1. Introduction
Growing interest in the design and construction of small modular reactors (SMRs) for nuclear power generation requires renewed scientific and engineering tools for monitoring and predicting the fate and behaviour, transport, and accumulation of radionuclides in potential receiving environments. Radioactive material released from a source (whether natural, anthropogenic, or nuclear technology-associated) varies in physio-chemical composition and bioavailability. Both the source and mechanics of particle release will affect particle size and behaviour [
1,
2]. Following an uncontrolled nuclear release, a radionuclide can enter an aqueous receiving environment by atmospheric deposition, in runoff from contaminated lands, via percolation or erosive forces, and/or through direct discharge to surface water. Transport and kinetics are two major processes that dictate how pollutants behave in water. Transport occurs by advection and diffusion [
3]. Dispersion patterns and concentrations are subject to the direction and velocity of the water, intensity of turbulent mixing, bed geometry, and composition of the water body. Transport mechanisms do not alter the chemical composition of materials or constituent particles. However, transport can influence chemical and biochemical reaction kinetics involved with the transformation of compounds into by-products and metabolites. These reactions, such as transport and dispersion, can be heterogeneous or homogeneous, reversible or irreversible [
3]. The rate of reactions is a function of multiple factors, including water and pollutant chemistry and concentration, as well as temperature. Riverine systems generally experience higher turbulence, diffusion, and advection than do lakes and reservoirs. Shallow lakes mix well vertically, but not horizontally, and deep lakes experience decreasing mixing rates with depth, except during seasonal turnover periods when nearly complete mixing occurs due to temperature-induced changes in water density [
4].
The concentration of radionuclides in water can occur either due to natural geochemical contributions or human interventions. The fate of radionuclides in water is influenced by dispersion patterns that reflect water velocity and flow rates, source discharge characteristics, mixing intensity, bed geometry and geochemistry, water temperature, sediment size, and sorption properties [
5,
6]. The predominant processes of radionuclide transport in rivers include hydraulic sub models considering the relationships among water, suspended solids, and bottom sediment dynamics, and another model considering radionuclides in different phases driven by these hydraulic processes [
7].
The behaviour and speciation of the radioactive isotope of strontium in the aquatic environment are understudied and under-reported in the scientific literature. The adsorption of radionuclides to sediments follows a first-order kinetics reaction, generally characterized by a faster initial adsorption rate followed by slower low-variable removal rates [
3,
8]. From previous research, we know that natural strontium will co-precipitate with calcium carbonate as an impurity in calcite precipitate [
9,
10,
11]. Co-precipitation may occur via homogeneous crystal inclusion, or scattered crystal occlusions, or by surface adsorption [
12]. The sorptive behaviour of radionuclides is similar to organic pollutants in the sense that they sorb to particulates but, being inorganic, are not subject to the same processes and mechanisms of decomposition.
Projected increases in air temperature and reduced precipitation volumes in the summer months are expected to produce greater evaporation of the water from the lakes, reservoirs, and rivers with lower rainfall contributions [
13]. These changes will result in the increasing salinity of the remaining water, which in turn affects the concentration of minerals by influencing solubility, adsorption, desorption to the sediments, and accumulation in living tissues. The bioaccumulation of strontium particles increases as water temperature increases [
14], and the balance in radionuclides and their distribution in aqueous phase, sorbed to sediments or particles, or bound as impurities in precipitates, are affected by both chemical and physical water quality properties [
15].
Strontium is a fission product that does not occur naturally, but rather is a result of nuclear reactions in power-generating facilities or testing and the use of nuclear weaponry. Chemically, Sr
2+ is similar to Ca
2+ and Ba
2+. In the human body, it mimics Ca
2+ and the body incorporates it into bone matter (Gupta & Walther, 2018). Because Sr
2+ has a hydrated ionic radius similar to Ca
2+, it substitutes readily for Ca
2+ [
16]. It easily mobilises during weathering, and like Ca
2+, has a high affinity for organics [
17,
18]. Like Ca
2+, Sr
2+ is water soluble and readily adsorbs to soils. As a function of its chemistry and solubility, Sr
2+ is highly bioavailable to plants, readily moving from soil moisture to plant material. In this way, it can also move easily into the food chain.
Strontium removal by calcium carbonate (CaCO
3) precipitation has been of interest to the water treatment industry for many years. In water treatment, lime–soda softening enhances calcium carbonate precipitation [
19]. In cold lime–soda softening, the formation of the calcite crystal dominates the precipitate with about 10–15% aragonite present in the crystal. In hot lime–soda softening, aragonite dominates in the precipitate [
12]. Aragonite is one of the three most commonly occurring crystal forms of CaCO
3, along with calcite and vaterite. Only aragonite and calcite are discussed in this work.
Limited data from mid-20th century laboratory experiments demonstrated that both calcite and aragonite can facilitate strontium removal from water. Based on those laboratory results, aragonite is the more effective of the two [
12,
20]. The solubility constants (pKs) for calcite and aragonite can be calculated, respectively, using the following equations (where T is absolute temperature in K) [
21,
22]
Since Sr2+ behaves similarly to Ca2+ and co-precipitates as an impurity along with CaCO3, understanding its behaviour in the selected cold region study environment of Saskatchewan, Canada, is useful in the assessment and mitigation planning in case of an accident from a future nuclear power plant. A calcium carbonate precipitation potential (CCPP) model can be empirically modified for strontium and validated for the assessment along with the logarithmic distribution law to estimate the amount of Sr2+ that can be precipitated along with CaCO3. Models that consider that both precipitation and mineral formation with impurities are governed by the environmental conditions occurring at the time of precipitation and can be used to produce reliable and useful data to select the site where the impact on the environment will not be detrimental and to establish mitigation measures in case an unplanned event occurs.
The objective of this research was to develop and validate a strontium transport model to provide the knowledge required to assess the fate and transport of strontium in surface waters. To that end, data from both experiments and longitudinal riverine water quality monitoring studies were assembled to modify the CCPP for strontium, and then the model was calibrated and validated to cold region surface water systems.
2. Materials and Methods
The results and calculated co-precipitation constant (λ) derived from field and laboratory experiments were used to modify a calcium carbonate precipitation potential (CCPP) model to provide a tool for environmental scientists, nuclear engineers, and regulatory agencies to better understand the mechanisms controlling the solubility, transport, and bioavailability of strontium. The λ was determined using wastewater from a cold-region water treatment plant (WTP) that accesses a ground water source containing naturally occurring strontium. Those data were then applied to revise the CCPP tool for estimating Sr2+ precipitation in surface water. Finally, the modified CCPP model for strontium was validated and used to demonstrate the potential impacts of Sr2+ release on surface water resources in the study region.
2.1. Study Region
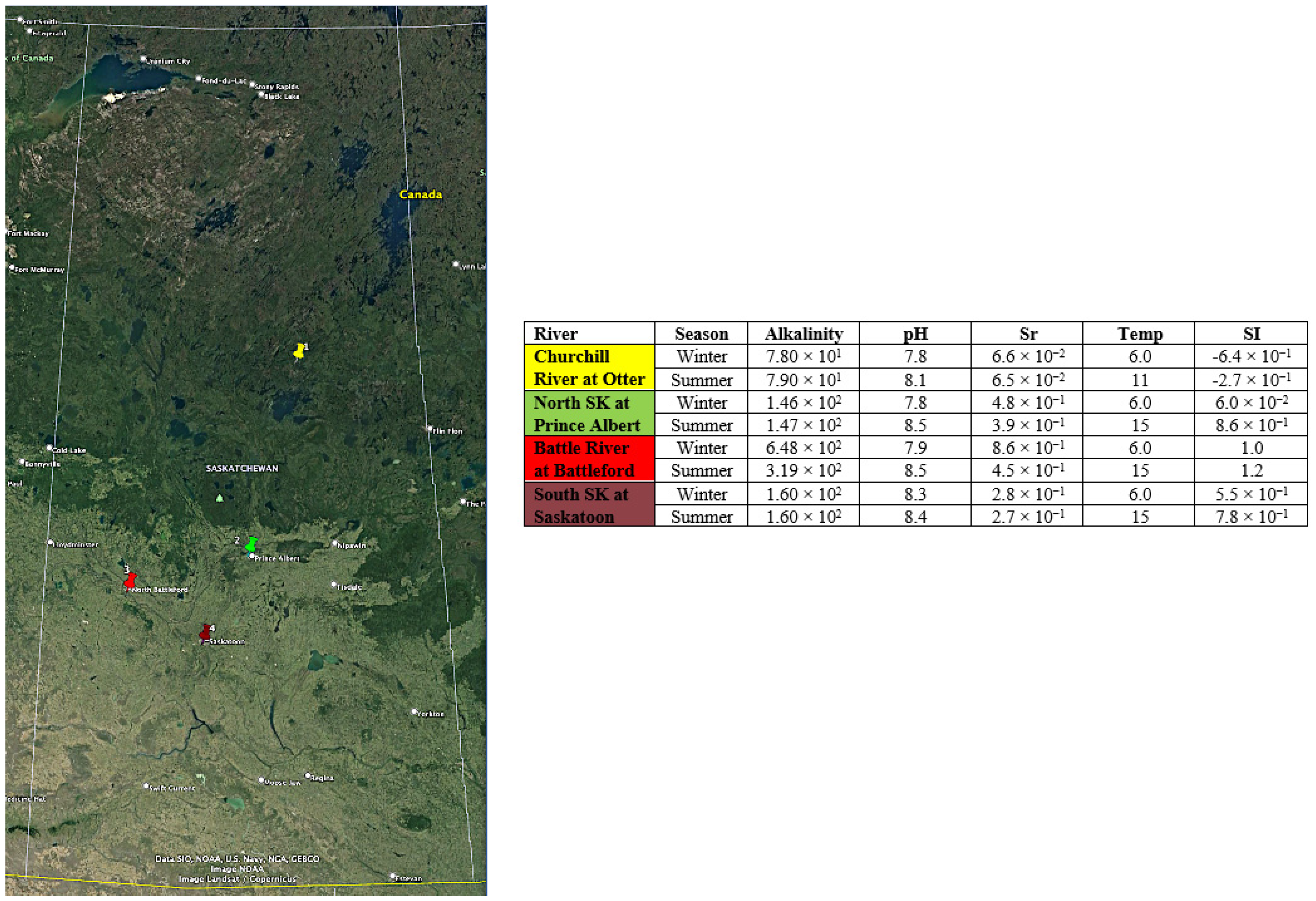
Saskatchewan is a Canadian prairie province, landlocked and climatically and ecologically diverse from north to south. In the north (proximate to the 60th parallel), the climate is classified as sub-arctic in a taiga ecosystem; central regions are more humid and ecologically boreal; and the south (proximate to the 49th parallel) is typified by semi-arid conditions and grasslands and parklands ecology (
Figure 1). The predominant Köppen–Geiger climate classification is subtype “Dfb” (Warm Summer Continental Climate).
Northern Saskatchewan includes deep geological deposits containing various metals, including some of the world’s largest and most abundant reserves of uranium and strontium. Across the full extent of the province, significant concentrations of radionuclides exist in the soils, subsurface structures and aquifer materials, riverine and lake sediments, and both surface and ground water supplies.
A multidisciplinary study was completed to assess the technical siting requirements of small modular nuclear reactors (SMRs) in Saskatchewan, including access to sufficient water quantity and quality under changing climate conditions [
25]. The electrical grid in Saskatchewan is small and SMR technology offers the diversity, flexibility of installation, and construction, which are appealing attributes that make it a potentially feasible technology for the province. Water quantity and quality accessibility is crucial for the construction and operation of an SMR; furthermore, it is particularly important for landlocked regions, regardless of location on the globe. Since regional climate models project that Saskatchewan is likely to experience an increased frequency of precipitation extremes, alternative or back-up water sources must be secured and must be protected from the potential contamination associated with SMR operations.
The water availability in northern Saskatchewan is highly favourable in terms of both water quantity and quality, both now and under projected climate change. However, the other technical requirements for SMR siting, such as transportation networks, access to the electrical grid, and high distribution losses, are not as favourable. Because some of the world’s largest deposits of uranium and strontium exist in northern Saskatchewan, both the geology and water contain background concentrations of radionuclides, and aquatic life, in particular, has adapted to and requires their presence. The majority of strontium in water exists in its dissolved form such that water quality analyses presume that total strontium is approximately equal to dissolved strontium in natural surface waters [
13]. In Saskatchewan waters, those concentrations range between 0.029 and 1.74 mg/L, with a median concentration of 0.34 mg/L. The environmental limits for the protection of aquatic life are well above those values, at 2.5 mg/L [
13].
2.2. Empirical Calibration of Calcium Carbonate Precipitation Potential Model for Sr
Corrosiveness indices are used in the water treatment industry to evaluate whether water will scale or erode infrastructure. Over time, several scaling indices tools have been developed, such as the Saturation Index, Langelier Saturation Index, and CCPP. The CCPP theoretically quantifies how much calcium carbonate might precipitate once the water reaches final equilibrium. Although the CCPP calculation is more complex than that of the other scaling indices, the benefits of CCPP far outweigh the additional analysis since CCPP not only quantifies the precipitate potential, but also offers theoretically precipitated Ca2+ values that can be used along with logarithmic distribution law to estimate the quantity of the other materials (impurities) that will co-precipitate with CaCO3. The logarithmic distribution coefficient, λ, in a co-precipitation process must be determined for each impurity of interest.
The CCPP empirical model is widely used in the water treatment industry but has not previously been applied to the fate and transport of radionuclides. The model was assembled in Microsoft Excel as per the instructions published by the Joint Task Group on Calcium Carbonate Saturation [
21]. The paper presented an adapted method of calculating and interpreting calcium carbonate saturation indexes from Method 2330 Calcium Carbonate Saturation published in the 17th edition of Standard Methods for the Examination of Water and Wastewater [
26].
The precipitation or dissolution of CaCO3 in water changes the initial concentration of Ca2+, alkalinity, and pH of water, in the process of reaching equilibrium. To evaluate CCPP, the pH of equilibrium (pHeq) must be calculated. Once the pHeq is determined, the CCPP can be determined. The model is adjusted to calculate the ionic strength of the water using the following three formulas.
The first is a molar ionic strength formula that is a function of the concentration of all ions present in that solution (Stumm & Morgan, 1981):
where:
I is ionic strength;
Xi is concentration of component i (gr.mol/L); and
Zi is ionic charge of species i.
As per the CCPP method, it is vital that the pH of equilibrium (pHeq) is determined appropriately, so that the equilibrium alkalinity (Alkeq) and CCPP for precipitate can be estimated. The calcium concentration incorporated in calcite or aragonite is estimated by multiplying the CCPP by a factor of 0.4 to reflect that there are 40 mg of Ca2+ in every 100 mg of CaCO3.
The equilibrium constant Ks’ is determined as a function of temperature, ionic strength, and thermodynamic constants K1 (first disassociation constant for carbonic acid), K2 (second dissociation constant for carbonic acid), Kw (disassociation constant for water), and Ksa (solubility product constant for aragonite).
To estimate how much strontium co-precipitates with calcium, the logarithmic distribution law equation is used:
By solving this equation, the concentration of strontium in solution (Sr
sol) or as a precipitate (SrPP) can be determined by:
From the experimental data, the
λ value is calculated by:
In this research, CCPP was used to estimate the amount of Ca2+ that will precipitate by the time the water reaches equilibrium. The CCPP calculates the CaCO3 that will precipitate at equilibrium along with the alkalinity and pH of the water at equilibrium. Having the initial values for Ca2+ and Sr2+, the estimated value of Ca2+ at equilibrium and the λ value for initial Sr2+ precipitation, one can estimate the quantity of Sr2+ that will co-precipitate with Ca2+ in the CaCO3 scale. This can be useful to estimate how much radioactive Sr2+ will precipitate. Using the water quality of nearby waterbody and wastewater quality analysis as well as water volumes, CCPP and the mass of co-precipitation impurities can be estimated at different sections of the waterbody. In this manner, a modified method for SrPP was developed and tested.
2.3. Water Quality and Media Analyses
Water and sediment samples were collected from the inlet and outlet of the wastewater holding ponds of a water treatment plant (WTP) in central Saskatchewan, as per Zanacic and McMartin [
9]. The water chemistry analyses included the quantification of conductivity, pH, total dissolved solids (TDS), total suspended solids (TSS), alkalinity, chloride, fluoride, ammonia, nitrate, nitrite, sulphate, and total and dissolved metals. The sediment samples were analysed for the presence and precipitation of strontium as well as total metals concentration. PREEQC (PH REdox EQuilibrium (in C language)) was used to calculate both the distribution species and saturation state and holding pond sediment samples.
The WTP wastewater samples were also exposed to pyrolusite media that were subsequently examined using X-ray photoelectron spectroscopy (XPS) and Raman spectrometry with a Reinshaw Raman Invia Reflex Microscope at the Saskatchewan Structural Sciences Centre at the University of Saskatchewan [
9]. XPS analysis elucidates the quantitative and chemical state of the media surface, while Raman spectroscopy examines the incorporation of impurities into precipitates. Here, Raman spectroscopy was employed to confirm whether Sr had been incorporated into the precipitate formed during the experiments, thus explaining any loss from the aqueous phase.
2.4. Model Validation Method
The CCPP and strontium precipitation potential tool was validated against well-monitored surface water systems in Saskatchewan. Each is a large river chosen for access to data as well as to determine the potential impacts and extent of impact in the event of an unplanned release associated with the design and operation of a potential future SMR facility.
The four river systems include the Churchill River in northern Saskatchewan, the North Saskatchewan River in central Saskatchewan, the Battle River in west central Saskatchewan, and the South Saskatchewan River in southern and central Saskatchewan. Four point-source monitoring stations were selected in proximity to high-affinity technical sites that could reasonably host an SMR based on geology, transportation, population, safety and security, and water needs (
Figure 2).
The water quality in each river was assessed for scaling potential using the saturation index method. Determining the scaling potential of the waters can help predict the extent of dissolved strontium transport in the water in case of an unplanned release.
3. Results and Discussion
The CCPP model was used to calculate precipitation potential using experimental and Water Security Agency of Saskatchewan database (WSA, 2022) information about water quality, including ion concentrations and temperature. The ion balance for all water samples was verified by PHREEQC software and was found to be less than 5%.
The results of experiments using water treatment plant wastewater containing naturally occurring strontium demonstrated that alkalinity is a rate and reaction completion-limiting factor for the formation and precipitation of carbonate minerals, CaCO
3 and/or CaMg(CO
3)
2 (
Table 1). Although dolomite precipitation was not observed, the loss of magnesium from the solution coupled with the results of PHREEQC saturation index calculations suggests that the precipitation of dolomite is highly likely. Analyses using Raman spectroscopy further showed that strontium co-precipitated along with calcium carbonate.
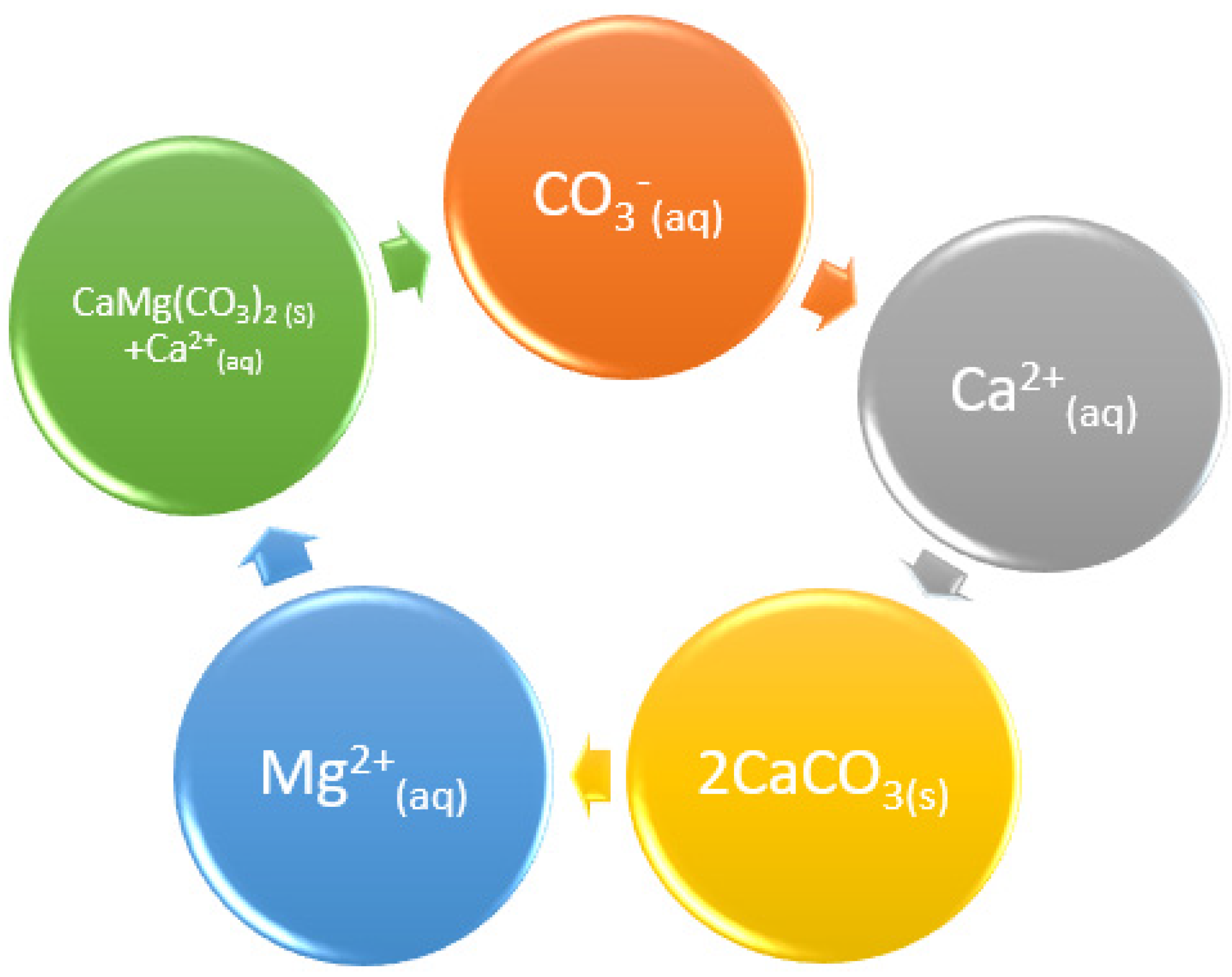
The XPS analysis indicated magnesium on the precipitates, along with calcium. Since the precipitation of calcium is initiated by calcium carbonate precipitation, followed by calcite precipitates and then dolomite, it is assumed that an equal amount of alkalinity is consumed in calcite and dolomite precipitation. In the continuity of precipitation reactions, the formation of dolomite and calcite reached equilibrium, where dissociation and precipitation occurred equally and simultaneously, as per the cycle in
Figure 3.
As the solution becomes saturated with alkalinity, it reacts with other ions that are reaching saturation point. In this case, the alkalinity reacts with the calcium and as the particles are moving through the water, adding energy, the calcium carbonate starts to precipitate. The calcium carbonate precipitates first since the calcium has greater reactivity than magnesium. As the calcium is removed from water, the magnesium attaches to the carbonate precipitate to form dolomite. In the process, calcium ions are released back into the aqueous solution. During this process, other reactive ions, such as strontium, also attach to the precipitates as impurities. Therefore, the precipitation of dolomite is accounted for when determining the logarithmic distribution coefficient (λ) in the Sr2+ co-precipitation process, along with CaCO3.
In calculating
λ, Equation (7) included strontium as the micro-component, and calcite calcium as the macro-component. In natural systems, depending on the environment and environmental conditions, it cannot be assumed that all of the calcium lost from water is incorporated into a CaCO
3 crystal, since other minerals such as dolomite and gypsum are also precipitating. For that reason, the calculation for
λ only considered calcium lost due to calcite precipitation. Water quality analyses of Ca and Sr in both raw water samples and those from experiments with pyrolusite media were used to calculate the CCPP with respect to both calcite and aragonite (
Table 2).
The raw water CCPP is 353 mg/L for calcite. In the water samples exposed to pyrolusite media for 6 hr, the CCPP for calcite exists in a tight range between 296 and 304 mg/L. As noted, the precipitation of strontium can be calculated using the logarithmic distribution law producing a calculated average (n = 6) of λ = 1.88 after 6 hr exposure. This λ value is specific to surface water at pH < 8.2 and a temperature of 6 °C. In ground water, the λ may differ due to the geochemistry and other environmental factors. However, it is a useful number for estimating the initial precipitation of Sr2+ from a water source from an unplanned release of wastewater into an aquatic receiving environment. The average reductions in strontium and calcium concentration in raw water were 1.65 and 150 mg/L, respectively. Determining the initial potential of strontium loss from water and deposition in the environment is important in determining the potential impact of a discharge on downstream users.
With the determination of a lab-derived
λ for a complex water source (i.e., WTP wastewater), the Sr precipitation potential (SrPP) method was applied to a large dataset of water quality in Saskatchewan and at locations deemed to have sufficient water quantity and supply to support an SMR facility (
Figure 2). The Churchill River in northern Saskatchewan has negative SI in both summer and winter, suggesting that there is no precipitation of calcium carbonate from the river. However, water in the Churchill River does display a tendency to dissolve calcium carbonate minerals. The North Saskatchewan River has a scaling tendency in summer (SI 0.86), but not in winter (SI 0.06). Both the South Saskatchewan River and Battle River have a scaling capacity in both summer and winter. The data for the locations at all four river systems include pH of saturation (pH
s), alkalinity at equilibrium (Alk
eq), CCPP, SrPP, and dissolved strontium that remains in the aqueous phase in the water column (
Table 3). If water quality is such that the CCPP and SrPP of the water are positive, precipitates will form. Likewise, if the water quality changes such that precipitation potentials are negative, precipitates are more likely to dissolve and contaminants more likely to remain in solution with the aqueous phase.
The results of in-stream water quality analyses indicate that the Churchill River, flowing over bedrock low in calcium and magnesium, has a negative CCPP in winter that is even more pronounced than in summer. The water chemistry reflects the chemistry of its surrounding and contact environments. As such, it has the potential to retain a higher concentration of dissolved strontium in the aqueous phase and thus distribute it over a longer time and distance. The North Saskatchewan River has a low CCPP and demonstrates greater stability in winter than in summer, as evidenced by the near-equilibrium alkalinity measured in situ. There, CCPP and SrPP are very low, indicating the stability of strontium concentrations. In the North Saskatchewan River, it is anticipated that dissolved strontium will remain in the water, allowing for transportation across longer periods of time and distance. In summer, the CCPP and SrPP are slightly higher in the North Saskatchewan River, indicating that the concentration of dissolved strontium will be slightly reduced. The South Saskatchewan River and Battle River have significant precipitation potential for both CCPP and SrPP and for both summer and winter.
The results suggest that CaCO3 precipitation is more pronounced in summer than in winter. This is due to the warmer water temperatures in summer, but more significantly, due to changes in pH. Increased summer pH values are primarily associated with algae in these waters. At increased pH, CaCO3 precipitates more readily, whereas at lower pH, the CaCO3 is dissolved. At increased temperature, the amount of CO2 in the water open to atmosphere decreases. The loss of CO2 from the water will result in the increased pH of the water. In addition, an increased temperature promotes water evaporation, thereby favouring the supersaturation of CaCO3 in the water.
The results also indicate that those water sources in the southern part of the province have positive scaling ability in both summer and winter. In the north, the scaling capacity is negative in summer and winter. This means that if an unintentional release of the radionuclides happens in waters that have the tendency to precipitate CaCO3, the precipitate has a higher probability to stay and become bound within the sediments. However, if an unintentional release occurs in waters with a corrosive tendency, any precipitation that occurs initially will be overridden by a dissolution action that releases the contaminant into solution with the aqueous phase.
Such variation in water quality suggests different contaminant fate and transport for pollutants that might be unintentionally released by an SMR facility. In the north, the lower alkalinity of the waters will result in the majority of strontium discharged to a receiving water will remain dissolved in water for longer periods of time, due, in large part, to the low precipitation potential of the water. Therefore, dissolved strontium is more available to the flora and fauna of the waters, as well as wildlife and surrounding aquatic environments. In the central part of Saskatchewan, where the elevated water pH in summer months is driven by algal blooms, the dissolved strontium that exists in summer may precipitate in winter due to the corrosiveness of the water driven by lower pH values. In the south, precipitated minerals will most likely remain suspended in the water and settle into riverine sediments over time, should flow regimes permit. In this case, the predominant form of strontium in the water will not be dissolved as is expected to, thereby having a lesser impact on ecosystems.
Surface water with calcium carbonate SI > 0 will facilitate the precipitation of calcium carbonate primarily in the form of calcite. Since the presence of dissolved strontium will also create a potential for strontium to co-precipitate, by applying the CCPP and logarithmic distribution law, losses of calcium and strontium from the water can be reasonably predicted. The decrease of dissolved strontium in cold water can be estimated using λ, which, for this experiment, is λ = 1.88. This value is used to estimate the initial co-precipitation of strontium in various surface water supplies and to identify locations and water sources that may be more susceptible to the significant transport of Sr downstream from an unplanned SMR discharge to an aqueous receiving environment. When water contains higher concentrations of particles, the interactions between particles and dissolved contaminants can further facilitate precipitation until the water quality is infinitely close to equilibrium.
As applied to these four locations and river systems in Saskatchewan, the results show that the chemistry of the water in the Churchill River has the highest capacity to transport dissolved strontium over longer distances than waters further south. In case of an instantaneous and large input of dissolved strontium into the water, those with higher CCPP and SrPP are better equipped to reduce downstream impacts due to the transport mechanisms associated with dissolved strontium. Thus, when identifying potential sites for SMR construction and operation, water with higher CCPP and SrPP characteristics will have a higher capacity to decrease the Sr concentration in a shorter period of time and in closer proximity to the source of the discharge than waters with lower CCPP and SrPP.