Dissolution of Ag Nanoparticles in Agricultural Soils and Effects on Soil Exoenzyme Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Characterization of Soil Samples
2.2. Synthesis of AgNPs and Characterization of AgNPs Colloids
2.3. Pot Experiments
2.4. Sampling and Analysis of Pore Water
2.5. Analysis of Ag Retained in the Soil Solid Matrix
2.6. Analysis of Soil Exoenzymes Activities
2.7. Statistical Analysis
3. Results
3.1. Characterization of Soil Samples
3.2. Characterization of AgNPs
3.3. Pore Water Concentrations of AgNPs in Soils
3.4. Concentration of Ag Retained in the Solid Matrix
3.5. Potential Availability of Ag Retained in the Solid Matrix
3.6. Analysis of Soil Exoenzymes Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Zhao, L.J.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef]
- Benoit, R.; Wilkinson, K.J.; Sauvé, S. Partitioning of silver and chemical speciation of free Ag in soils amended with nanoparticles. Chem. Cent. J. 2013, 7, 75–83. [Google Scholar] [CrossRef]
- Yu, S.; Yin, Y.; Liu, J. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in Plant Protection and Fertilization: Current State, Foreseen Applications, and Research Priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Gupta, N.; Upadhyaya, C.P.; Singh, A.; Abd-Elsalam, K.A.; Prasad, R. Applications of Silver Nanoparticles in Plant Protection. In Nanobiotechnology Applications in Plant Protection. Nanotechnology in the Life Sciences; Abd-Elsalam, K., Prasad, R., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kim, K.; Sung, W.S.; Moon, S.; Choi, J.; Kim, J.G.; Lee, D.G. Antifungal Effect of Silver Nanoparticles on Dermatophytes. J. Microbiol. Biotechnol. 2008, 18, 1482–1484. [Google Scholar]
- Jo, Y.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Kim, B.; Park, C.S.; Murayama, M.; Hochella, M.F. Discovery and Characterization of Silver Sulfide Nanoparticles in Final Sewage Sludge Products. Environ. Sci. Technol. 2010, 44, 7509–7514. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Trindade, T.; Duarte, A.C.; Pereira, E.; Koopmans, G.F.; Römkens, P.F.A.M. A framework to measure the availability of engineered nanoparticles in soils: Trends in soil tests and analytical tools. Trends Anal. Chem. 2016, 75, 129–140. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; van den Brink, N.; Nickel, C. Fate and Bioavailability of Engineered Nanoparticles in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Coutris, C.; Joner, E.J.; Oughton, D.H. Aging and soil organic matter content affect the fate of silver nanoparticles in soil. Sci. Total Environ. 2012, 420, 327–333. [Google Scholar] [CrossRef]
- Cornelis, G.; Pang, L.P.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 2013, 463, 120–130. [Google Scholar] [CrossRef]
- Lowry, G.V.; Espinasse, B.P.; Badireddy, A.R.; Richardson, C.J.; Reinsch, B.C.; Bryant, L.D.; Bone, A.J.; Deonarine, A.; Chae, S.; Therezien, M. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ. Sci. Technol. 2012, 46, 7027–7036. [Google Scholar] [CrossRef]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments. Acc. Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef]
- Klitzke, S.; Metreveli, G.; Peters, A.; Schaumann, G.E.; Lang, F. The fate of silver nanoparticles in soil solution—Sorption of solutes and aggregation. Sci. Total Environ. 2015, 535, 54–60. [Google Scholar] [CrossRef]
- Gao, X.Y.; Spielman-Sun, E.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. Time and Nanoparticle Concentration Affect the Extractability of Cu from CuO NP-Amended Soil. Environ. Sci. Technol. 2017, 51, 2226–2234. [Google Scholar] [CrossRef]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO Nanoparticle Dissolution and Toxicity to Wheat (Triticum aestivum) in Rhizosphere Soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Whitley, A.; Levard, C.; Oostveen, E.; Bertsch, P.M.; Matocha, C.J.; von der Kammer, F.; Unrine, J.M. Behavior of Ag nanoparticles in soil: Effects of particle surface coating, aging and sewage sludge amendment. Environ. Pollut. 2013, 182, 141–149. [Google Scholar] [CrossRef]
- Hedberg, J.; Oromieh, A.G.; Kleja, D.B.; Wallinder, I.O. Sorption and dissolution of bare and coated silver nanoparticles in soil suspensions—Influence of soil and particle characteristics. J. Environ. Sci. Health Part A 2015, 50, 891–900. [Google Scholar]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Navarro, D.A.; Kirby, J.K.; McLaughlin, M.J.; Waddington, L.; Kookana, R.S. Remobilisation of silver and silver sulphide nanoparticles in soils. Environ. Pollut. 2014, 193, 102–110. [Google Scholar] [CrossRef]
- Doolette, C.L.; McLaughlin, M.J.; Kirby, J.K.; Navarro, D.A. Bioavailability of silver and silver sulfide nanoparticles to lettuce (Lactuca sativa): Effect of agricultural amendments on plant uptake. J. Hazard. Mater. 2015, 300, 788–795. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hotze, E.M.; Bernhardt, E.S.; Dionysiou, D.D.; Pedersen, J.A.; Wiesner, M.R.; Xing, B.S. Environmental Occurrences, Behavior, Fate, and Ecological Effects of Nanomaterials: An Introduction to the Special Series. J. Environ. Qual. 2010, 39, 1867–1874. [Google Scholar] [CrossRef]
- Sagee, O.; Dror, I.; Berkowitz, B. Transport of silver nanoparticles (AgNPs) in soil. Chemosphere 2012, 88, 670–675. [Google Scholar] [CrossRef]
- Tavares, D.S.; Rodrigues, S.M.; Cruz, N.; Carvalho, C.; Teixeira, T.; Carvalho, L.; Duarte, A.C.; Trindade, T.; Pereira, E.; Römkens, P.F.A.M. Soil-pore water distribution of silver and gold engineered nanoparticles in undisturbed soils under unsaturated conditions. Chemosphere 2015, 136, 86–94. [Google Scholar] [CrossRef]
- Römkens, P.F.A.M.; Guo, H.Y.; Chu, C.L.; Liu, T.S.; Chiang, C.F.; Koopmans, G.F. Characterization of soil heavy metal pools in paddy fields in Taiwan: Chemical extraction and solid-solution partitioning. J. Soils Sediments 2009, 9, 216–228. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Cruz, N.; Coelho, C.; Henriques, B.; Carvalho, L.; Duarte, A.C.; Pereira, M.E.; Römkens, P.F.A.M. Risk assessment for Cd Cu Pb and Zn in urban soils: Chemical availability as the central concept. Environ. Pollut. 2013, 183, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Henriques, B.; Ferreira da Silva, E.; Pereira, M.E.; Duarte, A.C.; Römkens, P.F.A.M. Evaluation of an approach for the characterization of reactive and available pools of twenty potentially toxic elements in soils: Part I—The role of key soil properties in the variation of contaminants reactivity. Chemosphere 2010, 81, 1549–1559. [Google Scholar] [CrossRef]
- Fedotov, P.S.; Kördel, W.; Miró, M.; Peijnenburg, W.J.G.M.; Wennrich, R.; Huang, P. Extraction and Fractionation Methods for Exposure Assessment of Trace Metals, Metalloids, and Hazardous Organic Compounds in Terrestrial Environments. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1117–1171. [Google Scholar] [CrossRef]
- Hänsch, M.; Emmerling, C. Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J. Plant Nutr. Soil Sci. 2010, 173, 554–558. [Google Scholar] [CrossRef]
- Velicogna, J.R.; Ritchie, E.E.; Scroggins, R.P.; Princz, J.I. A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils. Nanotoxicology 2016, 10, 1144–1151. [Google Scholar] [CrossRef]
- Peyrot, C.; Wilkinson, K.J.; Desrosiers, M.; Sauvé, S. Effects of silver nanoparticles on soil enzyme activities with and without added organic matter. Environ. Toxicol. Chem. 2014, 33, 115–125. [Google Scholar] [CrossRef]
- Gaddam, D.P.; Devamma, N.; Prasad, T.N.V.K.V. Evaluation of the effect of indigenous mycogenic silver nanoparticles on soil exo-enzymes in barite mine contaminated soils. Appl. Nanosci. 2015, 5, 505–513. [Google Scholar] [CrossRef][Green Version]
- Shin, Y.J.; Kwak, J.I.; An, Y.J. Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere 2012, 88, 524–529. [Google Scholar] [CrossRef]
- Bascomb, C.L. Method for the determination of cation exchange capacity of calcareous and non-calcareous soils. J. Sci. Food Agric. 1964, 15, 821–823. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z.; Kowalski, Z. Silver nanoparticles synthesis with different concentrations of polyvinylpyrrolidone. Dig. J. Nanomater. Biostruct. 2012, 7, 1527–1534. [Google Scholar]
- Unrine, J.M.; Hunyadi, S.E.; Tsyusko, O.V.; Rao, W.; Shoults-Wilson, W.A.; Bertsch, P.M. Evidence for Bioavailability of Au Nanoparticles from Soil and Biodistribution within Earthworms (Eisenia fetida). Environ. Sci. Technol. 2010, 44, 8308–8313. [Google Scholar] [CrossRef]
- Shoutz-Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.L.; Unrine, J.M. Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida). Nanotoxicology 2011, 5, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- USEPA. Standard Operating Procedure for an In Vitro Bioaccessibility Assay for Lead in Soil EPA 9200; Environmental Protection Agency: Washington, DC, USA, 2008; pp. 1–86.
- Houba, V.J.G.; van der Lee, J.J.; Novozamsky, I. Part 5b: Soil analysis procedures. In Soil and Plant Analysis a Series of Syllabi; Houba, V.J.G., Novozamsky, I., Eds.; Department of Soil Science and Plant Nutrition of Wageningen Agricultural University: Wageningen, The Netherlands, 1995; 262p. [Google Scholar]
- Alef, K.; Nannipieri, P.; Trasar-Cepeda, C. Phosphatase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 335–344. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. β-Glucosidase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 350–352. [Google Scholar]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulase in a silt loam soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Protease activity. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 313–315. [Google Scholar]
- Inácio, M.; Pereira, V.; Pinto, M. The Soil Geochemical Atlas of Portugal: Overview and applications. J. Geochem. Explor. 2008, 98, 22–33. [Google Scholar] [CrossRef]
- Cornelis, G.; Kirby, J.K.; Beak, D.; Chittleborough, D.; McLaughlin, M.J. A method for determination of retention of silver and cerium oxide manufactured nanoparticles in soils. Environ. Chem. 2010, 7, 298–308. [Google Scholar] [CrossRef]
- Settimio, L.; McLaughlin, M.J.; Kirby, J.K.; Langdon, K.A.; Lombi, E.; Donner, E.; Scheckel, K.G. Fate and lability of silver in soils: Effect of ageing. Environ. Pollut. 2014, 191, 151–157. [Google Scholar] [CrossRef]
- VanderVoot, A.R.; Tappero, R.; Arai, Y. Residence time effects on phase transformation of nanosilver in reduced soils. Environ. Sci. Pollut. Res. 2014, 21, 7828–7837. [Google Scholar]
- Cruz, N.; Rodrigues, S.M.; Tavares, D.; Monteiro, R.J.R.; Carvalho, L.; Trindade, T.; Duarte, A.C.; Pereira, E.; Romkens, P.F.A.M. Testing single extraction methods and in vitro tests to assess the geochemical reactivity and human bioaccessibility of silver in urban soils amended with silver nanoparticles. Chemosphere 2015, 135, 304–311. [Google Scholar] [CrossRef]
- Hamon, R.E.; Parker, D.R.; Lombi, E. Advances in isotopic dilution techniques in trace element research: A review of methodologies, benefits and limitations. Adv. Agron. 2008, 99, 289–343. [Google Scholar]
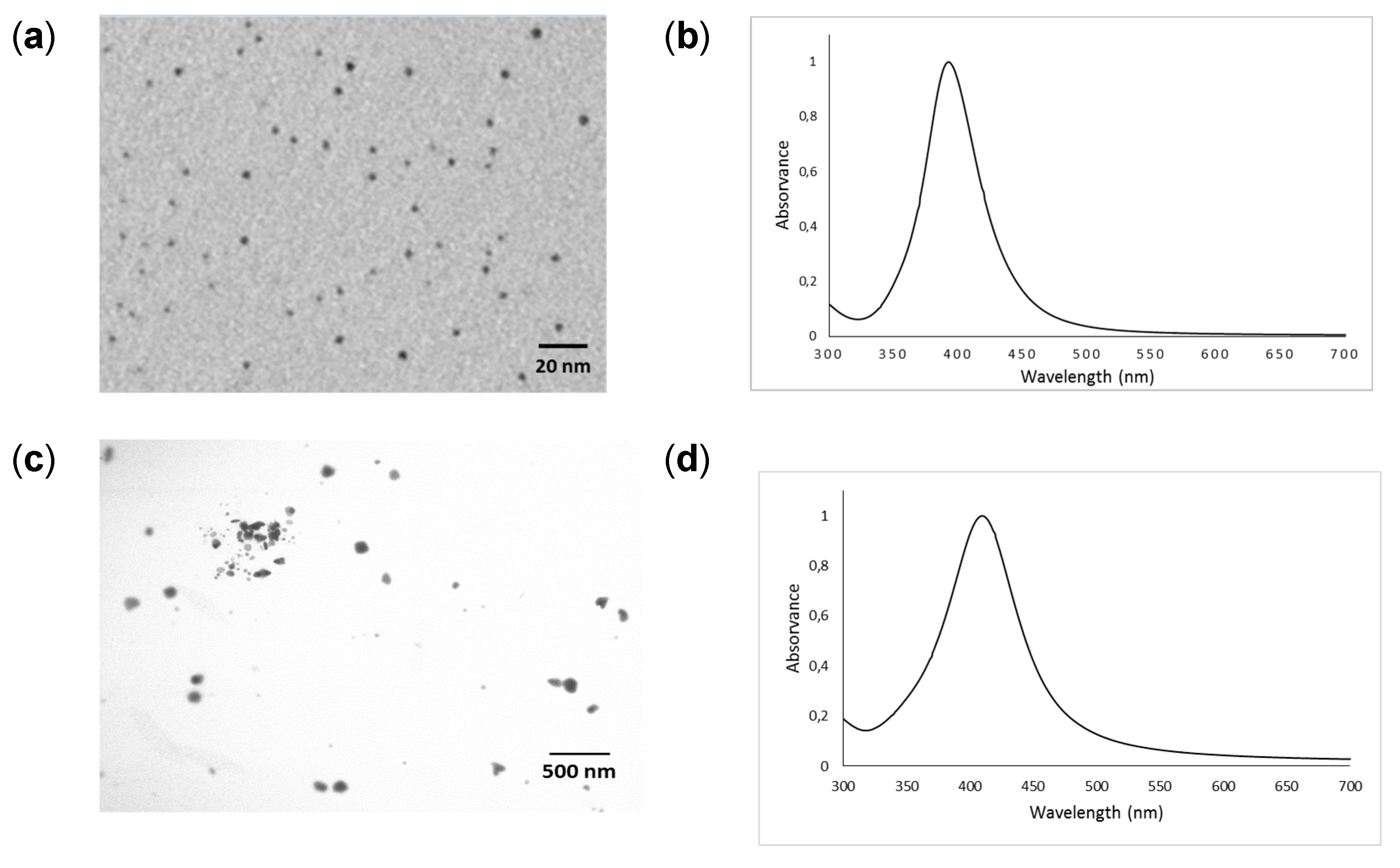
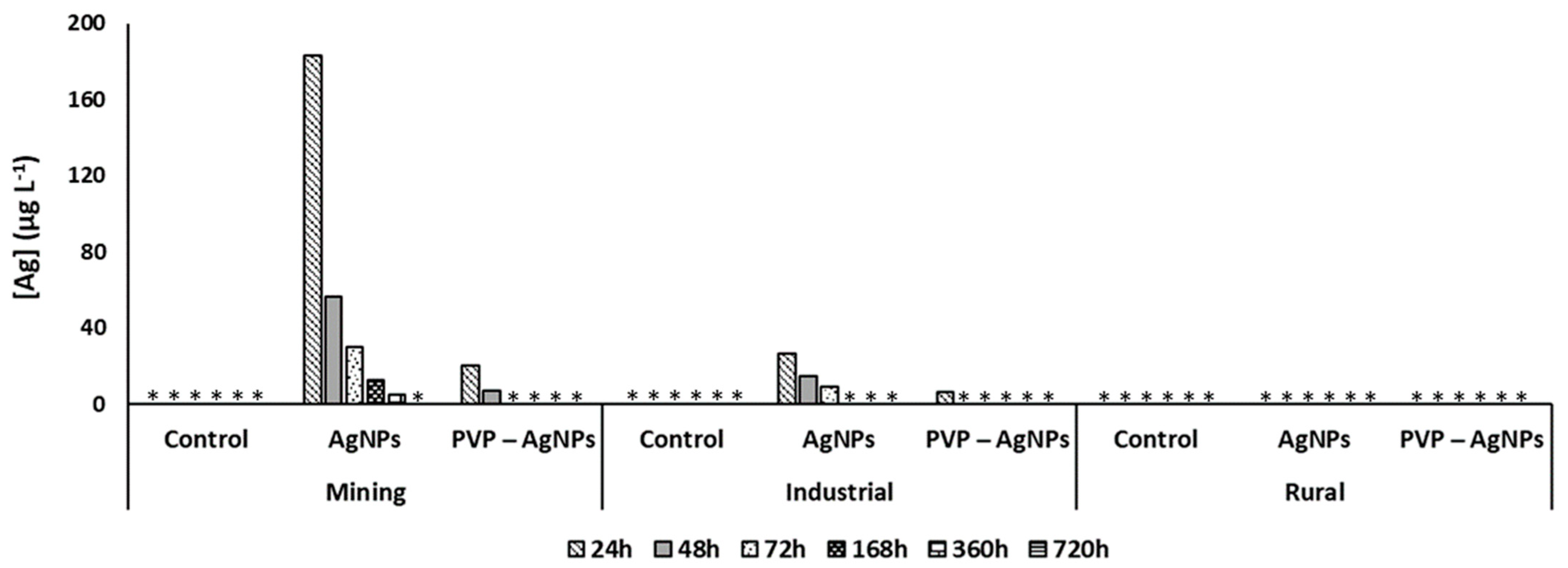
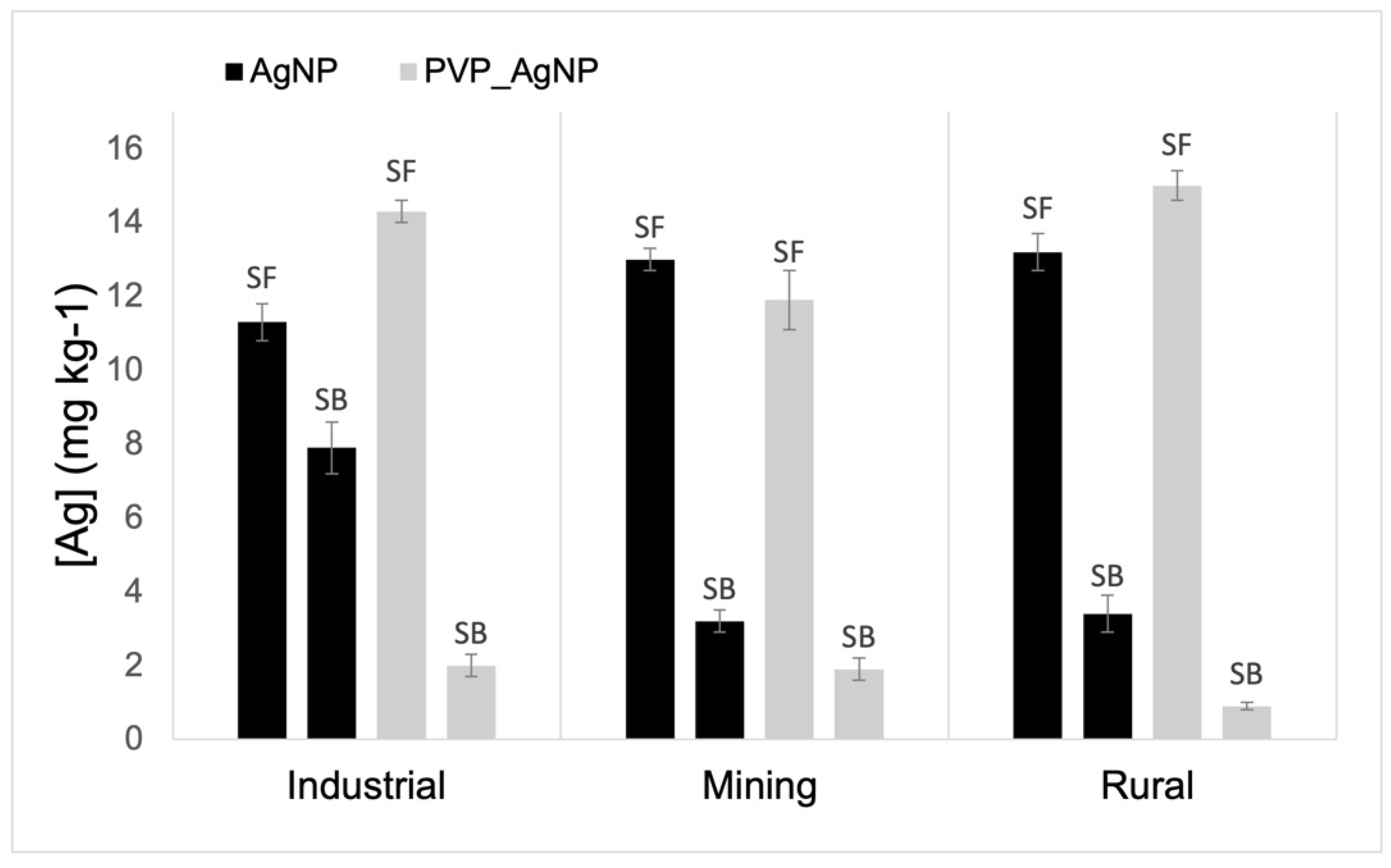


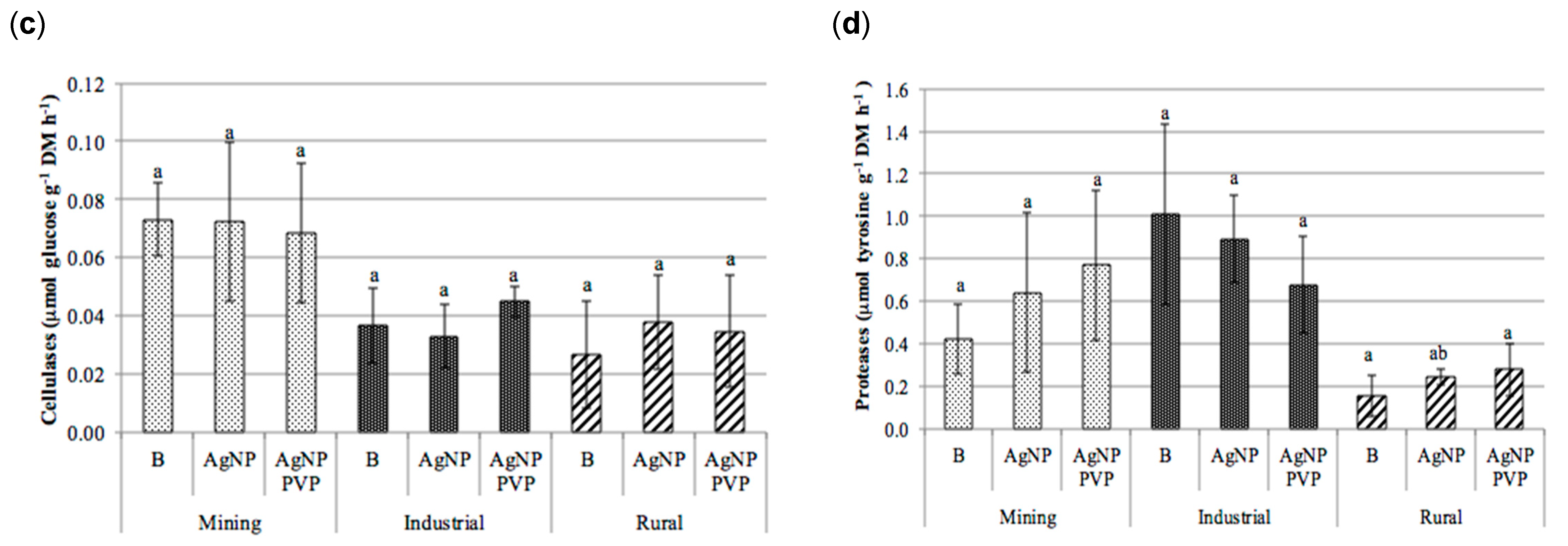
| Land Use | pH (CaCl2) | Org C | CEC | Clay | Al | Fe |
|---|---|---|---|---|---|---|
| % | meq/100 g | % | % | % | ||
| Mining | 4.4 | 1.2 | 16 | 19 | 1.3 | 3.7 |
| Industrial | 4.9 | 2.1 | 12 | 2.6 | 0.5 | 1.1 |
| Rural | 6.3 | 0.8 | 33 | 23 | 1.7 | 3.9 |
| F Factor | |||||
|---|---|---|---|---|---|
| Main Effects | D.f. | Acid Phosphatase | β-Glucosidase | Cellulases | Proteases |
| A: Type of soil | 2 | 163.68 *** | 259.31 *** | 38.01 *** | 42.12 *** |
| B: AgNP treatment | 2 | 8.58 *** | 1.10 n.s. | 0.32 n.s. | 0.42 n.s. |
| Interaction: AxB | 4 | 4.59 ** | 7.63 *** | 0.96 n.s. | 4.10 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, N.C.; Farto, M.; Mourinha, C.; Tavares, D.; Duarte, A.C.; Trindade, T.; Pereira, E.; Römkens, P.F.A.M.; Alvarenga, P.; Rodrigues, S.M. Dissolution of Ag Nanoparticles in Agricultural Soils and Effects on Soil Exoenzyme Activities. Environments 2021, 8, 22. https://doi.org/10.3390/environments8030022
Cruz NC, Farto M, Mourinha C, Tavares D, Duarte AC, Trindade T, Pereira E, Römkens PFAM, Alvarenga P, Rodrigues SM. Dissolution of Ag Nanoparticles in Agricultural Soils and Effects on Soil Exoenzyme Activities. Environments. 2021; 8(3):22. https://doi.org/10.3390/environments8030022
Chicago/Turabian StyleCruz, Nuno C., Márcia Farto, Clarisse Mourinha, Daniela Tavares, Armando C. Duarte, Tito Trindade, Eduarda Pereira, Paul F. A. M. Römkens, Paula Alvarenga, and Sónia M. Rodrigues. 2021. "Dissolution of Ag Nanoparticles in Agricultural Soils and Effects on Soil Exoenzyme Activities" Environments 8, no. 3: 22. https://doi.org/10.3390/environments8030022
APA StyleCruz, N. C., Farto, M., Mourinha, C., Tavares, D., Duarte, A. C., Trindade, T., Pereira, E., Römkens, P. F. A. M., Alvarenga, P., & Rodrigues, S. M. (2021). Dissolution of Ag Nanoparticles in Agricultural Soils and Effects on Soil Exoenzyme Activities. Environments, 8(3), 22. https://doi.org/10.3390/environments8030022











