Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes
Abstract
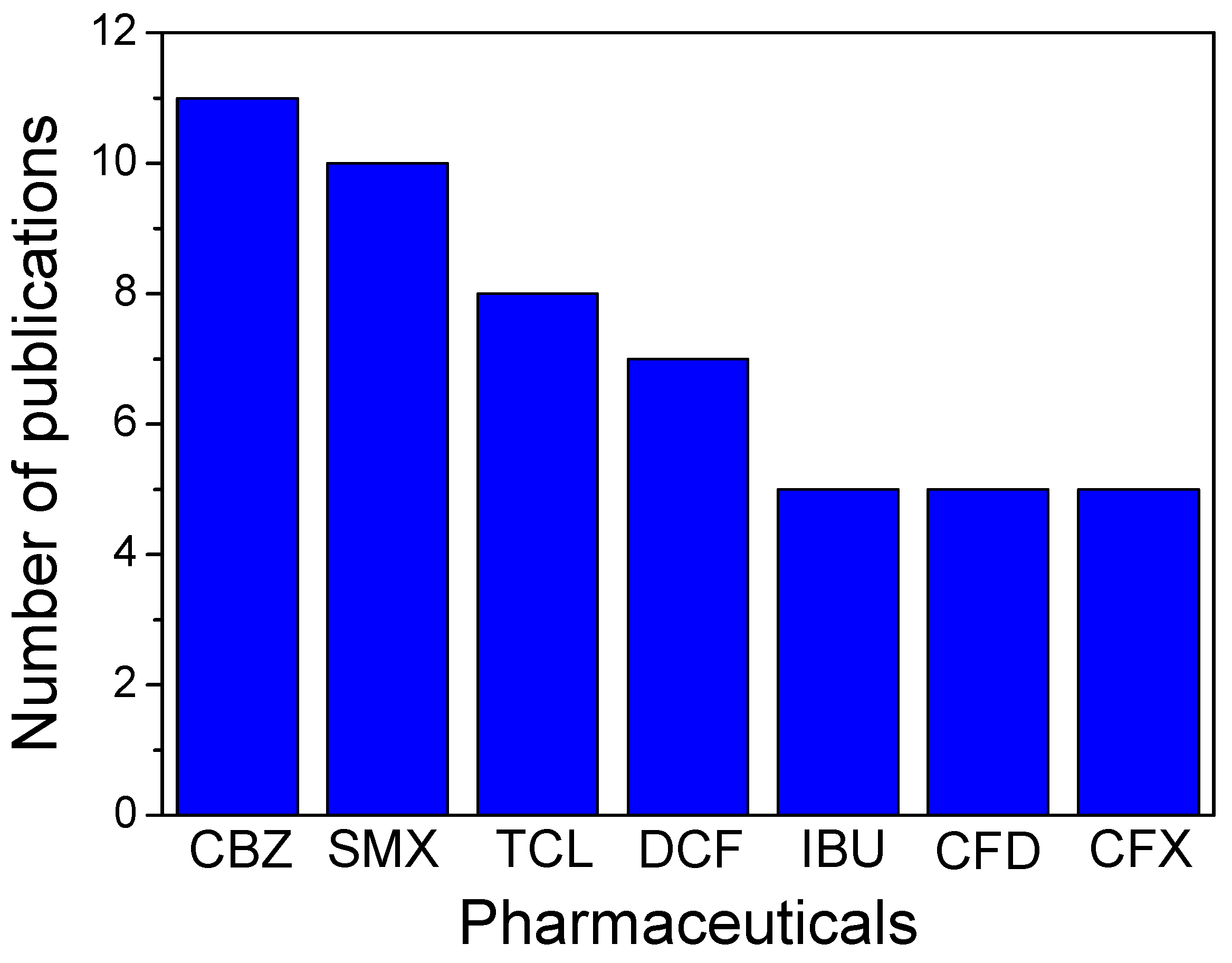
:1. Introduction
2. Contamination of Aquatic Environment by PhCs
3. Recent Developments of EOPs for Specific PhCs
3.1. Carbamazapine (CBZ)
3.2. Sulfamethoxazole (SMX)
3.3. Tetracycline (TCL)
3.4. Diclofenac (DCF)
3.5. Ibuprofen (IBU)
3.6. Ceftazidime (CFD)
3.7. Ciprofloxacin (CFX)
3.8. Other PhCs
4. Conclusions and Future Perspectives
- -
- The study of three-dimensional electrodes seems to be an exciting strategy to address the limitations of the active surface of the electrodes.
- -
- The further study of the (photo) electrochemical generation of hydrogen peroxide at the cathode, in combination with other processes, such as (Photo) Fenton reaction and UV/H2O2, will allow additional reaction mechanisms in the main volume (bulk) of the solution.
- -
- The gradual shift of investigations in the case of mixtures of drugs where the observed results mainly concern toxicity have not been studied in full detail.
- -
- The simultaneous study of the process for the removal of micro-pollutants, pathogens, and organic material in conditions that resemble the tertiary treatment of domestic wastewater or in real effluent.
- -
- Coupling with renewable energy sources and the study of integrated systems on a pilot scale under realistic conditions.
Funding
Conflicts of Interest
References
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process. Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.; Maza-Márquez, P.; Herrera, J.M.P.; Rodelas, B.; Osorio, F.; Pozo, C. Removal of anti-inflammatory/analgesic pharmaceuticals from urban wastewater in a pilot-scale A2O system: Linking performance and microbial population dynamics to operating variables. Sci. Total Environ. 2018, 643, 1481–1492. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.-M.; Yang, Q.; Yue, X.; Shen, T.-T.; Zheng, W.; Luo, K.; Sun, Y.-H.; Zeng, G.-M. Landfill leachate pretreatment by coagulation–flocculation process using iron-based coagulants: Optimization by response surface methodology. Chem. Eng. J. 2012, 200, 39–51. [Google Scholar] [CrossRef]
- Liu, G.-H.; Ye, Z.; Tong, K.; Zhang, Y.-H. Biotreatment of heavy oil wastewater by combined upflow anaerobic sludge blanket and immobilized biological aerated filter in a pilot-scale test. Biochem. Eng. J. 2013, 72, 48–53. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, M.; Pan, Y.; Zhang, K.; Lyu, L.; Wang, M.; Zhu, T. Performance analysis and optimization of ammonium removal in a new biological folded non-aerated filter reactor. Sci. Total Environ. 2019, 688, 505–512. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Madani, K.; Vial, C.; Sekki, A. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation. A comparative study. Desalination 2011, 272, 246–253. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Khan, N.A.; Ahmed, S.; Dhingra, A.; Singh, C.P.; Khan, S.U.; Mohammadi, A.A.; Changani, F.; Yousefi, M.; Alam, S.; et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020, 269, 122411. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.-W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Series Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Panizza, M. Organic Pollutants, Direct and Mediated Anodic Oxidation BT. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 1424–1428. ISBN 978-1-4419-6996-5. [Google Scholar]
- Dao, K.C.; Yang, C.-C.; Chen, K.-F.; Tsai, Y.-P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef] [Green Version]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; García, M.F.; Prados-Joya, G.; Perez, R.O. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Bush, K. Antimicrobial agents. Curr. Opin. Chem. Biol. 1997, 1, 169–175. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Jones, O.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Alighardashi, A.; Aghta, R.S.; Ebrahimzadeh, H. Improvement of Carbamazepine Degradation by a Three-Dimensional Electrochemical (3-EC) Process. Int. J. Environ. Res. 2018, 12, 451–458. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Sánchez-Martín, J. Electrochemical Advanced Oxidation of Carbamazepine on Boron-Doped Diamond Anodes. Influence of Operating Variables. Ind. Eng. Chem. Res. 2010, 49, 8353–8359. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Nacheva, P.M. Degradation of pharmaceutical compounds in water by oxygenated electrochemical oxidation: Parametric optimization, kinetic studies and toxicity assessment. Sci. Total Environ. 2019, 691, 417–429. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Mijaylova-Nacheva, P.; Avilés-Flores, M. Electrochemical carbamazepine degradation: Effect of the generated active chlorine, transformation pathways and toxicity. Chemosphere 2018, 192, 142–151. [Google Scholar] [CrossRef]
- García-Gómez, C.; Drogui, P.; Zaviska, F.; Seyhi, B.; Gortáres-Moroyoqui, P.; Buelna, G.; Neira-Sáenz, C.; Estrada-Alvarado, M.; Ulloa-Mercado, R. Experimental design methodology applied to electrochemical oxidation of carbamazepine using Ti/PbO2 and Ti/BDD electrodes. J. Electroanal. Chem. 2014, 732, 1–10. [Google Scholar] [CrossRef] [Green Version]
- García-Gómez, C.; Drogui, P.; Seyhi, B.; Gortáres-Moroyoqui, P.; Buelna, G.; Estrada-Alvgarado, M.; Alvarez, L.H. Combined membrane bioreactor and electrochemical oxidation using Ti/PbO2 anode for the removal of carbamazepine. J. Taiwan Inst. Chem. Eng. 2016, 64, 211–219. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Palo, P.; Dominguez, J.R.; González, T.; Sánchez-Martín, J.; Cuerda-Correa, E.M. Feasibility of electrochemical degradation of pharmaceutical pollutants in different aqueous matrices: Optimization through design of experiments. J. Environ. Sci. Health Part. A 2014, 49, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Ouarda, Y.; Tiwari, B.; Azaïs, A.; Vaudreuil, M.-A.; Ndiaye, S.D.; Drogui, P.; Tyagi, R.D.; Sauvé, S.; Desrosiers, M.; Buelna, G.; et al. Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment. Chemosphere 2018, 193, 160–169. [Google Scholar] [CrossRef]
- Ensano, B.M.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C. Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J. Hazard. Mater. 2018, 361, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Gul, S.; Steter, J.R.; Miwa, D.W.; Motheo, A.J. Route of electrochemical oxidation of the antibiotic sulfamethoxazole on a mixed oxide anode. Environ. Sci. Pollut. Res. 2015, 22, 15004–15015. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Coetsier, C.; Causserand, C.; Serrano, K.G. On the role of salts for the treatment of wastewaters containing pharmaceuticals by electrochemical oxidation using a boron doped diamond anode. Electrochimica Acta 2017, 231, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Niu, J.; Xu, J.; Li, Y.; Pan, Y. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochimica Acta 2013, 97, 167–174. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, C.-Y.; Lo, S.-L. Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J. Hazard. Mater. 2018, 366, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep. Purif. Technol. 2018, 195, 184–191. [Google Scholar] [CrossRef]
- Rodríguez-Nava, O.; Ramírez-Saad, H.; Loera, O.; González, I. Evaluation of the simultaneous removal of recalcitrant drugs (Bezafibrate, Gemfibrozil, Indomethacin and Sulfamethoxazole) and biodegradable organic matter from synthetic wastewater by electro-oxidation coupled with a biological system. Environ. Technol. 2016, 37, 1–21. [Google Scholar] [CrossRef]
- Sifuna, F.W.; Orata, F.; Okello, V.; Jemutai-Kimosop, S. Comparative studies in electrochemical degradation of sulfamethoxazole and diclofenac in water by using various electrodes and phosphate and sulfate supporting electrolytes. J. Environ. Sci. Health Part A 2016, 51, 954–961. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Belkheiri, D.; Fourcade, F.; Geneste, F.; Floner, D.; Aït-Amar, H.; Amrane, A. Feasibility of an electrochemical pre-treatment prior to a biological treatment for tetracycline removal. Sep. Purif. Technol. 2011, 83, 151–156. [Google Scholar] [CrossRef]
- Brinzila, C.; Pacheco, M.J.; Ciríaco, L.; Ciobanu, R.; Lopes, A. Electrodegradation of tetracycline on BDD anode. Chem. Eng. J. 2012, 209, 54–61. [Google Scholar] [CrossRef]
- Liang, S.; Lin, H.; Yan, X.; Huang, Q. Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products, and toxicity. Chem. Eng. J. 2018, 332, 628–636. [Google Scholar] [CrossRef]
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef]
- Yahiaoui, I.; Aissani-Benissad, F.; Fourcade, F.; Amrane, A. Removal of tetracycline hydrochloride from water based on direct anodic oxidation (Pb/PbO2 electrode) coupled to activated sludge culture. Chem. Eng. J. 2013, 221, 418–425. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.; Dionysiou, D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2019, 383, 123149. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical incineration of diclofenac in neutral aqueous medium by anodic oxidation using Pt and boron-doped diamond anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef]
- García-Montoya, M.F.; Gutiérrez-Granados, S.; Alatorre-Ordaz, A.; Galindo, R.; Ornelas, R.; Peralta-Hernández, J.M. Application of electrochemical/BDD process for the treatment wastewater effluents containing pharmaceutical compounds. J. Ind. Eng. Chem. 2015, 31, 238–243. [Google Scholar] [CrossRef]
- Ambuludi, S.L.; Panizza, M.; Oturan, N.; Ozcan, A.; Oturan, M.A. Kinetic behavior of anti-inflammatory drug ibuprofen in aqueous medium during its degradation by electrochemical advanced oxidation. Environ. Sci. Pollut. Res. 2012, 20, 2381–2389. [Google Scholar] [CrossRef]
- Ciríaco, L.; Anjo, C.; Correia, J.; Pacheco, M.J.; Lopes, A. Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochimica Acta 2009, 54, 1464–1472. [Google Scholar] [CrossRef]
- Li, X.; Ma, F.; Li, Y.; Zhang, H.; Min, J.; Zhang, X.; Yao, H. Enhanced mechanisms of electrocatalytic-ozonation of ibuprofen using a TiO2 nanoflower-coated porous titanium gas diffuser anode: Role of TiO2 catalysts and electrochemical action in reactive oxygen species formation. Chem. Eng. J. 2020, 389, 124411. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yin, L.; Niu, J.; Hou, L.-A. Insights of ibuprofen electro-oxidation on metal-oxide-coated Ti anodes: Kinetics, energy consumption and reaction mechanisms. Chemosphere 2016, 163, 584–591. [Google Scholar] [CrossRef]
- Duan, P.; Hu, X.; Ji, Z.; Yang, X.; Sun, Z. Enhanced oxidation potential of Ti/SnO2-Cu electrode for electrochemical degradation of low-concentration ceftazidime in aqueous solution: Performance and degradation pathway. Chemosphere 2018, 212, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Gao, S.; Li, X.; Sun, Z.; Hu, X. Preparation of CeO2-ZrO2 and titanium dioxide coated carbon nanotube electrode for electrochemical degradation of ceftazidime from aqueous solution. J. Electroanal. Chem. 2019, 841, 10–20. [Google Scholar] [CrossRef]
- Duan, P.; Yang, X.; Huang, G.; Wei, J.; Sun, Z.; Hu, X. La2O3-CuO2/CNTs electrode with excellent electrocatalytic oxidation ability for ceftazidime removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 119–128. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Sun, Z. Preparation and characterization of cerium-doped multiwalled carbon nanotubes electrode for the electrochemical degradation of low-concentration ceftazidime in aqueous solutions. Electrochimica Acta 2016, 199, 80–91. [Google Scholar] [CrossRef]
- Li, X.; Duan, P.; Lei, J.; Sun, Z.; Hu, X. Fabrication of Ti/TiO2/SnO2-Sb-Cu electrode for enhancing electrochemical degradation of ceftazidime in aqueous solution. J. Electroanal. Chem. 2019, 847, 113231. [Google Scholar] [CrossRef]
- Lan, Y.; Coetsier, C.; Causserand, C.; Serrano, K.G. An experimental and modelling study of the electrochemical oxidation of pharmaceuticals using a boron-doped diamond anode. Chem. Eng. J. 2018, 333, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Huang, C.; Li, H.; Chen, L.; Zhang, D.; Yang, Z. Electrochemical degradation of ciprofloxacin with a Sb-doped SnO2 electrode: Performance, influencing factors and degradation pathways. RSC Adv. 2019, 9, 29796–29804. [Google Scholar] [CrossRef] [Green Version]
- Wachter, N.; Aquino, J.M.; Denadai, M.; Barreiro, J.C.; Silva, A.J.; Cass, Q.B.; Rocha-Filho, R.C.; Bocchi, N. Optimization of the electrochemical degradation process of the antibiotic ciprofloxacin using a double-sided β-PbO2 anode in a flow reactor: Kinetics, identification of oxidation intermediates and toxicity evaluation. Environ. Sci. Pollut. Res. 2018, 26, 4438–4449. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.-T.; Yu, Y.-G. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Lei, J.; Liu, W.; Tong, M.; Liang, J. The degradation pathways of carbamazepine in advanced oxidation process: A mini review coupled with DFT calculation. Sci. Total Environ. 2021, 779, 146498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Fate of pharmaceuticals––Photodegradation by simulated solar UV-light. Chemosphere 2003, 52, 1757–1769. [Google Scholar] [CrossRef]
- Ginebreda, A.; Muñoz, I.; de Alda, M.L.; Brix, R.; López-Doval, J.C.; Barceló, D. Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, A.; Mendret, J.; Cazals, G.; Petit, E.; Brosillon, S. Ozonation as a pretreatment process for nanofiltration brines: Monitoring of transformation products and toxicity evaluation. J. Hazard. Mater. 2017, 338, 381–393. [Google Scholar] [CrossRef]
- Huo, Q.; Qi, X.; Li, J.; Liu, G.; Ning, Y.; Zhang, X.; Zhang, B.; Fu, Y.; Liu, S. Preparation of a direct Z-scheme α-Fe2O3/MIL-101(Cr) hybrid for degradation of carbamazepine under visible light irradiation. Appl. Catal. B Environ. 2019, 255, 117751. [Google Scholar] [CrossRef]
- Borea, L.; Ensano, B.M.B.; Hasan, S.W.; Balakrishnan, M.; Belgiorno, V.; de Luna, M.D.G.; Ballesteros, F.C.; Naddeo, V. Are pharmaceuticals removal and membrane fouling in electromembrane bioreactor affected by current density? Sci. Total Environ. 2019, 692, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2019, 250, 119553. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Kiruba, U.P.; Kumar, P.S.; Prabhakaran, C.; Aditya, V. Characteristics of thermodynamic, isotherm, kinetic, mechanism and design equations for the analysis of adsorption in Cd(II) ions-surface modified Eucalyptus seeds system. J. Taiwan Inst. Chem. Eng. 2014, 45, 2957–2968. [Google Scholar] [CrossRef]
- Ji, L.; Shao, Y.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of Monoaromatic Compounds and Pharmaceutical Antibiotics on Carbon Nanotubes Activated by KOH Etching. Environ. Sci. Technol. 2010, 44, 6429–6436. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalination Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Wu, K.; Si, X.; Jiang, J.; Si, Y.; Sun, K.; Yousaf, A. Enhanced degradation of sulfamethoxazole by Fe–Mn binary oxide synergetic mediated radical reactions. Environ. Sci. Pollut. Res. 2019, 26, 14350–14361. [Google Scholar] [CrossRef]
- Pretto, P.R.P.; Palácio, S.M.; de Campos, E.A.; Pazini, C.R.; Veit, M. Sulfamethoxazole photocatalytic degradation in a continuous flow reactor using artificial radiation. J. Environ. Chem. Eng. 2018, 6, 1926–1933. [Google Scholar] [CrossRef]
- Roy, K.; Moholkar, V.S. Sulfadiazine degradation using hybrid AOP of heterogeneous Fenton/persulfate system coupled with hydrodynamic cavitation. Chem. Eng. J. 2019, 386, 121294. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.-H.; Cha, S.M.; Yu, S. Degradation of sulfamethoxazole by ionizing radiation: Identification and characterization of radiolytic products. Chem. Eng. J. 2017, 313, 556–566. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human Health Consequences of Use of Antimicrobial Agents in Aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of hormones and antibiotics by nanofiltration membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Wei, D.; Hu, C. Photolysis of Chlortetracycline in aqueous solution: Kinetics, toxicity and products. J. Environ. Sci. 2012, 24, 254–260. [Google Scholar] [CrossRef]
- Bu, D.; Zhuang, H. Biotemplated synthesis of high specific surface area copper-doped hollow spherical titania and its photocatalytic research for degradating chlorotetracycline. Appl. Surf. Sci. 2013, 265, 677–685. [Google Scholar] [CrossRef]
- Grimm, J.; Bessarabov, D.; Sanderson, R. Review of electro-assisted methods for water purification. Desalination 1998, 115, 285–294. [Google Scholar] [CrossRef]
- Brinzila, C.I.; Monteiro, N.; Pacheco, M.J.; Ciríaco, L.; Siminiceanu, I.; Lopes, A. Degradation of tetracycline at a boron-doped diamond anode: Influence of initial pH, applied current intensity and electrolyte. Environ. Sci. Pollut. Res. 2014, 21, 8457–8465. [Google Scholar] [CrossRef] [PubMed]
- Frontistis, Z.; Meriç, S. The role of operating parameters and irradiation on the electrochemical degradation of tetracycline on boron doped diamond anode in environmentally relevant matrices. J. Chem. Technol. Biotechnol. 2018, 93, 3648–3655. [Google Scholar] [CrossRef]
- Scheurell, M.; Franke, S.; Shah, R.; Hühnerfuss, H. Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan. Chemosphere 2009, 77, 870–876. [Google Scholar] [CrossRef]
- Hartmann, J.; Bartels, P.; Mau, U.; Witter, M.; Tümpling, W.; Hofmann, J.; Nietzschmann, E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 2008, 70, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-Fenton Degradation of Diclofenac: Identification of Main Intermediates and Degradation Pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef]
- Davarnejad, R.; Soofi, B.; Farghadani, F.; Behfar, R. Ibuprofen removal from a medicinal effluent: A review on the various techniques for medicinal effluents treatment. Environ. Technol. Innov. 2018, 11, 308–320. [Google Scholar] [CrossRef]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total Environ. 2018, 619–620, 446–459. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2016, 177, 272–280. [Google Scholar] [CrossRef]
- Casas, M.E.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Dominguez, J.R.; Gonzalez, T.; Palo, P.; Sanchez-Martin, J.; Rodrigo, M.A.; Saez, C. Electrochemical Degradation of a Real Pharmaceutical Effluent. Water Air Soil Pollut. 2011, 223, 2685–2694. [Google Scholar] [CrossRef]
- Giri, A.S.; Golder, A.K. Ciprofloxacin degradation from aqueous solution by Fenton oxidation: Reaction kinetics and degradation mechanisms. RSC Adv. 2014, 4, 6738–6745. [Google Scholar] [CrossRef]
- Haddad, T.; Kümmerer, K. Characterization of photo-transformation products of the antibiotic drug Ciprofloxacin with liquid chromatography–tandem mass spectrometry in combination with accurate mass determination using an LTQ-Orbitrap. Chemosphere 2014, 115, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaim, F.; Mussa, Z.H.; Othman, M.R.; Abdullah, P. Removal of caffeine from aqueous solution by indirect electrochemical oxidation using a graphite-PVC composite electrode: A role of hypochlorite ion as an oxidising agent. J. Hazard. Mater. 2015, 300, 387–397. [Google Scholar] [CrossRef]
- Brocenschi, R.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of estrone using a boron-doped diamond anode in a filter-press reactor. Electrochimica Acta 2016, 197, 186–193. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Garcia-Segura, S.; Centellas, F.; Brillas, E. Electrochemical incineration of omeprazole in neutral aqueous medium using a platinum or boron-doped diamond anode: Degradation kinetics and oxidation products. Water Res. 2013, 47, 1803–1815. [Google Scholar] [CrossRef]
- Coledam, D.A.; Aquino, J.M.; Silva, B.F.; da Silva, A.J.; Rocha-Filho, R.C. Electrochemical mineralization of norfloxacin using distinct boron-doped diamond anodes in a filter-press reactor, with investigations of toxicity and oxidation by-products. Electrochimica Acta 2016, 213, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Coledam, D.A.; Pupo, M.M.; Silva, B.F.; da Silva, A.J.; Eguiluz, K.; Banda, G.; Aquino, J.M. Electrochemical mineralization of cephalexin using a conductive diamond anode: A mechanistic and toxicity investigation. Chemosphere 2017, 168, 638–647. [Google Scholar] [CrossRef]
- Da Silva, S.W.; Prado, J.M.D.; Heberle, A.N.A.; Schneider, D.E.; Rodrigues, M.A.S.; Bernardes, A.M. Electrochemical advanced oxidation of Atenolol at Nb/BDD thin film anode. J. Electroanal. Chem. 2019, 844, 27–33. [Google Scholar] [CrossRef]
- Díaz, E.; Stożek, S.; Patiño, Y.; Ordóñez, S. Electrochemical degradation of naproxen from water by anodic oxidation with multiwall carbon nanotubes glassy carbon electrode. Water Sci. Technol. 2019, 79, 480–488. [Google Scholar] [CrossRef]
- González, T.; Dominguez, J.R.; Palo, P.; Sanchez-Martin, J. Conductive-diamond electrochemical advanced oxidation of naproxen in aqueous solution: Optimizing the process. J. Chem. Technol. Biotechnol. 2010, 86, 121–127. [Google Scholar] [CrossRef]
- Periyasamy, S.; Muthuchamy, M. Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng. 2018, 6, 7358–7367. [Google Scholar] [CrossRef]
- Pinar, P.T.; Yardım, Y.; Şentürk, Z. Electrochemical oxidation of ranitidine at poly(dopamine) modified carbon paste electrode: Its voltammetric determination in pharmaceutical and biological samples based on the enhancement effect of anionic surfactant. Sens. Actuators B Chem. 2018, 273, 1463–1473. [Google Scholar] [CrossRef]
- Rahmani, A.; Seid-Mohammadi, A.; Leili, M.; Shabanloo, A.; Ansari, A.; Alizadeh, S.; Nematollahi, D. Electrocatalytic degradation of diuron herbicide using three-dimensional carbon felt/β-PbO2 anode as a highly porous electrode: Influencing factors and degradation mechanisms. Chemosphere 2021, 276, 130141. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process. Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Yazirdagi, M.; Kilinc, Z.; Konstantinou, I.; Katsaounis, A.; Mantzavinos, D. Boron-doped diamond electrooxidation of ethyl paraben: The effect of electrolyte on by-products distribution and mechanisms. J. Environ. Manag. 2017, 195, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Steter, J.R.; Rocha, R.S.; Dionisio, D.; Lanza, M.; Motheo, A. Electrochemical oxidation route of methyl paraben on a boron-doped diamond anode. Electrochimica Acta 2014, 117, 127–133. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Zheng, H.; Zhao, C.; Xiao, X.; Xu, Y.; Sun, W.; Wu, H.; Ren, M. Electrochemical treatment of chloramphenicol using Ti-Sn/γ-Al 2 O 3 particle electrodes with a three-dimensional reactor. Chem. Eng. J. 2017, 308, 1233–1242. [Google Scholar] [CrossRef]
- Turkay, O.; Barışçı, S.; Ulusoy, E.; Dimoglo, A. Electrochemical Reduction of X-ray Contrast Iohexol at Mixed Metal Oxide Electrodes: Process Optimization and By-product Identification. Water Air Soil Pollut. 2018, 229, 170. [Google Scholar] [CrossRef]
- Frontistis, Z. Sonoelectrochemical Degradation of Propyl Paraben: An Examination of the Synergy in Different Water Matrices. Int. J. Environ. Res. Public Health 2020, 17, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontistis, Z.; Mantzavinos, D.; Meriç, S. Degradation of antibiotic ampicillin on boron-doped diamond anode using the combined electrochemical oxidation—Sodium persulfate process. J. Environ. Manag. 2018, 223, 878–887. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Xu, Z.; Niu, J.; Hou, L.-A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Chen, J.; Fu, Z.; Niu, J. Bicarbonate enhancing electrochemical degradation of antiviral drug lamivudine in aqueous solution. J. Electroanal. Chem. 2019, 848, 113314. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, T.; Liu, H.; Wang, G.; Zhang, Y.; Cai, C. Direct and indirect electrochemical oxidation of ethanethiol on grey cast iron anode in alkaline solution. Electrochimica Acta 2020, 356, 136706. [Google Scholar] [CrossRef]
- Xia, Y.; Bian, X.; Xia, Y.; Zhou, W.; Wang, L.; Fan, S.; Xiong, P.; Zhan, T.; Dai, Q.; Chen, J. Effect of indium doping on the PbO2 electrode for the enhanced electrochemical oxidation of aspirin: An electrode comparative study. Sep. Purif. Technol. 2019, 237, 116321. [Google Scholar] [CrossRef]

| PhCs | Anode Electrode Material | Electrolyte | Removal Efficiency | Reference |
|---|---|---|---|---|
| Carbamazepine (CBZ) | Activated carbon powder (PAC) 3-D electrode | NaCl | 89.8% (10 min) | [19] |
| BDD | Na2SO4 | 100% (0.98 min) | [20] | |
| Nb/BDD | Na2SO4 | 82% (90 min) | [21] | |
| Nb/BDD | NaCl | 88.7% (12.45 min) | [22] | |
| Ti/PbO2 | NaSO4 | 88% (101 min) | [23] | |
| Ti/PbO2 (EOP coupled with eMBR) | NaSO4 | 99.99% (101 min) | [24] | |
| TiO2 meshes | KCl | 100% (45 min) | [25] | |
| BDD | NaSO4 | 50% (1 min) kapp = 0.73 min−1 | [26] | |
| Nb/BDD (EOP coupled with eMBR) | (NH4)2SO4, KH2PO4 | 97% (40 min) | [27] | |
| Aluminium | NH4-N, NO3-N, PO4-P | 50% (19 h) | [28] | |
| Sulfamethoxazole (SMX) | Nb/BDD | Na2SO4 | 86% (90 min) | [21] |
| Ti/Ru0.3Ti0.7O2 | NaCl | 98% (30 min) | [29] | |
| BDD | K2SO4 | 75% (60 min) | [30] | |
| Ti/SnO2-Sb/Ce-PbO2 | NaOH, Na2SO4, NaNO3, NaNO2, NaClO4, NH4Cl, H3PO4, HClO4 | 95% (60 min) | [31] | |
| Graphite | NaCl | 99.6% (30 min) kapp = 0.170 min−1 | [32] | |
| BDD | - | 72.9% (180 min) | [33] | |
| BDD | NaOH | 100% (50 min) | [34] | |
| Pt | Na2SO4 | 98% (90 min) | [35] | |
| Ti/SnO2-Sb/Er-PbO2 | Na2SO4 | kapp = 0.299 min−1 (9.2 min) | [36] | |
| Tetracycline (TCL) | Graphite felt | Na2SO4 | 99% | [37] |
| BDD | Na2SO4 | 99% (120 min) | [38] | |
| Magnéli phase Ti4O7 | H2SO4 | 98.4% (56.4 min) | [39] | |
| Ti/Ti4O7 | Na2SO4 | 97.2% (11.2 min) | [40] | |
| DSA (mixed metal oxide, Ti/RuO2–IrO2) | Na2SO4 | 100% (20 min) | [41] | |
| Pb/PbO2 | H2SO4 | 86.7% (DOC) (60 min) | [42] | |
| Ti4O7 | Na2SO4 | 97.2% (120 min) | [43] | |
| Diclofenac (DCF) | BDD | Na2SO4 | 100% (200 min) | [44] |
| Aluminium | NH4-N, NO3-N, PO4-P | 47% (6 h) | [28] | |
| Graphite | NaCl | 99.2% (60 min) | [32] | |
| BDD | - | 73.7% (180 min) | [33] | |
| BDD | methanol/phosphate buffer | 79.4% (175 min) | [45] | |
| Pt | Na2SO4 | 98% (90 min) | [35] | |
| Ibuprofen (IBU) | BDD | NaCl | 96% (TOC) (8 h) | [46] |
| BDD | Na2SO4 | 95% (COD) 92% (TOC) (6 h) | [47] | |
| TiO2 nanoflower-modified porous titanium gas diffuser (TiO2-NF @PTGD) | Na2SO4 | kapp = 0.0195 min−1 (30 min) | [48] | |
| Nb/BDD (EOP coupled with eMBR) | (NH4)2SO4, KH2PO4 | 93% (120 min) | [27] | |
| Ti/SnO2-Sb/Ce-PbO2 | Na2SO4 | 93.2% (60 min) kapp = 0.094 min−1 | [49] | |
| Ceftazidime (CFD) | Ti/SnO2-Cu | Na2SO4 | 90% (60 min) | [50] |
| CeO2-ZrO2/TiO2/CNT | Na2SO4 | 83.47% (60 min) | [51] | |
| La2O3-CuO2/CNTs | Na2SO4 | 90% (30 min) | [52] | |
| CeO2/MWCNTs | Na2SO4 | 100% (60 min) | [53] | |
| Ti/TiO2/SnO2-Sb-Cu | Na2SO4 | 97.65% (20 h) | [54] | |
| Ciprofloxacin (CFX) | BDD | K2SO4 | 100% (250 min) | [30] |
| BDD | K2SO4 | 100% (180 min) | [55] | |
| Sb-doped SnO2 | Na2SO4 | 100% (60 min) | [56] | |
| Ti-Pt/β-PbO2 | Na2SO4 | 100% (120 min) | [57] | |
| SnO2-Sb/Ti | Na2SO4 | 99.5% (120 min) | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bampos, G.; Petala, A.; Frontistis, Z. Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes. Environments 2021, 8, 85. https://doi.org/10.3390/environments8080085
Bampos G, Petala A, Frontistis Z. Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes. Environments. 2021; 8(8):85. https://doi.org/10.3390/environments8080085
Chicago/Turabian StyleBampos, Georgios, Athanasia Petala, and Zacharias Frontistis. 2021. "Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes" Environments 8, no. 8: 85. https://doi.org/10.3390/environments8080085
APA StyleBampos, G., Petala, A., & Frontistis, Z. (2021). Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes. Environments, 8(8), 85. https://doi.org/10.3390/environments8080085








