Abstract
The present study provides a solvent-free organic synthesis of quaternary ammonium salts: bis(2-hydroxyethyl)dimethylammonium taurate ([BHEDMA][Tau]) and bis(2-hydroxyethyl)dimethylammonium acetate ([BHEDMA][OAc]). These ionic compounds are promising materials for carbon dioxide capture processes, as mono sorbents, supplemental components in the conventional process of chemical absorption, and in the combined membrane approach for improving sorption efficiency. The synthesized compounds were characterized by 1H NMR and FT-IR spectroscopies and elemental analysis. Afterward, the sorption properties of the compounds were evaluated using the inverse gas chromatography (IGC) method, and their thermodynamic parameters were calculated in the temperature range of 303.15–333.15 K. The enthalpy change (∆sH) was less than 80 kJ·mol−1, indicated by the physical nature of sorption and also proved by FT-IR. Henry’s law constant in regard to carbon dioxide at 303.15 K was equal to 4.76 MPa for [BHEDMA][Tau], being almost 2.5 lower than for [BHEDMA][OAc] (11.55 MPa). The calculated carbon dioxide sorption capacity for [BHEDMA][Tau] and [BHEDMA][OAc] amounted to 0.58 and 0.30 mmol·g−1, respectively. The obtained parameters are comparable with the known solid sorbents and ionic liquids used for CO2 capture. However, the synthesized compounds, combining the advantages of both alkanolamines and ionic liquids, contain no fluorine in their structure and thus match the principles of environmental care.
1. Introduction
Nowadays, with the growing energy demands and ecological concerns, natural gas is considered an environmentally friendly source of energy while, at the same time, finding application as a raw chemical material. As a consequence, its annual consumption is growing rapidly worldwide. The strict necessity to purify natural gas from acidic impurities (sweetening process) is due to the carbon dioxide’s assistance in the formation process of gas hydrates, thus rendering the raw material incompatible for utilization in fuel cells and shortening the service life of the equipment [1,2].
The current industry-accepted CO2 removal technology is chemical absorption using aqueous solutions of alkanolamines such as monoethanolamine (MEA), diethanolamine (DEA), triethanolamine (TEA), diisopropanolamine (DIPA), diglycolamine (DGA), and methyldiethanolamine (MDEA). Although a widely used method, it has a number of disadvantages, for instance, loss of the sorbent caused by thermal degradation and potential release of dangerous substances. In addition, this method is energy intensive due to the absorbent regeneration procedure requiring quite high capital costs, owing to the complicated design of the equipment and thus the large square of the plants [3,4,5]. Hence, the enhancement of the CO2 removal process should be achieved by the development of new sorbents with high sorption capacity in relation to CO2 and/or development and improvement in separation techniques.
As aforementioned, sorption systems based on alkanolamines are the most commonly used media in the field of chemical sorption of acid gases, due to their ability to form weakly bound complexes with CO2 [6,7]. On the other hand, ionic liquids (ILs) have some unique properties: low vapor pressure, thermal stability, high sorption capacity with respect to acid gases, and the ability to customize their physicochemical properties by combining various cations and anions or introducing functional groups. This class of materials found an application in many fields: extraction [8], nanotechnology [9], electrochemistry [10], electrolysis [11], catalysis [12], gas separation [13,14], and absorption [15]. However, the majority of ILs with high CO2 sorption capacity contain fluorine anion (e.g., [BF4], [Tf2N], [PF6]). Therefore, in terms of designing the new sorbents for carbon dioxide capture, the development of novel quaternary ammonium compounds with fluorine-free anions acquires great significance. This type of compound combining properties of ionic liquids based, and alkanolamines are promising sorbents of carbon dioxide due to them possessing the advantages of both classes.
Along with the development of new materials, novel approaches providing the rejection of high energy-consuming chemical absorption techniques are of great high interest. Membrane-based processes [16,17], as reaction-free approaches, are promising methods to reduce energy consumption and increase the economic efficiency of natural gas treatment. A novel and unique hybrid method—membrane-assisted gas absorption (MAGA)—provides the separation without a phase transition in the single-volume mass-exchange apparatus and does not require heat supply or removal [18,19]. The application of liquid sorbents in that process increases the selectivity; meanwhile, the gas separation membrane provides regeneration of the sorbent in a continuous steady-state mode [20,21].
The present research focuses on the quaternary ammonium compounds based on alkanolamines with “natural” anions: bis(2-hydroxyethyl)dimethylammonium taurate and bis(2-hydroxyethyl)dimethylammonium acetate. The novelty of the study is the preparation and investigation of simply designed but effective fluorine-free sorbents for carbon dioxide capture. These compounds were obtained via a solvent-free procedure and characterized by conventional methods (1H NMR, IR spectroscopies, and elemental analysis). Sorption properties such as thermodynamic parameters of the sorption process and sorption capacities for CH4, N2, and CO2 were investigated using inverse gas chromatography (IGC) in the temperature range of 303.15–333.15 K. A comparison of the sorption properties of the synthesized ionic compounds with the literature data revealed the potential application of the novel compounds as effective carbon dioxide capture materials.
2. Materials and Methods
2.1. Chemicals and Materials
For this study, 2-chloroethanol (99 wt%), 2-aminoethanesulfonic acid (≥99%), and anion-exchange resin Amberlyst® A21 free base were purchased from Sigma-Aldrich (Darmstadt, Germany). Hydrochloric acid and acetic acid were purchased from CJSC Chimreaktiv (Nizhny Novgorod, Russia), while sodium hydroxide and diethyl ether (>99.5 vol.%) were purchased from JSC Vekos (Nizhny Novgorod, Russia). Diethyl ether was dried using standard procedure and stored over activated molecular sieves (4 Å) for at least 48 h. In addition, 2-dimethylaminoethanol (≥98 wt%) was provided by «Oka-Sintez» Ltd. (Dzerzhinsk, Russia) and used without any additional purification. Single pure gases were purchased from Monitoring LLC (St. Petersburg, Russia): methane (≥99.9 vol.%), carbon dioxide (≥99.99 vol.%), hydrogen sulfide (≥99.5 vol.%), and nitrogen (≥99.999 vol.%), helium (≥99.9999 vol.%). Millipore Direct-Q3 deionized water (DI) was used.
2.2. NMR Spectroscopy
1H NMR spectra were recorded on a DD2 400 MHz spectrometer (Agilent, Santa Clara, CA, USA) using D2O or DMSO-d6 as solvents. The residual solvent peak was used as an internal standard. The chemical shifts (δ) are reported in parts per million (ppm); J values are given in hertz (Hz).
2.3. IR Spectroscopy
FT-IR spectra were obtained at ambient temperature under an inert atmosphere of argon (99.9995%) by means of the IRAfinity-1 instrument (Shimadzu, Kyoto, Japan) additionally equipped with the HATR PIKE cell with a ZnSe glass. The solid compound was placed onto the glass and pressed by the sampler, then the spectrum was registered. For the operando in situ experiments, the solid compound sample was placed into a HATR cell equipped with a purge gas (CO2) system. The adsorption spectra of carbon dioxide on the samples were recorded after flushing with CO2 for 2 h with a flow rate of 10 mL min−1 at a pressure of 0.1 MPa. A minimum of 30 scans was signal-averaged with a resolution of 4 cm−1 within the 4000–700 cm−1 range.
2.4. Elemental Analysis
Elemental composition was obtained by means of the Vario EL cube elemental analyzer (Elementar, Langenselbold, Germany) with a supplier-stated precision of <0.1%.
2.5. Melting Point Determination
The determination of the melting point of the synthesized compounds was checked by the capillary method, which consists of determining the temperature at which a crystal of a substance passes into a molten state in a capillary. To determine the melting point, the finely powdered substance was dried in vacuo and over anhydrous silica gel for 24 h. Then, the substance was placed into a capillary, degassed, sealed from the second end, fixed in a glass vessel with silicone oil, and placed on a magnetic stirrer. The temperature was gradually increased by 10 K starting from 333.15 K to 373.15 K every 10 min. Then, the rate of heating was adjusted to about 1 K·min−1. The temperature at which the last particle passed into the liquid phase was defined as the melting point.
2.6. Moisture Content Determination
Moisture content was determined by Karl Fischer titration on an 831 KF Coulometer (Metrohm AG, Herisau, Switzerland) by the standard procedure. The moisture content did not exceed 0.2 wt%.
2.7. Sorption Properties Determination
The sorption properties of the obtained ionic compounds were evaluated using the inverse gas chromatography (IGC) method. The ionic compound, dissolved in ethanol in a ratio of 1:2 by weight, was added to the Silochrome-80 carrier sorbent. The solution was stirred at room temperature for 2 h, and then the solvent was removed under vacuum for 10 h while gradually increasing the temperature to 333.15 K. After that, the sorbent was loaded into a stainless-steel gas chromatographic column using a vacuum pump connected to the opposite side until the column was filled. The prepared column was placed into a Chromos GC-1000 gas chromatography system. Helium was used as a carrier gas. The column was vented under helium with a flow rate of 7.5 cm3 min−1 at 373.15 K for 8 h. The details of the analysis are given in Table 1. The measurements of N2, CH4, and CO2 sorption were performed at temperatures: 303.15, 313.15, 323.15, 333.15 K, and pressure of 0.132 and 0.142 MPa for bis(2-hydroxyethyl)dimethylammonium acetate and bis(2-hydroxyethyl)dimethylammonium taurate, respectively. In order to obtain the precise data and guarantee that reproducibility, each experiment included 10 runs, where the regeneration of ionic compound occurred spontaneously from run to run under the experimental conditions.

Table 1.
The operating conditions of the GC system.
Theoretical basis: The main objective of any sorption method, including IGC, is to determine the partition constant K of the solute between the vapor and polymer phases. In addition, constant K can be derived from data on the static sorption under equilibrium conditions [22] as follows:
where and are the solute concentrations in the polymer and vapor phases, respectively.
In chromatography, the concentration ratio is replaced with the ratio of time for which a sorbate molecule stays in the carrier-gas flow () to the time for which a sorbate molecule stays in the sorbed state (). In this case, the partition constant takes the form
To determine parameters and in an IGC experiment, it is necessary to measure the retention times of sorbed and nonsorbed components, and , respectively. In fact, the value of corresponds to the retention time of an “almost nonsorbed” or “poorly sorbed” component and is used to estimate the “dead volume” of the chromatograph. The “dead volume” is the volume of the eluent required to fill the pores of the sorbent and the space between its particles in the column.
where is the “dead volume”, cm3; —is the volumetric flow rate of the carrier gas, cm3 min−1.
On the basis of these times, we can calculate corrected retention volume [22].
where and are the elution times of the retained and non-retained components, respectively, s; is the volumetric flow rate of the carrier gas at the average pressure in the column and temperature , cm3 min−1.
where and are column temperature and room temperature, respectively, K; is defined by James–Martin correction factor [23] as follows:
where n and m are integers (1, 2, 3, …), and and are the pressure at the inlet and outlet of the column, respectively, atm.
The normalization of the corrected retention volume to mass of the mass of the ionic compound in the chromatographic column yields specific retention volume as follows:
where is the density of the ionic compound, g cm−3; is the mass of the ionic compound in the column, g.
Specific retention volume is one of the main experimental parameters used to calculate all the thermodynamic parameters on the basis of a simple relationship.
The dimensionless Henry’s law constant is then converted into the dimensional Henry’s law constant (KC) in units of mmol g−1 atm−1 [24].
where 𝑅 is the gas law constant.
The dimensional Henry’s law constant () in units of mmol g−1 atm−1 can be converted into the dimensional Henry’s law constant () in units of atm as follows:
where is the molar mass of the ionic compound, g mol−1.
2.8. Synthesis Procedure
Halide salt. The rational strategy for the high-yield solvent-free synthesis of alkylammonium halides is described elsewhere [25]. To prepare bis(2-hydroxyethyl)dimethylammonium chloride [BHEDMA][Cl] halide salt, an equimolar amount of 2-chloroethanol (50 mmol) was added to 2-dimethylaminoethanol (50 mmol) and placed in a round bottom flask. The reaction mixture was heated up to 343.15 K under reflux for 3 days (Scheme 1). The reaction was carried out under an inert gas (nitrogen) atmosphere. The resulting white solid was washed three times with diethyl ether to remove the rest of the unreacted compounds. Then, the solvent was decanted, and the final product was dried under vacuum at 333.15 K for 24 h. The product was obtained as white solid (yield 92%).
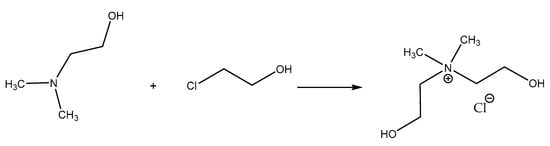
Scheme 1.
Synthesis of ionic compound bis(2-hydroxyethyl)dimethylammonium chloride [BHEDMA][Cl] (1).
Anion-exchange reaction. For the synthesis of the taurate or acetate ammonium salts, anion-exchange reactions using an activated free base anion exchange resin (Amberlyst® A21) were carried out. The resin’s activation proceeded as follows [21]: a weighed portion of Amberlyst A21 resin (10 g) was washed with a 10% mass of an aqueous solution of hydrochloric acid (50 g) 5 times using an ion exchanger with a porous filter. Afterward, the resin was washed with distilled water until pH = 7. Then, using the same ion exchanger, the resin was treated with 10% of the mass of an aqueous NaOH solution (50 g) 5 times and then was washed with distilled water until pH = 7 to obtain a hydroxyl anionic resin. Before use, it was dried to constant weight at room temperature and pressure in an inert gas stream (Scheme 2).
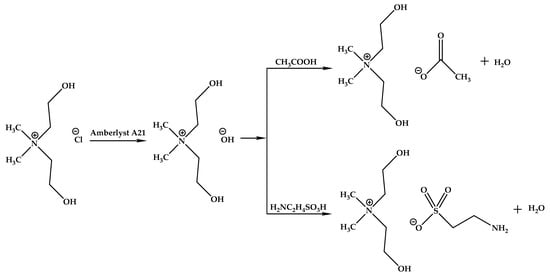
Scheme 2.
Synthesis of ionic compounds bis(2-hydroxyethyl)dimethylammonium with taurate [BHEDMA][Tau] (2) and acetate [BHEDMA][OAc] (3) anions.
To obtain bis(2-hydroxyethyl)dimethylammonium hydroxide 30% of the mass, an aqueous solution of bis(2-hydroxyethyl)dimethylammonium chloride was passed through a column with an OH− ion-exchange resin. Then, equimolar amounts of taurine or acetic acid were added to the resulting solution to obtain bis(2-hydroxyethyl)dimethylammonium taurate or acetate, respectively. The reaction mixture was stirred at room temperature for 4 h, after which the solution was decanted, and water was removed by an evaporator. The resulting product was dried under reduced pressure at room temperature for 24 h and continued drying with increasing the temperature gradually up to 343.15 K until the water content became less than 0.2 wt%.
[(CH3)2N(CH2CH2OH)2]+ [Cl]− (1). White solid, yield 92%. 1H NMR (400 MHz, DMSO-d6) δ 5.56–5.49 (m, 2H; OH), 3.85–3.75 (m, 4H; CH2-CH2OH), 3.49–3.44 (m, 4H; CH2-CH2OH), 3.13 (s, 6H; NCH3). FT-IR (KBr), ν (cm−1): 3280 (O-H), 3020, 2962, 2887 (C-H), 1471, 887 (N+–C), 1076, 1053, 956, 933 (C-O(H)). Elemental analysis: calc.: C, 42.48; H, 9.51; N, 8.26. Found: C, 42.4; H, 9.5; N 8.3.
[(CH3)2N(CH2CH2OH)2]+ [C2H6NSO3]− (2). White solid, yield 95%. 1H NMR (400 MHz, D2O) δ 4.11–4.06 (m, 4H; CH2-CH2OH), 3.63–3.59 (m, 4H; CH2-CH2OH), 3.43 (t, J = 6.6 Hz, 2H; S-CH2-CH2-NH2), 3.27 (t, J = 6.6 Hz, 2H; S-CH2-CH2-NH2), 3.24 (s, 6H; NCH3). FT-IR (KBr), ν (cm−1): 3387 (O-H), 3047, 2926, 2866 (C-H), 1473, 889 (N+–C), 1087, 1053, 954 (C-O(H)), 1637 (N–H), 1211 (S=O). Elemental analysis: calc.: C, 37.20; H, 8.58; N, 10.84. Found: C, 37.1; H, 8.5; N, 10.8. M.p.433.15 K.
[(CH3)2N(CH2CH2OH)2]+ [CH3C(O)O]− (3). Pale yellow solid, yield 95%. 1H NMR (400 MHz, D2O) δ 4.12–4.05 (m, 4H; CH2-CH2OH), 3.63–3.59 (m, 4H; CH2-CH2OH), 3.25 (s, 6H; NCH3), 2.09 (s, 1H; CH3). FT-IR (KBr), ν (cm−1): 3350 (O-H), 3028, 2958, (C-H), 1473, 887 (N+–C), 1080, 952 (C-O(H)), 1730 (C=O). Elemental analysis: calc.: C, 49.72; H, 9.91; N, 7.25. Found: C, 49.7; H, 9.9; N, 7.3. M.p. 418.15 K.
3. Results and Discussion
3.1. Spectral Characterization
The FT-IR spectra of all synthesized compounds are shown in Figure 1. As expected, and clearly seen in Figure 1, for all ionic compounds, a set of similar bands were observed. These include a broad absorption band of stretching vibrations of hydroxyl groups centered at around 3300 cm−1 and C–H stretching modes in hydroxyalkyl radicals located around 2800–3050 cm−1 [26,27,28]. Several characteristic vibrations for alcohol fragments could also be found in the spectra. For example, single or twin peaks at 1150–1050 cm−1 and peaks at 950–930 cm−1 assigned to C–O stretching vibrations [29] also appeared in the spectra.
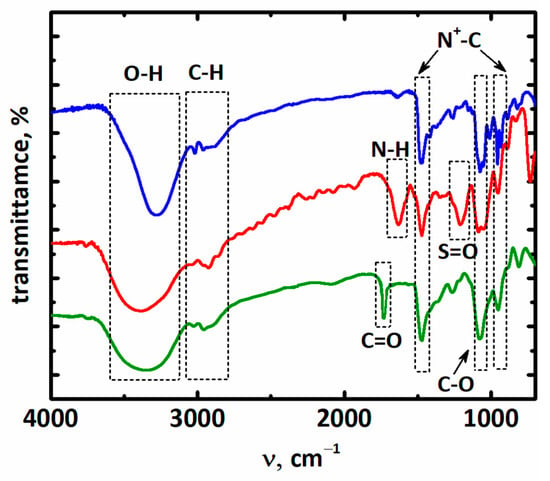
Figure 1.
The FT-IR spectra of [BHEDMA][Cl] (1, blue), [BHEDMA][Tau] (2, red, +56% transmittance from baseline), and [BHEDMA][OAc] (3, green 87% transmittance from baseline).
In addition, some new peaks corresponded to the taurate and acetate anion and appeared in the spectra of compounds 2 and 3. Thus, the absorption bands at 1637 cm−1 (asym. N–H), 1211 cm−1 (stretching S=O) [30] (red spectrum), and band at 1730 cm−1 dedicated to C=O vibration [31] (green spectrum) could be seen in the spectra of compound 2 and 3, respectively.
The 1H NMR spectrum of compound 1 (Figure 2) contained a multiplet corresponding to hydroxyl protons in the range of 5.49–5.56 ppm. These peaks were not observed in the spectra of compounds 2 and 3 since these spectra were recorded in deuterated water. All spectra contained the singlets of methyl protons: at 3.13 ppm for 1, at 3.24 ppm for 2, and at 3.25 ppm for 3. The multiplets of methylene protons emerged at 3.85–3.75 and 3.49–3.44 ppm (compound 1), at 4.06–4.11 and 3.63–3.59 ppm (compound 2), and 4.12–4.05 and 3.63–3.59 (compound 3). Beyond that, 1H NMR spectra of compounds 2 and 3 contained signals that correspond to the protons of anions. Thus, the methylene protons of the taurate anion emerged as triplets at 3.27 and 3.43 ppm (Figure 3). However, the protons of the amino group (taurate anion) were invisible in the spectrum. This observation could be explained by deuterium exchange with protons of deuterated solvent [32]. Three methyl protons of acetate anion appeared as a singlet at 2.09 ppm (Figure 4).
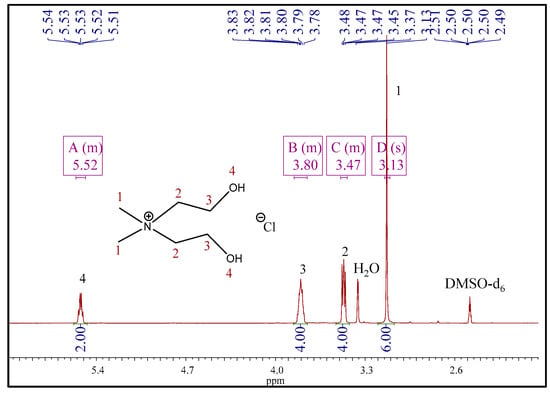
Figure 2.
The 1H NMR spectra of [BHEDMA][Cl] (1).
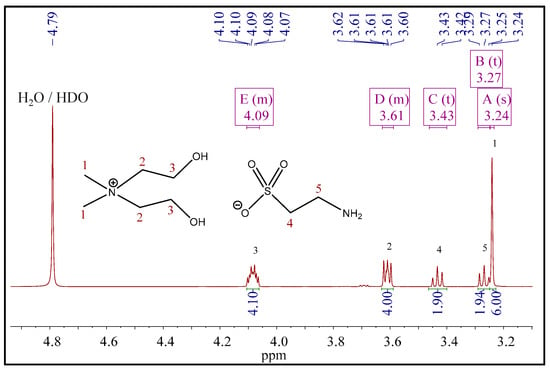
Figure 3.
The 1H NMR spectra of [BHEDMA][Tau] (2).
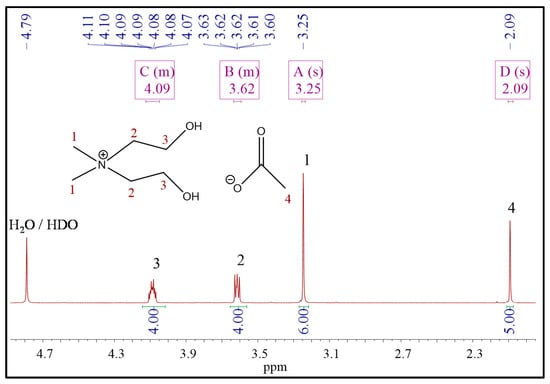
Figure 4.
The 1H NMR spectra of [BHEDMA][OAc] (3).
3.2. Sorption Characteristics
To investigate the sorption properties of the obtained ionic compounds, the IGC analysis at temperatures of 303.15, 313.15, 323.15, 333.15 K, and pressures close to atmospheric was carried out.
Figure 5a,b show the obtained Henry’s law constants as the function of temperature. Henry’s law constant values of [BHEDMA][Tau] with respect to nitrogen fall in the range 10.02–11.59 MPa, and with respect to methane, they were 11.07–13.50 MPa. In the case of [BHEDMA][OAc], the values were even higher, 30.36–97.00 MPa for nitrogen and 28.14–92.00 MPa for methane, indicating that the amount of captured gases was quite low. Meanwhile, Henry’s law constant values of [BHEDMA][Tau] with respect to carbon dioxide were in the range of 4.76–7.80 MPa, and in the case of [BHEDMA][OAc], they were 11.55–27.38 MPa. As seen, [BHEDMA][Tau] had a lower value of Henry’s law constants in comparison with [BHEDMA][OAc], thus providing higher carbon dioxide sorption capacity. Taking into account the same cation for both compounds, one can conclude that the anion structure (composition and configuration) affects the sorption properties vastly, although further investigation did not find out any interaction between sorbent and sorbate resulting from a chemical reaction.
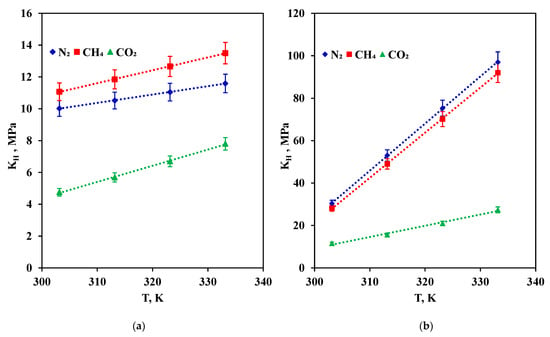
Figure 5.
Henry’s law constants for synthesized compounds: (a) [BHEDMA][Tau]; (b) [BHEDMA][OAc].
The thermodynamic parameters of CO2 sorption by [BHEDMA][Tau] and [BHEDMA][OAc] were derived from the correlation of Henry’s law constant, applying the known thermodynamic relations [33]. The Gibbs energy is given by Equation (11) as follows:
where is the standard-state pressure, MPa; R is the universal gas constant, J·mol−1·K−1. The dependence of the constant of Henry’s law on pressure can be neglected since in this experiment the pressure range is narrow [33].
Using this approximation and Equations (11)–(13), the thermodynamic parameters of the sorption of carbon dioxide by an ionic compound were estimated. The values of the Gibbs energy, enthalpy, and entropy of sorption are given in Table 2.

Table 2.
Thermodynamic properties of [BHEDMA][Tau] and [BHEDMA][OAc].
The dependence of values show a linear correlation with 1/T (Figure 6), which means that within the studied temperature range, the sorption enthalpy is independent of temperature. Additionally, in Figure 6, it is indicated that carbon dioxide is more than three times soluble in [BHEDMA][Tau] than in [BHEDMA][OAc].
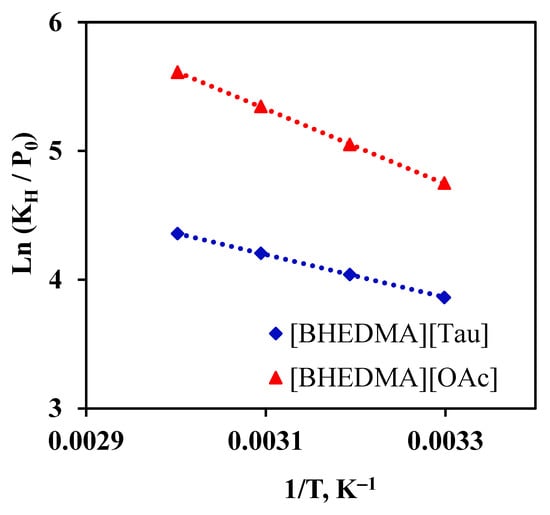
Figure 6.
Dependence of from a reverse temperature, , for the sorption of CO2 by ionic compounds. The dots represent the experimentally obtained values, and the dashed lines are the trend lines.
As is seen from Table 2, the values of are positive and increase with increasing temperature in the same manner for both ionic compounds making the sorption process is exothermic. As known, the value less than 80 kJ·mol−1 corresponds to the physisorption, while within the range of 80–200 kJ·mol−1, it corresponds to chemisorption [34]. Hence, the values of in the present study indicate the adsorption processes of CO2 on [BHEDMA][Tau] and [BHEDMA][OAc] are physisorption processes. The negative values of suggest a decrease in disorderliness during the adsorption process of CO2 because the molecules are adsorbed on the active centers, depressing the degree of freedom and leading to the tendency of the system to be ordered [35].
The physical nature of the absorption process was proven also by FTIR spectroscopy. For this, the operando in situ experiment was carried out. As clearly seen in Figure 7, no shifts of main peaks of compounds, as well as of C=O band, in carbon dioxide occurred, thus indicating no chemical interaction occurred between ionic compounds and gas.
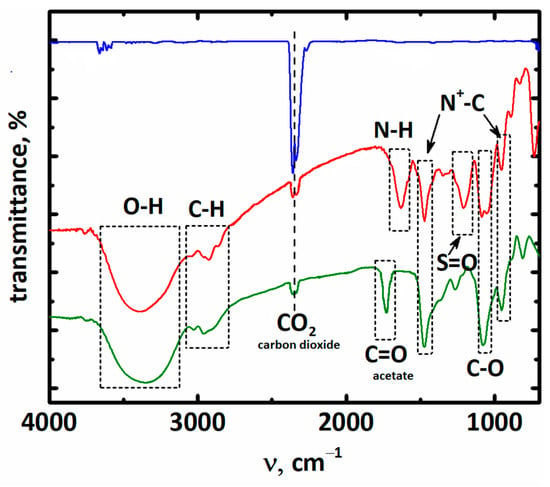
Figure 7.
The FT-IR spectra of carbon dioxide (blue), [BHEDMA][Tau]+CO2 (red, 67% transmittance from baseline), and [BHEDMA][OAc]+CO2 (3, green, 97% transmittance from baseline).
The comparison of Henry’s law constant’s values of carbon dioxide for synthesized compounds and of some known ionic liquids are presented in Table 3. As seen, for [BHEDMA][OAc], the values of Henry’s law constants are higher, and for [BHEDMA][Tau], it is comparable to all the values shown. As seen from the literature, the ILs containing fluorine have a high absorption capacity [36,37,38,39,40,41,42]. This can be explained by the influence of the anion fluorine groups on the solubility of CO2. It was shown in [43,44] that an increase in the number of fluorine atoms increases the carbon dioxide sorption capacity of the ionic liquid. However, due to environmental reasons, ILs with no fluorine are preferable for application [44].

Table 3.
Henry’s law constant CO2 for ionic compound at 303.15 K.
As seen in Table 4, the sorption capacity of [BHEDMA][Tau] was 0.58 mmol·g−1, almost twice higher than of [BHEDMA][OAc] (0.30 mmol·g−1). Compared with some known solid CO2 sorbents, the sorption capacity of [BHEDMA] [Tau] was about two times higher than of solid silica sorbents. Compared with carbon-containing, organometallic solid sorbents and zeolites, the compound synthesized in this work with the taurate anion showed comparable or slightly lower values of the sorption capacity for carbon dioxide. This means that [BHEDMA][Tau], which functions as a standalone sorbent, will most likely be able to compete with the currently known solid CO2 sorbents after the modification of its sorption qualities.

Table 4.
CO2 uptake by solid sorbents.
Nowadays, the scientific community aims at the search and development of “green” materials and technologies and the development of effective and environmentally friendly fluorine-free sorbents with high sorption capacity, which are quite preferable for further investigation and use. Thus, the synthesized [BHEDMA][Tau] and [BHEDMA][OAc] investigated in this study are promising adsorbents for CO2.
4. Conclusions
In the present study, the solvent-free synthesis of two quaternary ammonium ionic compounds—bis(2-hydroxyethyl)dimethylammonium taurate and bis(2-hydroxyethyl)dimethylammonium acetate—was performed and described in detail. The obtained compounds were characterized by spectroscopic methods (FTIR, 1H NMR), elemental analysis, and the inverse gas chromatography (IGC) method. It was shown that Henry’s law constant value determined for [BHEDMA][Tau] with respect to carbon dioxide ranged from 4.76 to 7.80 MPa, which was much lower than for nitrogen (10.02–11.59 MPa), and for methane (11.07–13.50 MPa) in the temperature range 303.15–333.15 K. In the case of [BHEDMA][OAc], the same parameter equaled 11.55 to 27.38 MPa regarding carbon dioxide, 30.36–97.00 MPa for nitrogen, and 28.14–92.00 MPa for methane within the same temperatures. The calculated carbon dioxide sorption capacity for [BHEDMA] [Tau] and [BHEDMA][OAc] amounted to 0.58 and 0.30 mmol·g−1, respectively. For both compounds, the sorption process was found to have a physical nature ( 80 kJ·mol−1). Taking into account the same cation of both compounds, one can suggest the structure of anion making the most significant contribution to the sorption properties. Thus, the presence of an amino group and extra-oxygen in the taurate structure increased the sorption efficiency while avoiding chemical interaction, which was proven by FT-IR. In addition to some known solid adsorbents, the sorption capacities for CO2 of the synthesized compounds were of the same order. Meanwhile, the Henry’s law constants for [BHEDMA][Tau] had comparable values regarding the known ionic liquids but the absence of the fluorine atoms in its structure provides an advantage, making [BHEDMA][Tau] a good sorbent even as a mono material. Additionally, the synthesized compounds can be used as an additional component in the conventional process of chemical absorption by alkanolamines as well as in the combined membrane approach for improving sorption efficiency. Further studies will focus on this approach and its direct application in membrane-assisted gas absorption and natural gas sweetening.
Author Contributions
Methodology, O.V.K. and M.E.A.; synthesis of materials and IR spectroscopy, M.E.A.; NMR spectroscopy, A.V.N.; IHC analysis, D.M.Z. and A.N.P.; data curation, A.N.P., A.V.V. and A.A.A.; writing—original draft preparation, M.E.A.; writing—original draft preparation, M.E.A., O.V.K. and A.A.A.; project administration, I.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
The main part and findings of the reported study were funded by RFBR, Project Number 20-38-90207. The part related to the NMR characterization was financially supported Ministry of Science and Higher Education of the Russian Federation as part of the scientific project the Laboratory of Smart Materials and Technologies (LSMT) Project Number FSSM-2021-0013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bahadori, A. Natural Gas Processing: Technology and Engineering Design; Gulf Professional Publishing: Houston, TX, USA, 2014; pp. 1–872. [Google Scholar] [CrossRef]
- Mazyan, W.; Ahmadi, A.; Ahmed, H.; Hoorfar, M. Market and technology assessment of natural gas processing: A review. J. Nat. Gas Sci. Eng. 2016, 30, 487–514. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Memb. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Anselmi, H.; Mirgaux, O.; Bounaceur, R.; Patisson, F. Simulation of Post-Combustion CO2 Capture, a Comparison among Absorption, Adsorption and Membranes. Chem. Eng. Technol. 2019, 42, 797–804. [Google Scholar] [CrossRef]
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chemie-Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, P.; Kundu, S.; Patel, S.; Setiawan, A.; Atkin, R.; Parthasarthy, R.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids. Renew. Sustain. Energy Rev. 2019, 105, 268–292. [Google Scholar] [CrossRef]
- Minea, A.A.; Murshed, S.M.S. A review on development of ionic liquid based nanofluids and their heat transfer behavior. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Atlaskina, M.E.; Kazarina, O.V.; Mochalova, A.E.; Vorotyntsev, I.V. Synthesis of Monomeric Ionic Liquids Based on 4-Vinylbenzyl Chloride as Precursors of a Material for the Selective Layer of Gas Separation Membranes. Membr. Membr. Technol. 2021, 3, 36–42. [Google Scholar] [CrossRef]
- Atlaskina, M.E.; Markov, A.N.; Kazarina, O.V. Calorimetric Study of Ionic Liquids Based on 4-Vinylbenzyl Triethylammonium with Chloride and Tetrafluoroborate Anion. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 062146. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Belyaev, E.S. Deep gas cleaning of highly permeating impurities using a membrane module with a feed tank. Pet. Chem. 2011, 51, 595–600. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Murav’ev, D.V. Fine gas purification to remove slightly penetrating impurities using a membrane module with a feed reservoir. Dokl. Chem. 2006, 411, 243–245. [Google Scholar] [CrossRef]
- Petukhov, A.N.; Atlaskin, A.A.; Kryuchkov, S.S.; Smorodin, K.A.; Zarubin, D.M.; Petukhova, A.N.; Atlaskina, M.E.; Nyuchev, A.V.; Vorotyntsev, A.V.; Trubyanov, M.M.; et al. A highly-efficient hybrid technique–Membrane-assisted gas absorption for ammonia recovery after the Haber-Bosch process. Chem. Eng. J. 2020, 421, 127726. [Google Scholar] [CrossRef]
- Kryuchkov, S.S.; Petukhov, A.N.; Atlaskin, A.A. Experimental Evaluation of the Membrane-Assisted Gas Absorption Technique Efficiency Using an Aqueous Solution Of PEG-400 for the Ammonia Capture. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 052071. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Yanbikov, N.R.; Smorodin, K.A.; Petukhov, A.N.; Trubyanov, M.M.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of acid gases removal process by membrane-assisted gas absorption using imidazolium ionic liquids solutions absorbent. Sep. Purif. Technol. 2020, 239, 116578. [Google Scholar] [CrossRef]
- Vorotyntsev, I.V.; Atlaskin, A.A.; Trubyanov, M.M.; Petukhov, A.N.; Gumerova, O.R.; Akhmetshina, A.I.; Vorotyntsev, V.M. Towards the potential of absorbing pervaporation based on ionic liquids for gas mixture separation. Desalin. Water Treat. 2017, 75, 305–313. [Google Scholar] [CrossRef]
- Belov, N.A.; Safronov, A.P.; Yampolskii, Y.P. Inverse-gas chromatography and the thermodynamics of sorption in polymers. Polym. Sci.-Ser. A 2012, 54, 859–873. [Google Scholar] [CrossRef]
- Kawakami, M.; Kagawa, S. Measurements of the Solubility Coefficients of Gases and Vapors in Natural Rubber by Gas Chromatographic Technique. Bull. Chem. Soc. Jpn. 1978, 51, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.M.W.; Handan Tezel, F. Adsorption separation of CF4, O2, CO2, and COF2 from an excimer gas mixture. Sep. Purif. Technol. 2021, 258, 117659. [Google Scholar] [CrossRef]
- Kazarina, O.V.; Agieienko, V.N.; Nagrimanov, R.N.; Atlaskina, M.E.; Petukhov, A.N.; Moskvichev, A.A.; Nyuchev, A.V.; Barykin, A.V.; Vorotyntsev, I.V. A rational synthetic approach for producing quaternary ammonium halides and physical properties of the room temperature ionic liquids obtained by this way. J. Mol. Liq. 2021, 344, 117925. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Ganguly, S.; Swamy, H.R.; Oxton, I.A. Infrared studies of the phase transitions of alkylammonium halides, RNH3X, and bis-(alkylammonium) tetrahalogenometallates(II), (RNH3)2MX4, (R = alkyl, M = metal, X = Cl or Br). J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1981, 77, 1825–1836. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1872–1892. [Google Scholar] [CrossRef]
- Snavely, D.L.; Dubsky, J. Near-IR spectra of polyethylene, polyethylene glycol, and polyvinylethyl ether. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2575–2579. [Google Scholar] [CrossRef]
- Miyazawa, T.; Fukushima, K.; Ideguchi, Y. Molecular vibrations and structure of high polymers. III. Polarized infrared spectra, normal vibrations, and helical conformation of polyethylene glycol. J. Chem. Phys. 1962, 37, 2764–2776. [Google Scholar] [CrossRef]
- Goodarzi, A.; Khanmohammadi, M.; Ebrahimi-Barough, S.; Azami, M.; Amani, A.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, A.; Farzin, A.; Ai, J. Alginate-Based Hydrogel Containing Taurine-Loaded Chitosan Nanoparticles in Biomedical Application. Arch. Neurosci. 2019, 6, e86349. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Shahkhatuni, A.A.; Shahkhatuni, A.G.; Mamyan, S.S.; Ananikov, V.P.; Harutyunyan, A.S. Proton–deuterium exchange of acetone catalyzed in imidazolium-based ionic liquid–D2O mixtures. RSC Adv. 2020, 10, 32485–32489. [Google Scholar] [CrossRef]
- Jalili, A.H.; Rahmati-Rostami, M.; Ghotbi, C.; Hosseini-Jenab, M.; Ahmadi, A.N. Solubility of H2S in Ionic Liquids [bmim][PF6], [bmim][BF4], and [bmim][Tf2N]. J. Chem. Eng. Data 2009, 54, 1844–1849. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, H.; Tang, X.; Deng, H.; Liu, H. Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem. Eng. J. 2012, 200, 399–404. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Ma, X.; Zeng, Y. Adsorption equilibrium and thermodynamics of CO2 and CH4 on carbon molecular sieves. Appl. Surf. Sci. 2017, 396, 870–878. [Google Scholar] [CrossRef]
- Lee, B.-C.; Outcalt, S.L. Solubilities of Gases in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2006, 51, 892–897. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Sensenich, B.; Scurto, A.M. High-pressure phase equilibria of {carbon dioxide (CO2) + n-alkyl-imidazolium bis(trifluoromethylsulfonyl)amide} ionic liquids. J. Chem. Thermodyn. 2010, 42, 305–311. [Google Scholar] [CrossRef]
- Yunus, N.M.; Mutalib, M.I.A.; Man, Z.; Bustam, M.A.; Murugesan, T. Solubility of CO2 in pyridinium based ionic liquids. Chem. Eng. J. 2012, 189, 94–100. [Google Scholar] [CrossRef]
- Ying, H.; Baltus, R.E. Experimental measurement of the solubility and diffusivity of CO2 in room-temperature ionic liquids using a transient thin-liquid-film method. Ind. Eng. Chem. Res. 2007, 46, 8166–8175. [Google Scholar] [CrossRef]
- Ramdin, M.; Amplianitis, A.; Bazhenov, S.; Volkov, A.; Volkov, V.; Vlugt, T.J.H.; de Loos, T.W. Solubility of CO2 and CH4 in Ionic Liquids: Ideal CO2 /CH4 Selectivity. Ind. Eng. Chem. Res. 2014, 53, 15427–15435. [Google Scholar] [CrossRef]
- Camper, D.; Scovazzo, P.; Koval, C.; Noble, R. Gas solubilities in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2004, 43, 3049–3054. [Google Scholar] [CrossRef]
- Kumełan, J.; Pe, Ä. Solubility of CO2 in the Ionic Liquids [bmim][CH3SO4] and [bmim][PF6]. Engineering 2006, 51, 1802–1807. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Aki, S.N.V.K.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving carbon dioxide solubility in ionic liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef] [PubMed]
- Harlick, P.J.E.; Sayari, A. Applications of Pore-Expanded Mesoporous Silica. 5. Triamine Grafted Material with Exceptional CO2 Dynamic and Equilibrium Adsorption Performance. Ind. Eng. Chem. Res. 2007, 46, 446–458. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Ghosh, A.; Subrahmanyam, K.S.; Krishna, K.S.; Datta, S.; Govindaraj, A.; Pati, S.K.; Rao, C.N.R. Uptake of H2 and CO2 by graphene. J. Phys. Chem. C 2008, 112, 15704–15707. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Arstad, B.; Fjellvåg, H.; Kongshaug, K.O.; Swang, O.; Blom, R. Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption 2008, 14, 755–762. [Google Scholar] [CrossRef]
- Jadhav, P.D.; Chatti, R.V.; Biniwale, R.B.; Labhsetwar, N.K.; Devotta, S.; Rayalu, S.S. Monoethanol Amine Modified Zeolite 13X for CO2 Adsorption at Different Temperatures. Energy Fuels 2007, 21, 3555–3559. [Google Scholar] [CrossRef]
- Katoh, M.; Yoshikawa, T.; Tomonari, T.; Katayama, K.; Tomida, T. Adsorption Characteristics of Ion-Exchanged ZSM-5 Zeolites for CO2/N2 Mixtures. J. Colloid Interface Sci. 2000, 226, 145–150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).