Abstract
This review systematically outlines the recent advances in the application of circular bioeconomy technologies for converting agricultural wastewater to value-added resources. The properties and applications of the value-added products from agricultural wastewater are first summarized. Various types of agricultural wastewater, such as piggery wastewater and digestate from anaerobic digestion, are focused on. Next, different types of circular technologies for recovery of humic substances (e.g., humin, humic acids and fulvic acids) and nutrients (e.g., nitrogen and phosphorus) from agricultural wastewater are reviewed and discussed. Advanced technologies, such as chemical precipitation, membrane separation and electrokinetic separation, are evaluated. The environmental benefits of the circular technologies compared to conventional wastewater treatment processes are also addressed. Lastly, the perspectives and prospects of the circular technologies for agricultural wastewater are provided.
1. Introduction
Clean drinking water is a crucial substance needed by all living beings to survive. Contaminated drinking water can result in the spread of various diseases [1,2]. Thus, the United Nations placed clean drinking water on its priority list of Sustainable Development Goals (SDG-6: Clean Water and Sanitation) for achieving overall sustainability. To achieve this goal, each country decided its quality standards to ensure cleanliness and hygiene. Water collected from the river and groundwater, or reused wastewater from industry and agriculture, water must be filtered or processed before the distribution. In general, water pollutants may originate from point sources or dispersed sources. Dispersed source pollutants are uncontrollable, carrying plant nutrition, pathogenic organisms, organic-inorganic chemicals and pesticides, due to their unconfined locations. The major dispersed sources include urban stormwater drainage and illegal effluent, i.e., especially from agriculture and industries wastewater. To avoid pollution and obtain more available water, it is essential to enforce proper land use plans and develop more eco-efficient technologies for wastewater treatment.
Wastewater is any type of water that has undergone some sort of human intervention and is then not able to be disposed of into surface or ground waters without certain treatment. Agricultural wastewater is fundamentally the excess water that emerges from fields into basins, furrows, border strips and flooded areas during irrigation. Such types of agricultural wastewater are also referred to as “irrigation tailwater”. Another source of agricultural wastewater is from the harvesting of plants and preparation of those plants for processing foods, which is usually composed of fats/oils, nutrients, disease causing bacteria and viruses, high biochemical oxygen demand and suspended solids [3]. In other words, agricultural wastewater consists of different forms of contaminants, and it should be treated properly before being disposed of in the environment. If the wastewater is disposed of inappropriately, it would cause eutrophication in surface and ground waters, leading to heavy trade waste charges.
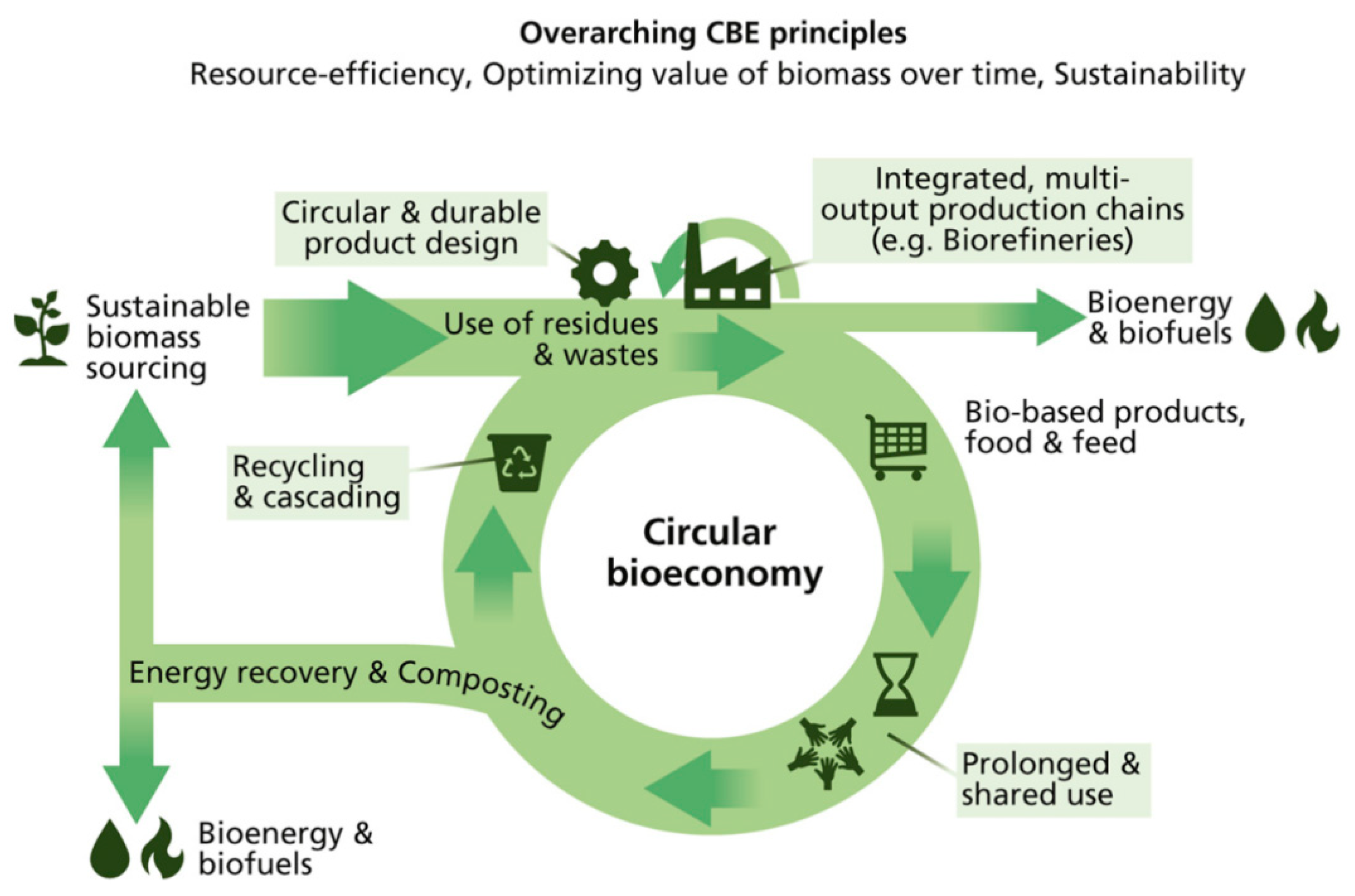
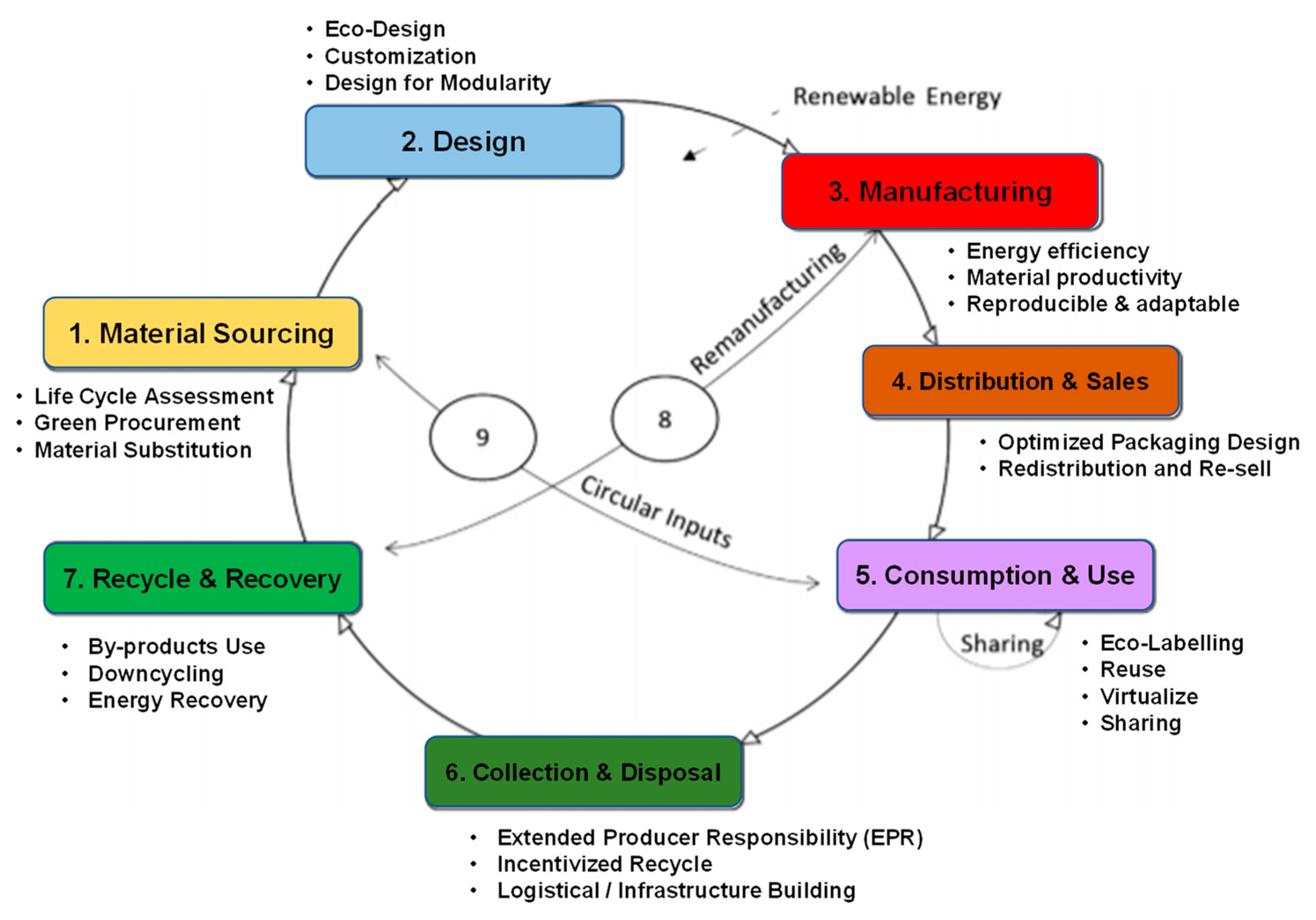
In general, agricultural wastewater includes several major sources: (1) husbandry wastewater, (2) aquaculture wastewater, (3) liquid digestate from anaerobic digestion using agricultural wastes and (4) surface runoff from farmlands. Owing to the different physicochemical properties of the sources, the subsequent treatment processes are different accordingly. For farming processes, pollution is generated due to surface runoff, which contains pesticides, animal feed stocks, slurries and organic residues. Nitrogen and phosphorus are the key pollutants in agricultural runoff which are applied to agricultural land in the form of pesticides, manures, effluent or sludge [4,5]. Apart from nitrogen and phosphorus, humic substances are the additional pollutants found with heavy agricultural runoff. However, the surface runoff from farmlands is difficult to monitor and measure. Instead, husbandry wastewater, aquaculture wastewater and liquid digestate are easy to collect and utilize, which provides opportunities to realize a circular economy. Table 1 compiles the physicochemical characteristics of different agricultural point–source wastewaters reported in the literature. In general, agricultural point–source wastewaters contain a number of elements, such as organic carbons (humic substances), volatile fatty acids, nutrients (e.g., phosphorus and nitrogen) and metal ions. These components can be considered to be raw materials of various chemical compounds. The United Nations announced the concept of closing the loop of circular economy in 2014, which would decouple economic growth and environmental degradation. The concept of a circular bioeconomy (CBE) aims to create a sustainable and resource-efficient world with a low carbon footprint [6]. As shown in Figure 1, the life of bio-based production can be prolonged to become the source of biomass and bioenergy with integrated and multi-output production chains. Energy efficiency and resource sustainability are the main concepts in CBE, and thus CBE pushes the practitioner towards an energy-efficient and renewable method of agriculture production. Phosphorus has usually been recovered and separated by precipitation in the form of crystalline struvite, and can be utilized as fertilizers in the agriculture department. Thus, the valuable resources in agricultural wastewater should be recycled and reused for environmental sustainability.

Table 1.
Physicochemical characteristics of different agricultural wastewaters reported in the literature. a.
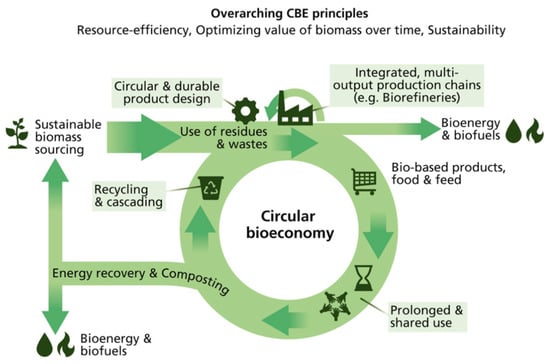
Figure 1.
The concept of the circular bioeconomy (CBE) and its elements. Adapted from the ref. [6].
This review describes and evaluates the performances of different circular technologies for converting agricultural wastewater into value-added resources. These substances can be utilized for beneficial activities, such as plant growth, metabolism and seed germination. We firstly summarize the characteristics of potential value-added products from agricultural wastewater, such as humic substances and nutrients. Then, we discuss the advances in the application of CBE technologies for converting agricultural wastewater to value-added products. We also summarize the environmental and economic benefits of these circular technologies. Lastly, we illustrate the perspectives and prospects of the circular technologies for agricultural wastewater towards CBE system.
2. Value-Added Products from Agricultural Wastewater: Properties and Applications
2.1. Organic (Humic) Substances
One of the main compositions in agricultural wastewater is organic and/or humic substances, which are usually classified as natural organic matter (NOM) [15]. NOM are organic compounds produced directly from biological and chemical degradation of animal and plant remnants. They are a complex mixture of organic materials including viruses, bacteria, polysaccharides, proteins and humic elements (such as fulvic acids and humic acids). Water with these substances may become yellow-brownish, odorous and have foul taste, which makes water undrinkable [16]. Meanwhile, NOM could cause a number of technical issues in water treatment [17], such as membrane fouling and the formation of dihaloacetonitrile (toxic to human health) during chlorination processes. NOM have various shapes, sizes and molecular weights, and can be classified into two broad categories: (1) hydrophobic compounds and (2) hydrophilic compounds. Hydrophobic compounds are made up of aromatic carbon, phenolic structures and conjugated carbon double bonds, and consist of mostly humic and fulvic substances. Hydrophilic compounds are made up of aliphatic carbon structures, nitrogen structures and other compounds, such as proteins, carboxylic acids and carbohydrates. Table 2 summarizes the classifications and characteristics of organic substances that are commonly found in agricultural wastewater. For instance, humic substances are the most chemically active compounds in soil that undergo anion and cation exchange with soil. These humic substances are mixtures including phenolic and carboxylic substituents, which form humic acid. The humic acid is highly reactive with ions, such as Mg2+, Ca2+, Fe2+, and Fe3+, due to its reactivity to form chelating agent metal complexes.

Table 2.
Classifications and characteristics of natural organic matter that are commonly found in agricultural wastewater.
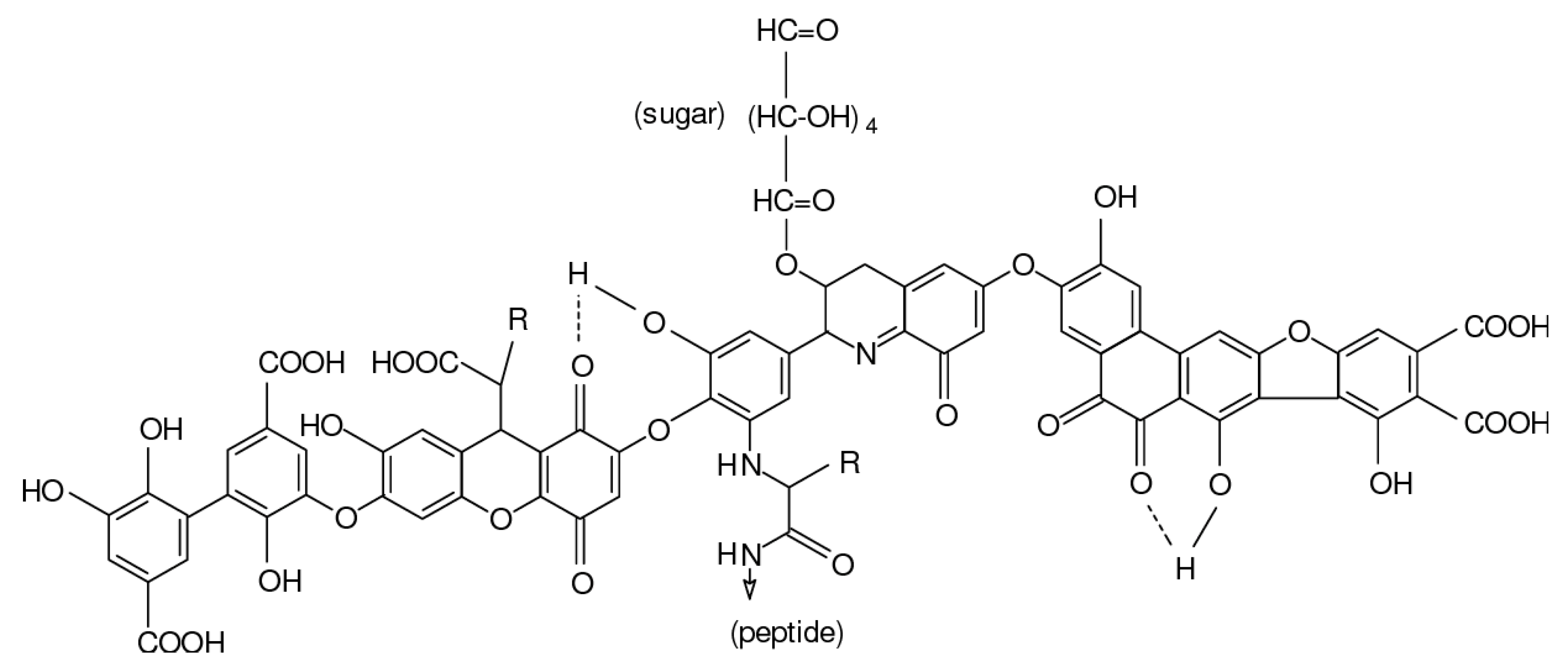
Humic acids consist of humic substances that are organic matter residing in sediments of vegetable decay and residue, terrestrial soil as well as natural water. Humic acid is also one of the major substances that need to be recovered from agricultural wastewater. This compound is an amphiphilic substance and is partially soluble in water, completely soluble in alkaline solutions and insoluble in acidic solutions. This property makes it widely used in medicine, pharmaceuticals, pollution remediation and agriculture. As a weak acid, humic acids are found in substances used as an electrolyte and contains carboxylic (-C(=O)OH) and phenolic groups. It is also a parent form of another organic compound called fulvic acid. They differ in their oxygen and carbon contents, acidity, molecular weight and polymerization. The way to form humic acid is through natural and sustainable fermentation using an empty fruit bunch of palm trees as a substrate. It can also be produced by chemical methods such as polymerization and condensation reactions [33]. Figure 2 depicts the model molecular structure of humic acid predicted by Stevenson in 1982 [34]. The structure of humic acid is complex, with many binding sites where several interactions can take place between humic acid and the environmental media (e.g., soil and water). Each binding site of the compound has a different form of stereochemistry, which is determined by the interactions and mechanisms with other compounds [35].
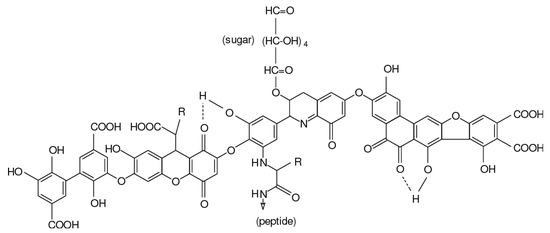
Figure 2.
Model structure of humic acid according to Stevenson [34].
Humic acid is closely associated with plant nutrition as it binds to the roots and shoots of plants to help them receive water and nutrients. It can also progress activities with heavy metal complexation, anti-inflammation and anti-viruses [36]. As a result, the deficiency of humic acid hinders farmers and gardeners from providing their crops with optimum nutrition. Rose et al. [37] conducted a meta-analysis by reviewing the plant growth responses to humic substances and found that the growth of both the roots and shoots could be enhanced by 15−20% after introducing humic acid to the plants. The key mechanism is that the stability of the humic substance–micronutrient complex brings micronutrients to the plants [37]. The sources of humic acid are critical when choosing elements for plant growth. Humic substances extracted from peat and coal are less effective than those extracted from compost and soil. Those derived from compost and soil have a positive effect on plant growth due to their chemical formula and structure. The amide functional group in the humic substances depletes more quickly in the soil and compost, showing that the rate of its biological activities through decomposition is faster. On the other hand, when using humic acid as an organic fertilizer for plant growth, the recovery of humic acid from agricultural wastewater becomes more important. Various ways to recover humic acid from agricultural wastewater have been developed [38]. Nonetheless, humic acid is not the only nutrient that plants need. Phosphates and ammonium are also important for farming. The information on nitrogen- and phosphorus-based nutrients and the methods to recover value-added products from agricultural wastewater are described in the coming chapter.
2.2. Nitrogen- and Phosphorus-Based Nutrients
Besides humic substances, there are many other value-added substances that are necessary to be recovered or removed from agricultural wastewater, such as nitrogen and phosphorus. Nitrogen (e.g., ammonium) and phosphorus (e.g., phosphates) are important for farming and have many external uses, such as fish and animal feeds and biofuels. For instance, several studies have reported that about 5–7% of global energy production is consumed for the mining of phosphorus-containing rocks [39,40,41,42]. However, research has reported that inefficient utilization of phosphorus will be the main cause of increased eutrophication and leakage of fertilizers, detergent and sewage containing phosphorus into water bodies [43]. This provides evidence to highlight the importance and significance of recovering nutrients from agricultural wastewater [44]. These nutrients are commonly removed and then recovered through processes known as nitrification and phosphate struvite precipitation, with the aid of several other methods.
The demand for nitrogen, especially nitrogen-based fertilizers, has rapidly increased. Currently, these nitrogen-based fertilizers are manufactured through the Haber–Bosch process to produce ammonia or other nitrogen-based materials. The Haber–Bosch process chemically combines nitrogen and hydrogen to form ammonia using catalysts and reproves in the production of plant fertilizers. The nitrogen recovered from wastewater gets diffused into the air as N2 gas. For this process, a lot of energy is required due to food production, and the requirement of nitrogen is also high. For wastewater, nitrogen recovery generally requires an energy-intensive process to be converted into fertilizers, which means that technologies used for the recovery have to be properly chosen [45].
Phosphorus is a non-renewable resource that is commonly mined underground to be used as fertilizers. Phosphorus also comes in limited quantities and cannot be replaced by any other element, which makes it even more crucial that it be efficiently recovered. It is an element that is vital for human life and food production. Therefore, the use of phosphorus is essential because of its role in fertilizer; it converts nutrients into disposable building blocks that are vital for the plants to grow [46]. In addition to this element’s use in fertilizers, it is a crucial component of adenosine triphosphate, i.e., the form of energy in plants produced during photosynthesis.
3. Recovery of Humic Substances from Agricultural Wastewater
There are bare databases on the recovery of humic acid since it possesses a large and versatile structure with 187 carbon atoms. Nevertheless, several approaches can be found in the literature to remove (bare recovery) humic substances from agricultural wastewater: (1) chemical method with NaOH and KOH such as alkaline solvents and (2) physical separation methods, such as coagulation–flocculation and membrane filtration [47]. The coagulation–flocculation method is conducted using a coagulant to separate solid particles from the liquid, which destabilizes pollutants through a reaction with the water and gets rid of the solid particles. Membrane filtration methods depend on their pore size and functions to separate the humic substance. Li et al. [48] indicated that alkaline pretreatment, ultrafiltration separation and subsequent anaerobic digestion are considered to be effective for the recovery or removal of humic substances. Kliaugaitė et al. [49] also indicated that electrochemical methods should provide a valid option for the recovery of humic substances and could potentially emulate other coagulation methods and conventional oxidation. Several important processes are illustrated to remove or recover humic substances from agricultural wastewater.
3.1. Coagulation and Flocculation
Coagulation and flocculation are the common methods for agricultural wastewater and raw water treatments. There are two types of coagulation methods that can be used: chemical coagulation and electrocoagulation. Both methods can remove humic substances from wastewater, but they have different mechanisms. Coagulation and flocculation play a huge role in wastewater treatment by separating solid particles from the liquid. After coagulation happens, the process of floatation occurs to grow floc, and then sedimentation filtration can be conducted for the final stage of recovery. In chemical coagulation, specific coagulants are used and react in water to form hydrolysis products (such as Al(OH)3). These coagulants are usually aluminum- or iron-based salts. Equation (1) represents the coagulation mechanism of aluminum sulfate released into the water, which reacts with calcium bicarbonate to form aluminum hydroxide precipitates.
Al2(SO4)3 + 3 Ca(HCO3)2 → 2 Al(OH)3 + 3 CaSO4 + 6 CO2
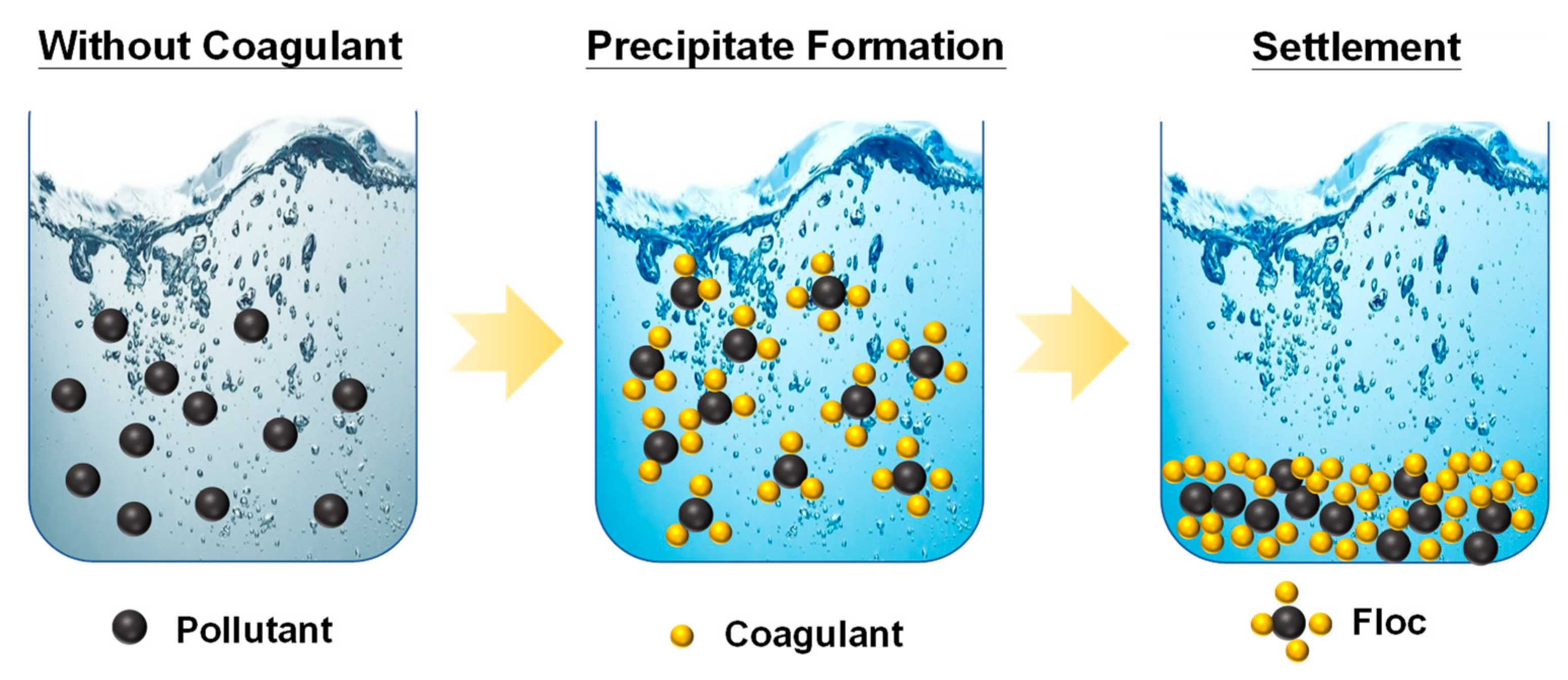
The most widely used aluminum coagulants include aluminum chloride, aluminum sulfate and sodium aluminate. Common iron salts used as coagulants include ferrous sulfate, ferric sulfate, ferric chloride sulfate and ferric chloride. The metal cation from these salts and the hydroxide from the water can reduce repulsion forces between the colloids (see Figure 3) and trap particles with their coagulant, which makes them destabilize the pollutants [50]. The performance of the coagulants depends on the pH of the solution, the mixing intensity and the dose of coagulants. Finally, the trapped particles settle to the bottom and form a bottom layer of sludge. However, a significant amount of sludge forms as a result of the coagulants reacting with humic substances in the water. These sludges can be quite hazardous at times, which make them difficult to dispose of. It can also be extremely difficult to separate the formed sludge from the water due to their heavy water content.
Figure 3.
Principles of chemical coagulation used for removing colloidal pollutants in wastewater.
Electrocoagulation is an alternative technique to chemical coagulation. It has been proven to be an efficient method for the removal of contaminants from agricultural wastewater. This method also destabilizes pollutants without the use of chemicals. Oils, suspended solids, heavy metals and greasy particles can be recovered thoroughly. Instead of utilizing salts as coagulants, electrical currents are passed through the water and provide the electromotive force required for such chemical reactions to take place. By introducing the electrical current into the water, this process can undermine dissolved, emulsified and suspended contaminants in the aqueous medium. In general, aluminum electrodes are used in electrocoagulation with the cathodic (Equation (2)) and anodic (Equation (3)) reactions. One of the main limitations of electrocoagulation is electrode passivation, which occurs when the metal used in the process coats the anode. This phenomenon is disadvantageous to electrocoagulation because once the metal coats the anode it limits the number of metal ions and amount of current that can be further passed through into the water [51].
Cathodic reaction: 2H+ + 2e− → H2
Anodic reaction (or any other metal present): Al → Al3+ + 3e−
Both chemical coagulation and electrocoagulation treatment are followed by flocculation afterwards. Technically, coagulation takes place successively because the events keep cycling. Flocculation is when the fine, destabilized particles from coagulation collide together and then accumulate to form clumps, which are technically recognized as “flocs” [52]. Compared to coagulation, flocculation is a relatively slow process that involves gentle mixing of the destabilized particles in the water. This is also regarded as an electro-neutralization process where the positively charged and destabilized particles are adsorbed onto negatively charged substances, which are the ones that result in the color, clay, turbidity and other particles. The particles must be gently mixed so that they have the opportunity to adsorb instead of splitting further apart within the water in harsh conditions. The rate of flocculation is directly proportional to the velocity gradient of mixing [53]. After flocculation, the particles either sink to the bottom sedimentation or float up to the top for further recovery processes.
3.2. Membrane Filtration
Membrane filtration processes are one of the most feasible and efficient methods due to a number of advantages, such as relatively low energy consumption and compactness [54]. Membrane filtration is a crucial process in wastewater treatment because it can be used to separate humic acid without any chemical contaminations [55]. Membrane filtration methods generally include microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO). These membrane processes have different features and serve different purposes of application [56]. Table 3 describes the various physicochemical parameters, such as pressure, pore size and operational cost of different membrane filtration processes. MF involves the largest pore size among the four processes along with low operational costs; therefore, it is mainly used within the dairy and wastewater treatment industry. It is used for purification, separation and concentration of large macromolecules. UF utilizes a fairly high operational pump pressure compared to MF, and thus UF membranes are used for reusing and recycling wastewater that does not contain any solid material. UF is preferred over other traditional wastewater treatment methods because no chemicals are used. The quality of the treated water remains constant and the plant size is closely packed and it is capable of removing 90–100% of the pathogens from the water [57]. However, the pressure can occasionally be too high for smaller flocculation particles, and this is difficult to regulate and sample them accurately since flocs come in a variety of different sizes. In general, MF and UF are used to remove particles, turbidity, microorganisms and natural organic matter [58,59]. Prisciandaro et al. [54] suggested that the UF process should be more efficient than the MF processes, because MF membranes are only effective when the process is conducted in a tubular configuration.

Table 3.
Operating factors of different pressure-driven membranes for wastewater treatment.
NF has a small pore size as well as high operational costs. NF membranes cleave matter that can easily pass through UF membranes but cannot pass through RO membranes. It is considered to be one of the most promising technologies in this field for separating neutral and charged solutes dissolved in aqueous solutions [66]. NF membranes also have the unique ability to separate charged solutes in electrolytes in their process. RO is largely used in brackish water treatment for removing numerous types of ions, molecules and bacteria from different solutions. RO is also widely used in the potable water production industry; however, it requires higher operational costs. Due to its non-porous characteristics, only small substances can fit through, separating the molecules and ions by their sizes via applying pressure. A specialty of RO is that it is a self-cleansing system; it can self-clean through cross-flow where fluid passes through the membrane and the large molecules that get rejected from the membrane get swished away [67]. Through these membranes, the flow rate of the water passing can be measured by Equation (4):
where Δp (Nm−2) stands for the pressure drop; η (N s m−2) stands for the dynamic viscosity of the fluid; l (m) stands for the pore length; Q (m3s−1) stands for the volumetric flow rate; d (m) stands for the diameter of the pore. The pore diameter is vital in the flow rate of the water, indicating that since different membranes differ in pore size by a factor of 10, their water flux differs by a factor of 100 [68].
Membrane fouling is a phenomenon where foreign particles/impurities deposit and block the pores of membranes. It occurs when many substances accumulate on the surface of the membrane as well as the membrane pores, and results in the retrogression of the membrane’s performance when recovering chemicals from the water. The side effects of membrane fouling include the formation of disinfection byproducts, which can cause the water to diminish in quality as well as causing severe flux decline [69]. If the membrane fouling is more serious, it usually requires severe cleaning and even membrane replacement. Therefore, the prevention of membrane fouling usually results in a high operational cost for membrane filtration processes. NOM, such as humic acid, fulvic acid and tannic acid, often results in a consequential amount of membrane fouling when recovered from agricultural wastewater. This occurs because other materials, such as silica, clay, iron, oil and sulfur, are also present in agricultural wastewater. Other nutrients contained in agricultural wastewater are also classified within the NOM and are the main cause of membrane fouling [70]. Various approaches were developed to mitigate membrane fouling, such as coagulation, activated carbon adsorption and oxidation. These processes are mostly served as a pretreatment before membrane filtration to prevent the accumulation of substances on the pores of membranes [71]. Zhu et al. [58] investigated the effect of humic acid on membrane fouling and found that, other than the molecular weight loss and mineralization of the structure of humic acid, the other changes that occur in its structure after photocatalytic oxidation play a vital role in preventing membrane fouling. Photocatalytic oxidation works well for mitigating membrane fouling because it introduces oxygen-containing functional groups into the structure of humic acid. This can assist in increasing its hydrophilicity and eventually reducing its interactions with the membrane and its foulant [72].
3.3. Exchange Adsorption
Adsorption techniques are known to be one of the most efficient techniques for the removal of organic contaminants (or NOM) from wastewater. Compared to electrochemical methods or biological methods, adsorption is more reliable due to its simplicity, low operational costs and a limited amount of maintenance and supervision. Activated carbon is one of the most common adsorbents used in pollution control in the industries. It can be used for the removal of non-degradable organic and inorganic compounds from various sources of water, such as groundwater, process water and drinking water. In cases of removing pollutants from large and varied quantities, using other adsorbents—such as salicylic acid, silica or activated aluminum—may be an alternative option [63]. For manufacturing adsorbents (e.g., activated carbon), the raw ingredients can be found in the localities, such as agricultural wastes, natural materials and industrial wastes [73].
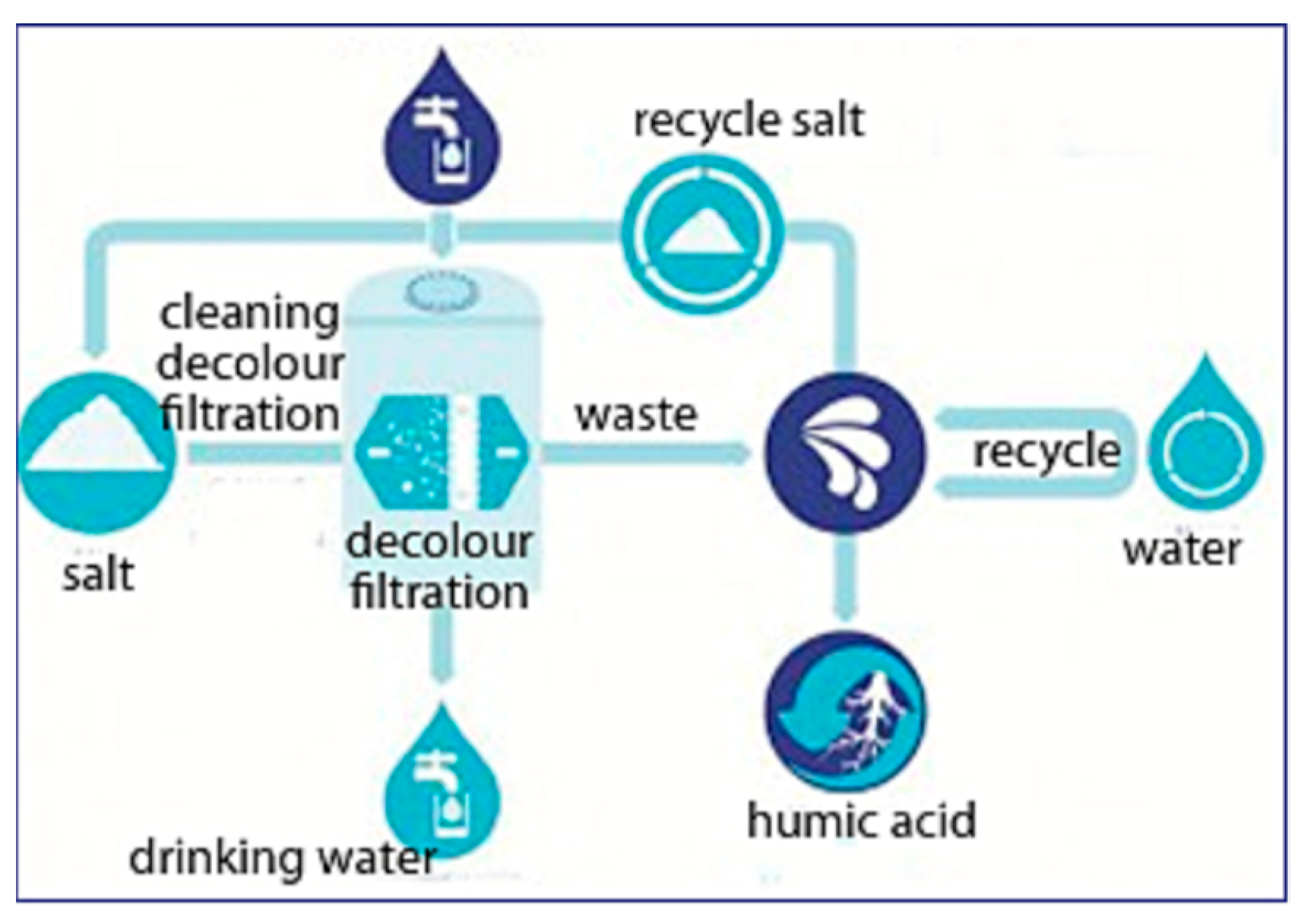
Adsorption is a surface phenomenon and is the mass transfer process that occurs when a substance is transferred to a solid from a liquid phase and bounded either chemically or physically. Therefore, the structure of the adsorbent surface plays a critical role in surface reactivity and adsorption capacity. When a solid with a reactive or ionic surface makes contact with a highly adsorbable solute, the intermolecular forces of attraction between the liquid and the solid surface allow the liquid to be deposited on the porous surface. The temperature, pH and column design also affect the adsorption efficiency. Recent solutions use exchange adsorption methods to remove and recover humic acid from wastewater [74]. Undergoing NF and then electrodialysis to regenerate both anions and cations, humic acid can at least be separated from other salts present in the surroundings. Nonetheless, there have been a couple of new inventions and ideas by scientists who have been trying to recover humic acid. In 2015, two companies, i.e., Royal Haskoning DHV (an engineering company) and Vitens (a water supply company) jointly invented a novel technology, called “HumVi” as shown in Figure 4, to recover humic acid in its natural state as a fertilizer. The HumVi technology originates in Holland and is used to reclaim humic acid in its purest form and other salts from agricultural wastewater [75]. There is not much information or many studies on this technology; however, the concept of the process flow and designs can be utilized in future research.
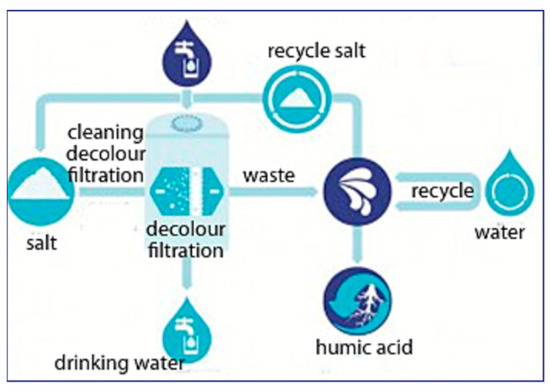
Figure 4.
Process flow diagram of the HumVi technology to recover humic acid from wastewater. Adapted from ref. [75].
3.4. Limitations and Opportunities
Since organic substances can be divided into a large variety of properties, it is difficult to recover the organic matter using a single method [76]. Conventionally, treatment processes can be used to remove humic substances from water, such as chemical coagulation, electrocoagulation, flocculation and membrane filtration followed by flotation and sedimentation. However, although the above methods are efficient in NOM removal, it is still hard to recover humic substances in a high concentration and purity because of their complex structures. From the technological point of view, due to its complex structure and great molecular size, we believe that the sorption-based separation should be more efficient in terms of energy expenditure, as compared to the pressure-driven and/or electrokinetic separation.
Furthermore, the recovered humic acid still has several limitations in its usage, especially in water and soil research. The main limitation is that it is still difficult to precisely identify the structure and characteristics of humic acid. Its characteristics are highly dependent on the structure, such as amphiphilic properties, solubility, pH dependence, metal chelation and hydrophobic interactions [77]. Malcolm and MacCarthy [78] collected samples of humic acid from five different sources, analyzed them through cross polarization and NMR spectroscopy, and then studied the relation between humic acids’ characteristics and the environmental media (such as streams and other water bodies). The results indicated that there were distinctive differences between the interactions of each sample of humic acid. With various sources and structures of humic acid, it is vital and necessary to identify a technique to separate and recover humic acid from agricultural water. It is important to estimate the potential and amount of humic acid that can be recovered. One of the simple ways to analyze the concentration of humic acid (or other NOM) is using ultraviolet-visible (UV-VIS) spectroscopy through coagulation. According to the principle of the Beer–Lambert Law, the UV-VIS absorbance obtained through specific wavelengths is directly proportional to the concentration of the substance [79]. Other indirect methods for analyzing the quantity of humic acid are the increase in surface area and solubility. However, varying the water-holding capacity of different sources of humic acids may interfere with the accuracy [80].
4. Recovery of Nutrients from Agricultural Wastewater
Several separation mechanisms can be used to remove and/or recover nitrogen and phosphorus, e.g., chemical crystallization, electrokinetic separation and bioconversion. Here, we illustrate the advances of several potential circular technologies for recovering nutrients from agricultural wastewater.
4.1. Struvite Precipitation
Struvite, known as magnesium ammonium phosphate hexahydrate (MgNH4PO4·6H2O) is a precipitated mineral containing ammonium, magnesium and phosphate ions [53]. Struvite is a form of white crystal which originates from either a neutral or alkali source. Nutrients, such as nitrogen and phosphorus, are recovered from wastewater through precipitation as crystalline struvite. The struvite crystallization method is a promising technique for reducing levels of water pollution due to the low utilization efficiency of phosphorus. Once phosphorus is recovered, it can be used in chemical industries immediately [81]. This crystalline struvite can be used as a fertilizer or a general raw material in the same industry [82]. As well as the recovery of phosphorus, struvite precipitation is an efficient technology for the removal of ammonium from acidic wastewater [83]. Equation (5) represents the chemical equation for the basic formation of struvite. This is one of the most common processes that happen through either precipitation or crystallization. Drawing the focus on the recovery of phosphorus, struvite formation is a relatively new and beneficial method for wastewater companies and industries, since the recovered phosphorus is beneficial for other agricultural use. However, one of the limitations of struvite precipitation is that, as seen in Equation (5), there is a release of hydrogen ions in the water when magnesium and ammonium react; therefore, the pH of water decreases. The pH of the solution is an extremely important factor for this process because an insufficient pH level generates the conversion of ammonium ions to gaseous ammonia, thereby reducing the nitrogen concentration in water and altering the Mg/N/P ratio [84].
Mg2+ + NH4+ + HnPO43−n + 6 H2O ⇔ MgNH4PO4·6H2O + n H+ (n = 0,1,2)
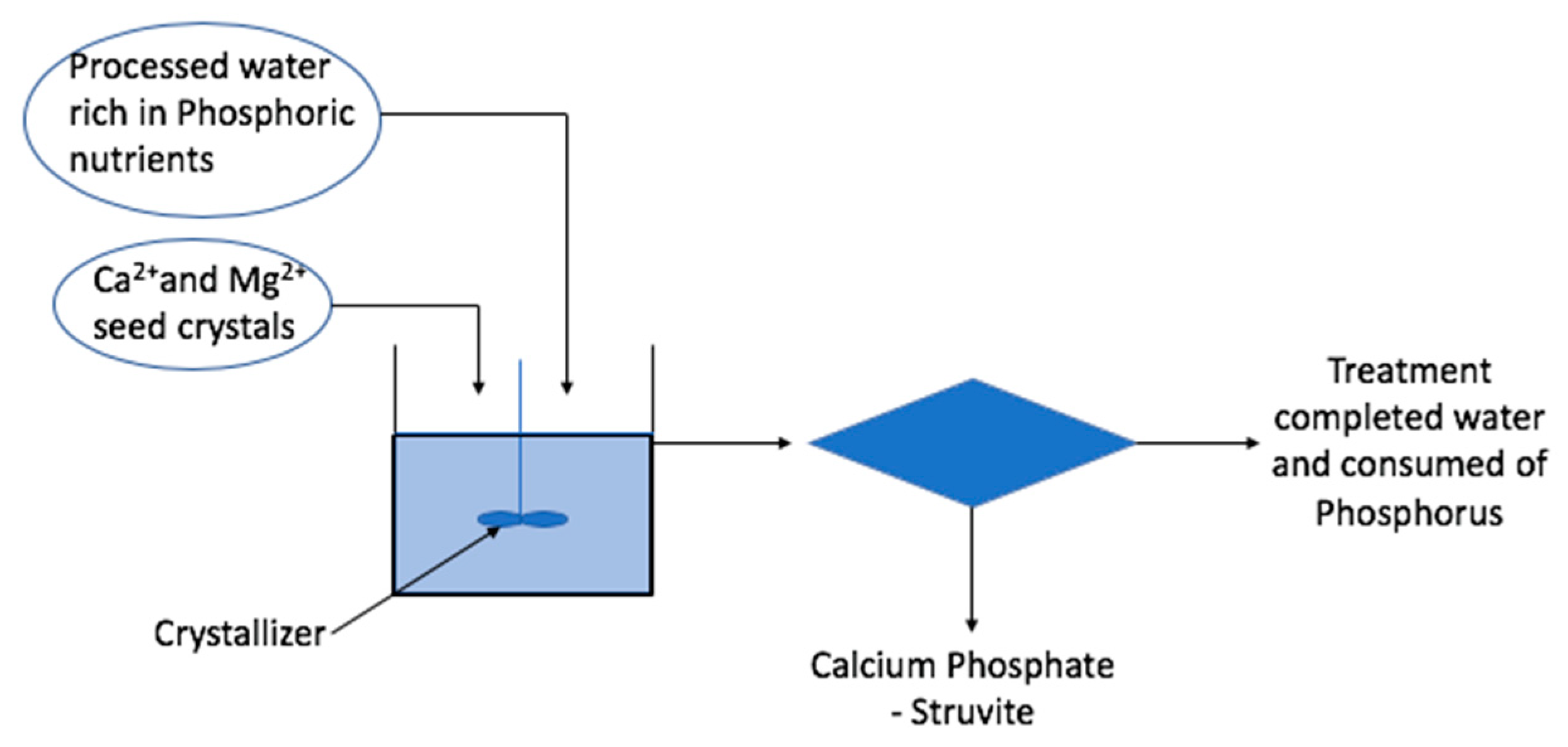
Figure 5 illustrates the schematic diagram of nitrogen and phosphorus recovery from wastewater. The pH of the solution is mainly responsible for the rate of this reaction. The multivalent metal ions, e.g., calcium, aluminum and iron, are commonly used in precipitation. In the precipitation mechanism, lime/calcium hydroxide is introduced to raise the pH of the solution and leads to the formation of calcium carbonate. Struvite crystallization proceeds in a two-stage process. The first stage is nucleation and the second stage is crystal growth. These two stages allow the struvite to develop from its generation stage to its development stage. Nucleation occurs when Mg2+, NH4+ and PO43− collide under proper pH conditions and form a saturated solution from the mixing of the ions. The mixing velocities play a critical role in the quantity of struvite crystal formation, which further affects the recovery ratio of phosphorus from wastewater.
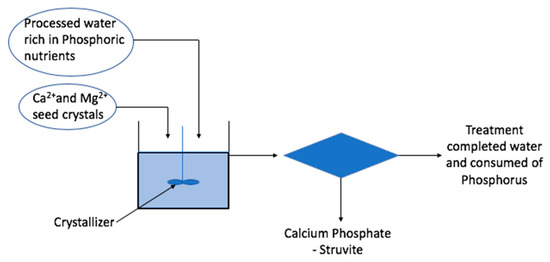
Figure 5.
Schematic diagram for recovery of nitrogen and phosphorus in liquid phase from wastewater.
Struvite precipitation is considered the most adequate process in terms of phosphorus recovery (around 80–99%), and thus it can be applicable for fertilizer industries [85,86,87,88]. Laridi and Auclair [89] attempted to increase the performance of struvite precipitation by introducing ferric chloride and other flocculants into the wastewater and observed a greater recovery of phosphorus. Furthermore, the struvite precipitation can be combined with a pre-concentration process using membrane separation; for instance, the recovery of nitrogen using membrane–precipitation processes can be as high as 99–100% with energy consumption of 10 kWh/m3 [90]. In addition to membrane separation, other techniques can be combined with a subsequent struvite precipitation for nutrient recovery, such as air stripping of ammonia from anaerobic digestion [85,91] and ion-exchange adsorption [92].
4.2. Electrochemical Separation
Electrochemical methods are processes where an electric field is implemented between the anode and cathode to separate pollutants from the wastewater [93]. Electrochemical separation can remove organic compounds, inorganic ions and microorganisms. Two major types of electrochemical methods, electro-redox reactions and electrokinetic separation, have been developed for wastewater treatment and reuse. The electro-redox reactions include electrocoagulation, electroflotation, electro-Fenton, disinfection, mineralization of organic pollutants, recovery of metals and extraction of cyanides and sulfides. For instance, both electrocoagulation and electroflotation are used for the removal of suspended particles. Electro-Fenton enables the local collection of catalysts which reduce sludge formation. The energy consumption of electro-Fenton for 60 min using the current of 1 A was ~63.6 kWh/kg without the filtration of the substances [94,95]. For electrokinetic separation processes, a number of devices, such as electrodialysis, electrodeionization and capacitive deionization, have been applied for desalination (or so-called demineralization) and for the generation of energy by salinity gradients [96]. In the case of electrodeionization, the energy consumption of desalination for brackish water (e.g., from a salinity of 5 g/L down to 0.5 g/L) was approximately 0.354–0.657 kWh/m3 [96,97]. Similarly, capacitive deionization uses porous chemically modified electrodes to separate ions from wastewater with the recovery of electric energy [98,99].
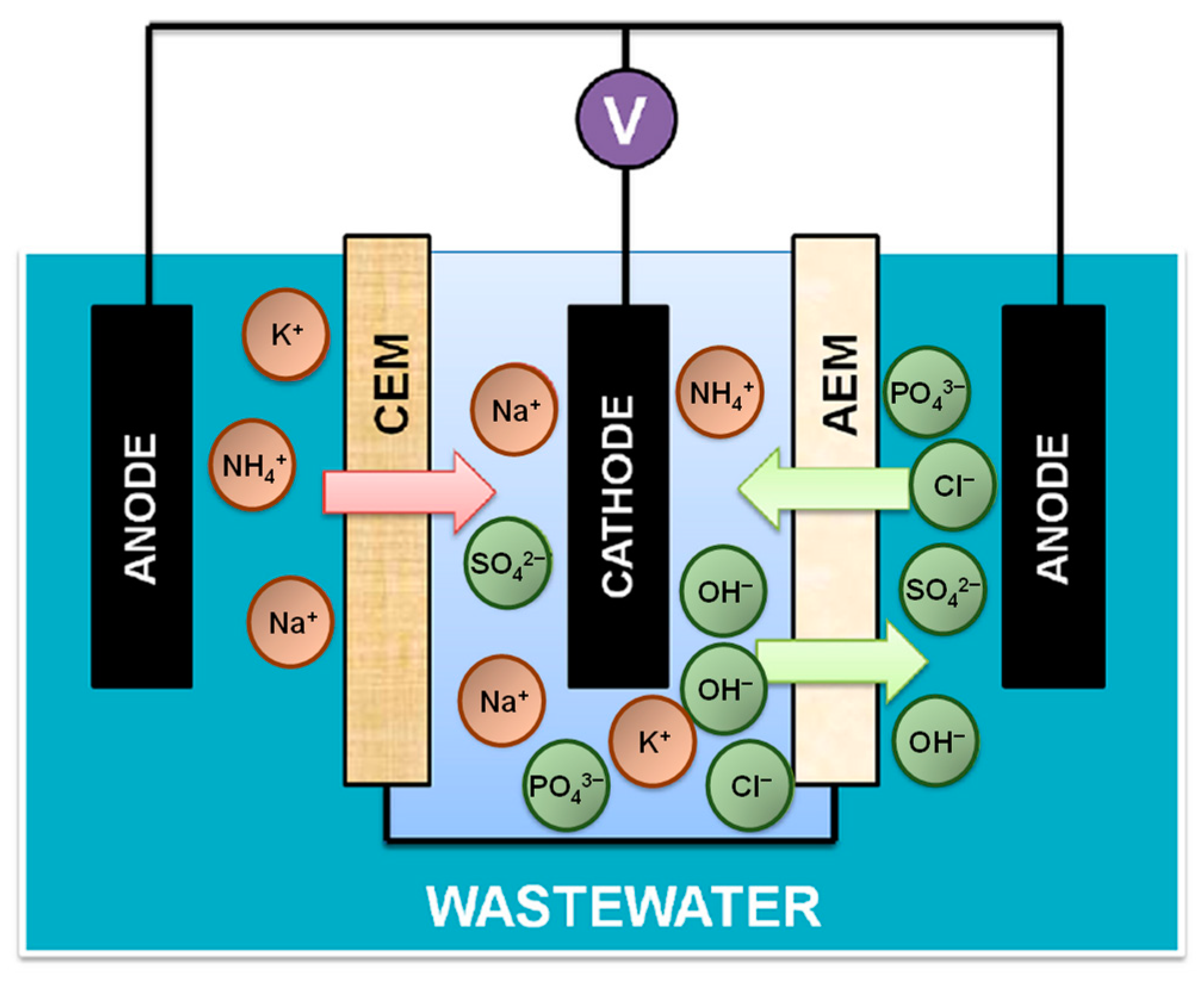
Electrochemical devices can be combined with biological elements, such as microbial fuel cell, to enhance the recovery of electric energy. With the opportunities for energy recovery, a microbial fuel cell (e.g., 750 Ω external resistor, 0.3 mA current production, and the anode potential controlled to +100 mV vs. SHE0) requires only a low energy consumption of 0.317 kWh per m3 of wastewater [100]. However, electro-biochemical technologies are sometimes unsatisfactory, but they still have their perks [101]. Recently, Zhang et al. [102] developed a novel Removing-Recovering Bio-Electrochemical System (“R2-BES”) technology to recover organic nutrients, such as nitrogen and phosphorus, from wastewater. The R2-BES system, as shown in Figure 6, involves the oxidation of organic compounds to lure the ammonium ions to circulate away from the wastewater via electricity generation. The system also involves processes of electrolysis, where hydroxide ions are produced through the cathodic reactions and those ions then replace the phosphate ions from wastewater. Moreover, the removal and recovery of phosphorus requires specific conditions, such as high pH and reduction at the cathode. As well as removing nitrogen and phosphorus through those mechanisms, when extra external voltage is applied, there is removal of COD and other nutrients increase through the amalgamation into existing treatment facilities [102]. Overall, this system is a form of microbial interaction accompanied by solid electron donors and acceptors. This method is considered environmentally friendly because it uses bioenergy from the wastewater itself, and it does not consume any external energy from the environment. However, one of the main limitations in R2-BES is that it can be time consuming since it is still a challenge to recover both nitrogen and phosphorus simultaneously.
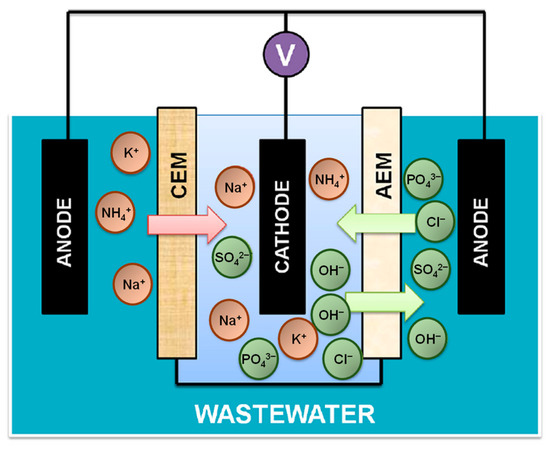
Figure 6.
Schematic diagram of Removing-Recovering Bio-Electrochemical System (R2-BES) for recovery of nitrogen and phosphorus from wastewater. Abbreviations: CEM (cation exchange membrane); AEM (anion exchange membrane). Adapted from the ref. [102].
4.3. Microalgae Uptake
An alternative method of recovering value-added nutrients from agricultural wastewater is by utilizing microalgae. Microalgae, a group of primary producers, are a diverse group of eukaryotic microorganisms that can be found in freshwater or marine sediments. They also exhibit better opportunities than other technology to be an economical resource for efficient removal of metals and other high-value nutrients from wastewater [103]. Microalgae can uptake both nitrogen and phosphorus, as well as a more remote generation of biomass which can be used as a further source of producing energy-rich and high-value compounds. They are considered economical because they are an alternative method to high-resolution and professional technologies that would require more funding. The method also minimizes the emission of greenhouse gases and saves energy throughout the process, which makes it relatively environmentally friendly [104]. However, there are several limitations; for instance, the adaptability of microalgae in wastewater is difficult to evaluate due to the complex configurations of different forms of wastewater. Thus, efficient design and optimization of the microalgae uptake have yet to be found [105].
5. Environmental Benefits for Deployment of Circular Technologies
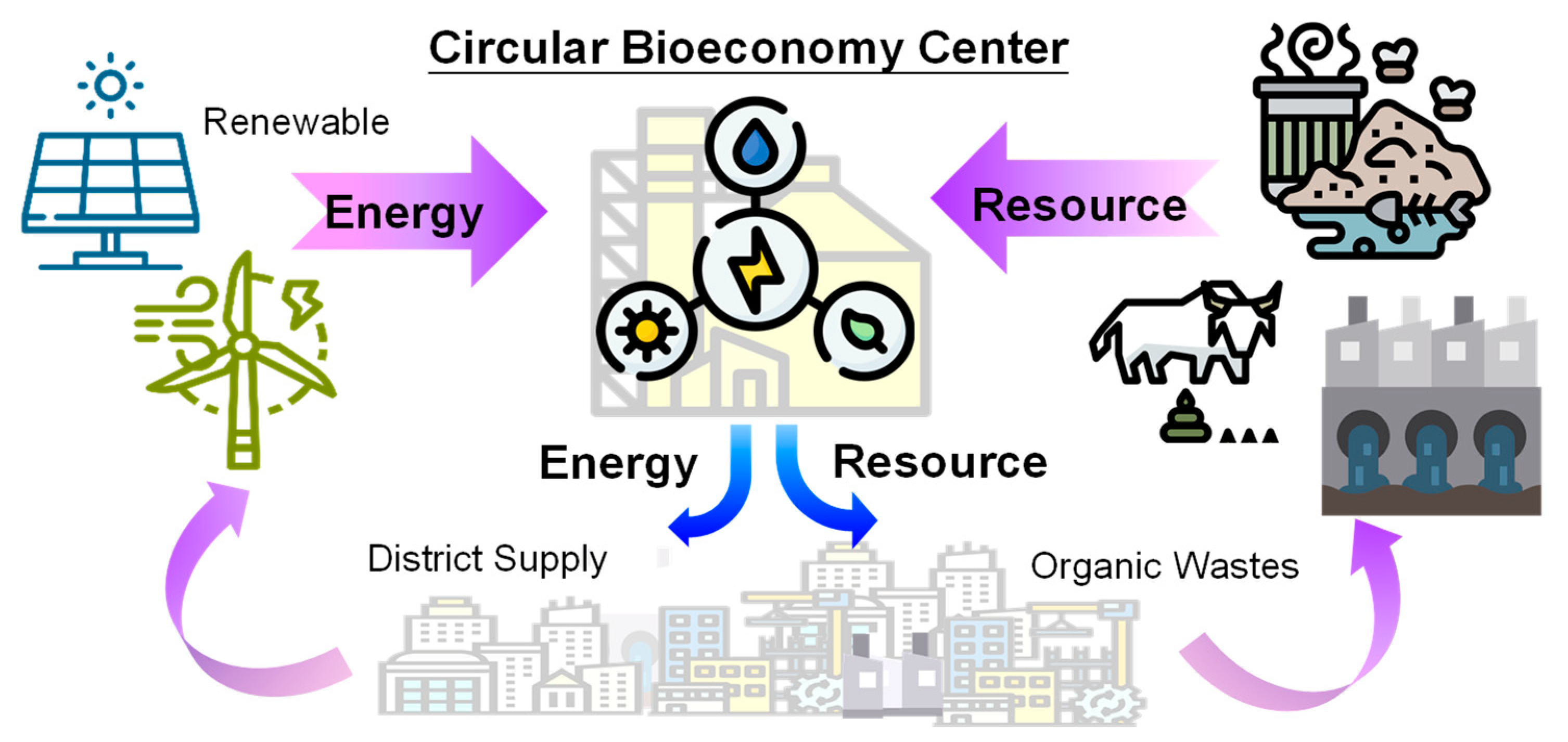
Recovering resources from agriculture wastewater not only reduces the environmental impacts but also gives a chance to achieve a resource-efficient and sustainable world. Nowadays, the shortages of water, energy and resources are worldwide crises due to pollution, extreme weather and population increase. As a result, recovering value-added resources from agriculture wastewater can assist in addressing the optimization of water, energy and food nexus, while realizing a CBE system, as shown in Figure 7. Selecting suitable circular techniques for agricultural wastewater is important to optimize the environmental benefits.
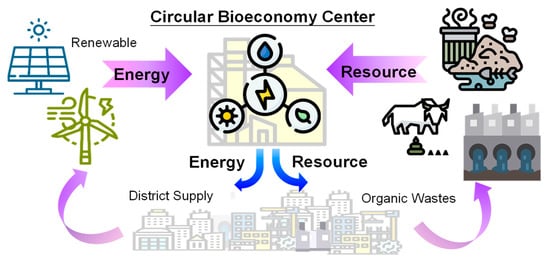
Figure 7.
Concept of a circular bioeconomy to optimize and sustain the water, energy and food elements.
Firstly, removing nutrients out of the effluent would reduce pollution risk and produce reclaimed water. Humic acid itself has several negative environmental impacts when it is present in wastewater. The environmental impacts include the effect of the water’s color, odor and taste. Humic acid reacts with the chlorine present in the surroundings, producing toxic materials, and it also aids in the production of complexes which contain heavy metal ions [106]. The removal of humic acid is meant to create a positive impact on the environment. As mentioned above, the binding of humic acid with pollutants in the water destabilizes them so they can be removed. The biological method where the microalgae are used to remove nutrients from wastewater minimizes environmental impact by reducing energy consumption [107]. Secondly, the nitrogen/phosphorus in wastewater is known for its various environmental downsides, such as eutrophication. The nitrogen that enters the waste streams always gets released into the atmosphere as nitrogen gas. Moreover, when nitrogen is removed biologically, nitrogen dioxide is emitted in the gas phase. The emission of nitrogen dioxide is an intense concern since nitrogen dioxide plays a significant role in the greenhouse gas footprint of the water and atmosphere chains. As an alternative to consuming electricity and destroying organic matter (including nitrogen), scientists have come up with a relatively environmentally friendly method to generate methane directly from wastewater which produces a reusable substance that can be used in fertilizers [45].
Because of increasing population, demand for phosphorus application in agricultural production is increasing rapidly throughout the globe. However, phosphorus is a nonrenewable resource and phosphate rock is rapidly depleted [108]. Although applications of phosphorus for fertilization improve the soil fertility and agriculture yield, it also leads to environmental damage. For example, for phosphate rock mining, several heavy metals and radionuclides may be exposed in crop fields and may be found in water bodies worldwide [109]. Therefore, the recovery of phosphorus from the agriculture wastewater is required to enhance the efficiency of the nutrient and resource recycling process. During the recovery of phosphorus, various methods take place to aid the process since phosphorus can be recovered from a range of different mediums/sources. When it is recovered from a liquid medium, the environmental impacts are either very acute or positive because the energy demand for the certain technology is fairly low. On the contrary, when phosphorus is recovered from sewage sludge, the energy demand as well as the gaseous emission is quite high. It also leads to a large amount of heavy metal contamination. Lastly, the recovery of ash has the least environmental impact—if any, it is positive. This method has the greatest potential for recycling phosphorus [110].
6. Perspectives and Prospects
Sustainable Development Goals (SDGs) proposed by the United Nations in 2015 aimed to improve environment quality while enhancing social equality and human well-being. Among SDGs, clean water and sanitation (SDG-6), decent work and economic growth (SDG-8), sustainable cities and communities (SDG-11) and responsible consumption and production (SDG-12) highlighted the importance of resource efficiency and waste management for a circular economy. Thus, advanced research and development for circular technologies are required. The environmental impact evaluation and economic analysis should be conducted with various techniques for integration process in the future. The route to successful implementation of bioeconomy is establishing the new business model to carry out the concept throughout the world. Here, we point out several priority research directions for the development and deployment of circular technologies for agricultural wastewater in the future.
6.1. Development of Circular Technologies in Accordance with Green Chemistry Principles
The circular technologies should be economically viable, environmentally friendly and engineering efficient [111]. Green Chemistry Principles (GCP) have been developed to prevent chemical hazard, benefit the economy and sustain the natural environment. The 12 GCPs can achieve the SDGs by waste prevention, energy and resource efficacy and safety assurance; for instance, Chen et al. [112] provided several strategies on the implementation of GCPs toward the circular economy. With the GCP concept, the production of value-added products from agricultural wastewater should meet the GCPs’ requirement. The electrical-driven technologies, such as membrane and electrochemical separation, are more acceptable than others using chemicals. Therefore, future research should focus on the energy efficiency design for energy-based techniques. Otherwise, for CBE, it is critical to redesign the traditional technologies for recovering resources from agricultural wastewater as a green chemical process and developing alternative technology.
6.2. Comprehensive Technology Evaluation: Integration for Innovation
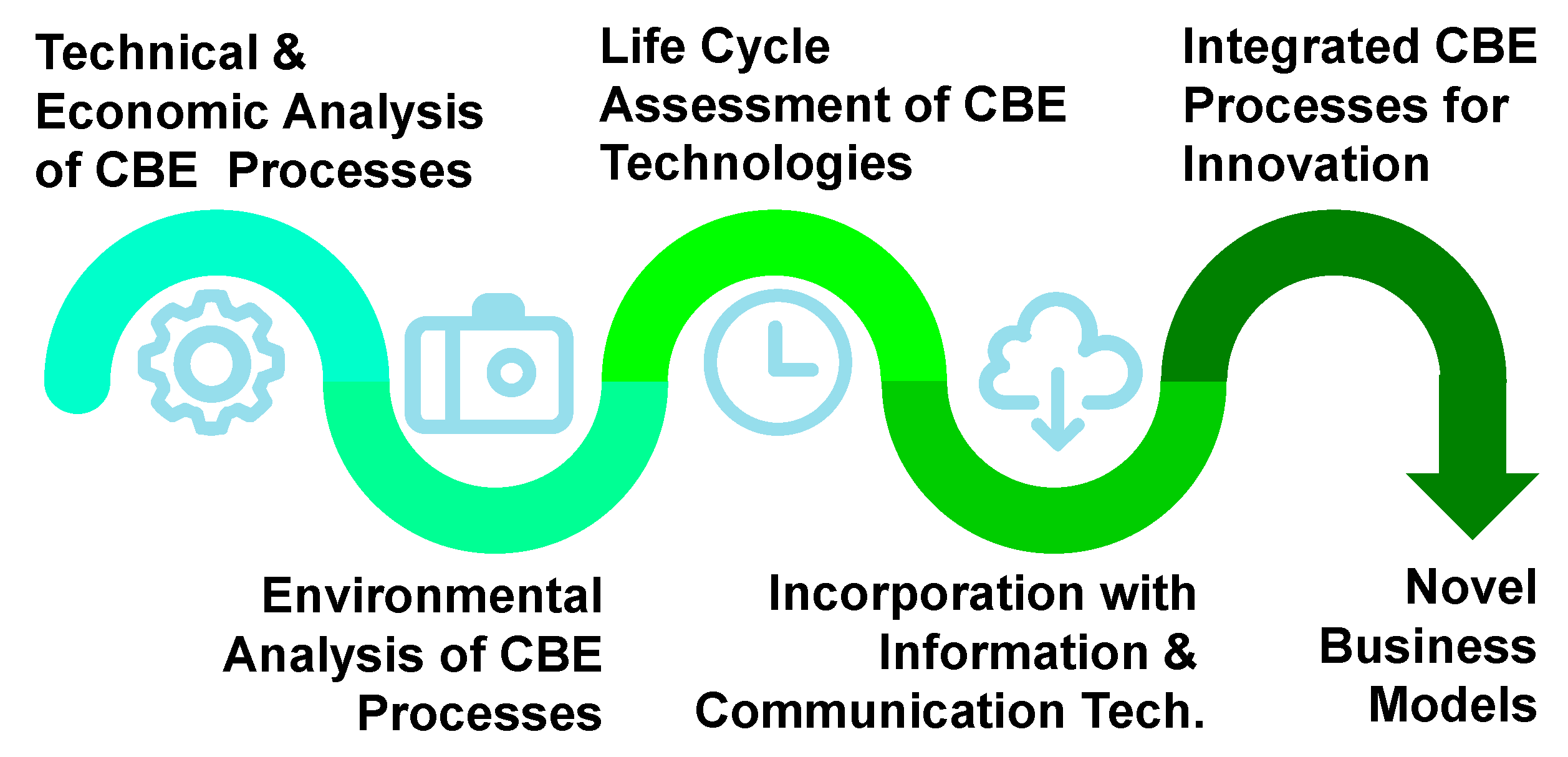
The technologies for resource recovery mentioned in the second part of this article are processes to separate compounds individually. However, in order to reuse the value-added product recovered as feedstock, integrated processes can provide multiple outcomes for precise separation and resource recovery. To achieve that, the technical and economic analyses of each of these technologies are required, such as productivity, energy-intensive and cost–benefit assessment. Although the CBE technologies provide much environmental benefit, the environmental impacts from the recovering process itself also need to be considered and analyzed, e.g., the energy consumption, greenhouse gas emission, by-product and derivation. Life cycle assessment (LCA) is a method to evaluate the environmental impacts of a product or a process [113]. The results for LCA can be used to compare different conditions or design of technologies with different input or output. For these methods, the optimal integration process with various technologies can be set up as Figure 8. On the other hand, to implement the CBE concept and integration process successfully, information and communication technology can provide solutions in many aspects. For instance, Demestichas and Daskalakis [114] conducted an extensive academic literature review on prominent information and communications technology solutions paving the way towards a CBE. The most popular technologies include internet of things for data collection, artificial intelligence for data analysis, 5G for data transmission with digital platforms and software tools. Furthermore, Francesca, Ans and Siri [115] investigated the digital platform organization, called “circularity broker”, for the transfer and recovery of discarded resources from food waste by network and circular supply chain research.
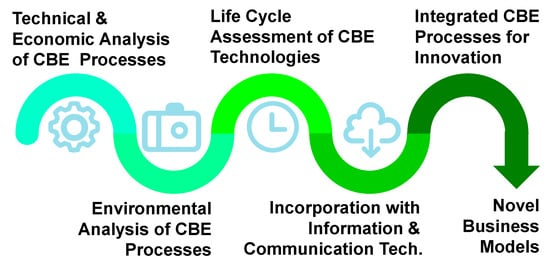
Figure 8.
Pathway of process integration for innovation. Abbreviations: CBE (circular bioeconomy).
6.3. Innovative Business Models for Green Circularity
CBE is a key element of the green economy, which is resource-focused on saving material costs, improving security of supply to promote economic growth by creating new businesses and circularity work as well as reducing environmental impacts [116,117]. It solves the linear economic problems by new circular resource flows, decoupling economic growth from resource consumption. Kalmykova et al. [117] constructed the Circular Economy Strategies Database that is applied in each part of the value chain in the circular economy, as shown in Figure 9. Industrial symbiosis is one of the successful implementations of the circular economy to share and circularly input the resource. In addition to developing technology for improving energy efficiency and reducing resource consumption, a new business model is more critical for enterprises to gain economic benefits and sustainable development. A growing amount of research is focused on business model innovation [118,119]. For consumers and public buyers, the green certification and green finance, e.g., carbon negative products and green bonus, can also be a component in the business model and support the development of CBE.
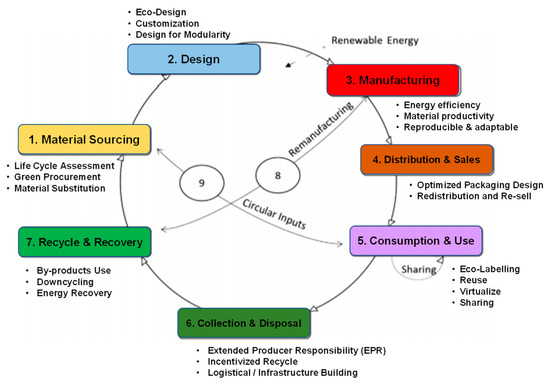
Figure 9.
Resource flow and strategies database in a value chain of circular economy. Adapted and modified from the ref. [117].
7. Conclusions
The recovery of humic acid and other high-value added nutrients depends largely on the situation and technique used to recover or remove the nutrients. Analysis of recovery will vary from structure to structure because humic acid has such versatile formations. Research databases state that the scarcity and demand for clean water will keep increasing due to growing world population, improving living standards, variance in consumption patterns and development in agricultural irrigation norms. The efficiency of the methods for recovering nutrients from agricultural wastewater needs to be improved. Scarcity of clean water is not limited to only drinking water; it occurs not only because the methods that have been analyzed in this paper have limitations, but because of other issues, such as mismanagement of water resources. There are various methods which are utilized in order to recover high-value added nutrients such as nitrogen and phosphorus. These methods consist of biological, chemical and physical methods. However, all of these methods, though they may be efficient in their own ways, have scope for improvement. With GCPs and SDGs, more detailed research of these technologies is needed. For economic feasibility and environmental benefits, integration process and life cycle assessment should be conducted in the future. Finally, development of innovative business models is the key to successfully and sustainably implementing the circular bioeconomy.
Author Contributions
Conceptualization, N.M. and S.-Y.P.; methodology, N.M.; software, N.M.; validation, K.J.S., Y.-I.L. and Y.S.; formal analysis, N.M.; investigation, N.M.; resources, K.J.S. and S.-Y.P.; data curation, Y.-I.L.; writing—original draft preparation, N.M.; writing—review and editing, K.J.S. and Y.-I.L.; visualization, Y.S.; supervision, S.-Y.P.; project administration, S.-Y.P.; funding acquisition, S.-Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology (MOST) of Taiwan (ROC) under Grant Number MOST 109-2636-M-002-013.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Sincere appreciation goes to the Ministry of Science and Technology (MOST) of Taiwan (ROC) under Grant Number MOST 109-2636-M-002-013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Filippis, G.; Piscitelli, P.; Castorini, I.F.; Raho, A.M.; Idolo, A.; Ungaro, N.; Lacarbonara, F.; Sgaramella, E.; Laghezza, V.; Chionna, D.; et al. Water Quality Assessment: A Quali-Quantitative Method for Evaluation of Environmental Pressures Potentially Impacting on Groundwater, Developed under the M.I.N.O.Re. Project. Int. J. Environ. Res. Public Health 2020, 17, 1385. [Google Scholar] [CrossRef]
- Bhutiani, R.; Kulkarni, D.B.; Khanna, D.R.; Gautam, A. Water Quality, Pollution Source Apportionment and Health Risk Assessment of Heavy Metals in Groundwater of an Industrial Area in North India. Expo. Health 2015, 8, 3–18. [Google Scholar] [CrossRef]
- Samer, M. Biological and chemical wastewater treatment processes. Wastewater Treat. Eng. 2015. [Google Scholar] [CrossRef]
- Gosch, L.; Liu, H.J.; Lennartz, B. Performance of a Woodchip Bioreactor for the Treatment of Nitrate-Laden Agricultural Drainage Water in Northeastern Germany. Environments 2020, 7, 71. [Google Scholar] [CrossRef]
- Serio, F.; Miglietta, P.P.; Lamastra, L.; Ficocelli, S.; Intini, F.; De Leo, F.; De Donno, A. Groundwater nitrate contamination and agricultural land use: A grey water footprint perspective in Southern Apulia Region (Italy). Sci. Total Environ. 2018, 645, 1425–1431. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Z.; Yuan, T.; Yu, Y.; Huang, W.; Lei, Z.; Zhang, Z. Enhanced dry anaerobic digestion of swine excreta after organic nitrogen being recovered as soluble proteins and amino acids using hydrothermal technology. Biomass. Bioenergy 2018, 108, 120–125. [Google Scholar] [CrossRef]
- Baral, K.R.; Arthur, E.; Olesen, J.E.; Petersen, S.O. Predicting nitrous oxide emissions from manure properties and soil moisture: An incubation experiment. Soil Biol. Biochem. 2016, 97, 112–120. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmati, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.H.; Martinez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.B.; Dong, R.J.; Ahring, B.K.; Zhang, W.Q. Properties of plant nutrient: Comparison of two nutrient recovery techniques using liquid fraction of digestate from anaerobic digester treating pig manure. Sci. Total Environ. 2016, 544, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Silkina, A.; Fuentes-Grunewald, C.; Wood, E.E.; Ndovela, V.L.S.; Oatley-Radcliffe, D.L.; Lovitt, R.W.; Llewellyn, C.A. Valorising nutrient-rich digestate: Dilution, settlement and membrane filtration processing for optimisation as a waste-based media for microalgal cultivation. Waste Manag. 2020, 118, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Nunez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Treatment of aquaculture effluent with Chlorella vulgaris and Tetradesmus obliquus: The effect of pretreatment on microalgae growth and nutrient removal efficiency. Ecol. Eng. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Huang, X.F.; Ye, G.Y.; Yi, N.K.; Lu, L.J.; Zhang, L.; Yang, L.Y.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Marquez, E.E.; Zarazua, G.M.S.; Bueno, J.D.P. Prospects for the Use of Electrooxidation and Electrocoagulation Techniques for Membrane Filtration of Irrigation Water. Environ. Process. Int. J. 2020, 7, 391–420. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 19. [Google Scholar] [CrossRef]
- Cui, X.; Choo, K.-H. Natural Organic Matter Removal and Fouling Control in Low-Pressure Membrane Filtration for Water Treatment. Environ. Eng. Res. 2014, 19, 1–8. [Google Scholar] [CrossRef]
- Aziz, H.A. Trends on Natural Organic Matter in Drinking Water Sources and its Treatment. 2014. Available online: https://www.researchgate.net/publication/275601642_Trends_on_Natural_Organic_Matter_in_Drinking_Water_Sources_and_its_Treatment/figures?lo=1 (accessed on 15 July 2020).
- Britannica. Humic Acid. Available online: https://www.britannica.com/science/humic-acid (accessed on 8 July 2020).
- PubChem. Fulvic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fulvic-acid (accessed on 15 July 2020).
- Britannica. Aromatic Acid. Available online: https://www.britannica.com/science/aromatic-acid (accessed on 8 July 2020).
- Libretexts. Names of Formulas of Organic Compounds. 2019. Available online: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/03%3A_Chemical_Compounds/3.7%3A__Names_of_Formulas_of_Organic_Compounds (accessed on 15 July 2020).
- Libretexts. Aldehydes and Ketones: Structure and Names. 2019. Available online: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/14%3A_Organic_Compounds_of_Oxygen/14.09_Aldehydes_and_Ketones%3A_Structure_and_Names (accessed on 15 July 2020).
- Brown, W.H.; March, J. Aldehyde. 2018. Available online: https://www.britannica.com/science/aldehyde (accessed on 18 July 2020).
- Britannica, E. Proteins. Available online: http://abyss.uoregon.edu/~js/glossary/proteins.html (accessed on 18 July 2020).
- Xu, L.; He, Z.; Zhang, H.; Wu, S.; Dong, C.; Fang, Z. Production of aromatic amines via catalytic co-pyrolysis of lignin and phenol-formaldehyde resins with ammonia over commercial HZSM-5 zeolites. Bioresour. Technol. 2020, 320, 124252. [Google Scholar] [CrossRef]
- Helmenstine, A.M. What is the Chemical Formula of Sugar? 2019. Available online: https://www.thoughtco.com/chemical-formula-of-sugar-604003 (accessed on 18 July 2020).
- Wilson, N.K. Alpha Hydroxy Acids. Skin Aging Handbook. 2009. Available online: https://www.sciencedirect.com/topics/neuroscience/hydroxy-acids (accessed on 18 July 2020).
- Infoplease. The Chemistry of Biology: Carbohydrates. 2016. Available online: https://www.infoplease.com/math-science/chemistry/the-chemistry-of-biology-carbohydrates (accessed on 20 July 2020).
- EDinformatics. 1999. Available online: https://www.edinformatics.com/math_science/what_are_polysaccharides.htm (accessed on 20 July 2020).
- PubChem. Purine. 2005. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Purine (accessed on 20 July 2020).
- PubChem. Pyrimidine. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pyrimidine (accessed on 20 July 2020).
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Peña-Méndez, E.M.; Havel, J.; Patočka, J. Humic substances—Compounds of still unknown structure: Applications in agriculture, industry, environment, and biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Laird, D.A. Triazine Soil Interactions. The Triazine Herbicides. 2008. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/humic-acid (accessed on 20 July 2020).
- Hamad, M.M.; Tantawy, M.F.A. Effect of different Humic Acids Sources on the Plant Growth, Calcium and Iron Utilization by Sorghum. Egypt. J. Soil Sci. 2018, 58, 291–307. [Google Scholar]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances; Elsevier: Amsterdam, The Netherlands, 2014; pp. 37–89. [Google Scholar]
- Nikbakht, A.; Kafi, M.; Babalar, M.; Xia, Y.P.; Luo, A.; Etemadi, N.A. Effect of Humic Acid on Plant Growth, Nutrient Uptake, and Postharvest Life of Gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef]
- Johnson, D.B. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Jarvenin, G. Precipitation and Crystallization Processes. 2009. Available online: http://www.cresp.org/NuclearChemCourse/monographs/09_Jarvinen_FuelCycleSep%20CrystPrec12-08fin_3_2_09.pdf (accessed on 20 July 2020).
- Cedric Damour, M.B.; Boillereaux, L.; Grondin-Perez, B.; Chabriat, J.-P. Energy Efficiency Improvement of an Industrial Crystallization Process Using Linearizing Control. J. Cryst. Process Technol. 2011, 2, 44–54. [Google Scholar] [CrossRef]
- Ohtake, H. Phosphorus recovery and reuse from wastewater. 2018. Available online: https://iwa-network.org/phosphorus-recovery-and-reuse-from-wastewater/ (accessed on 22 July 2020).
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Fahad, S.; Datta, R.; Abbas, M.; Rahi, A.A.; Brtnicky, M.; Holatko, J.; Tarar, Z.H.; et al. Alleviation of Cadmium Adverse Effects by Improving Nutrients Uptake in Bitter Gourd through Cadmium Tolerant Rhizobacteria. Environments 2020, 7, 54. [Google Scholar] [CrossRef]
- Van der Hoek, J.; Duijff, R.; Reinstra, O. Nitrogen Recovery from Wastewater: Possibilities, Competition with Other Resources, and Adaptation Pathways. Sustainability 2018, 10, 4605. [Google Scholar] [CrossRef]
- Novak, J.M.; Sigua, G.C.; Ducey, T.F.; Watts, D.W.; Stone, K.C. Designer Biochars Impact on Corn Grain Yields, Biomass Production, and Fertility Properties of a Highly-Weathered Ultisol. Environments 2019, 6, 64. [Google Scholar] [CrossRef]
- Pan, S.Y.; Snyder, S.W.; Lin, Y.J.; Chiang, P.C. Electrokinetic desalination of brackish water and associated challenges in the water and energy nexus. Environ. Sci. Water Res. Technol. 2018, 4, 613–638. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jin, Y.; Zou, S.; Li, C. Recovery of sludge humic acids with alkaline pretreatment and its impact on subsequent anaerobic digestion. J. Chem. Technol. Biotechnol. 2014, 89, 707–713. [Google Scholar] [CrossRef]
- Kliaugaitė, D.; Yasadi, K.; Euverink, G.-j.; Bijmans, M.F.M.; Racys, V. Electrochemical removal and recovery of humic-like substances from wastewater. Sep. Purif. Technol. 2013, 108, 37–44. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Balderas-Hernández, P.; Bilyeu, B. Electrocoagulation: Fundamentals and Prospectives Electrochemical Water and Wastewater Treatment. 2018. Available online: https://www.sciencedirect.com/topics/engineering/chemical-coagulation (accessed on 20 July 2020).
- Rios, G.B.; Almeraya, F.; Herrera, M.T.A. Electrode Passivation in the Electrocoagulation Process. Port. Electrochim. Acta 2005, 23, 17–34. [Google Scholar] [CrossRef]
- Twort, A.C.; Ratnayaka, D.D.; Brandt, M.J. (Eds.) Storage, Clarification and Filtration of Water. In Water Supply; Butterworth-Heinemann: London, UK, 2000; p. 267-XVII. [Google Scholar]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Storage, Clarification and Chemical Treatment. In Twort’s Water Supply; Butterworth-Heinemann: Oxford, UK, 2017; pp. 323–366. [Google Scholar]
- Prisciandaro, M.; Salladini, A.; Barba, D. Membrane filtration of surface water for the removal of humic substances. Chem. Eng. Trans. Ser. 2008, 14, 437–442. [Google Scholar]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and characterization of low fouling novel hybrid ultrafiltration membranes based on the blends of GO−TiO2 nanocomposite and polysulfone for humic acid removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
- Teow, Y.H. Characterization and Performance Evaluation of Ultrafiltration Membrane for Humic Acid Removal. Indian J. Sci. Technol. 2016, 9. [Google Scholar] [CrossRef]
- Theobald, D. What is Ultrafiltration and What Are Ultrafiltration Processes in Wastewater? 2015. Available online: https://www.watertechonline.com/what-is-ultrafiltration-and-what-are-ultrafiltration-processes-in-wastewater/ (accessed on 20 July 2020).
- Zhu, R.C.; Diaz, A.J.; Shen, Y.; Qi, F.; Chang, X.M.; Durkin, D.P.; Sun, Y.X.; Solares, S.D.; Shuai, D.M. Mechanism of humic acid fouling in a photocatalytic membrane system. J. Membr. Sci. 2018, 563, 531–540. [Google Scholar] [CrossRef]
- Conidi, C.; Macedonio, F.; Argurio, P.; Cassano, A.; Drioli, E. Performance of Reverse Osmosis Membranes in the Treatment of Flue-Gas Desulfurization (FGD) Wastewaters. Environments 2018, 5, 71. [Google Scholar] [CrossRef]
- Lenntech, Micro filtration System (MFS). Available online: https://www.lenntech.com/microfiltration.htm (accessed on 20 July 2020).
- PureAqua. Ultrafiltration UF Systems. Available online: https://www.pureaqua.com/ultrafiltration-uf-systems/ (accessed on 20 July 2020).
- MRWA, Membrane Filtration. Minnesota Rural Water Association. Available online: https://pdf4pro.com/amp/view/membrane-filtration-mrwa-56d3d8.html (accessed on 11 July 2020).
- EMIS. Adsorption Techniques. 2010. Available online: https://emis.vito.be/en/techniekfiche/adsorption-techniques (accessed on 12 July 2020).
- Samco. Reverse Osmosis vs Nanofiltration Membrane Process: What Is the Difference? 2017. Available online: https://www.samcotech.com/reverse-osmosis-vs-nanofiltration-membrane-process-what-is-the-difference/ (accessed on 20 July 2020).
- Pan, S.Y.; Haddad, A.Z.; Kumar, A.; Wang, S.W. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Shang, W.J.; Wang, D.X.; Wu, L.; Tu, C.H. Characterization and applications of nanofiltration membranes: State of the art. Desalination 2009, 236, 316–326. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Khan, S.J.; Davies, P.A. Performance evaluation of reverse osmosis (RO) pre-treatment technologies for in-land brackish water treatment. Desalination 2017, 406, 44–50. [Google Scholar] [CrossRef]
- Sakarinen, E. Humic acid Removal by Chemical Coagulation, Electrocoagulation and Ultrafiltration. In Plastics Technology; Arcada University of Applied Sciences: Helsinki, Finland, 2016. [Google Scholar]
- Watanabe, Y.; Kimura, K.; Yamamura, H.; Yonekawa, H.; Suzuki, T. Mechanism and Control of Membrane Fouling in Water Purification. In Proceedings of the 8th International Symposium on Water Supply Technology, Kobe, Japan, 10–12 June 2009. [Google Scholar]
- Gao, K.; Li, T.; Liu, J.X.; Dong, B.Z.; Chu, H.Q. Ultrafiltration membrane fouling performance by mixtures with micromolecular and macromolecular organics. Environ. Sci. Water Res. Technol. 2019, 5, 277–286. [Google Scholar] [CrossRef]
- Dhawan, G.K. Solutions to Membrane Fouling. 2007. Available online: http://www.watertreatmentguide.com/membrane_fouling_solutions.htm (accessed on 12 July 2020).
- Yigit, Z.; Inan, H. A Study of the Photocatalytic Oxidation of Humic Acid on Anatase and Mixed-phase Anatase–Rutile TiO2 Nanoparticles. Waterairsoil Pollut. Focus 2009, 9, 237–243. [Google Scholar] [CrossRef]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In Organic Pollutants—Monitoring, Risk and Treatment; Intech: London, UK, 2013; pp. 167–194. [Google Scholar]
- Cornelissen, E. Recovering Salt and Humic Acids in Drinking Water Production. 2015. Available online: https://kwrwater.nl/en/actueel/recovering-salt-humic-acids-drinking-water-production/ (accessed on 13 July 2020).
- Sector, D.W. Royal HaskoningDHV and Vitens Start Worldwide Marketing of Technology to Recover Humic Acid at Drinking Water Plants. 2015. Available online: https://www.dutchwatersector.com/news/royal-haskoningdhv-and-vitens-start-worldwide-marketing-of-technology-to-recover-humic-acid-at (accessed on 13 July 2020).
- Nissinen, T.K.; Miettinen, I.T.; Martikainen, P.J.; Vartiainen, T. Molecular size distribution of natural organic matter in raw and drinking waters. Chemosphere 2001, 45, 865–873. [Google Scholar] [CrossRef]
- De Melo, B.A.; Motta, F.L.; Santana, M.H. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.L.; Maccarthy, P. Limitations in the use of commercial humic acids in water and soil research. Environ. Sci. Technol. 1986, 20, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Photophysics. Handbook of Material Weathering, Fifth edition. 2013. Available online: https://www.sciencedirect.com/topics/engineering/beer-lambert-law (accessed on 20 July 2020).
- EcoCatalysts. 2018. Available online: https://www.ecocatalysts.com.au/humic-acids-just-how-important-are-they/ (accessed on 15 July 2020).
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Kofina, A.N.; Koutsoukos, P.G. Spontaneous precipitation of struvite from synthetic wastewater solutions. Cryst. Growth Des. 2005, 5, 489–496. [Google Scholar] [CrossRef]
- Li, Z.; Ren, X.; Zuo, J.; Liu, Y.; Duan, E.; Yang, J.; Chen, P.; Wang, Y. Struvite precipitation for ammonia nitrogen removal in 7-aminocephalosporanic acid wastewater. Molecules 2012, 17, 2126–2139. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Hobbs, E.V.-J.P.; Parsons, S.A. Phosphorus recovery from wastewater by strucite crystallisation: A review. Crit. Rev. Environ. Sci. Technol. 2009, 396, 433–477. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Dutta, S. Evaluating Water Quality to Prevent Future Disasters. Separation Science and Technology. 2019. Available online: https://www.sciencedirect.com/topics/chemical-engineering/precipitation-chemical (accessed on 16 July 2020).
- Tsoutsos, T. Modelling hydrolysis and fermentation processes in lignocelluloses-to-bioalcohol production. Bioalcohol Production. 2010. Available online: https://www.sciencedirect.com/topics/engineering/dilute-acid-hydrolysis (accessed on 16 July 2020).
- Cheng, W. Carbon Fluxes in the Rhizosphere. 2007. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/assimilation-efficiency (accessed on 17 July 2020).
- Laridi, R.; Auclair, J.C.; Benmoussa, H. Laboratory and pilot-scale phosphate and ammonium removal by controlled struvite precipitation following coagulation and flocculation of swine wastewater. Environ. Technol. 2005, 26, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, P.; Saravanan, A. Sustainable Wastewater Treatments in Textile Sector. In Sustainable Fibres and Textiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–346. [Google Scholar]
- Ghyselbrecht, K.; Monballiu, A.; Somers, M.H.; Sigurnjak, I.; Meers, E.; Appels, L.; Meesschaert, B. Stripping and scrubbing of ammonium using common fractionating columns to prove ammonium inhibition during anaerobic digestion. Int. J. Energy Environ. Eng. 2018, 9, 447–455. [Google Scholar] [CrossRef]
- Couper, J.R.; Penney, W.R.; Fair, J.R.; Walas, S.M. (Eds.) Chapter 15—Adsorption and Ion Exchange. In Chemical Process Equipment, 2nd ed.; Gulf Professional Publishing: Burlington, NJ, USA, 2005; pp. 523–554. [Google Scholar]
- Ghimire, U.; Jang, M.; Jung, S.P.; Park, D.; Park, S.J.; Yu, H.; Oh, S.E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies 2019, 12, 883. [Google Scholar] [CrossRef]
- Duran Moreno, A.; Frontana-Uribe, R.M.; Zamora, R.R. Electro-fenton as a feasible advanced treatment process to produce reclaimed water. Water Sci. Technol. 2004, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Sillanpää, M.E.T.; Shestakova, M. Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Butterworth-Heinemann, an Imprint of Elsevier: Oxford, UK, 2017. [Google Scholar]
- Pan, S.Y.; Snyder, S.W.; Ma, H.W.; Lin, Y.J.; Chiang, P.C. Development of a Resin Wafer Electrodeionization Process for Impaired Water Desalination with High Energy Efficiency and Productivity. ACS Sustain. Chem. Eng. 2017, 5, 2942–2948. [Google Scholar] [CrossRef]
- Dykstra, J.E.; van der Wal, S.P.A.; Biesheuvel, P.M. Energy consumption in capcitive deionization—Constant current versus constant voltage operation. Water Res. 2018, 143, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Wu, A.; Yamanaka, Y.; Nealson, K.H.; Bretschger, O. Identifying the microbial communities and operational conditions for optimized wastewater treatment in microbial fuel cells. Water Res 2013, 47, 7120–7130. [Google Scholar] [CrossRef]
- Chopra, A.; Sharma, A.K.; Kumar, V. Overview of Electrolytic treatment: An alternative technology for purification of wastewater. Arch. Appl. Sci. Res. 2011, 3, 1–5. [Google Scholar]
- Zhang, F.; Li, J.; He, Z. A new method for nutrients removal and recovery from wastewater using a bioelectrochemical system. Bioresour. Technol. 2014, 166, 630–634. [Google Scholar] [CrossRef]
- Madhurya Ray, C.B. Microalgae: A Way Forward Approach towards Wastewater Treatment and BioFuel Production. 2019. Available online: https://www.sciencedirect.com/topics/neuroscience/microalgae (accessed on 17 July 2020).
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients from Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Farhad Asgharyan, M.K.N.; Bagher Anvaripour, I.D. The Effect of Different Electrodes on Humic Acid Removal by Electrocoagulation. 2018. Available online: http://ijogst.put.ac.ir/article_65739.html (accessed on 18 July 2020).
- Junying Liu, Y.S.; Ruan, R.; Liu, Y. Removal of humic acid from composted hog waste by the white-rot fungus, Phanerochaete chrysosporium. Water Sci. Technol. 2015, 72, 92–98. [Google Scholar]
- Wang, X.L.; Xiong, J.B.; He, Z.L. Activated dolomite phosphate rock fertilizers to reduce leaching of phosphorus and trace metals as compared to superphosphate. J. Environ. Manag. 2020, 255, 109872. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for Recovering Nutrients from Wastewater: A Critical Review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Rashid, S.S.; Liu, Y.Q.; Zhang, C. Upgrading a large and centralised municipal wastewater treatment plant with sequencing batch reactor technology for integrated nutrient removal and phosphorus recovery: Environmental and economic life cycle performance. Sci. Total Environ. 2020, 749, 141465. [Google Scholar] [CrossRef]
- Demestichas, K.; Daskalakis, E. Information and Communication Technology Solutions for the Circular Economy. Sustainability 2020, 12, 7272. [Google Scholar] [CrossRef]
- Ciulli, F.; Kolk, A.; Boe-Lillegraven, S. Circularity Brokers: Digital Platform Organizations and Waste Recovery in Food Supply Chains. J. Bus. Ethics 2020, 167, 299–331. [Google Scholar] [CrossRef]
- D’Amato, D.; Droste, N.; Allen, B.; Kettunen, M.; Lahtinen, K.; Korhonen, J.; Leskinen, P.; Matthies, B.D.; Toppinen, A. Green, circular, bio economy: A comparative analysis of sustainability avenues. J. Clean. Prod. 2017, 168, 716–734. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Sadagopan, M.; Rosado, L. Circular economy—From review of theories and practices to development of implementation tools. Resour. Conserv. Recycl. 2018, 135, 190–201. [Google Scholar] [CrossRef]
- Chen, L.H.; Hung, P.Y.; Ma, H.W. Integrating circular business models and development tools in the circular economy transition process: A firm-level framework. Bus. Strategy Environ. 2020, 29, 1887–1898. [Google Scholar] [CrossRef]
- Lopez, F.J.D.; Bastein, T.; Tukker, A. Business Model Innovation for Resource-efficiency, Circularity and Cleaner Production: What 143 Cases Tell Us. Ecol. Econ. 2019, 155, 20–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).