Abstract
Little is known about the effect of nitrogen (N) application via biochar on the removal of trace elements by crops, and the effects with chemical fertilizers are inconsistent. We determined, from a previous study, the influence of increased N addition via biochars produced from switchgrass (SGB) and poultry litter (PLB) on cadmium (Cd) removal by ryegrass. The biochar rates of 0, 0.5, 1, 2, and 4% w/w were applied to a Cd-contaminated soil before seeding in a potting experiment with a complete randomized block design (CRBD). Ryegrass yield and N and Cd removed by harvest were strongly related (p < 0.05). The ryegrass yields increased up to 1% of PLB, and Cd removal was also the highest at 1% of PLB. The biomass of ryegrass roots increased with Cd accumulation (p < 0.05). Overall, the Cd transfer factor (TF) from ryegrass roots to shoots increased when up to 206 ± 38 kg N ha−1 was removed in ryegrass shoots (p < 0.0001). The application of PLB up to 1% might be a viable option since it is a practical rate for handling operations requiring less volume of material than SGB. Additionally, the Cd concentration in the aboveground forage remained acceptable for grazing cattle. Future studies are encouraged to evaluate different sources of N fertilizers affecting Cd uptake on cash crops.
1. Introduction
Human activities have gradually transferred many toxic metals from the earth’s crust to the environment, resulting in the spread and contamination of toxic metals in the ecosystem [1,2,3,4]. The metals originate from many sources including mining activities, industrial waste disposals, paints, and gasoline additives that lead to physical and chemical processes such as leaching and oxidation thus causing the accumulation of metals in the soil. Toxic metal pollution has become a serious problem worldwide in the last decade. Cadmium (Cd) is considered the most toxic element among toxic metals for living organisms and has been detected in some agricultural lands [1]. Cadmium accumulates in plants and animals and threatens their health when entering through the food chain [5,6]. Thus, it is important but challenging to remediate Cd-contaminated soils worldwide.
The Tar Creek area is designated as a superfund site located in the tri-state regions of Oklahoma, Kansas, and Missouri by US Environmental Protection Agency (EPA), and is one of the most polluted sites in the world [7]. A Superfund site is an abandoned hazardous waste site in the United States subject to the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA), which allows federal funding to be spent on investigating and remediating the environmental contamination at the designated site. The Tar Creek area was mined for approximately 70 years starting from the beginning of the 1900s. Large quantities of coarse material contaminated with Cd were left on the ground surface in piles due to the mining and milling operations. Such a coarse material is gravel-like waste well known as “chat pile.” The large size and the considerable amount of chat piles resulted in Cd contamination in the surrounding areas due to wind blowing and the chat materials used as gravel. Reclamations of contaminated lands have been attempted by the US Government and the Quapaw Tribe. Although the status of Cd contamination in the Tar Creek has been addressed in numerous studies [8,9,10], with some of them elucidating the use of biochars for Cd remediation and phytoavailability reduction [11], there is still a lack of research evaluating the effect of nitrogen (N) application via biochars on the accumulation of Cd by crops.
Nitrogen is the most important essential nutrient for plants, and N application is the biggest contributor to biomass production [12]. Although some studies suggested that Cd concentration in plants decreased as their biomass increased by N application [13,14], other works concluded that N fertilizer not only improved plants’ biomass but also increased their Cd concentration [12,15,16,17]. In an experiment conducted by Symanowicz et al. [18], N fertilization significantly contributed to increase Cd accumulation in the aboveground portion (shoots) of eastern galega. Thus, the effect of N application on Cd accumulation remains controversial [12]. In general, N fertilization often decreases soil pH, which increases the solubility and mobility of Cd in the soil [19,20]. Ji et al. [12] also highlighted a more direct effect of N fertilization in Cd uptake by suggesting a mechanism in which the active uptake and influx rate of Cd2+ into the roots of Italian ryegrass (Lolium multifolorum Lam.) were enhanced by the greater affinity of the membrane transporter to Cd2+ as a consequence of the urea application.
Although the studies mentioned above have evaluated the effect of N on Cd bioavailability and plant uptake, all of them were performed through the addition of chemical fertilizers. Therefore, the evaluation of N addition using biochar aiming the same goal makes our research unique since there might be a compound effect of biochar properties, such as alkalinity and surface functional groups, also affecting Cd uptake, not just the N addition itself.
Perennial ryegrasses (Lolium perenne) are widely used in grazed pastures due to its potential to reduce nitrate (NO3−) leaching [21], which makes such grass a potential scavenger of soil N. Ryegrass is also known for its efficient phytoaccumulation of toxic metals such as Cd [11]. These characteristics make ryegrass a promising tool to study the N and Cd accumulation relationship in plant tissues. It is a gramineous grass with fast growth, high yield potential, strong resistance to toxic metals, and able to normally grow in tailing areas where toxic metal pollution is severe and the environment is harsh [12]. Moreover, Mongkhonsin et al. [22] pointed out that ryegrass shoots had the highest Cd enrichment of up to fourfold of what is commonly found in other plant species that are hyperaccumulators (Solanum nigrum and Indian mustard); and Italian ryegrass had the strongest tolerance and accumulation capacity of Cd among eight C3 herbage grass species. Therefore, the perennial ryegrass with high biomass yields is also an appropriate species to use for the remediation of Cd-contaminated soils [23].
Our objective was to determine the influence of increased N addition via biochar derived from two feedstocks on Cd removal by ryegrass. This study is derived from the work of Antonangelo and Zhang [11] and data were reanalyzed to evaluate the N and Cd removal instead of their simple uptake. Since current literature is undoubtedly deficient in such information, we believe that our research has the potential to expand from small-scale to field-scale studies. This will serve as a multi-purpose biochar application to increase forage biomass and Cd uptake for phytoremediation purposes, at the same time immobilizing a certain amount of bioavailable Cd in the soil, as observed with the previous study of Antonangelo and Zhang [11], thus avoiding its leaching to the groundwater.
This would be a pioneering work for other studies focusing on N rates from biochars and/or the combination of biochar + N fertilizers to increase toxic metal phytoaccumulation while immobilizing the metal in the soil-plant system. This multi-purpose aspect contributes to the sustainable use of resources, since only immobilization is not enough if there is a need for disposal, land application, or landfill. Hence, if animal grazing is needed in such contaminated areas, the risk of intoxication could be still avoided depending on the metal accumulation in shoots. A sustainable attitude (multi-purpose) like this might be useful for contaminated lands, such as the Tar Creek superfund site.
2. Materials and Methods
2.1. Biochar Production and Characteristics
The biochars used were converted from switchgrass (SG; Panicum virgatum) and poultry litter (PL) via slow pyrolysis at 700 °C. The feedstocks obtainment and the detailed description of their conversion into biochar products can be found in Antonangelo and Zhang [11]. Switchgrass- and poultry litter–derived biochars are referred to as SGB and PLB, respectively. The biochars coarse materials were ground with a mortar and pastel gently before sieved through 1 mm for further physicochemical analyses [24] and through 0.25 mm for the potting experiment. A full characterization of SGB and PLB including all physicochemical properties such as moisture, ash content, particle-size distribution, elemental composition, surface functional groups, chemical attributes, specific surface area, cation exchange capacity (CEC), and morphology can be found in [24]. For this study, it is useful to emphasize the ash, total carbon (TC), total nitrogen (TN), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) contents, as presented in Table 1.

Table 1.
Physicochemical properties of biochar derived from switchgrass (SGB) and poultry litter.
2.2. Soil Sampling and Analysis
Cadmium contaminated soil samples were collected with a shovel (0 to 15 cm) from a residential yard near chat piles located in Picher, Ottawa County, Oklahoma. Soil sample preparation for analyses and for the potting experiment was described in Antonangelo and Zhang [11]. Before and after the pot experiment, soil samples were analyzed for DTPA–extractable Cd. Before the potting experiment, the total Cd content was determined by an inductively coupled plasma-atomic emission spectroscopy, ICP-AES (SPECTRO Analytical Instruments GmbH, Boschstr. 10, 47533 Kleve, Germany) after digestion by concentrated HNO3 and H2O2 using EPA method 3050B [25]. The TN, and total and DTPA-extractable Cd before the potting experiment were respectively 1.7 ± 0.1 g kg−1, 9 ± 0.6 mg kg−1, and 1.84 ± 0.03 mg kg−1. The total Cd content was about tenfold the maximum found in normal Oklahoma soils [26]. The DTPA-extractable Cd has been considered the most efficient method to predict the Cd phytoavailability to grasses, such as rice (Oryza sativa) [27] and perennial ryegrass [11]. Still, the threshold of DTPA-extractable Cd found by Wu et al. [27] was 0.03 to 0.16 mg kg−1 depending on the soil organic matter (SOM) content, which is more than 10 times lower than the content found in the Tar Creek soil of this study.
2.3. Potting Experiment
Plastic pots were filled with 1.2 kg of 2 mm sieved soils and amended with 0.0 (control), 0.5, 1.0, 2.0, and 4.0% (w/w) of 0.25 mm sieved SGB and PLB. The biochar-amended soils were incubated for approximately 30 days at 75% of field capacity before ryegrass sowing in each pot at 30 kg ha−1. Ryegrass was grown for ~75 days in the biochar amended soils in an environmentally controlled growth chamber. The pots were arranged in a complete randomized block design (CRBD) with 3 replicates (n = 3) and rotated weekly to eliminate spatial variability in the chamber. All procedures and analyses conducted during the potting experiment are detailed in Antonangelo and Zhang [11].
2.4. Nitrogen and Cadmium Analysis in Ryegrass Shoots and Roots
After harvesting, ryegrass shoots and roots were separated, washed with D.I. water, and oven-dried to constant weight at 105 °C, and their weight was recorded. Dried plant materials were ground using a mechanical grinder and further analyses were followed. Ground plant materials were analyzed for Cd using nitric acid digestion and TN using dry combustion and determination via LECO, as previously described for soil. For Cd in plant tissues, 0.5 g of ground plant materials were predigested for 1h with 10 mL of trace metal grade HNO3 in the HotBlock™ Environmental Express block digester (Environmental Express, 2345A Charleston Regional Parkway, Charleston, SC, USA), and the digestion products were then heated to 115 °C for 2 h and diluted with D.I. water to 50 mL [28]. Finally, the digested samples were filtered and analyzed for Cd by an ICP-AES.
The N and Cd concentration determined in plant tissues (uptake) was multiplied by their respective biomass to eliminate any dilution effect caused by over yield and the N and Cd in ryegrass shoots and/or roots were then treated as “removed” (shoots, when harvested) and/or “accumulated”: ).
The Cd transfer factor (TF) was calculated by considering the Cd removal and accumulation following: .
2.5. Statistical Analysis
Ryegrass N and Cd accumulation in ryegrass tissues and Cd TF from ryegrass shoots to roots, as described above, were subjected to a two-way ANOVA following the CRBD and the average of the treatments (biochar rates and biochar from different feedstocks) compared by the Tukey test at 5% (p ≤ 0.05). Analysis of covariance (ANCOVA) was performed for the linear regressions between ryegrass yield and N accumulation and between N and Cd accumulations to plot the linear models with both biochars combined or for each biochar separately, depending on the results. Data analysis was performed using SAS version 9.4.
The maximum N removal from which the highest Cd TF is reached was determined by fitting the segmented linear-plateau response model using the NLIN (nonlinear) procedure of SAS version 9.4. Pearson’s simple correlation for specific variables has also been carried out. Regression analyses and Pearson correlation were performed with the whole dataset of measurements (all replicate data) at α = 0.05 for both model fitting and equation coefficients. Graphs were plotted with the assistance of Excel.
3. Results
3.1. Ryegrass Production, and Cadmium × Nitrogen Accumulation Relationship
Ryegrass N and Cd accumulation are shown in Table 2. A two-way ANOVA was conducted to test the differences among biochar rates and between the two different feedstock-derived biochars for a given rate. This was because the interaction rate (R) × biochar (B) was significant (p < 0.05) in all cases: shoots, roots, and shoots + roots (Table 2). As highlighted in the previous work of Antonangelo and Zhang [11], the ryegrass shoots, roots, and whole plant (shoots + roots) yielded better when treated with PLB presenting, on average, an increase of 64, 51, and 59% respectively when compared to those treated with SGB (Table 2). Similarly, N and Cd accumulation had an overall increase of 88, 60, and 84%, and 71, 30, and 40% respectively, when PLB was applied (Table 2). At 0.5 and 1% of PLB application, the ryegrass shoots presented the highest N and Cd accumulation (Table 2), accompanied by their highest yields. This is the first indication that Cd accumulation increases as the ryegrass yield increase with N accumulation. The approximately 35 g Cd removed ha−1 in ryegrass shoots after PLB application at 0.5 and 1% (Table 2) is equivalent to a concentration of 3.4 ± 0.1 mg kg−1 (dry matter, DM) in our study, which is still far below the maximum tolerable concentration of 10 mg kg−1 for animal grazing [29]. The Cd accumulation in ryegrass plant parts decreased with biochar application rates regardless the feedstock from which the biochar was produced (Table 2). However, it must be pointed out that such reduction is not only a consequence of the reduced N removal and ryegrass yields at 4% of biochar amendment but also due to a direct effect of the biochar properties with the potential to immobilize Cd in the soil, amongst them surface functional groups, alkalinity, organic carbon (OC), and CEC [24].

Table 2.
Nitrogen (N) and cadmium (Cd) accumulation in ryegrass shoots, roots, and shoots + roots as a function of biochar (B) application rates (R).
Table 3 shows the ANCOVA of linear regressions between ryegrass yield and N accumulation and between N and Cd accumulations plotted in Figure 1. Conversely to ANOVA, the interactions of biochar × yield and biochar × nitrogen from ANCOVA were not significant in ryegrass shoots and roots (p > 0.05), except for the whole plant when evaluating the biochar × nitrogen interaction in the relationship between N and Cd accumulations (p < 0.05) (Table 3). Therefore, most relationships were plotted with both biochars combined (Figure 1a–e).

Table 3.
Analyses of covariance (ANCOVA) from linear regression models between nitrogen (N) and ryegrass yield and between cadmium (Cd) and N accumulations.
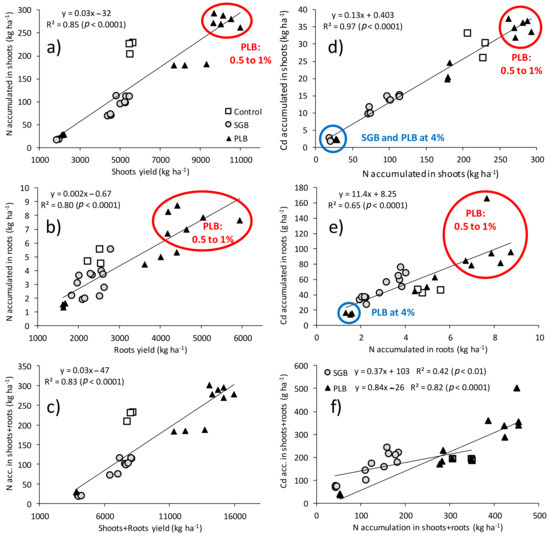
Figure 1.
Relationships between ryegrass yield and N accumulation (a–c) and between nitrogen (N) and cadmium (Cd) accumulations in ryegrass shoots and roots (d–f) as functions of biochar application rates; acc.—accumulated.
Figure 1 clearly shows that N accumulation increases as a function of biochar application rates regardless of its feedstock, and such an accumulation contributes to ryegrass yield increases in shoots and roots. Concomitantly, the Cd accumulation of those plant parts follows their yields increment as well (Figure 1). This agrees with the findings of [12,15,16,17,18] who observed greater yields with increased N rates and consequently greater Cd uptake in the studied plants. In fact, N is the most limiting nutrient as far as yield increase is concerned and, in the case of our study, the same evaluations carried out for P and K showed a positive correlation with ryegrass yields but no relationship was found between P and K with Cd accumulation in any plant part (data not shown). Opposite to the increase of Cd accumulation in ryegrass plant parts, the Cd concentration in ryegrass shoots and roots, when their yields are not accounted for, decreased considerably with biochar application due to immobilization effect of phytoavailable Cd as a direct consequence of biochar properties, as observed in the previous study [11].
Curiously, the relationship between the yield of ryegrass roots and Cd accumulation was highly positive regardless of the biochar (Figure 2). There was an increment of 23 mg Cd accumulated ha−1 for every kg of ryegrass roots increase (Figure 2). The maximum accumulation in the range of 80 to 160 g Cd ha−1 is related to the PLB at 0.5 and 1% application rates (Table 2, and Figure 1 and Figure 2). Feng et al. [30] also reported an increase in root yields (DM) of two ryegrass varieties when the levels of phytoavailable Cd were higher. This might be attributed to the fact that root density is higher in the presence of this toxic metal. However, Feng et al. [30] also observed that ryegrass roots although presenting higher enrichment ability for Cd, its transportability to shoots was poor. Not surprisingly, the accumulation of Cd in ryegrass roots was much higher than in shoots in the case of our study (Table 2 and Figure 1e). This larger accumulation of Cd in the roots is also a cause of the lower Cd transference to the shoots [31,32], as will be discussed further.
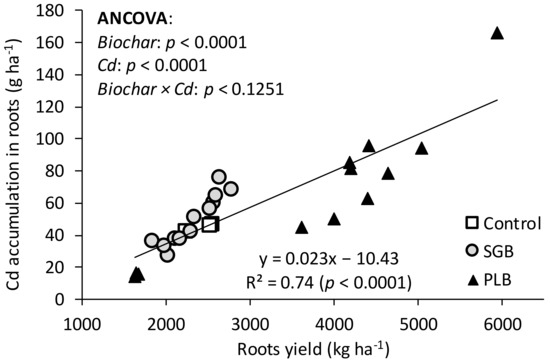
Figure 2.
Relationship between root yields and cadmium (Cd) accumulation in ryegrass roots as a function of biochar application rates. ANCOVA: analysis of covariance. When the interaction biochar × Cd is p ≥ 0.05, results of both biochars must be combined in a single linear regression.
3.2. Cadmium Bioavailability
Overall, DTPA-extractable Cd concentrations were higher for the control and decreased as the rates of biochars increased [11]. Previous studies also suggested that biochars incorporation to soils effectively decreased the Cd availability as assessed by DTPA [33,34,35,36,37,38]. In this study, we correlated the DTPA-extractable Cd from the biochar-treated soils with Cd and accumulation in ryegrass plant parts (Table 4). Some studies revealed that the dilution effect caused by the increase of plant biomass also contributed to the decrease of Cd concentrations in plants cultivated in biochar-treated soils [33,34,35], which suggests that such decrease is not a consequence of biochar application. However, the positive correlations (and no correlations) found for DTPA-extractable Cd in the soil and Cd accumulation in ryegrass plant parts, when the biomass is accounted for, for both biochars, ensure the data reliability obtained in this study (Table 4). The absence of correlation suggests that DTPA-extractable Cd is better correlated with its uptake in plant parts, as shown in the previous study [11].

Table 4.
Pearson correlations between DTPA-extractable Cd contents in the soil (x-axis) and Cd accumulation in ryegrass shoots and roots.
3.3. Cadmium Transfer Factor
Table 5 shows the Cd transfer factor (TF) from ryegrass roots to shoots. The TF of accumulated Cd was similar to that observed by Antonangelo and Zhang [11] when evaluating the simple Cd uptake TF. Therefore, the TF decreased as the application of both biochars increased (Table 5). A lower TF indicates higher Cd accumulation in roots and lower transference to aboveground plant parts, which lowers the risk to the primary consumer [33,39]. Other studies have also reported the preferential accumulation of Cd in the roots of grasses rather than in shoots, such as ryegrass [31] and rice [32]. In our study, it is evident that the accumulated Cd was higher in the roots of ryegrass than in the shoots even for the control. On average, TF as a function of PLB application was higher than that of SGB, which is consistent with the fact that ryegrass shoots yielded better under the PLB amendment; which was also closely related to the N removal, thus resulted in higher Cd removal aboveground when compared to SGB. The maximum Cd TF was reached when 206 ± 38 kg N ha−1 was removed in ryegrass shoots (Figure 3). This is close to a TF of 0.5 obtained between the control and the lowest rate of PLB application rate (0.5%).

Table 5.
The transfer factor (TF) of accumulated Cd from ryegrass roots to shoots as a function of biochar (B) application rates (R).
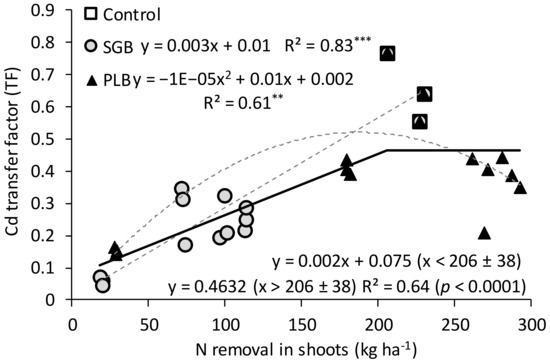
Figure 3.
Linear and quadratic regression models (gray dashed lines) for Cd transfer factor and N removal in ryegrass shoots as a function of applications of switchgrass (SGB) and poultry litter-derived biochars (PLB). The black solid line represents a linear regression with a plateau model combining both biochar-treated soils (SGB + PLB). The black dashed vertical line indicates the joint point (plateau point) of N removal where the Cd transfer factor ceases to increase. **: p < 0.01. ***: p < 0.001.
The linear with plateau model was converged from the whole dataset of measurements and for both biochars combined, which is probably a result of the simple linear regression obtained for SGB and a quadratic regression for PLB (Figure 3). The highest point of which the linear line reaches its maximum in the SGB is close to the maximum point reached with the quadratic line drawn in the PLB before the Cd TF values begin to reduce. Therefore, a maximum point (plateau or joint point), was obtained for both biochars combined thus indicating an optimum N removal in ryegrass shoots in which Cd removal also reached its limit (Figure 3).
4. Discussion
Our research demonstrated that Cd accumulation in ryegrass plant parts is proportionally affected by N uptake and ryegrass yields. Nitrogen fertilization may indeed change the phytoavailability of Cd in the growth medium and its absorption by plants [40]. Additionally, several studies have shown that N fertilization increases Cd concentration in plant tissues [16,41,42]. One of the reasons may rely on a possible synergic effect between nitrate (NO3−) and Cd [42,43,44,45] while the ratio between NO3− and ammonium-NH4+ might influence the accumulation of Cd in plants as well [46]. Commonly, NH4+ easily undergoes the nitrification process in aerobic environments and forms NO3−, which is taken up by plants, and such a process releases H+ in the soil solution thus reducing its pH and increasing the Cd bioavailability. In this sense, more research is encouraged to test nitrate and ammoniacal sources of nitrogen fertilizers to evaluate their impact on toxic elements removal. Additionally, evaluating if biochars produced from different feedstocks are a better source of NO3− or NH4+ would contribute to the better understanding of metal remediation practices if biochars can immobilize the metal and at the same time increase the toxic element removal by providing N efficiently while keeping metal concentration below the threshold for animal grazing.
A higher accumulation of Cd was found in the ryegrass roots as compared with the shoots. Since the root is the first plant part in contact with Cd in contaminated soil and its structural component likely accumulates the largest amount of Cd present in the plant tissues [47,48]. This is a physiological strategy in which plants phytostabilize the metal in the roots to prevent toxic elements from reaching the xylem, being transported to the shoots, and damaging the photosynthetic apparatus of plants [46] This prevention of Cd transport to the shoots may occur through the synthesis of chelants or even physical barriers that prevent the Cd movement in the apoplast [49]. Therefore, more attention must be paid to plants metabolism and molecular mechanisms to reveal the direct role of organic amendments, such as biochar, in future phytoremediation studies, as pointed out by Liu et al. [50]. For example, the recent study of Peco et al. [51] has found Biscutella auriculata L., a wild herbaceous species that grows on pastureland, as a new Cd-tolerant plant capable of activating efficient metal-sequestering mechanisms in the root surfaces and leaves, and of inducing phytochelatins in both parts, besides stimulating antioxidative defenses in roots.
5. Conclusions
Cadmium removal was the highest at the 1% PLB rate accompanied by the highest ryegrass yield. However, the Cd concentration in grazable forage remained acceptable. The Cd transfer factor from ryegrass roots to shoots increased when up to 206 ± 38 kg N ha−1 was removed in ryegrass shoots. Application of up to 1% PLB is a viable option, since it is a practical rate for handling operations requiring less volume of material than SGB.
Author Contributions
Conceptualization, J.A.; methodology, J.A. and H.Z.; validation, J.A. and H.Z.; formal analysis, J.A.; investigation, J.A. and H.Z.; resources, H.Z.; data curation, J.A.; writing—original draft preparation, J.A.; writing—review and editing, J.A. and H.Z.; visualization, J.A.; supervision, H.Z.; project administration, H.Z.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Oklahoma Agricultural Experiment Station.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data were generated at an environmentally controlled growth chamber located at CERL (Controlled Environmental Research Lab) Central, Oklahoma State University, main campus. Derived data supporting the findings of this study are available from the corresponding author J.A. on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeng, X.; Xu, H.; Lu, J.; Chen, Q.; Li, W.; Wu, L.; Tang, J.; Ma, L. The Immobilization of Soil Cadmium by the Combined Amendment of Bacteria and Hydroxyapatite. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jacquiod, S.; Cyriaque, V.; Riber, L.; Al-Soud, W.A.; Gillan, D.C.; Wattiez, R.; Sørensen, S.J. Long-term industrial metal contamination unexpectedly shaped diversity and activity response of sediment microbiome. J. Hazard. Mater. 2018, 344, 299–307. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.J.; Martínez-López, S.; Martínez-Martínez, L.B.; Pérez-Sirvent, C. Importance of the oral arsenic bioaccessibility factor for characterising the risk associated with soil ingestion in a mining-influenced zone. J. Environ. Manag. 2013, 116, 10–17. [Google Scholar] [CrossRef]
- Sinha, S.; Mishra, R.K.; Sinam, G.; Mallick, S.; Gupta, A.K. Comparative Evaluation of Metal Phytoremediation Potential of Trees, Grasses, and Flowering Plants from Tannery-Wastewater-Contaminated Soil in Relation with Physicochemical Properties. Soil Sediment Contam. Int. J. 2013, 22, 958–983. [Google Scholar] [CrossRef]
- He, M.; Shi, H.; Zhao, X.; Yu, Y.; Qu, B. Immobilization of Pb and Cd in Contaminated Soil Using Nano-Crystallite Hydroxyapatite. Procedia Environ. Sci. 2013, 18, 657–665. [Google Scholar] [CrossRef]
- Niu, L.; Yang, F.; Xu, C.; Yang, H.; Liu, W. Status of metal accumulation in farmland soils across China: From distribution to risk assessment. Environ. Pollut. 2013, 176, 55–62. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Hu, S.C.; Drake, K.D.; Jim, R. Potential health impacts of heavy-metal exposure at the Tar Creek Superfund site, Ottawa County, Oklahoma. Environ. Geochem. Health 2008, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Compton, H.; Basta, N. Field Test of In Situ Soil Amendments at the Tar Creek National Priorities List Superfund Site. J. Environ. Qual. 2007, 36, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.E.; Henke, W.; Davis, C.; Mottaleb, M.A.; Campbell, J.H.; Mcaliley, L.R. Quantitative analysis of the extent of heavy-metal contamination in soils near Picher, Oklahoma, within the Tar Creek Superfund Site. Chemosphere 2017, 172, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gouzie, D. Potential Remediation Methods and Their Applicability to the Tri-State Mining District, USA. In Proceedings of the GSA Annual Meeting, Indianapolis, IN, USA, 4–7 November 2018. [Google Scholar]
- Antonangelo, J.A.; Zhang, H. Heavy metal phytoavailability in a contaminated soil of northeastern Oklahoma as affected by biochar amendment. Environ. Sci. Pollut. Res. 2019, 26, 33582–33593. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Gao, L.; Chen, W.; Su, J.; Shen, Y. Urea application enhances cadmium uptake and accumulation in Italian ryegrass. Environ. Sci. Pollut. Res. 2020, 27, 34421–34433. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Singh, B.; Karwasra, S.P.S. Yield and uptake response of lettuce to cadmium as influenced by nitrogen application. Fertil. Res. 1988, 18, 49–56. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Liu, Y.; Xue, W.-L.; Chen, R.-X.; Du, S.-T.; Jin, C.-W. Slow-release nitrogen fertilizers can improve yield and reduce Cd concentration in pakchoi (Brassica chinensis L.) grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 25074–25083. [Google Scholar] [CrossRef]
- Wei, S.; Ji, D.; Twardowska, I.; Li, Y.; Zhu, J. Effect of different nitrogenous nutrients on the cadmium hyperaccumulation efficiency of Rorippa globosa (Turcz.) Thell. Environ. Sci. Pollut. Res. 2015, 22, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, C.; Hu, P.; Luo, Y.; Wu, L.; Sale, P.; Tang, C. Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii. Environ. Sci. Pollut. Res. 2016, 23, 1246–1253. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Deng, Y.; Zhong, F.; Xu, B.; Hu, L.; Wang, M.; Wang, G. Comparison of ammonium fertilizers, EDTA, and NTA on enhancing the uptake of cadmium by an energy plant, Napier grass (Pennisetum purpureum Schumach). J. Soils Sediments 2017, 17, 2786–2796. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalesa, S.; Jaremko, D.; Niedbała, M. Effect of nitrogen application and year on concentration of Cu, Zn, Ni, Cr, Pb and Cd in herbage of Galega orientalis Lam. Plant Soil Environ. 2016, 61, 11–16. [Google Scholar] [CrossRef]
- Benyas, E.; Owens, J.; Seyedalikhani, S.; Robinson, B. Cadmium Uptake by Ryegrass and Ryegrass–Clover Mixtures under Different Liming Rates. J. Environ. Qual. 2018, 47, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Q.; Hu, L.; Tang, J.; Xu, L.; Yang, X.; Yong, J.W.H.; Chen, X. Legumes Can Increase Cadmium Contamination in Neighboring Crops. PLoS ONE 2012, 7, e42944. [Google Scholar] [CrossRef][Green Version]
- Malcolm, B.J.; Moir, J.L.; Cameron, K.C.; Di, H.J.; Edwards, G.R. Influence of plant growth and root architecture of Italian ryegrass (Lolium multiflorum) and tall fescue (Festuca arundinacea) on N recovery during winter. Grass Forage Sci. 2015, 70, 600–610. [Google Scholar] [CrossRef]
- Mongkhonsin, B.; Nakbanpote, W.; Meesungnoen, O.; Prasad, M.N.V. Adaptive and Tolerance Mechanisms in Herbaceous Plants Exposed to Cadmium. Cadmium Toxic. Toler. Plants 2019, 73–109. [Google Scholar]
- Han, S.; Li, X.; Amombo, E.; Fu, J.; Xie, Y. Cadmium Tolerance of Perennial Ryegrass Induced by Aspergillus aculeatus. Front. Microbiol. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Antonangelo, J.A.; Zhang, H.; Sun, X.; Kumar, A. Physicochemical properties and morphology of biochars as affected by feedstock sources and pyrolysis temperatures. Biochar 2019, 1, 325–336. [Google Scholar] [CrossRef]
- Church, C.; Spargo, J.; Fishel, S. Strong Acid Extraction Methods for “Total Phosphorus” in Soils: EPA Method 3050B and EPA Method 3051. Agric. Environ. Lett. 2017, 2, 160037. [Google Scholar] [CrossRef]
- Richards, J.R.; Schroder, J.L.; Zhang, H.; Basta, N.T.; Wang, Y.; Payton, M.E. Trace Elements in Benchmark Soils of Oklahoma. Soil Sci. Soc. Am. J. 2012, 76, 2031–2040. [Google Scholar] [CrossRef]
- Wu, J.; Song, Q.; Zhou, J.; Wu, Y.; Liu, X.; Liu, J.; Zhou, L.; Wu, Z.; Wu, W. Cadmium threshold for acidic and multi-metal contaminated soil according to Oryza sativa L. Cadmium accumulation: Influential factors and prediction model. Ecotoxicol. Environ. Saf. 2021, 208, 111420. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W. Sampling, Handling, and Analyzing Plant Tissue Samples. SSSA Book Ser. Soil Test. Plant Anal. 2018, 3, 389–427. [Google Scholar]
- Rigby, H.; Smith, S.R. The significance of cadmium entering the human food chain via livestock ingestion from the agricultural use of biosolids, with special reference to the UK. Environ. Int. 2020, 143, 105844. [Google Scholar] [CrossRef]
- Feng, D.; Huang, C.; Xu, W.; Qin, Y.; Li, Y.; Li, T.; Yang, M.; He, Z. Difference of Cadmium Bioaccumulation and Transportation in Two Ryegrass Varieties and the Correlation between Plant Cadmium Concentration and Soil Cadmium Chemical Forms. Wirel. Pers. Commun. 2020, 110, 291–307. [Google Scholar] [CrossRef]
- Jarvis, S.C.; Jones, L.H.P.; Hopper, M.J. Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil 1976, 44, 179–191. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, X.; Zhao, Z.; He, Q.; Wang, S.; Zhu, Y.; Yan, Y.; Liu, X.; Sun, K.; Zhao, Y.; et al. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ. Pollut. 2016, 218, 513–522. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.R.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.-S.; Li, Z.-G.; Liu, Y.; Chen, F.; Dai, K. Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Valdez-González, J.C.; López-Chuken, U.J.; Guzmán-Mar, J.L.; Flores-Banda, F.; Hernández-Ramírez, A.; Hinojosa-Reyes, L. Saline irrigation and Zn amendment effect on Cd phytoavailability to Swiss chard (Beta vulgaris L.) grown on a long-term amended agricultural soil: A human risk assessment. Environ. Sci. Pollut. Res. 2014, 21, 5909–5916. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Effects of organic and inorganic amendments on bio-accumulation and partitioning of Cd in Brassica juncea and Ricinus communis. Ecol. Eng. 2015, 74, 93–100. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Chen, R.; Fu, G.; Chen, T.; Tao, L. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ. Exp. Bot. 2016, 122, 141–149. [Google Scholar] [CrossRef]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J. Hazard. Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef]
- Luo, B.F.; Du, S.T.; Lu, K.X.; Liu, W.J.; Lin, X.Y.; Jin, C.W. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2012, 63, 3127–3136. [Google Scholar] [CrossRef]
- Hu, J.; Wu, S.; Wu, F.; Leung, H.M.; Lin, X.; Wong, M.H. Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 2013, 93, 1359–1365. [Google Scholar] [CrossRef]
- Nogueirol, R.C.; Monteiro, F.A.; Junior, J.C.D.S.; Azevedo, R.A. NO3−/NH4+ proportions affect cadmium bioaccumulation and tolerance of tomato. Environ. Sci. Pollut. Res. 2018, 25, 13916–13928. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Ribeiro, P.C.; Tezotto, T.; Azevedo, R.A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculik, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2010, 62, 21–37. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Wang, L.; Xiao, Y. Influences of rice straw biochar and organic manure on forage soybean nutrient and Cd uptake. Int. J. Phytoremediat. 2020, 23, 53–63. [Google Scholar] [CrossRef]
- Peco, J.D.; Campos, J.A.; Romero-Puertas, M.C.; Olmedilla, A.; Higueras, P.; Sandalio, M.L. Characterization of mechanisms involved in tolerance and accumulation of Cd in Biscutella auriculata L. Ecotoxicol. Environ. Saf. 2020, 201, 110784. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).