Modeling Cultural Keystone Species for the Conservation of Biocultural Diversity in the Afroalpine
Abstract
1. Introduction
2. Methods
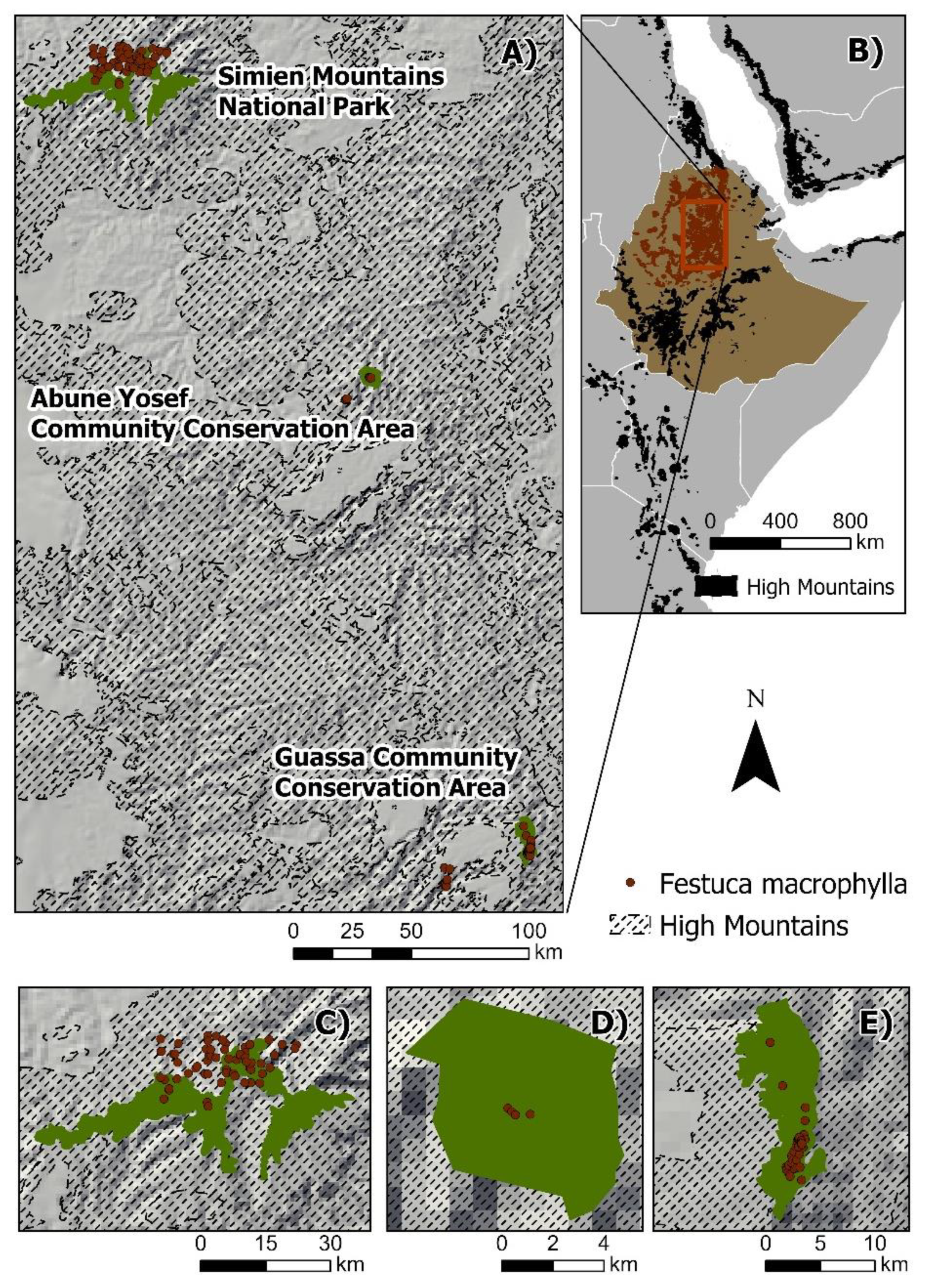
2.1. Area of Study
2.2. Species Occurrence Data
2.3. Environmental Predictors
2.4. Model Selection
3. Results
4. Discussion
4.1. Evaluating the Extinction Debt of Guassa Grass
4.2. A Community-Based Conservation Network for Afroalpine Biocultural Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.; Anschlag, K.; Baker, B.; Finch, O.D.; Diekkrueger, B.; Wundram, D.; Schroeder, B.; Pape, R.; Lundberg, A. Mountain ecosystem response to global change. Erdkunde 2011, 65, 189–213. [Google Scholar] [CrossRef]
- Klein, J.A.; Tucker, C.M.; Steger, C.E.; Nolin, A.; Reid, R.; Hopping, K.A.; Yeh, E.T.; Pradhan, M.S.; Taber, A.; Molden, D.; et al. An Integrated community and ecosystem-based approach to disaster risk reduction in mountain systems. Environ. Sci. Policy 2019, 94, 143–152. [Google Scholar] [CrossRef]
- Sayre, R.; Frye, C.; Karagulle, D.; Krauer, J.; Breyer, S.; Aniello, P.; Wright, D.J.; Payne, D.; Adler, C.; Warner, H.; et al. A new high-resolution map of world mountains and an online tool for visualizing and comparing characterizations of global mountain distributions. Mt. Res. Dev. 2018, 38, 240–249. [Google Scholar] [CrossRef]
- Karagulle, D.; Frye, C.; Sayre, R.; Breyer, S.; Aniello, P.; Vaughan, R.; Wright, D. Modeling global Hammond landform regions from 250-m elevation data. Trans. GIS 2017, 21, 1040–1060. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.O.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Viviroli, D.; Dürr, H.H.; Messerli, B.; Meybeck, M.; Weingartner, R. Mountains of the world 2019, water towers for humanity: Typology, mapping, and global significance. Water Resour. Res. 2007, 43, 7. [Google Scholar]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Broennimann, O.; Hughes, G.; Alkemade, J.R.M.; Midgley, G.F.; Corsi, F. Vulnerability of African mammals to anthropogenic climate change under conservative land transformation assumptions. Glob. Chang. Biol. 2006, 12, 424–440. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Glob. Chang. Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above-and belowground linkages shape responses of mountain vegetation to climate change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Gehrke, B.; Linder, H.P. Species richness, endemism and species composition in the tropical Afroalpine flora. Alp. Bot. 2014, 124, 165–177. [Google Scholar] [CrossRef]
- Enquist, C.A.F. Predicted regional impacts of climate change on the geographical distribution and diversity of tropical forests in Costa Rica. J. Biogeogr. 2002, 29, 519–553. [Google Scholar] [CrossRef]
- Stévart, T.; Dauby, G.; Lowry, P.P.; Blach-Overgaard, A.; Droissart, V.; Harris, D.J.; Mackinder, B.A.; Schatz, G.E.; Sonké, B.; Sosef, M.S.; et al. A third of the tropical African flora is potentially threatened with extinction. Sci. Adv. 2019, 5, eaax9444. [Google Scholar] [CrossRef]
- Mairal, M.; Namaganda, M.; Gizaw, A.; Chala, D.; Brochmann, C.; Catalán, P. Multiple mountain-hopping colonization of sky-islands on the two sides of Tropical Africa during the Pleistocene: The afroalpine Festuca grasses. J. Biogeogr. 2021, 48, 1858–1874. [Google Scholar] [CrossRef]
- Yalden, D.; Largen, M. The endemic mammals of Ethiopia. Mammal Rev. 1992, 22, 115–150. [Google Scholar] [CrossRef]
- Brochmann, C.; Gizaw, A.; Chala, D.; Kandziora, M.; Eilu, G.; Popp, M.; Pirie, M.D.; Gehrke, B. History and evolution of the afroalpine flora: In the footsteps of Olov Hedberg. Alp. Bot. 2021, 132, 65–87. [Google Scholar] [CrossRef]
- Conway, D. From headwater tributaries to international river: Observing and adapting to climate variability and change in the Nile basin. Glob. Environ. Chang. 2005, 15, 99–114. [Google Scholar] [CrossRef]
- Anderson, R.P.; Martınez-Meyer, E. Modeling species’ geographic distributions for preliminary conservation assessments: An implementation with the spiny pocket mice (Heteromys) of Ecuador. Biol. Conserv. 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Chang. Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Arcos, J.M.; Bécares, J.; Villero, D.; Brotons, L.; Rodríguez, B.; Ruiz, A. Assessing the location and stability of foraging hotspots for pelagic seabirds: An approach to identify marine Important Bird Areas (IBAs) in Spain. Biol. Conserv. 2012, 156, 30–42. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Fajardo, J.; Lessmann, J.; Bonaccorso, E.; Devenish, C.; Munoz, J. Combined use of systematic conservation planning, species distribution modelling, and connectivity analysis reveals severe conservation gaps in a megadiverse country (Peru). PLoS ONE 2014, 9, e114367. [Google Scholar] [CrossRef]
- Ferrier, S.; Watson, G.; Pearce, J.; Drielsma, M. Extended statistical approaches to modeling spatial pattern in biodiversity: The north-east New South Wales experience I. Species-level modeling. Biodiv. Conserv. 2002, 11, 2275–2307. [Google Scholar] [CrossRef]
- Kremen, C.; Cameron, A.; Moilanen, A.; Phillips, S.J.; Thomas, C.D.; Beentje, H.; Dransfield, J.; Fisher, B.L.; Glaw, F.; Good, T.C.; et al. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 2008, 320, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Brockington, D. Fortress Conservation: The Preservation of the Mkomazi Game Reserve, Tanzania; Indiana University Press: St. Bloomington, IN, USA, 2002. [Google Scholar]
- West, P.; Igoe, J.; Brockington, D. Parks and peoples: The social impact of protected areas. Annu. Rev. Anthropol. 2006, 35, 251–277. [Google Scholar] [CrossRef]
- Berkes, F. Community-based conservation in a globalized world. Proc. Natl. Acad. Sci. USA 2007, 104, 15188–15193. [Google Scholar] [CrossRef] [PubMed]
- Palomo, I.; Montes, C.; Martin-Lopez, B.; González, J.A.; Garcia-Llorente, M.; Alcorlo, P.; Mora, M.R.G. Incorporating the social–ecological approach in protected areas in the Anthropocene. BioScience 2014, 64, 181–191. [Google Scholar] [CrossRef]
- Cumming, G.S.; Allen, C.R.; Ban, N.C.; Biggs, D.; Biggs, H.C.; Cumming, D.H.; De Vos, A.; Epstein, G.; Etienne, M.; Maciejewski, K.; et al. Understanding protected area resilience: A multi-scale, social-ecological approach. Ecol. Appl. 2015, 25, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Brueckner-Irwin, I.; Armitage, D.; Courtenay, S. Applying a social-ecological well-being approach to enhance opportunities for marine protected area governance. Ecol. Soc. 2019, 24, 3. [Google Scholar] [CrossRef]
- Maffi, L. Linguistic, cultural, and biological diversity. Annu. Rev. Anthropol. 2005, 34, 599–617. [Google Scholar] [CrossRef]
- Kassam, K.A. Diversity as if nature and culture matter: Bio-cultural diversity and Indigenous peoples. Int. J. Divers. Organ. Commun. Nations Annu. Rev. 2008, 8, 87–95. [Google Scholar] [CrossRef]
- Hanspach, J.; Haider, L.J.; Oteros-Rozas, E.; Olafsson, A.S.; Gulsrud, N.M.; Raymond, C.M.; Torralba, M.; Martín-López, B.; Bieling, C.; Garcia-Martin, M.; et al. Biocultural approaches to sustainability: A systematic review of the scientific literature. People Nat. 2020, 2, 643–659. [Google Scholar] [CrossRef]
- Harmon, D. Indicators of the world’s cultural diversity. In Proceedings of the Fourth World Congress on National Parks and Protected Areas, Caracas, Venezuela, 10–21 February 1992; pp. 1–33. [Google Scholar]
- Loh, J.; Harmon, D. A global index of biocultural diversity. Ecol. Indic. 2005, 5, 231–241. [Google Scholar] [CrossRef]
- Stepp, J.R.; Castaneda, H.; Cervone, S. Mountains and biocultural diversity. Mt. Res. Dev. 2005, 25, 223–227. [Google Scholar] [CrossRef]
- Taylor, P.J. Unruly Complexity; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Crane, T.A. Of models and meanings: Cultural resilience in social–ecological. Ecol. Soc. 2010, 15, 4. [Google Scholar] [CrossRef]
- Cristancho, S.; Vining, J. Culturally defined keystone species. Hum. Ecol. Rev. 2004, 11, 153–164. [Google Scholar]
- Garibaldi, A.; Turner, N. Cultural keystone species: Implications for ecological conservation and restoration. Ecol. Soc. 2004, 9, 1. [Google Scholar] [CrossRef]
- McBrearty, S.; Brooks, A.S. The revolution that wasn’t: A new interpretation of the origin of modern human behavior. J. Hum. Evol. 2000, 39, 453–563. [Google Scholar] [CrossRef]
- Ambrose, S.H. Paleolithic technology and human evolution. Science 2001, 291, 1748–1753. [Google Scholar] [CrossRef]
- Vogelsang, R.; Bubenzer, O.; Kehl, M.; Meyer, S.; Richter, J.; Zinaye, B. When hominins conquered highlands—An acheulean site at 3000 m asl on mount dendi/Ethiopia. J. Paleolit. Archaeol. 2018, 1, 302–313. [Google Scholar] [CrossRef]
- Ossendorf, G.; Groos, A.R.; Bromm, T.; Tekelemariam, M.G.; Glaser, B.; Lesur, J.; Schmidt, J.; Akçar, N.; Bekele, T.; Beldados, A.; et al. Middle Stone Age foragers resided in high elevations of the glaciated Bale Mountains, Ethiopia. Science 2019, 365, 583–587. [Google Scholar] [CrossRef]
- Ashenafi, Z.T.; Leader-Williams, N. Indigenous common property resource management in the central highlands of Ethiopia. Hum. Ecol. 2005, 33, 539–563. [Google Scholar] [CrossRef]
- Steger, C.; Nigussie, G.; Alonzo, M.; Warkineh, B.; Van Den Hoek, J.; Fekadu, M.; Evangelista, P.H.; Klein, J.A. Knowledge coproduction improves understanding of environmental change in the Ethiopian highlands. Ecol. Soc. 2020, 25, 2. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, G.M.; Lazo, P.X.; Célleri, R.; Wilcox, B.P.; Crespo, P. Runoff from tropical alpine grasslands increases with areal extent of wetlands. Catena 2015, 125, 120–128. [Google Scholar] [CrossRef]
- Hurni, H. Degradation and conservation of the resources in the Ethiopian highlands. Mt. Res. Dev. 1988, 8, 123–130. [Google Scholar] [CrossRef]
- Hurni, H.; Stahli, P. Simen Mountains, Ethiopia Vol II: Climate and the Dynamics of Altitudinal Belts from the Last Cold Period to the Present Day; Geographische Gesellschaft Bern und Geographica Bernensia: Bern, Switzerland, 1982. [Google Scholar]
- EWCA. State of Conservation Report of the World Natural Heritage Site, Simen Mountains National Park (Ethiopia); Ethiopian Wildlife Conservation Authority: Addis Ababa, Ethiopia, 2015.
- Nemomissa, S.; Puff, C. Flora and vegetation of the Simen Mountains National Park, Ethiopia. Biol. Skr. 2001, 54, 335–348. [Google Scholar]
- Puff, C.; Nemomissa, S. Plants of the Simen: A Flora of the Simen Mountains and Surroundings, Northern Ethiopia; National Botanic Garden: Meise, Belgium, 2005; Volume 37. [Google Scholar]
- Jacob, M.; Frankl, A.; Hurni, H.; Lanckriet, S.; Ridder, M.; Guyassa, E.; Beeckman, H.; Nyssen, J. Land cover dynamics in the Simien Mountains (Ethiopia), half a century after establishment of the National Park. Reg. Environ. Chang. 2016, 17, 777–787. [Google Scholar] [CrossRef]
- Nievergelt, B.; Good, T.; Guttinger, R. A survey of the flora and fauna of the Simen Mountains National Park, Ethiopia. Special Issue of Walia. J. Ethiop. Wildl. Nat. Hist. Soc. 1998. [Google Scholar]
- Gebremedhin, B.; Flagstad, O.; Bekele, A.; Chala, D.; Bakkestuen, V.; Boessenkool, S.; Popp, M.; Gussarova, G.; Schrøder-Nielsen, A.; Nemomissa, S.; et al. DNA Metabarcoding Reveals Diet Overlap between the Endangered Walia Ibex and Domestic Goats—Implications for Conservation. PLoS ONE 2016, 11, e0159133. [Google Scholar] [CrossRef]
- Yihune, M.; Bekele, A.; Tefera, Z. Human-wildlife conflict in and around the Simen Mountains National Park, Ethiopia. Ethiop. J. Sci. 2009, 32, 57–64. [Google Scholar]
- Alemayehu, K.; Dessie, T.; Gizaw, S.; Haile, A.; Mekasha, Y. Population dynamics of Walia ibex (Capra walie) at Simen Mountains National Park, Ethiopia. Afr. J. Ecol. 2011, 49, 292–300. [Google Scholar] [CrossRef]
- Girma, E.; Tesfay, G.; Bauer, H.; Ashenafi, Z.T.; de Iongh, H.; Marino, J. Community Resource Uses and Ethiopian Wolf Conservation in Mount Abune Yosef. Environ. Manag. 2015, 56, 684–694. [Google Scholar]
- Ashenafi, Z.T. Common Property Resource Management of an Afroalpine Habitat: Supporting a Population of the Critically Endangered Ethiopian wolf (Canis simensis). Ph.D. Thesis, University of Kent, Canterbury, UK, 2001. [Google Scholar]
- Gebrehiwot, K.; Dessalegn, T.; Woldu, Z.; Demissew, S.; Teferi, E. Soil organic carbon stock in Abune Yosef afroalpine and sub-afroalpine vegetation, northern Ethiopia. Ecol. Process. 2018, 7, 6. [Google Scholar] [CrossRef]
- Fischer, A.; Wakjira, D.T.; Weldesemaet, Y.T.; Ashenafi, Z.T. On the interplay of actors in the co-management of natural resources—A dynamic perspective. World Dev. 2014, 64, 158–168. [Google Scholar] [CrossRef]
- Billi, P. Geomorphological landscapes of Ethiopia. In Landscapes and Landforms of Ethiopia, World Geomorphological Landscapes; Billi, P., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Gebrehiwot, K.; Teferi, E.; Woldu, Z.; Fekadu, M.; Desalegn, T.; Demissew, S. Dynamics and drivers of land cover change in the Afroalpine vegetation belt: Abune Yosef mountain range, Northern Ethiopia. Environ. Dev. Sustain. 2020, 23, 10679–10701. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Amatulli, G.; Domisch, S.; Tuanmu, M.N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Sci. Data 2018, 5, 180040. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Baco, A.R.; Brewin, P.E.; Clark, M.R.; Consalvey, M.; Hall-Spencer, J.; Rowden, A.A.; Schlacher, T.; Stocks, K.I.; Rogers, A.D. Predicting global habitat suitability for stony corals on seamounts. J. Biogeogr. 2009, 36, 1111–1128. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.W.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Evangelista, P.H.; Kumar, S.; Stohlgren, T.J.; Jarnevich, C.S.; Crall, A.W.; Norman, J.B., III; Barnett, D.T. Modelling invasion for a habitat generalist and a specialist plant species. Divers. Distrib. 2008, 14, 808–817. [Google Scholar] [CrossRef]
- Wakie, T.T.; Evangelista, P.H.; Jarnevich, C.S.; Laituri, M. Mapping current and potential distribution of non-native Prosopis juliflora in the Afar region of Ethiopia. PLoS ONE 2014, 9, e112854. [Google Scholar] [CrossRef]
- Davis, A.P.; Gole, T.W.; Baena, S.; Moat, J. The impact of climate change on indigenous arabica coffee (Coffea arabica): Predicting future trends and identifying priorities. PLoS ONE 2012, 7, e47981. [Google Scholar] [CrossRef]
- Evangelista, P.; Young, N.; Burnett, J. How will climate change spatially affect agriculture production in Ethiopia? Case studies of important cereal crops. Clim. Chang. 2013, 119, 855–873. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Saupe, E.E.; Barve, V.; Myers, C.E.; Soberón, J.; Barve, N.; Hensz, C.M.; Peterson, A.T.; Owens, H.L.; Lira-Noriega, A. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Model. 2012, 237, 11–22. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S.J. The art of modeling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with MaxEnt. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Ashenafi, Z.T.; Leader-Williams, N.; Coulson, T. Consequences of human land use for an Afroalpineecological community in Ethiopia. Conserv. Soc. 2012, 10, 209–216. [Google Scholar]
- Wesche, K. The importance of occasional droughts for afroalpine landscape ecology. J. Trop. Ecol. 2003, 19, 197–208. [Google Scholar] [CrossRef]
- Hedberg, O. Features of Afroalpine Plant Ecology; Almqvist & Wiksells Boktryckeri: Uppsala, Sweden, 1964. [Google Scholar]
- Phillips, S. Poaceae (Gramineae). In Flora of Ethiopia and Eritrea; Hedberg, I., Edwards, S., Eds.; The National Herbarium, Addis Ababa University: Addis Ababa, Ethiopia; Uppsala, Sweden, 1995; Volume 7, pp. 3–6. [Google Scholar]
- Buytaert, W.; Cuesta Camacho, F.; Tobón, C. Potential impacts of climate change on the environmental services of humid tropical alpine regions. Glob. Ecol. Biogeogr. 2011, 20, 19–33. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Kidane, Y.; Stahlman, R.; Beierkuhnlein, C. Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob. Ecol. Conserv. 2019, 19, e00670. [Google Scholar] [CrossRef]
- Chala, D.; Brochmann, C.; Psomas, A.; Ehrich, D.; Gizaw, A.; Masao, C.A.; Bakkestuen, V.; Zimmermann, N.E. Good-bye to tropical alpine plant giants under warmerclimates? Loss of range and genetic diversity in Lobelia rhynchopetalum. Ecol. Evol. 2016, 6, 8931–8941. [Google Scholar] [CrossRef]
- Rosell, S. Regional perspective on rainfall change and variability in the central highlands of Ethiopia, 1978–2007. Appl. Geogr. 2011, 31, 329–338. [Google Scholar] [CrossRef]
- Fashing, P.J.; Nguyen, N.; Venkataraman, V.V.; Kerby, J.T. Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: Variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 2014, 155, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Groth, J.; Ide, T.; Sakdapolrak, P.; Kassa, E.; Hermans, K. Deciphering interwoven drivers of environment-related migration—A multisite case study from the Ethiopian highlands. Glob. Environ. Chang. 2020, 63, 102094. [Google Scholar] [CrossRef]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Brooks, T.M.; Pimm, S.L.; Oyugi, J.O. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv. Biol. 1999, 13, 1140–1150. [Google Scholar] [CrossRef]
- Ferraz, G.; Russell, G.J.; Stouffer, P.C.; Bierregaard, R.O.; Pimm, S.L.; Lovejoy, T.E. Rates of species loss from Amazonian forest fragments. Proc. Natl. Acad. Sci. USA 2003, 100, 14069–14073. [Google Scholar] [CrossRef]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef]
- Cousins, S.A. Extinction debt in fragmented grasslands: Paid or not? J. Veg. Sci. 2009, 20, 3–7. [Google Scholar] [CrossRef]
- DeAngelis, D.L.; Yurek, S. Spatially explicit modeling in ecology: A review. Ecosystems 2017, 20, 284–300. [Google Scholar] [CrossRef]
- Carroll, C.; Noss, R.F.; Paquet, P.C.; Schumaker, N.H. Extinction debt of protected areas in developing landscapes. Conserv. Biol. 2004, 18, 1110–1120. [Google Scholar] [CrossRef]
- Bulman, C.R.; Wilson, R.J.; Holt, A.R.; Bravo, L.G.; Early, R.I.; Warren, M.S.; Thomas, C.D. Minimum viable metapopulation size, extinction debt, and the conservation of a declining species. Ecol. Appl. 2007, 17, 1460–1473. [Google Scholar] [CrossRef]
- Admassie, Y. Twenty Years to Nowhere. Property Rights, Land Management and Conservation in Ethiopia; Red Sea Press: Trenton, NJ, USA, 2000. [Google Scholar]
- UNDP. Community and Regional Leadership Dialogue on Protecting Choke Mountain. 2019. Available online: https://www.et.undp.org/content/ethiopia/en/home/presscenter/articles/2019/community-and-regional-leadership-dialogue-on-protecting-choke-m0.html (accessed on 21 December 2021).
- UNEP-WCMC; I.U.C.N. Protected Planet: The World Database on Protected Areas (WDPA). 2021. Available online: www.protectedplanet.net (accessed on 21 December 2021).
- DeCaro, D.; Stokes, M. Social psychological principles of community based conservation and conservancy motivation: Attaining goals within an autonomy supportive environment. Conserv. Biol. 2008, 22, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre, R. Community-based tourism as a sustainable solution to maximise impacts locally? The Tsiseb Conservancy case, Namibia. Dev. South. Afr. 2010, 27, 757–772. [Google Scholar] [CrossRef]
- Marks, S.A. Back to the future: Some unintended consequences of Zambia’s community-based wildlife program (ADMADE). Afr. Today 2001, 48, 121–141. [Google Scholar] [CrossRef]
- Silva, J.A.; Mosimane, A. “How could I live here and not be a member?”: Economic versus social drivers of participation in Namibian conservation programs. Hum. Ecol. 2014, 42, 183–197. [Google Scholar] [CrossRef]
- Sheppard, D.J.; Moehrenschlager, A.; McPherson, J.M.; Mason, J.J. Ten years of adaptive community-governed conservation: Evaluating biodiversity protection and poverty alleviation in a West African hippopotamus reserve. Environ. Conserv. 2010, 37, 270–282. [Google Scholar] [CrossRef]
- Dyer, J.; Stringer, L.; Dougill, A.; Leventon, J.; Nshimbi, M.; Chama, F.; Kafwifwi, A.; Muledi, J.; Kaumbu, J.-M.; Falcao, M.; et al. Assessing participatory practices in community-based natural resource management: Experiences in community engagement from southern Africa. J. Environ. Manag. 2014, 137, 137–145. [Google Scholar] [CrossRef]
- Ostrom, E.; Dietz, T.; Dolšak, N.; Stern, P.C.; Stonich, S.; Weber, E.U. The Drama of the Commons; National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Seixas, S.C.; Berkes, F. Community-based enterprises: The significance of partnerships and institutional linkages. Int. J. Commons 2009, 4, 183–212. [Google Scholar] [CrossRef]
- Galvin, K.A.; Beeton, T.A.; Luizza, M.W. African community-based conservation. Ecol. Soc. 2018, 23, 3. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Iverson, L.R.; Fosgitt, J.; Handler, S.D.; Dallman, M.; Thomasma, S.; Hutnik, B.; Swanston, C.W. Assessing stand-level climate change risk using forest inventory data and species distribution models. J. For. 2017, 115, 222–229. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species distribution modelling to support forest management. A literature review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]


| Variable | Description | Ecological Significance | Percent Contribution |
|---|---|---|---|
| BIO10 | Mean temperature of the warmest quarter | Afroalpine vegetation is found in cold climates [24] | 36.7 |
| BIO16 | Total precipitation of wettest quarter | The wettest months are typically June through September. Afroalpine vegetation is found in wet conditions [93]. | 21 |
| BIO17 | Total precipitation of driest quarter | The driest months are typically December through February. Desiccation stress and fire occurrence during these months are thought to favor grasses over shrubs in the Afroalpine [94]. | 15.6 |
| SLOPE | Rate of change of elevation in magnitude | Guassa grass is thought to prefer steep to moderately steep slopes [68]. | 9.9 |
| BIO2 | Mean diurnal range: mean of monthly (max temp − min temp) | Afroalpine vegetation has evolved under conditions of high daily temperature fluctuations, known as “summer every day and winter every night” [95,96]. Diurnal fluctuations are typically greater during the dry season. | 9.4 |
| BIO19 | Total precipitation of coldest quarter | Afroalpine vegetation is thought to persist in areas of low temperature and high moisture [68,97]. | 7.4 |
| Name | Unsuitable | Low Suitability | Moderate Suitability | Suitable | Highly Suitable |
|---|---|---|---|---|---|
| Existing Protected Areas | |||||
| Simien Mountains National Park | 81 km2 (16.5%) | 113 km2 (23.0%) | 132 km2 (26.9%) | 134 km2 (27.3%) | 31 km2 (6.3%) |
| Guassa Community Conservation Area | 6 km2 (7.5%) | 7 km2 (8.8%) | 15 km2 (18.8%) | 30 km2 (37.5%) | 22 km2 (27.5%) |
| Abune Yosef Community Conservation Area | 14 km2 (29.8%) | 8 km2 (17.0%) | 19 km2 (40.4%) | 6 km2 (12.8%) | 0 km2 (0%) |
| Potential Protected Areas | |||||
| Mount Choke | 21 km2 (6.6%) | 49 km2 (15.5%) | 35 km2 (11.0%) | 35 km2 (11.0%) | 177 km2 (55.8%) |
| South Wollo | 59 km2 (8.1%) | 40 km2 (5.5%) | 59 km2 (8.1%) | 278 km2 (38.1%) | 294 km2 (40.3%) |
| Mount Guna | 6 km2 (6.7%) | 6 km2 (6.7%) | 33 km2 (37.1%) | 34 km2 (38.2%) | 10 km2 (11.2%) |
| Ankober | 7 km2 (6.1%) | 8 km2 (7.0%) | 30 km2 (26.3%) | 65 km2 (57.0%) | 4 km2 (3.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chengere, S.A.; Steger, C.; Gebrehiwot, K.; Nemomissa, S.; Dullo, B.W. Modeling Cultural Keystone Species for the Conservation of Biocultural Diversity in the Afroalpine. Environments 2022, 9, 156. https://doi.org/10.3390/environments9120156
Chengere SA, Steger C, Gebrehiwot K, Nemomissa S, Dullo BW. Modeling Cultural Keystone Species for the Conservation of Biocultural Diversity in the Afroalpine. Environments. 2022; 9(12):156. https://doi.org/10.3390/environments9120156
Chicago/Turabian StyleChengere, Shambel Alemu, Cara Steger, Kflay Gebrehiwot, Sileshi Nemomissa, and Bikila Warkineh Dullo. 2022. "Modeling Cultural Keystone Species for the Conservation of Biocultural Diversity in the Afroalpine" Environments 9, no. 12: 156. https://doi.org/10.3390/environments9120156
APA StyleChengere, S. A., Steger, C., Gebrehiwot, K., Nemomissa, S., & Dullo, B. W. (2022). Modeling Cultural Keystone Species for the Conservation of Biocultural Diversity in the Afroalpine. Environments, 9(12), 156. https://doi.org/10.3390/environments9120156






