Abstract
In this study we compared the heavy metal concentration found in different tissues and eggs of the loggerhead sea turtle and evaluated the potential ecotoxicological risk for this important species. Eighteen heavy metal elements were determined in different tissues (liver, gonads, fat, kidney, heart, brain, and spleen) of nine individuals of Caretta caretta found stranded along the coasts of Messina (Sicily, Italy) and in the shell and yolk of six eggs from the island of Linosa (Sicily, Italy). For the analysis of the heavy metals, we used the analytical procedures in accordance with the EPA 200.8 method supplemented by EPA 6020b with three replicates for each measurement. The elements analysed showed different organotropism even if the liver showed higher levels of bioaccumulation. Turtles’ tissues showed the highest values of iron in the liver, followed by zinc in the heart and arsenic in the kidney. Regarding eggs, zinc, iron, and barium were dominant in the yolk and iron, boron, and copper in the eggshell. From the analyses carried out the worrying levels of arsenic and cadmium in the kidneys and liver of C. caretta raise questions about the risk related to exposure to these non-essential elements. This study highlights the importance of multi-element biomonitoring by increasing knowledge on the biodistribution of 18 heavy metals and the related potential risks for C. caretta. We also exploring for the first time the presence of several heavy metals in the eggs and their possible implication for the survival of the species.
1. Introduction
Marine pollution has increased in recent decades due to the production of waste and pollutants generated by different human activities (industrial and domestic) [1].
Heavy metals are among the most common pollutants in the marine environment, and, despite the increasing number of regulations adopted by governments, this contamination continues to increase and persist [2].
It is well known that some of these, defined as essential, can be toxic if present above certain concentrations [3], whereas others (non-essential) can be toxic even at low concentrations [4]. One of the most studied aspects that determines the increases in their ecotoxicological potential in marine organisms is the biomagnification, that is, the accumulation of pollutants along the food chain [2,5].
According to their chemical characteristics (e.g., lipophilia), heavy metals tend to bioaccumulate and biodegrade differently in tissues [6] and, unlike organic pollutants such as pesticides, are not biodegradable. Among the sentinel species that best reflect both organic [7,8] and inorganic contamination, we can consider C. caretta for its biological characteristics; thanks to the species’ longevity, diet, long stays in foraging areas, and migrations [6,9], it has high capacities to accumulate high amounts of contaminants in its tissues and for these reasons should be considered an ideal bioindicator of good marine ecological status [2,10].
In the literature, several researchers have found correlations between physiological parameters and the presence of heavy metals, suggesting that heavy metal pollution can affect the health of sea turtles [11,12,13,14]. Moreover, it has been observed that the increase in such contamination could adversely interfere with fertilization and the success of hatching [15,16]. The study of heavy metals in C. caretta is fundamental to evaluating their potentially toxic effects and estimating the type and degree of exposure. In fact, systemic toxic effects, such as teratogenicity, cytotoxicity, and genotoxicity [5], can cause multiple damages to the reproductive, immune, and nervous systems, behaviours, and cause carcinogenesis [17]. Decreased immune capacity, for example, can contribute to the development of infections, such as those produced by viruses, e.g., fibropapilloma [17]. In addition, some heavy metals, known to be endocrine disruptors, alter the concentrations of gonads, adrenal, and thyroid steroid hormones [17]. Moreover, heavy metals not only trigger co-selection processes but also increase the level of tolerance to antibiotics, thanks to the co-regulation of resistance genes [18], as recently observed by Alduina et al. [19] in samples of C. caretta.
According to Anan et al. [20] and Torrent et al. [21], differences in concentration profiles in various tissues indicate sources of contamination related to diet and geographical area. In this context, the Mediterranean Sea can be considered a contamination hotspot as it is a semi-closed basin surrounded by 22 countries whose human activities and heavy maritime traffic lead to high pollution [2].
Several studies in the Mediterranean have reported data on the concentrations of these inorganic pollutants in C. caretta and discussed their potential toxic effects. These were carried out in northwestern Adriatic Sea by Franzellitti et al. [22]; in the Adriatic and Ionian Sea by Storelli et al. [23]; in Tyrrenean by Maffucci et al. [24] and by Esposito et al. [10]; in the Northwestern Adriatic Sea by Andreani et al. [25]; in the Alboran Sea by García-Fernández et al. [17], Jerez et al. [3], and Martínez-López et al. [5]; in the Balearic Sea by Novillo et al. [6]; and in the Northeast Mediterranean Sea (on the southern coast of Turkey) by Yipel et al. [2].
The objective of the present paper was: (i) to determine the concentrations of 18 heavy metals in some tissues of C. caretta stranded along the northwest coast of Sicily; (ii) to improve the knowledge of these contaminants and their potential impact on this important species; (iii) to describe for the first time the presence of these 18 heavy metals in the loggerhead sea eggs collected in a nest from the island of Linosa.
2. Materials and Methods
2.1. Sampling and Pre-Treatment
Nine individuals of C. caretta were found dead stranded in 2021 along the coasts of Messina (Sicily, Italy). After the necroscopic analysis at the Teaching Veterinary Hospital of the University of Messina (first aid centre for sea turtles), the animals were dissected to obtain samples of the following tissues: liver, gonads, fat, kidney, heart, brain, and spleen. Unfortunately, not all individuals were sexed, and the curved length of the carapace was between 46–58 cm. During the autopsy of dead sea turtles, the status of the samples (post-mortem examination) was evaluated by veterinary specialists, in this way only individuals and tissues not in decomposition (stage A according to the Body Condition (BC) reported in Flint et al. [26]) were sampled in order to not affect the evaluation and comparison of the results.
The eggs, on the other hand, were collected during the monitoring activities of the nests conducted in the Marine Protected Area of the “Isole Pelagie” on the beach of “Pozzolana di Ponente” (35°86′33″ N, 12°85′48″ E) of Linosa (Mediterranean Sea). In particular, the six unhatched eggs analysed came from three different nests (two per nest) and were collected in the summer of 2018 and 2019.
The collection of eggs was carried out in strict compliance with the recommendations of the “Ministry of Environment and Protection of Territory and Sea” (Prot. n.17054 of 25 July 2018).
A total of 33 samples of tissue and eggs were weighed, homogenised, and stored separately in sterile polypropylene tubes and frozen at −20 °C until lyophilisation. All the tools used were made of disposable plastic material or duly rinsed with different cycles of washing in water.
Acid mineralisation of the samples was then performed with the CEM Mars 5 microwave system equipped with 12 containers: ~0.45 g of each freeze-dried sample was digested completely using a mixture containing 8 mL of nitric acid (HNO3 ≥ 69.0%, TraceSELECT™ for trace analysis, Fluka™) and 2 mL of hydrogen peroxide (H2O2 ≥ 30.0%, TraceSELECT™ Ultra for trace analysis, Fluka™) in Teflon containers according to the EPA method 3052.
For each mineralisation cycle, samples, blanks, and certified materials were prepared (quality control samples: NIST-1566b: Oyster Tissue and DORM-4: Fish protein). Acid digestion was carried out using a computer-controlled heating system up to 180 °C.
After cooling, the samples were brought to a volume of 20 mL with Milli-Q water (resistivity > 18.2 MΩ cm), stored at 4 °C, and filtered with PP syringes fitted with cellulose acetate filters (pore size: 0.45 μm, diameter: 25 mm, AISIMÔ). Finally, samples were analysed in inductively coupled plasma mass spectrometry (Agilent 7800 ICP-MS with autosampler SPS4).
2.2. Analysis of Heavy Metals on Turtle Samples C. caretta
The analytical procedure used was in accordance with the EPA 200.8 method supplemented by EPA 6020b and three replicates were performed for each measurement. To each analytical sample, including calibration samples, a constant volume of mixed internal standard consisting of Bi, Ho, ln, 6li, Sc, Tb, Y, and Rh in HNO3 at a concentration of 200 ppb was added during the analysis, through the peristaltic pump of ICP-MS.
To check for any contamination of the measuring system, several rinse blank solutions (1–5% HNO3 and 0.3–1% HCl) were included in the worklist, and in the specific case of mercury, to avoid the memory effect due to its presence in the samples, gold solutions were interspersed (200 ppb of Au in HNO3 to 7%).
The multielement calibration for the determination of the heavy metal of the present work has been carried out starting from the standard solutions of the single analytes (initial concentration: 1000 ppm). The analytes sought were selenium (Se), cobalt (Co), tin (Sn), vanadium (V), iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), chromium (Cr), mercury (Hg), cadmium (Cd), boron (B), barium (Ba), lead (Pb), antimony (Sb), nickel (Ni), silver (Ag), and arsenic (As).
The calibration range used was 0–160 ppb for all elements except mercury, for which more dilute concentrations were selected in the range of 0 to 4 ppb. The correlation coefficients showed high values (R2 = 0.9999 for each element).
A parallel analysis of procedural blanks and certified reference material (CRM) ensured quality and accuracy checks that showed less than 10% relative standard deviation. The recovery percentages (R) for each element were obtained through the analysis of the CRM (Table S1 in Supplementary Materials).
The limit of detection (LOD) and limit of quantification (LOQ) were calculated as the concentration equivalent to the mean (10 repeated blank measurements) of the selected analytical mass signals +3 (LOD) or +10 (LOQ) the standard deviation (SD) (Table S1 in Supplementary Materials).
The concentration levels in different tissues and eggs of Caretta caretta are shown in Table 1 (V, Co, Cu, B, Mn, Zn, Fe, Se, Cr, Ni, Sn, As, Ba, Hg, Ag, Sb, Cd, Pb). All concentration values are reported in dry weight (d.w.) and the acronym BLQ refers to values below the limit of quantification as reported by Faust et al. [27] and Morão et al. [28]. The concentration values of other works mentioned for comparison purposes and originally expressed in wet weight were converted to dry weight, assuming a percentage of the water content for each tissue as reported by Ross et al. [4]. This transformation is usually adopted by several authors to facilitate a comparison of data [6,17].

Table 1.
Mean concentration (µg/g d.w.) ± SD and concentration range (minimum and maximum) measured for each tissue and element analysed. BLQ = below quantification limit: <LOQ.
3. Results
Heavy metal concentrations in the tissues and eggs of Caretta caretta are reported in Table 1.
Selenium and copper showed high affinity for the liver with average concentrations of 64.57 µg/g d.w. and 37.33 µg/g d.w. respectively. Similarly, the highest concentrations of iron were observed in the liver, while zinc showed higher levels in the heart, followed by the spleen, liver, and kidneys (Table 1).
As for manganese, the highest concentrations found were in the liver, followed by gonads and kidneys, while tin was below the detection limit for all samples analysed. Similarly, nickel and chromium were below the detection limit, except Ni for gonads and kidneys (mean values of 0.57 and 0.7 µg/g d.w., respectively) and Cr in the liver and spleen (with an average value for both of 0.12 µg/g d.w.) (Table 1).
The highest mean concentrations of cobalt were observed in kidneys, gonads, and eggshells (Table 1). Differently, the highest levels of boron were found in the eggshell (8.12 µg/g d.w.), followed by fat tissues (7.63 µg/g d.w.), heart (6.55 µg/g d.w.) and liver (5.24 µg/g d.w.), while the highest vanadium mean was found in the liver (0.46 µg/g d.w.).
All tissues analysed showed significant concentrations of arsenic (Figure 1) with higher values in the brain (185.81 µg/g d.w.), kidneys (166.17 µg/g d.w.), gonads (112.36 µg/g d.w.), liver (77.27 µg/g d.w.), and heart (70.58 µg/g d.w.).
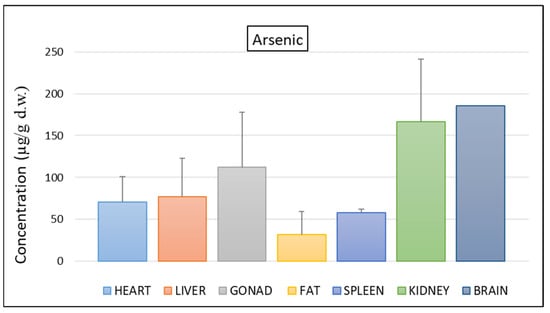
Figure 1.
Mean concentration ± SD of arsenic in the tissues of nine individuals of C. caretta.
A similar trend was observed for cadmium (Figure 2), which showed the highest concentration levels in kidneys (39.96 µg/g d.w.), liver (10.21 µg/g d.w.), and gonads (8.14 µg/g d.w.).
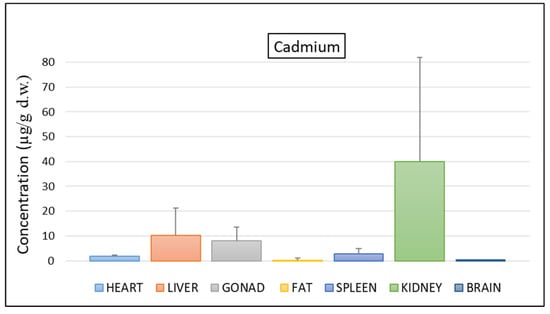
Figure 2.
Mean concentration ± SD of cadmium in the tissues of nine individuals of C. caretta.
The highest levels of mercury were found in the liver (mean 1.05 µg/g d.w., maximum 2.88 µg/g d.w.), followed by the kidney (mean 0.44 µg/g d.w., maximum value 0.84 µg/g d.w.).
Regarding barium, the highest concentration level was found in the yolk (11.30 µg/g d.w., maximum value 18.21 µg/g d.w.), followed by the brain (7.06 µg/g d.w.), eggshell (1.86 µg/g d.w.), and liver (0.52 µg/g d.w.), while in all other tissues it was below the detection limit.
The highest concentration of silver was recorded in the liver (0.45 µg/g d.w., maximum 0.94 µg/g d.w.), while lead was found only in the kidneys (0.19 µg/g d.w.) and liver (0.12 µg/g d.w.). Antimony was detected in low concentrations and only in the liver (0.02 µg/g d.w.), fat, and kidneys (both 0.01 µg/g d.w.).
4. Discussion
The results suggest a biodistribution of the elements in the different tissues, for instance, Hg, Ag, Sb, V, Cu, Mn, Fe, Se, and Cr were more abundant in the liver; Co, Cd, As, Ni, and Pb in kidneys; Zn in the heart; B in the eggshell; and Ba in the egg yolk (Table 1). The high standard deviation values compared to the average concentration levels could be justified by the high individual variability of exposure to sources of contamination in unknown areas of their origin and permanence. Moreover, in the case of non-essential elements, this high deviation may be due to the inability of individuals of C. caretta to strictly regulate these levels of contamination, as observed for cadmium by García-Fernández et al. [17]. However, between the different elements and tissues, the orders of magnitude of concentration levels are generally different.
Unlike other heavy metals, it has been highlighted that essential elements, such as Se, Cu, Fe, Zn, and Mn, can be regulated by C. caretta through homeostatic processes in a balance between metabolic needs and the prevention of toxic effects [24,25]. In this way, the concentration levels were found to vary depending on the element and tissue analysed (Table 1).
The results of Se, Zn and Cu were similar to those reported in other works for the liver of C. caretta in the Mediterranean Sea [2,3,17,22,23,24,25], while iron levels were significantly higher than in previous studies [2]. As for manganese, the average levels were similar to those found by Andreani et al. [25], except for the gonads that had values twice as high.
Comparing the concentration values of chromium and nickel with those found in other works for the same tissues [2,5,29] and eggs [30,31] it has been noted that those found in this study were lower. Considering that high Cr values could lead to cytotoxic and genotoxic effects [32] and high Ni values could have potentially negative effects [33], the present paper’s levels do not seem to be worrying.
The concentration values of cobalt and vanadium were comparable to those found in the tissues of some individuals in Chelonia mydas [27] and in eggs of C. caretta from Florida [30,31], while they were higher than the levels found in South Africa [34]. As regards boron, the average levels found in eggs were lower than those found in Florida by Alam and Brim [31] and in Mexico in eggs of Lepidochelys olivacea by Cortés-Gómez et al. [14].
In general, there are no studies on the toxicity limits for these elements, but considering the average levels found in the different works as physiological limits, it is assumed that the average concentrations of this study, which are close to these levels, shall not constitute a risk to the health of sea turtles.
Ba, Hg, Ag, Sb, Cd, Pb, and As are non-essential heavy metals of great concern due to their potential toxicity and have been found at different concentration levels (Table 1).
The arsenic levels found in the liver and kidneys were higher than those recorded in individuals of C. caretta stranded near Murcia (Spain) [5]. High concentrations of arsenic in kidneys and brain in adults may be indicative of chronic exposure [3], while high concentrations in the liver may be directly related to diet [35]. Although the information on arsenic toxicity thresholds in sea turtles is scarce [5], it should be considered that the inorganic forms of arsenic, usually present in the 2–10%, are those with the highest toxicity and tend to accumulate more in the liver [5,35], in which concentrations above 130 µg/g can lead to widespread liver degeneration in C. caretta [21]. The average levels found in our study seem to be slightly lower than those responsible for liver damage. However, the high concentrations found in turtle tissues support the hypothesis that the Mediterranean Sea area may be a hotspot for arsenic pollution.
As reported in other studies and found in this study, cadmium tends to bioaccumulate more in kidneys and liver [6,17,24]. In addition, it is estimated that, in case of chronic exposure, the renal concentration of cadmium (poorly eliminated) would be higher than that of the liver [17]. According to the above, in our work, two out of three turtles would have suffered chronic exposure to Cd (34.52 µg/g d.w. in kidneys vs. 4.34 µg/g d.w. in the liver and 84.27 µg/g d.w. in kidneys vs. 15.43 µg/g d.w. in the liver), while for the third turtle, contamination levels were lower and showed a less marked difference (1.08 µg/g in kidneys versus 2.04 µg/g d.w. in the liver).
For gonads, levels of Cd were higher than reported by Andreani et al. [25] (1.3 µg/g d.w.). The obtained results are worrying considering that in the freshwater turtle Chrysemys picta the maternal transfer of cadmium from gonadal tissues to eggs has been observed [36]. To this is added that the accumulation of Cd in the liver, the site of synthesis of vitellogenins, can also contribute to the contamination of the yolk through the transport of the blood flow of Cd from the liver to the gonads and then to the embryo [36].
In general, Cd concentrations found in this study were similar to those found in other individuals of C. caretta from other areas of the Mediterranean basin [17,22,23,24,29,35]. Concerning toxic effects, a cadmium chloride exposure experiment on C. picta suggested that cadmium concentrations of 11.59 µg/g d.w. in the liver and 21.17 µg/g d.w. in kidneys were high enough to adversely affect the health of sea turtles such as C. caretta [23]. In this way, despite the high concentrations detected for these tissues, the accumulation of Cd in renal tissue may be less toxic due to the presence of metallothionein, which plays a fundamental role in the preservation and detoxification of metals [25].
Generally, the highest levels of mercury were found in the liver [10,23,24], as observed in our study. The second most contaminated tissue were the kidneys whose average of 0.44 µg/g d.w. was equal to that recorded by Jerez et al. [3], who found the highest levels of Hg for this tissue. The presence of mercury in kidneys indicates increased exposure to inorganic salts of this metal, since, as an organometal, it would have higher lipid affinities. In general, the values recorded were close to those found in other works on C. caretta in the Mediterranean [2,3,10,23,24] and do not seem to be high enough to affect the health of sea turtles, as noted by Storelli and Marcotrigiano [35]. The highest concentration of barium, found in the yolk, was higher than those in Florida [30,31] and in Brazil [37].
Regarding silver, there was only one study carried out on C. caretta in South Africa [34], where the mean levels of Ag found in egg contents and eggshell were 0.94 × 10−3 and 2.3 × 10−3, respectively. These values were lower than those we found in the yolk and eggshell (Table 1), which were of the same order of magnitude as those recorded by Lam et al. [33] in Hong Kong in C. mydas eggs.
In our study, although at relatively low concentrations, silver was present in all samples, except in the brain and fat, with the highest value recorded for liver (0.45 µg/g d.w.).
However, higher concentrations were recorded in Japan by Anan et al. [20] in the liver of C. mydas (3.2 μg/g d.w.) and E. imbricata (1.4 μg/g d.w.). The low concentration levels found in lead indicated that the matrices analysed were less contaminated than in other studies [6,10,17,23], in which was observed that for these matrices threshold levels of concern are 1.67 µg/g d.w [10,23]. These results further confirm the decrease in Pb pollution in the Mediterranean, due to the limitation of its use as an additive in gasoline [10]. Antimony was also detected in low concentrations, as opposed to Turkey, where higher levels were recorded in the renal tissue of C. caretta and C. mydas [29]. For all the heavy metals investigated, the differences found concerning the other works are probably due to the different contamination in adult turtles’ foraging and nesting environments.
5. Conclusions
In this study, the presence of 18 heavy metals in tissues and eggs of C. caretta was determined and the concentration levels were compared with other similar studies carried out both inside and outside the Mediterranean. Since we were the first to analyse the presence of these contaminants in eggs, we did not have the opportunity to compare them with others. This highlighted the opportunity and importance of extending the analyses to the eggs and possibly also to the young immediately after hatching.
The results show high concentrations of toxic elements, such as cadmium and arsenic, in all tissues of the individuals analysed, with levels of concern in the liver, kidney, and gonads. Conversely, comparing heavy metals and tissues whose concentrations are similar to those found in the literature suggests that exposure to these metals has not changed significantly over time. The potentially negative effects of heavy metals dispersed in marine ecosystems require continuous biomonitoring and the evaluation of their geographical and biological distribution.
In this context, the investigations on C. caretta are of considerable interest both for the conservation of this species, both as excellent indicators of pollution by heavy metals in marine ecosystems that allow assessing the current state of pollution and supporting appropriate environmental policies and regulations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments9070088/s1, Table S1: Percentage of recovery, LOD, and LOQ averages for each element.
Author Contributions
Conceptualization, D.S., M.A. and A.M.; Data curation, D.S., G.D., S.M. and A.M.; Formal analysis, D.S., V.G., G.D., S.M. and A.M.; Funding acquisition, D.S.; Investigation, all authors; Methodology, D.S., V.G., G.D., S.M. and A.M.; Project administration, D.S., M.A. and A.M.; Resources, D.S., A.P., R.M., V.A., S.C., G.C., C.V., I.C., G.V. and V.G.; Software, D.S., G.D. and S.M.; Supervision, M.A., V.A., S.C., V.G., G.D., S.M. and A.M.; Validation, D.S., V.G., G.D. and S.M.; Visualization, D.S., M.A. and A.M.; Writing—Original draft, D.S.; Writing—Review and editing, D.S., M.A. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The collection of eggs was carried out in strict compliance with the recommendations of the “Ministry of Environment and Protection of Territory and Sea” (Prot. n.17054 of 25 July 2018).
Data Availability Statement
Not applicable.
Acknowledgments
The work was financially supported by the University of Palermo (Dipartimento STEBICEF) and ARPA Sicilia (UOS Divisione Analitica 2). This work was carried out in the frame of a PhD project that has been supported financially.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yipel, M.; Tekeli, I.O.; Işler, C.T.; Altuğ, M.E. Heavy metal distribution in blood, liver and kidneys of Loggerhead (Caretta caretta) and Green (Chelonia mydas) sea turtles from the Northeast Mediterranean Sea. Mar. Pollut. Bull. 2017, 125, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Jerez, S.; Motas, M.; Cánovas, R.; Talavera, J.; Almela, R.M.; del Río, A.B. Accumulation and tissue distribution of heavy metals and essential elements in loggerhead turtles (Caretta caretta) from Spanish Mediterranean coastline of Murcia. Chemosphere 2010, 78, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.A.; Guzmán, H.M.; Potvin, C.; van Hinsberg, V.J. A review of toxic metal contamination in marine turtle tissues and its implications for human health. Reg. Stud. Mar. Sci. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Martínez-López, E.; Herrero, D.; López-Berenguer, G.; Peñalver, J. Total Arsenic Concentrations in Sea Turtle Tissues from the Mediterranean Coast of Spain. Bull. Environ. Contam. Toxicol. 2021, 107, 820–826. [Google Scholar] [CrossRef]
- Novillo, O.; Pertusa, J.; Tomás, J. Exploring the presence of pollutants at sea: Monitoring heavy metals and pesticides in loggerhead turtles (Caretta caretta) from the western Mediterranean. Sci. Total Environ. 2017, 598, 1130–1139. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Barreca, S.; Buscemi, S.; Caracappa, S.; Gentile, A.; Persichetti, M.F.; Pace, A. Chasing phthalates in tissues of marine turtles from the Mediterranean sea. Mar. Pollut. Bull. 2018, 127, 165–169. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Vecchioni, L.; Cambera, I.; Visconti, G.; Melfi, R.; Arizza, V.; Piccionello, A.P.; Buscemi, S.; Pace, A. Can phthalates move into the eggs of the loggerhead sea turtle Caretta caretta? The case of the nests on the Linosa Island in the Mediterranean Sea. Mar. Pollut. Bull. 2021, 168, 112395. [Google Scholar] [CrossRef]
- Caracappa, S.; Persichetti, M.F.; Piazza, A.; Caracappa, G.; Gentile, A.; Marineo, S.; Crucitti, D.; Arculeo, M. Incidental catch of loggerhead sea turtles (Caretta caretta) along the Sicilian coasts by longline fishery. PeerJ 2018, 6, e5392. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; De Roma, A.; Sansone, D.; Capozzo, D.; Iaccarino, D.; di Nocera, F.; Gallo, P. Non-essential toxic element (Cd, As, Hg and Pb) levels in muscle, liver and kidney of loggerhead sea turtles (Caretta caretta) stranded along the southwestern coasts of Tyrrhenian sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108725. [Google Scholar] [CrossRef]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of Blood Mercury Levels to Health Parameters in the Loggerhead Sea Turtle (Caretta caretta). Environ. Health Perspect. 2007, 115, 1421–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Gómez, A.A.; Romero, D.; Santos, J.; Rivera-Hernández, J.R.; Girondot, M. Inorganic elements in live vs dead nesting olive ridley marine turtles in the Mexican Pacific: Introducing a new statistical methodology in ecotoxicology. Sci. Total Environ. 2020, 761, 143249. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Gómez, A.A.; Romero, D.; Girondot, M. Carapace asymmetry: A possible biomarker for metal accumulation in adult olive Ridleys marine turtles? Mar. Pollut. Bull. 2018, 129, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Gómez, A.A.; Morcillo, P.; Guardiola, F.A.; Espinosa, C.; Esteban, M.A.; Cuesta, A.; Girondot, M.; Romero, D. Molecular oxidative stress markers in olive ridley turtles (Lepidochelys olivacea) and their relation to metal concentrations in wild populations. Environ. Pollut. 2018, 233, 156–167. [Google Scholar] [CrossRef]
- Sinaei, M.; Bolouki, M. Metals in Blood and Eggs of Green Sea Turtles (Chelonia mydas) from Nesting Colonies of the Northern Coast of the Sea of Oman. Arch. Environ. Contam. Toxicol. 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Dennis, M.M.; Poppenga, R.; Conan, A.; Hill, K.; Hargrave, S.; Maroun, V.; Stewart, K.M. Leatherback sea turtle (Dermochelys coriacea) hatch success and essential and nonessential metals in eggs and embryos from nests in St. Kitts (2015). Mar. Pollut. Bull. 2020, 161 Pt A, 111726. [Google Scholar] [CrossRef]
- García-Fernández, A.J.; Gómez-Ramírez, P.; Martínez-López, E.; Hernández-García, A.; María-Mojica, P.; Romero, D.; Jiménez, P.; Castillo, J.J.; Bellido, J.J. Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the southwestern Mediterranean (Spain). Ecotoxicol. Environ. Saf. 2009, 72, 557–563. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [Green Version]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta caretta a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Anan, Y.; Kunito, T.; Watanabe, I.; Sakai, H.; Tanabe, S. Trace element accumulation in hawksbill turtles (Eretmochelys imbricata) and green turtles (Chelonia mydas) from Yaeyama Islands, Japan. Environ. Toxicol. Chem. 2001, 20, 2802–2814. [Google Scholar] [CrossRef]
- Torrent, A.; González-Díaz, O.; Monagas, P.; Orós, J. Tissue distribution of metals in loggerhead turtles (Caretta caretta) stranded in the Canary Islands, Spain. Mar. Pollut. Bull. 2004, 49, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Franzellitti, S.; Locatelli, C.; Gerosa, G.; Vallini, C.; Fabbri, E. Heavy metals in tissues of loggerhead turtles (Caretta caretta) from the northwestern Adriatic Sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.; Storelli, A.; D’Addabbo, R.; Marano, C.; Bruno, R.; Marcotrigiano, G. Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: Overview and evaluation. Environ. Pollut. 2005, 135, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, F.; Caurant, F.; Bustamante, P.; Bentivegna, F. Trace element (Cd, Cu, Hg, Se, Zn) accumulation and tissue distribution in loggerhead turtles (Caretta caretta) from the Western Mediterranean Sea (southern Italy). Chemosphere 2005, 58, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Andreani, G.; Santoro, M.; Cottignoli, S.; Fabbri, M.; Carpenè, E.; Isani, G. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci. Total Environ. 2008, 390, 287–294. [Google Scholar] [CrossRef]
- Flint, M.; Patterson-Kane, J.; Mills, P.; Limpus, C. A Veterinarian’s Guide for Sea Turtle Post Mortem Examination and Histological Investigation; The University of Queensland: Brisbane, Australia, 2009; ISBN 978-1-8649995-9-4. [Google Scholar]
- Faust, D.R.; Hooper, M.J.; Cobb, G.P.; Barnes, M.; Shaver, D.; Ertolacci, S.; Smith, P.N. Inorganic elements in green sea turtles (Chelonia mydas): Relationships among external and internal tissues. Environ. Toxicol. Chem. 2014, 33, 2020–2027. [Google Scholar] [CrossRef]
- Morão, I.F.; Lemos, M.F.; Félix, R.; Vieira, S.; Barata, C.; Novais, S.C. Stress response markers in the blood of São Tomé green sea turtles (Chelonia mydas) and their relation with accumulated metal levels. Environ. Pollut. 2021, 293, 118490. [Google Scholar] [CrossRef]
- Kaska, Y.; Celik, A.; Bag, H.; Aureggi, M.; Özel, K.; Elçi, A.; Kaska, A.; Elçi, L. Heavy metal monitoring in stranded sea turtles along the Mediterranean coast of Turkey. Fresenius Environ. Bull. 2004, 13, 769–776. [Google Scholar]
- Stoneburner, D.L.; Nicora, M.N.; Blood, E.R. Heavy Metals in Loggerhead Sea Turtle Eggs (Caretta caretta): Evidence to Support the Hypothesis That Demes Exist in the Western Atlantic Population. S. Am. J. Herpetol. 1980, 14, 171–175. [Google Scholar] [CrossRef]
- Alam, S.K.; Brim, M.S. Organochlorine, PCB, PAH, and metal concentrations in eggs of loggerhead sea turtles (Caretta caretta) from northwest Florida, USA. J. Environ. Sci. Health Part B 2000, 35, 705–724. [Google Scholar] [CrossRef]
- Wise, S.S.; Xie, H.; Fukuda, T.; Thompson, W.D.; Wise, J.P. Hexavalent chromium is cytotoxic and genotoxic to hawksbill sea turtle cells. Toxicol. Appl. Pharmacol. 2014, 279, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, J.C.; Tanabe, S.; Chan, S.K.; Lam, M.H.; Martin, M.; Lam, P.K. Levels of trace elements in green turtle eggs collected from Hong Kong: Evidence of risks due to selenium and nickel. Environ. Pollut. 2006, 144, 790–801. [Google Scholar] [CrossRef] [PubMed]
- du Preez, M.; Nel, R.; Bouwman, H. First report of metallic elements in loggerhead and leatherback turtle eggs from the Indian Ocean. Chemosphere 2018, 197, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.; Marcotrigiano, G. Heavy metal residues in tissues of marine turtles. Mar. Pollut. Bull. 2003, 46, 397–400. [Google Scholar] [CrossRef]
- Rie, M.; Lendas, K.; Callard, I. Cadmium: Tissue distribution and binding protein induction in the painted turtle, Chrysemys picta. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 41–51. [Google Scholar] [CrossRef]
- Souza, N.L.N.; Carneiro, M.T.W.D.; Pimentel, E.F.; Frossard, A.; Freire, J.B.; Endringer, D.C.; Júnior, P.D.F. Trace elements influence the hatching success and emergence of Caretta caretta and Chelonia mydas. J. Trace Elem. Med. Biol. 2018, 50, 117–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).