicate amyloplasts. Vertical red lines indicate some of the measures used to determine average thickness. Detail of palisade (E–H) and spongy (I–L) cells. Notice the presence of large intercellular spaces (asterisks) and the increase in amyloplasts after VEG treatments (arrows). (M): Average thickness of spongy (SM) and palisade (PM) mesophyll. (Data are mean ± SE; n = 20; *** p < 0.001).
4. Discussion
Investigations confirm that E124 and E120 exert marked toxic effects on rapidly developing aquatic systems [18]. Unexpectedly, similar effects are also observed after exposure to a vegan red pigment preparation, a vegetal product introduced in the food market to replace artificial or animal red dyes, considered unsafe. According to the analyses carried out, vegan red is as unsafe for aquatic animals as E120 and E124 since it alters 10 of the 15 endpoints analyzed (Table 1).

Table 1.
Summary of the effects registered on in vitro cells, Artemia salina nauplii, and Cucumis sativus seedlings after exposure to vegan red VEG, E120, or E124. Values significantly increased (+), decreased (−), or were unchanged (0) compared to the controls (p ˂ 0.05). Cell viability (MTT); hatchsing at 24 or 48 h (hatch); developmental rate, 5- and 10-days post-hatching (develop); mortality (mort); germination (germ); root and shoot (rt, sh); thickness of spongy (SM) and palisade (PM) mesophyll.
The first striking evidence is that vegan red favored vitality in the cells in vitro. In multicellular models, the effects were contrasting. In A. salina, vegan red delayed hatching and early development but accelerated later development. In C. sativus it reduced epigeal and total biomass but induced leaves cell hyperplasia and hypertrophy and increased the number of amyloplasts.
Both radish (Raphanus sativus) and black currant (Ribes nigrum) extracts, present in the vegan red preparation, may be responsible for the observed stimulatory effects. The former contains antifungal proteins [29], the latter is rich in antioxidative polyphenols [30], and both contain anthocyanins, natural colorants with marked oxygen radical absorbing capacity [31]. All together, these components may protect cells and favor growth. In effect, in C. sativus, leaves showed marked hyperplasia of mesophyll.
On the other hand, it is reported that Raphanus sativus and Ribes nigrum have marked antiproliferative effects and induce apoptosis in cancer cells [32,33]. Similar effects probably were exerted on early Artemia embryos, thus delaying hatching and early development, and in Cucumis, reducing shoot biomass and total leaf area.
The apparent incongruences in response to vegan red cannot be explained by differences in absorption that likely occurred in the three models via cell surface. Anthocyanins, in fact, penetrate roots [34] and in vivo and in vitro cells and organs [21,35], including the A. salina cyst chorion [36]. Unfortunately, no data were found on seed integument permeability. Toxicodynamics also would share many similarities since in both vegetal [34,37] and animal [38] cells, anthocyanins remain active once inside the cell [39] and protect against chemical and physical stressors by controlling inflammation and redox state.
An intriguing hypothesis partly explaining the effects of vegan red comes from the evidence that anthocyanins have an osmoregulatory effect on plants [40]. In C. sativus leaves, anthocyanins contained in the vegan red preparation would have unbalanced the osmotic potential and induced the mesophyll cells’ observed marked hypertrophy. If a similar anthocyanins effect also occurs in animal models [41], the consequent deregulation of the osmotic gradient in cysts would reduce the ability of the hatchlings to emerge from the embryonic membranes [42], thus explaining the delayed hatching [41].
Besides demonstrating the marked toxicity of vegan red on the three aquatic models, the present investigation collected further evidence of E124 and E120 toxicity.
By reducing in vivo cell viability, E124 confirms its cytotoxicity and the effects already observed in fast proliferating cancer cell lines [43]. In A. salina, the dye induced an unexpected initial increase in the number of hatched cysts, already observed in D. rerio [8]. Results also confirm the previously observed increase in mortality [8] and add new evidence: i.e., strangely enough, death occurred only among nauplii at stages L4–L5. Hatch interference and mortality account for the presence of L5 nauplii on day 5 (L4 in controls) and L3 and L4 nauplii on day 10 (while in controls most nauplii are at the L5 and L6 stages). The high mortality was never associated with significant visible alterations in gross morphology. Anticipation/delay in growth, however, are indicative of profound effects on metabolism and naupliar physiology. In a recent paper, we demonstrate that all red dyes increase ROS, while E120 and E124 reduced the susceptibility to oxidative stress [9].
In C. sativus, seedling elongation was unaffected by E124 despite the long-term exposure (15 days). However, the increased shoot/root length ratio, indicating a negative effect of E124 on root development, and the reduction in the total leaf area support the hypothesis that the dye has a genotoxic effect and inhibits cell division [7,8,44,45]. A limitation in root development may affect the whole plant’s growth, altering the plant’s ability to absorb water and uptake nutrients [8]. These modifications may, in turn, determine alterations in leaf anatomy, compromising plant functionality. Martinez et al. [46] recently demonstrated that nutrient deficiency causes thickening and alterations in cherry tomato organs. In fact, compared to vegan red and E120, the leaf structure of E124-treated seedlings highlights a pluri-stratified palisade tissue in which the cells are densely tightly packed and crammed to each other to form a compact layer where intercellular spaces are not visible. Moreover, a very thin cuticle is observed. This leads to the conclusion that E124 interfered with mesophyll, causing marked hyperplasia and hypertrophy, which are usually interpreted as a defense response [47,48].
New evidence provided by this study is that E124 made the roots shorter but thicker, thus reducing the shoot/root ratio and altering the carbon distribution between epigeal and hypogeal portions of plants. Results also confirm that the dye does not affect seed germination [8].
E120 was also confirmed to be just as unsafe as the synthetic E124. Though without effects on in vitro cells, E120 reduced hatching and accelerated development in A. salina nauplii up to a stage, L8, never observed in controls. Mortality also increased, confirming previous data [8]. In C. sativus, the dye-induced effects are comparable to those induced by E124, just less marked. The mechanism of action of E120 is not completely clarified. It is a grounded extract obtained from an insect and, after purification, is rich in carminic acid, carmines, and small residues of unbound aluminum cations. Toxicity is proven in long-term experiments on mammals [14], plants, and micro-crustaceans, and carminic acid would be the main responsible [45,49].
A final consideration should be made on the relatively high concentration of colorants used in these experiments [8,9]. It should be considered that the concentrations of dyes are significantly high in polluted areas (up to 0.5 gr/L; [1]), that many dyes may be present at the same time, and last but not least, that the organisms are exposed chronically, from ovo/seed to death, with multiple routes of access. The effects, therefore, may be significantly more severe than expected based on the responses obtained from exposure to a single, relatively high dose. The lack of precise information on the manufacturing processes of vegan red does not allow to separate the dye effects from those of the minor components potentially present in the preparation. These, however, are also eliminated with the dyes, reach the aquatic environments and eventually contribute to animal and plant toxicity.
5. Conclusions
The present investigation demonstrated that a commercial vegan red dye exerts marked toxicity on three models: in vitro cells, embryo/larval Artemia salina, and Cucumis sativus seedlings. Therefore, this potential substitute for E124 and E120 cannot be considered safe for the environment, especially if the concentration in freshwater will rise in the future. Further investigations are required to clarify the mechanism of action in different models so as to foresee the effects of long-term exposure on animals and plants occupying different levels of the trophic chain.
Author Contributions
Conceptualization, C.M.M., P.S. and C.A. (Carmen Arena); methodology, M.D.L., E.V., M.S., A.R., S.D.B., C.F. and T.C.; formal analysis, M.D.L., E.V., A.R., S.D.B., C.F. and T.C.; Investigation, M.D.L., E.V., M.S., A.R., S.D.B., C.F., T.C, B.A., C.A. (Carmen Arena), C.M.M., P.S. and C.A. (Claudio Agnisola); writing—original draft preparation, C.M.M., P.S., C.A. (Carmen Arena), C.A. (Claudio Agnisola) and B.A.; writing—review and editing, C.M.M., P.S., C.A. (Carmen Arena), C.A. (Claudio Agnisola), B.A., I.F. and G.N.; data curation, M.D.L., E.V., M.S., A.R., S.D.B., C.F., I.F., T.C., G.N., B.A., C.A. (Carmen Arena), C.A. (Claudio Agnisola), C.M.M. and P.S.; visualization, C.M.M., P.S., C.A. (Carmen Arena), B.A. and C.A. (Claudio Agnisola); supervision, C.M.M., P.S. and C.A. (Carmen Arena). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by research Funds from the University of Naples to C.M.M., C.A. (Carmen Arena), B.A. and M.D.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Raffaele Panzuto and Margherita Diana for the help and their contribution to the lab work and data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nojavan, S.; Tahmasebi, Z.; Bidarmanesh, T.; Behdad, H.; Nasiri-Aghdam, M.; Mansori, S.; Pourahadi, A. Electrically enhanced liquid-phase microextraction of three textile azo dyes from wastewater and plant samples. J. Sep. Sci. 2013, 36, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Natural Food Colors Market Size, Share & Trends Analysis Report by Product (Curcumin, Carotenoids, Anthocyanin, Carmine, Chlorophyllin), by Application, by Region, and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/natural-food-colors-market (accessed on 12 May 2018).
- Lepri, L.; Desideri, P.G.; Coas, V. Separation and identification of water-soluble food dyes by ion-exchange and soap thin-layer chromatography. J. Chromatogr. A 1978, 161, 279–286. [Google Scholar] [CrossRef]
- Perez-Urquiza, M.; Ferrer, R.; Beltran, J.L. Determination of sulfonated azo dyes in river water samples by capillary zone electrophoresis. J. Chromatogr. A 2000, 883, 277–283. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced oxidation processes for the removal of food dyes in wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Weiss, B.; Williams, J.H.; Margen, S.; Abrams, B.; Caan, B.; Citron, L.J.; Cox, C.; McKibben, J.; Ogar, D.; Schultz, S. Behavioral responses to artificial food colors. Science 1980, 207, 1487–1489. [Google Scholar] [CrossRef]
- Santana, G.M.; do Anjos Sousa, J.J.; Peron, A.P. Action of Ponceau 4R (E-124) food dye on root meristematic cells of Allium cepa L. Acta Sci. Biol. Sci. 2015, 37, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effect of four food dyes on development of three model species, Cucumis sativus, Artemia salina and Danio rerio, assessment of potential risk for environment. Environ. Pollut. 2019, 253, 1126–1135. [Google Scholar] [CrossRef]
- Napolitano, G.; Motta, C.M.; Agnisola, C.; Venditti, P.; Fasciolo, G.; Ferrandino, I.; Capriello, T.; Vitale, E.; Costanzo, G.; Avallone, B.; et al. Commercial red food dyes preparations modulate the oxidative state in three model organisms (Cucumis sativus, Artemia salina and Danio rerio). Environments 2022, 9, 63. [Google Scholar] [CrossRef]
- Shaw, D.W. Allergic contact dermatitis from carmine. Dermatitis. 2009, 20, 292–295. [Google Scholar] [CrossRef]
- Wüthrich, B.; Kägi, M.K.; Stücker, W. Anaphylactic reactions to ingested carmine (E120). Allergy 1997, 52, 1133–1137. [Google Scholar] [CrossRef]
- Quirce, S.; Cuevas, M.; Olaguibel, J.; Tabar, A.I. Occupational asthma and immunologic responses induced by inhaled carmine among employees at a factory making natural dyes. J. Allergy Clin. Immunol. 1994, 93, 44–52. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific Opinion on the re-evaluation of Ponceau 4R (E124) as a food additive. EFSA J. 2009, 7, 1328. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of cochineal, carminic acid, carmines (E 120) as a food additive. EFSA J. 2015, 13, 4288. [Google Scholar] [CrossRef]
- Brauch, J.E. Underutilized fruits and vegetables as potential novel pigment sources. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Sawston, UK, 2016; pp. 305–335. [Google Scholar] [CrossRef]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins Protect against Kainic Acid-induced Excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef]
- Manju, M.; Akbarsha, M.A.; Oommen, O.V. In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol. Biochem. 2012, 38, 309–318. [Google Scholar] [CrossRef]
- Araújo, M.C.; Dias, F.L.; Takahashi, C.S. Potentiation by turmeric and curcumin of gamma-radiation-induced chromosome aberrations in Chinese hamster ovary cells. Teratog. Carcinog. Mutagen. 1999, 19, 9–18. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin, an in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Wachter, B.; Syrowatka, M.; Obwaller, A.; Walochnik, J. In vitro efficacy of curcumin on Trichomonas vaginalis. Wien. Klin. Wochenschr. 2014, 126, 32–36. [Google Scholar] [CrossRef]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef]
- Krishnaraju, A.V.; Rao, T.V.; Sundararaju, D.; Vanisree, M.; Tsay, H.S.; Subbaraju, G.V. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. Int. J. Appl. Sci. Eng. 2005, 3, 125–134. [Google Scholar]
- Forte, M.; Di Lorenzo, M.; Iachetta, G.; Mita, D.G.; Laforgia, V.; De Falco, M. Nonylphenol acts on prostate adenocarcinoma cells via estrogen molecular pathways. Ecotoxicol. Environ. Saf. 2019, 180, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hannas, B.R.; Das, P.C.; Li, H.; LeBlanc, G.A. Intracellular conversion of environmental nitrate and nitrite to nitric oxide with resulting developmental toxicity to the crustacean Daphnia magna. PLoS ONE 2010, 5, e12453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicol. Environ. Saf. 1981, 5, 382–387. [Google Scholar] [CrossRef]
- Motta, C.M.; Cerciello, R.; De Bonis, S.; Mazzella, V.; Cirino, P.; Panzuto, R.; Ciaravolo, M.; Simoniello, P.; Toscanesi, M.; Trifuoggi, M.; et al. Potential toxicity of improperly discarded exhausted photovoltaic cells. Environ. Pollut. 2016, 216, 786–792. [Google Scholar] [CrossRef]
- Copf, T.; Rabet, N.; Celniker, S.E.; Averof, M. Posterior patterning genes and the identification of a unique body region in the brine shrimp Artemia franciscana. Development 2003, 130, 5915–5927. [Google Scholar] [CrossRef] [Green Version]
- Noman, A.; Ali, Q.; Maqsood, J.; Iqbal, N.; Javed, M.T.; Rasool, N.; Naseem, J. Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 2018, 195, 175–189. [Google Scholar] [CrossRef]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J. Small cysteine-rich antifungal proteins from radish, their role in host defense. Plant Cell 1995, 7, 573–588. [Google Scholar] [CrossRef] [Green Version]
- Landbo, A.K.; Meyer, A.S. Enzyme-assisted extraction of antioxidative phenols from black currant juice press residues (Ribes nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Martin, A.; Wang, H.; Denisova, N.; Bickford, P.C.; Joseph, J.A. Short-term dietary supplementation of blueberry polyphenolics, beneficial effects on aging brain performance and peripheral tissue function. Nutr. Neurosci. 2000, 3, 383–397. [Google Scholar] [CrossRef]
- Beevi, S.S.; Mangamoori, L.N.; Subathra, M.; Edula, J.R. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods Hum. Nutr. 2010, 65, 200–209. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, Y.L.; Kong, B.; Liu, Q.; Xia, X. Radical scavenging activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on gastric cancer cell proliferation via induction of apoptosis. J. Funct. Foods 2012, 4, 382–390. [Google Scholar] [CrossRef]
- Westfall, A.; Sigurdson, G.T.; Rodriguez-Saona, L.E.; Giusti, M.M. Ex Vivo and In Vivo Assessment of the penetration of topically applied anthocyanins utilizing ATR-FTIR/PLS regression models and HPLC-PDA-MS. Antioxidants 2020, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.D.C.; Flores, C.S.; He, J.; Tian, Q.; Schwartz, S.J.; Giusti, M.M. Characterization and preliminary bioactivity determination of Lechler fruit anthocyanins. Food Chem. 2011, 128, 717–724. [Google Scholar] [CrossRef]
- Colak, N.; Zengin, A.Y.; Ayaz, F.A. The effect of anthocyanin-rich bilberry extract on the antioxidant system in roots of barley (Hordeum vulgare L.) cultivars under ionizing radiation. Acta Physiol. Plant. 2015, 37, 187. [Google Scholar] [CrossRef]
- Glińska, S.; Bartczak, M.; Oleksiak, S.; Wolska, A.; Gabara, B.; Posmyk, M.; Janas, K. Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol. Environ. Saf. 2007, 68, 343–350. [Google Scholar] [CrossRef]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-κB and phosphatidylinositol 3-kinase/Akt pathway. J. Agric. Food Chem. 2008, 56, 8969–8974. [Google Scholar] [CrossRef]
- Płatosz, N.; Bączek, N.; Topolska, J.; Szawara-Nowak, D.; Skipor, J.; Milewski, S.; Wiczkowski, W. Chokeberry anthocyanins and their metabolites ability to cross the blood-cerebrospinal fluid barrier. Food Chem. 2021, 346, 128730. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 2002, 37, 103–127. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanevello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef]
- Trotman, C.N.A.; Mansfield, B.C.; Tate, W.P. Inhibition of emergence, hatching, and protein biosynthesis in embryonic Artemia salina. Dev. Biol. 1980, 80, 167–174. [Google Scholar] [CrossRef]
- Lye, H.M.; Chiew, J.C.; Siddique, M.M. Cytotoxic effect of commonly used food dyes on human hepatoma cell line. Int. Food Res. J. 2018, 25, 1457–1463. [Google Scholar]
- Dwivedi, K.; Kumar, G. Genetic damage induced by a food coloring dye (sunset yellow) on meristematic cells of Brassica campestris L. J. Environ. Public Health 2015, 2015, 319727. [Google Scholar] [CrossRef] [Green Version]
- e Silva, A.P.S.; de Sousa Silva, T.; dos Santos, A.D.A.; Ribeiro, K.G.; Marques, M.M.M.; de Almeida, P.M.; Peron, A.P. Toxicity of carmine cochineal and caramel iv dyes to terrestrial plants and micro-crustaceans. Water Air Soil Pollut 2020, 231, 313. [Google Scholar] [CrossRef]
- Martinez, H.E.P.; Maia, J.T.L.S.; Ventrella, M.C.; Milagres, C.; Cecon, P.R.; Clemente, J.M.; Garbin, C.Z. Leaf and stem anatomy of cherry tomato under calcium and magnesium deficiencies. Braz. Arch. Biol. Technol. 2020, 63, e20180670. [Google Scholar] [CrossRef]
- Gamboa-Tec, N.; Kú-González, A.; Gutiérrez-Pacheco, L.C.; Castaño, E.; López-Ochoa, L. Anatomic alterations and hyperplasia induced by Euphorbia mosaic virus Yucatan Peninsula isolate at the meshophyll. Rev. Mex. Fitopatol. 2018, 36, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.D.T.; Meira, R.M.S.A.; Ferreira, F.A.; Sant’Anna-Santos, B.F.; Ferreira, L.R. Morphological responses of different eucalypt clones submitted to glyphosate drift. Environ. Exp. Bot. 2007, 59, 11–20. [Google Scholar] [CrossRef]
- Merinas-Amo, R.; Martínez-Jurado, M.; Jurado-Güeto, S.; Alonso-Moraga, Á.; Merinas-Amo, T. Biological effects of food coloring in in vivo and in vitro model systems. Foods 2019, 8, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).