Exploring the Influence of Industrial and Climatic Variables on Communities of Benthic Macroinvertebrates Collected in Streams and Lakes in Canada’s Oil Sands Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Data
2.3. Statistical Analyses
3. Results
3.1. What Were the Most Commonly Selected Variables?
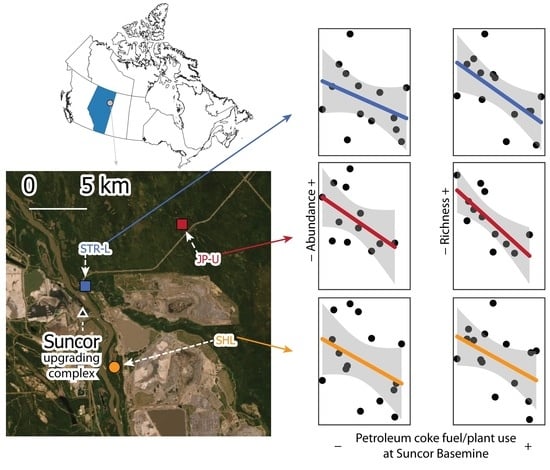
3.2. Industrial and Climatic Variables Selected at Primary Sites
3.3. Industrial and Climatic Variables Selected at Secondary Sites
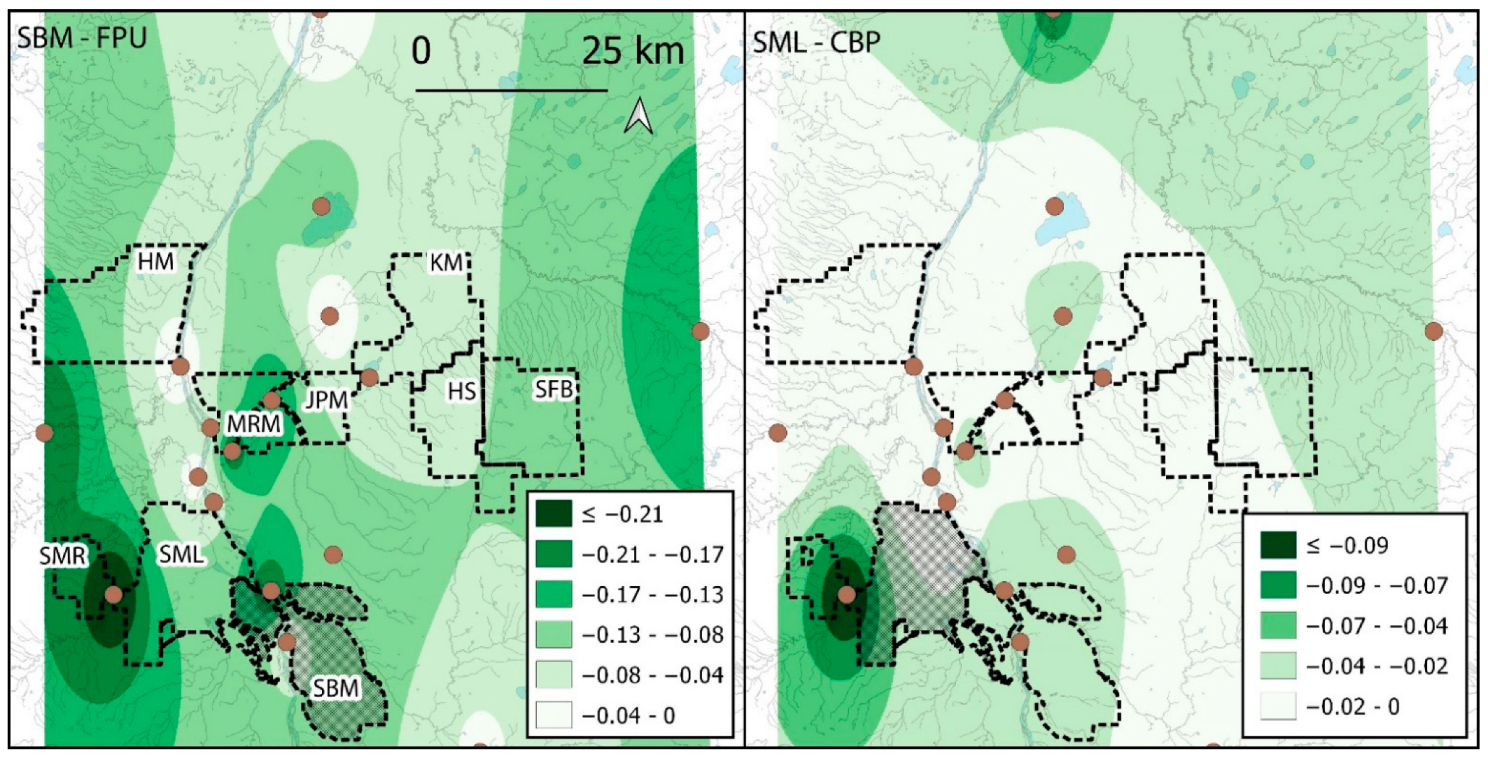
3.4. Spatial Patterns of Industrial Influences
3.5. Potential Influence of Climatic and Land Disturbance Variables
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Roberts, D.R.; Hazewinkel, R.O.; Arciszewski, T.J.; Beausoleil, D.; Davidson, C.J.; Horb, E.C.; Sayanda, D.; Wentworth, G.R.; Wyatt, F.; Dubé, M.G. An integrated knowledge synthesis of regional ambient monitoring in Canada’s oil sands. Integr. Environ. Assess. Manag. 2021, in press. [Google Scholar] [CrossRef]
- Horb, E.C.; Wentworth, G.R.; Makar, P.A.; Liggio, J.; Hayden, K.; Boutzis, E.I.; Beausoleil, D.L.; Hazewinkel, R.O.; Mahaffey, A.C.; Sayanda, D. A decadal synthesis of atmospheric emissions, ambient air quality, and deposition in the oil sands region. Integr. Environ. Assess. Manag. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Murray, W.A. The 1981 Snowpack Survey in the AOSERP Study Area; Alberta Oil Sands Environmental Research Program: Edmonton, AB, Canada, 1981. [Google Scholar] [CrossRef]
- Shelfentook, W. An Inventory System for Atmospheric Emissions in the AOSERP Study Area; Alberta Oil Sands Environmental Research Program: Edmonton, AB, Canada, 1978. [Google Scholar] [CrossRef]
- Cooke, C.A.; Kirk, J.L.; Muir, D.C.G.; Wiklund, J.A.; Wang, X.; Gleason, A.; Evans, M.S. Spatial and temporal patterns in trace element deposition to lakes in the Athabasca oil sands region (Alberta, Canada). Environ. Res. Lett. 2017, 12, 124001. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Hazewinkel, R.R.O.; Dubé, M.G. A critical review of the ecological status of lakes and rivers from Canada’s Oil Sands Region. Integr. Environ. Assess. Manag. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shotyk, W.; Zaccone, C.; Noernberg, T.; Pelletier, R.; Bicalho, B.; Froese, D.G.; Davies, L.; Martin, J.W. Airborne Petcoke Dust is a Major Source of Polycyclic Aromatic Hydrocarbons in the Athabasca Oil Sands Region. Environ. Sci. Technol. 2016, 50, 1711–1720. [Google Scholar] [CrossRef]
- Gopalapillai, Y.; Kirk, J.L.; Landis, M.S.; Muir, D.C.G.; Cooke, C.A.; Gleason, A.; Ho, A.; Kelly, E.; Schindler, D.; Wang, X.; et al. Source Analysis of Pollutant Elements in Winter Air Deposition in the Athabasca Oil Sands Region: A Temporal and Spatial Study. ACS Earth Space Chem. 2019, 3, 1656–1668. [Google Scholar] [CrossRef]
- Chibwe, L.; Muir, D.C.G.; Gopalapillai, Y.; Shang, D.; Kirk, J.L.; Manzano, C.A.; Atkinson, B.; Wang, X.; Teixeira, C. Long-term spatial and temporal trends, and source apportionment of polycyclic aromatic compounds in the Athabasca Oil Sands Region. Environ. Pollut. 2021, 268, 115351. [Google Scholar] [CrossRef]
- Klemt, W.H.; Kay, M.L.; Wiklund, J.A.; Wolfe, B.B.; Hall, R.I. Assessment of vanadium and nickel enrichment in Lower Athabasca River floodplain lake sediment within the Athabasca Oil Sands Region (Canada). Environ. Pollut. 2020, 265, 114920. [Google Scholar] [CrossRef] [PubMed]
- Jang, H. Characterization of Oil Sands Fly Ash. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2004. [Google Scholar]
- Holloway, P.C. Vanadium Recovery from Oil Sands Fly Ash. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2003. [Google Scholar]
- Gomez Bueno, C.O.; Rempel, G.L.; Spink, D.R. Physical and Chemical Characterization of Athabasca Tar Sands Fly Ash. CIM Bull. 1980, 73, 147–151. [Google Scholar]
- Stachiw, S.; Bicalho, B.; Grant-Weaver, I.; Noernberg, T.; Shotyk, W. Trace elements in berries collected near upgraders and open pit mines in the Athabasca Bituminous Sands Region (ABSR): Distinguishing atmospheric dust deposition from plant uptake. Sci. Total Environ. 2019, 670, 849–864. [Google Scholar] [CrossRef]
- Shotyk, W.; Bicalho, B.; Cuss, C.W.; Duke, M.J.M.; Noernberg, T.; Pelletier, R.; Steinnes, E.; Zaccone, C. Dust is the dominant source of “heavy metals” to peat moss (Sphagnum fuscum) in the bogs of the Athabasca Bituminous Sands region of northern Alberta. Environ. Int. 2016, 92, 494–506. [Google Scholar] [CrossRef]
- Phillips-Smith, C.; Jeong, C.H.; Healy, R.M.; Dabek-Zlotorzynska, E.; Celo, V.; Brook, J.R.; Evans, G. Sources of particulate matter components in the Athabasca oil sands region: Investigation through a comparison of trace element measurement methodologies. Atmos. Chem. Phys. 2017, 17, 9435–9449. [Google Scholar] [CrossRef]
- Mamun, A.A.; Celo, V.; Dabek-Zlotorzynska, E.; Charland, J.P.; Cheng, I.; Zhang, L. Characterization and source apportionment of airborne particulate elements in the Athabasca oil sands region. Sci. Total Environ. 2021, 788, 147748. [Google Scholar] [CrossRef] [PubMed]
- Ahad, J.M.E.; Pakdel, H.; Labarre, T.; Cooke, C.A.; Gammon, P.R.; Savard, M.M. Isotopic Analyses Fingerprint Sources of Polycyclic Aromatic Compound-Bearing Dust in Athabasca Oil Sands Region Snowpack. Environ. Sci. Technol. 2021, 55, 5887–5897. [Google Scholar] [CrossRef]
- Manzano, C.A.; Muir, D.; Kirk, J.; Teixeira, C.; Siu, M.; Wang, X.; Charland, J.P.; Schindler, D.; Kelly, E.; Zhang, Y.F. Temporal variation in the deposition of polycyclic aromatic compounds in snow in the Athabasca Oil Sands area of Alberta. Environ. Monit. Assess. 2016, 188, 542. [Google Scholar] [CrossRef]
- Kurek, J.; Kirk, J.L.; Muir, D.C.G.G.; Wang, X.; Evans, M.S.; Smol, J.P. Legacy of a half century of Athabasca oil sands development recorded by lake ecosystems. Proc. Natl. Acad. Sci. USA 2013, 110, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.S.; Studabaker, W.B.; Patrick Pancras, J.; Graney, J.R.; Puckett, K.; White, E.M.; Edgerton, E.S. Source apportionment of an epiphytic lichen biomonitor to elucidate the sources and spatial distribution of polycyclic aromatic hydrocarbons in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total Environ. 2019, 654, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Liggio, J.; Li, S.M.; Hayden, K.; Taha, Y.M.; Stroud, C.; Darlington, A.; Drollette, B.D.; Gordon, M.; Lee, P.; Liu, P.; et al. Oil sands operations as a large source of secondary organic aerosols. Nature 2016, 534, 91–94. [Google Scholar] [CrossRef]
- Landis, M.S.; Studabaker, W.B.; Pancras, J.P.; Graney, J.R.; White, E.M.; Edgerton, E.S. Source apportionment of ambient fine and coarse particulate matter polycyclic aromatic hydrocarbons at the Bertha Ganter-Fort McKay community site in the Oil Sands Region of Alberta, Canada. Sci. Total Environ. 2019, 666, 540–558. [Google Scholar] [CrossRef]
- Landis, M.S.; Pancras, J.P.; Graney, J.R.; Stevens, R.K.; Percy, K.E.; Krupa, S. Receptor Modeling of Epiphytic Lichens to Elucidate the Sources and Spatial Distribution of Inorganic Air Pollution in the Athabasca Oil Sands Region. Dev. Environ. Sci. 2012, 11, 427–467. [Google Scholar] [CrossRef]
- Wang, X.L.; Watson, J.G.; Chow, J.C.; Kohl, S.D.; Chen, L.W.A.; Sodeman, D.A.; Legge, A.H.; Percy, K.E. Measurement of Real-World Stack Emissions with a Dilution Sampling System. In Alberta Oil Sands: Energy, Industry and the Environment; Percy, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 171–192. [Google Scholar]
- Shotyk, W.; Bicalho, B.; Cuss, C.; Donner, M.; Grant-Weaver, I.; Javed, M.B.; Noernberg, T. Trace elements in the Athabasca Bituminous Sands: A geochemical explanation for the paucity of environmental contamination by chalcophile elements. Chem. Geol. 2021, 581, 120392. [Google Scholar] [CrossRef]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Munkittrick, K.R.; Scrimgeour, G.J.; Dubé, M.G.; Wrona, F.J.; Hazewinkel, R.R. Using adaptive processes and adverse outcome pathways to develop meaningful, robust, and actionable environmental monitoring programs. Integr. Environ. Assess. Manag. 2017, 13, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Suter, G.W.; Norton, S.B.; Cormier, S.M. A methodology for inferring the causes of observed impairments in aquatic ecosystems. Environ. Toxicol. Chem. Int. J. 2002, 21, 1101–1111. [Google Scholar] [CrossRef]
- Posthuma, L.; Dyer, S.D.; de Zwart, D.; Kapo, K.; Holmes, C.M.; Burton, G.A., Jr. Eco-epidemiology of aquatic ecosystems: Separating chemicals from multiple stressors. Sci. Total Environ. 2016, 573, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.N.; Schindler, D.W.; Hodson, P.V.; Short, J.W.; Radmanovich, R.; Nielsen, C.C. Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proc. Natl. Acad. Sci. USA 2010, 107, 16178–16183. [Google Scholar] [CrossRef]
- Culp, J.M.; Droppo, I.G.; di Cenzo, P.; Alexander, A.C.; Baird, D.J.; Beltaos, S.; Bickerton, G.; Bonsal, B.; Brua, R.B.; Chambers, P.A.; et al. Ecological effects and causal synthesis of oil sands activity impacts on river ecosystems: Water synthesis review. Environ. Rev. 2021, 29, 315–327. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bayne, E.; Beausoleil, D.; Dennett, J.; Fisher, J.T.; Hazewinkel, R.O.; Sayanda, D.; Wyatt, F.; Dubé, M.G. A synthetic review of terrestrial biological research from the Alberta oil sands region: Ten years of published literature. Integr. Environ. Assess. Manag. 2021, in press. [Google Scholar] [CrossRef]
- Fennell, J.; Arciszewski, T.J. Current knowledge of seepage from oil sands tailings ponds and its environmental influence in northeastern Alberta. Sci. Total Environ. 2019, 686, 968–985. [Google Scholar] [CrossRef]
- Plumlee, G.S.; Logsdon, M.J.; Filipek, L.F. The environmental geology of mineral deposits. In The Environmental Geochemistry of Mineral Deposits: Part A: Processes, Techniques, and Health Issues Part B: Case Studies and Research Topics; Society of Economic Geologists: Littleton, CO, USA, 1997; pp. 71–116. [Google Scholar] [CrossRef]
- Dowdeswell, L.; Dillon, P.; Ghoshal, S.; Miall, A.; Rasmussen, J.; Smol, J.P. A Foundation for the Future: Building an Environmental Monitoring System for the Oil Sands; Environment Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Evans, M.S.; McMaster, M.; Muir, D.C.G.; Parrott, J.; Tetreault, G.R.; Keating, J. Forage fish and polycyclic aromatic compounds in the Fort McMurray oil sands area: Body burden comparisons with environmental distributions and consumption guidelines. Environ. Pollut. 2019, 255, 113135. [Google Scholar] [CrossRef]
- Birks, S.J.; Cho, S.; Taylor, E.; Yi, Y.; Gibson, J.J. Characterizing the PAHs in surface waters and snow in the Athabasca region: Implications for identifying hydrological pathways of atmospheric deposition. Sci. Total Environ. 2017, 603–604, 570–583. [Google Scholar] [CrossRef]
- Yi, Y.; Birks, S.J.; Cho, S.; Gibson, J.J. Characterization of organic composition in snow and surface waters in the Athabasca Oil Sands Region, using ultrahigh resolution Fourier transform mass spectrometry. Sci. Total Environ. 2015, 518, 148–158. [Google Scholar] [CrossRef]
- RAMP. Regional Aquatics Monitoring in Support of the Joint Oil Sands Monitoring Plan Final 2015 Program Report. 2016. Available online: http://www.ramp-alberta.org/ramp/results/report.aspx (accessed on 8 November 2021).
- Gerner, N.V.; Koné, M.; Ross, M.S.; Pereira, A.; Ulrich, A.C.; Martin, J.W.; Liess, M. Stream invertebrate community structure at Canadian oil sands development is linked to concentration of bitumen-derived contaminants. Sci. Total Environ. 2017, 575, 1005–1013. [Google Scholar] [CrossRef]
- Roy, J.W.; Bickerton, G.; Frank, R.A.; Grapentine, L.; Hewitt, L.M. Assessing risks of shallow riparian groundwater quality near an oil sands tailings pond. Groundwater 2016, 54, 545–558. [Google Scholar] [CrossRef]
- Parsons, B.G.; Watmough, S.A.; Dillon, P.J.; Somers, K.M. A bioassessment of lakes in the Athabasca Oil Sands Region, Alberta, using benthic macroinvertebrates. J. Limnol. 2010, 69, 105–117. [Google Scholar] [CrossRef][Green Version]
- Alexander, A.C.; Levenstein, B.; Sanderson, L.A.; Blukacz-Richards, E.A.; Chambers, P.A. How does climate variability affect water quality dynamics in Canada’s oil sands region? Sci. Total Environ. 2020, 732, 139062. [Google Scholar] [CrossRef] [PubMed]
- Blanar, C.A.; Hewitt, M.; McMaster, M.; Kirk, J.; Wang, Z.; Norwood, W.; Marcogliese, D.J. Parasite community similarity in Athabasca River trout-perch (Percopsis omiscomaycus) varies with local-scale land use and sediment hydrocarbons, but not distance or linear gradients. Parasitol. Res. 2016, 115, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Culp, J.M.; Glozier, N.E.; Baird, D.J.; Wrona, F.J.; Brua, R.B.; Ritcey, A.L.; Peters, D.L.; Casey, R.; Choung, C.B.; Curry, C.J.; et al. Assessing Ecosystem Health in Benthic Macroinvertebrate Assemblages of the Athabasca River Main Stem, Tributaries and Peace-Athabasca Delta; Oil Sands Monitoring, Technical Report Series No. 1.7; Environment and Parks: Edmonton, AB, Canada, 2018; 82p, Available online: https://open.alberta.ca/publications/9781460140314 (accessed on 8 November 2021).
- McMaster, M.E.; Tetreault, G.R.; Clark, T.; Bennett, J.; Cunningham, J.; Evans, M. Aquatic ecosystem health assessment of the athabasca river mainstem oil sands area using white sucker health. WIT Trans. Ecol. Environ. 2018, 215, 411–420. [Google Scholar] [CrossRef]
- Pilote, M.; André, C.; Turcotte, P.; Gagné, F.; Gagnon, C. Metal bioaccumulation and biomarkers of effects in caged mussels exposed in the Athabasca oil sands area. Sci. Total Environ. 2018, 610–611, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, G.R.; Bennett, C.J.; Clark, T.W.; Keith, H.; Parrott, J.L.; McMaster, M.E. Fish Performance Indicators Adjacent to Oil Sands Activity: Response in Performance Indicators of Slimy Sculpin in the Steepbank River, Alberta, Adjacent to Oil Sands Mining Activity. Environ. Toxicol. Chem. 2020, 39, 396–409. [Google Scholar] [CrossRef]
- Suzanne, C.L. Effects of Natural and Anthropogenic Non-Point Source Disturbances on the Structure and Function of Tributary Ecosystems in the Athabasca oil Sands Region. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2015. [Google Scholar]
- Droppo, I.G.; di Cenzo, P.; Power, J.; Jaskot, C.; Chambers, P.A.; Alexander, A.C.; Kirk, J.; Muir, D. Temporal and spatial trends in riverine suspended sediment and associated polycyclic aromatic compounds (PAC) within the Athabasca oil sands region. Sci. Total Environ. 2018, 626, 1382–1393. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Munkittrick, K.R. Development of an adaptive monitoring framework for long-term programs: An example using indicators of fish health. Integr. Environ. Assess. Manag. 2015, 11, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.C.; Chambers, P.A. Assessment of seven Canadian rivers in relation to stages in oil sands industrial development, 1972–2010. Environ. Rev. 2016, 24, 484–494. [Google Scholar] [CrossRef]
- Schwalb, A.N.; Alexander, A.C.; Paul, A.J.; Cottenie, K.; Rasmussen, J.B. Changes in migratory fish communities and their health, hydrology, and water chemistry in rivers of the Athabasca oil sands region: A review of historical and current data. Environ. Rev. 2015, 23, 133–150. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Munkittrick, K.R.; Kilgour, B.W.; Keith, H.M.; Linehan, J.E.; McMaster, M.E. Increased size and relative abundance of migratory fishes observed near the Athabasca oil sands. Facets 2017, 2, 833–858. [Google Scholar] [CrossRef]
- Kilgour, B.W.; Munkittrick, K.R.; Hamilton, L.; Proulx, C.L.; Somers, K.M.; Arciszewski, T.; McMaster, M. Developing Triggers for Environmental Effects Monitoring Programs for Trout-Perch in the Lower Athabasca River (Canada). Environ. Toxicol. Chem. 2019, 38, 1890–1901. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; McMaster, M.E. Potential influence of sewage phosphorus and wet and dry deposition detected in fish collected in the athabasca river north of fort mcmurray. Environments 2021, 8, 14. [Google Scholar] [CrossRef]
- Downes, B.J. Back to the future: Little-used tools and principles of scientific inference can help disentangle effects of multiple stressors on freshwater ecosystems. Freshw. Biol. 2010, 55, 60–79. [Google Scholar] [CrossRef]
- Somers, K.M.; Kilgour, B.W.; Munkittrick, K.R.; Arciszewski, T.J. An Adaptive Environmental Effects Monitoring Framework for Assessing the Influences of Liquid Effluents on Benthos, Water, and Sediments in Aquatic Receiving Environments. Integr. Environ. Assess. Manag. 2018, 14, 552–566. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: New York, NY, USA, 2015; ISBN 3319194259. [Google Scholar]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef]
- McMillan, P. Regularized Regression Methods and Neural Networks for Modeling Fish Population Health with Water Quality Variables in the Athabasca Oil Sands Region. Master’s Thesis, University of Guelph, Guelph, ON, USA, 2021. [Google Scholar]
- Arciszewski, T.J.; Roberts, D.R.; Munkittrick, K.R.; Scrimgeour, G.J. Challenges and Benefits of Approaches Used to Integrate Regional Monitoring Programs. Front. Environ. Sci. 2021, 9, 256. [Google Scholar] [CrossRef]
- Hey, A.J.G.; Tansley, S.; Tolle, K.M. The Fourth Paradigm: Data-Intensive Scientific Discovery; Microsoft Research: Redmond, WA, USA, 2009; Volume 1. [Google Scholar]
- Suter, G.W., II. Ecological Risk Assessment; CRC Press: Boca Ration, FL, USA, 2016; ISBN 1420012568. [Google Scholar]
- RAMP. Technical Design and Rationale. Available online: http://www.ramp-alberta.org/UserFiles/File/RAMP_Design_&_Rationale.pdf (accessed on 8 November 2021).
- AER. ST53: Alberta Energy Regulator Statistical Report 53. Available online: https://www.aer.ca/providing-information/data-and-reports/statistical-reports/st53 (accessed on 8 July 2021).
- AER. ST39: Alberta Energy Regulator. Statistical Report 39. Available online: https://www.aer.ca/providing-information/data-and-reports/statistical-reports/st39 (accessed on 30 May 2021).
- ABMI. ABMI—Wall-to-Wall Human Footprint Inventory. Available online: https://abmi.ca/home/data-analytics/da-top/da-product-overview/Human-Footprint-Products/HF-inventory.html (accessed on 5 May 2021).
- WBEA. Historical Environmental Monitoring Data|Wood Buffalo Environmental Association. Available online: https://wbea.org/historical-monitoring-data/ (accessed on 5 May 2021).
- Friedman, J.; Hastie, T.; Tibshirani, R.; Narasimhan, B.; Tay, K.; Simon, N.; Qian, J. Package ‘glmnet.’ CRAN R Repositary. 2021. Available online: https://CRAN.R-project.org/package=glmnet (accessed on 8 November 2021).
- McNaughton, C.S.; Vandenberg, J.; Thiede, P. Reanalysis of aerial deposition of metals and polycyclic aromatic compounds to snow in the Athabasca Oil Sands Region of Alberta Canada. Sci. Total Environ. 2019, 682, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Wen, D.; Zhang, L.; Wu, Z.; Qiu, X.; Yang, F.; Harner, T. Deposition Mapping of Polycyclic Aromatic Compounds in the Oil Sands Region of Alberta, Canada and Linkages to Ecosystem Impacts. Environ. Sci. Technol. 2018, 52, 12456–12464. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Karimi, A.; Malekshahian, M. Characterization, gasification, activation, and potential uses for the millions of tonnes of petroleum coke produced in Canada each year. Can. J. Chem. Eng. 2014, 92, 1618–1626. [Google Scholar] [CrossRef]
- Barrie, L.A.; Kovalick, J. A Wintertime Investigation of the Deposition of Pollutants around an Isolated Power Plant in Northern Alberta. Alberta Oil Sands Environmental Research Program: Edmonton, AB, Canada, 1980. [Google Scholar] [CrossRef]
- Landis, M.S.; Pancras, J.P.; Graney, J.R.; White, E.M.; Edgerton, E.S.; Legge, A.; Percy, K.E. Source apportionment of ambient fine and coarse particulate matter at the Fort McKay community site, in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total Environ. 2017, 584, 105–117. [Google Scholar] [CrossRef]
- Willis, C.E.; Kirk, J.L.; St Louis, V.L.; Lehnherr, I.; Ariya, P.A.; Rangel-Alvarado, R.B. Sources of Methylmercury to Snowpacks of the Alberta Oil Sands Region: A Study of in Situ Methylation and Particulates. Environ. Sci. Technol. 2018, 52, 531–540. [Google Scholar] [CrossRef]
- Rangel-Alvarado, R.B.; Willis, C.E.; Kirk, J.L.; St Louis, V.L.; Amyot, M.; Bélanger, D.; Ariya, P.A. Athabasca oil sands region snow contains efficient micron and nano-sized ice nucleating particles. Environ. Pollut. 2019, 252, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Furimsky, E. Gasification of oil sand coke: Review. Fuel Process. Technol. 1998, 56, 263–290. [Google Scholar] [CrossRef]
- Parker, K. Electrostatic precipitators. In Particle Technology and Applications; Sunggyu, L., Henthorn, K.H., Eds.; CRC Press (Taylor & Francis Group): London, UK, 2012; pp. 281–300. [Google Scholar]
- Kirk, J.L.; Muir, D.C.G.G.; Gleason, A.; Wang, X.; Lawson, G.; Frank, R.A.; Lehnherr, I.; Wrona, F. Atmospheric deposition of mercury and methylmercury to landscapes and waterbodies of the athabasca oil sands region. Environ. Sci. Technol. 2014, 48, 7374–7383. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.N.; Short, J.W.; Schindler, D.W.; Hodson, P.V.; Ma, M.; Kwan, A.K.; Fortin, B.L. Oil sands development contributes polycyclic aromatic compounds to the Athabasca River and its tributaries. Proc. Natl. Acad. Sci. USA 2009, 106, 22346–22351. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Wren, C.D.; Stephenson, G.L. The effect of acidification on the accumulation and toxicity of metals to freshwater invertebrates. Environ. Pollut. 1991, 71, 205–241. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. Improving ecological monitoring. Trends Ecol. Evol. 2010, 25, 200–201. [Google Scholar] [CrossRef]
- Suncor Coke Boiler Replacement Project—Oil Sands|Suncor. Available online: https://www.suncor.com/en-CA/about-us/oil-sands/process/coke-boiler-replacement-project (accessed on 9 November 2021).
- Cho, S.; Sharma, K.; Brassard, B.W.; Hazewinkel, R. Polycyclic aromatic hydrocarbon deposition in the snowpack of the Athabasca oil sands region of Alberta, Canada. Water Air Soil Pollut. 2014, 225, 1910. [Google Scholar] [CrossRef]
- Smit, P.P.A. Dust concentrations around coal stockpiles. Sci. Total Environ. 1980, 15, 207–216. [Google Scholar] [CrossRef]
- Smitham, J.B.; Nicol, S.K. Physico-chemical principles controlling the emission of dust from coal stockpiles. Powder Technol. 1991, 64, 259–270. [Google Scholar] [CrossRef]
- Cong, X.C.; Yang, S.L.; Cao, S.Q.; Chen, Z.L.; Dai, M.X.; Peng, S.T. Effect of aggregate stockpile configuration and layout on dust emissions in an open yard. Appl. Math. Model. 2012, 36, 5482–5491. [Google Scholar] [CrossRef]
- US EPA. Chapter 13: Miscellaneous Sources, AP 42, Fifth Edition, Volume I|Clearinghouse for Emission Inventories and Emissions Factors|Technology Transfer Network. Available online: https://www3.epa.gov/ttn/chief/ap42/ch13/index.html (accessed on 23 August 2021).
- Summers, J.C.; Kurek, J.; Kirk, J.L.; Muir, D.C.G.; Wang, X.; Wiklund, J.A.; Cooke, C.A.; Evans, M.S.; Smol, J.P. Recent Warming, Rather than Industrial Emissions of Bioavailable Nutrients, Is the Dominant Driver of Lake Primary Production Shifts across the Athabasca Oil Sands Region. PLoS ONE 2016, 11, e0153987. [Google Scholar] [CrossRef]
- Barton, D.R.; Wallace, R.R. Ecological Studies of the Aquatic Invertebrates of the Alberta Oil Sands Environmental Research Program Study Area, Northeastern Alberta; Alberta Oil Sands Environmental Research Program: Edmonton, AB, Canada, 1980. [Google Scholar] [CrossRef]
- Hartland-Rowe, R.C.; Davies, R.W.; McElhone, H.; Crowther, R. The Ecology of Macro Benthic Invertebrate Communities in Hartley Creek, Northeastern Alberta; Alberta Oil Sands Environmental Research Program: Edmonton, AB, Canada, 1979. [Google Scholar] [CrossRef]
- McMaster, M.E.; Tetreault, G.R.; Clark, T.; Bennett, J.; Cunningham, J.; Ussery, E.J.; Evans, M. Baseline white sucker health and reproductive endpoints for use in assessment of further development in the alberta oil sands. Int. J. Environ. Impacts Manag. Mitig. Recover. 2020, 3, 219–237. [Google Scholar] [CrossRef]
- Wasiuta, V.; Kirk, J.L.; Chambers, P.A.; Alexander, A.C.; Wyatt, F.R.; Rooney, R.C.; Cooke, C.A. Accumulating Mercury and Methylmercury Burdens in Watersheds Impacted by Oil Sands Pollution. Environ. Sci. Technol. 2019, 53, 12856–12864. [Google Scholar] [CrossRef]
- Parrott, J.L.; Marentette, J.R.; Hewitt, L.M.; McMaster, M.E.; Gillis, P.L.; Norwood, W.P.; Kirk, J.L.; Peru, K.M.; Headley, J.V.; Wang, Z.; et al. Meltwater from snow contaminated by oil sands emissions is toxic to larval fish, but not spring river water. Sci. Total Environ. 2018, 625, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.C.; Chambers, P.A.; Jeffries, D.S. Episodic acidification of 5 rivers in Canada’s oil sands during snowmelt: A 25-year record. Sci. Total Environ. 2017, 599–600, 739–749. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; Hazewinkel, R.R.; Munkittrick, K.R.; Kilgour, B.W. Developing and applying control charts to detect changes in water chemistry parameters measured in the Athabasca River near the oil sands: A tool for surveillance monitoring. Environ. Toxicol. Chem. 2018, 37, 2296–2311. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Xiong, Y.; Du, K. Source apportionment of airborne particulate matters over the Athabasca oil sands region: Inter-comparison between PMF modeling and ground-based remote sensing. Atmos. Environ. 2020, 221, 117103. [Google Scholar] [CrossRef]
- Simpson, I.J.; Blake, N.J.; Barletta, B.; Diskin, G.S.; Fuelberg, H.E.; Gorham, K.; Huey, L.G.; Meinardi, S.; Rowland, F.S.; Vay, S.A.; et al. Characterization of trace gases measured over alberta oil sands mining operations: 76 speciated C2-C10 volatile organic compounds (VOCs), CO2, CH4, CO, NO, NO2, NOy, O3 and SO2. Atmos. Chem. Phys. 2010, 10, 11931–11954. [Google Scholar] [CrossRef]
- Howell, S.G.; Clarke, A.D.; Freitag, S.; McNaughton, C.S.; Kapustin, V.; Brekovskikh, V.; Jimenez, J.L.; Cubison, M.J. An airborne assessment of atmospheric particulate emissions from the processing of Athabasca oil sands. Atmos. Chem. Phys. 2014, 14, 5073–5087. [Google Scholar] [CrossRef]
- Makar, P.A.; Akingunola, A.; Aherne, J.; Cole, A.S.; Aklilu, Y.A.; Zhang, J.; Wong, I.; Hayden, K.; Li, S.M.; Kirk, J.; et al. Estimates of exceedances of critical loads for acidifying deposition in Alberta and Saskatchewan. Atmos. Chem. Phys. 2018, 18, 9897–9927. [Google Scholar] [CrossRef]
- Studabaker, W.B.; Krupa, S.; Jayanty, R.K.M.; Raymer, J.H. Measurement of Polynuclear Aromatic Hydrocarbons (PAHs) in Epiphytic lichens for Receptor Modeling in the Alberta Oil Sands Region. In Alberta Oil Sands: Energy, Industry, and the Environment; Percy, K.E.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 391–425. ISBN 9781622768226. [Google Scholar]
- Tokarek, T.W.; Odame-Ankrah, C.A.; Huo, J.A.; McLaren, R.; Lee, A.K.Y.; Adam, M.G.; Willis, M.D.; Abbatt, J.P.D.; Mihele, C.; Darlington, A.; et al. Principal component analysis of summertime ground site measurements in the Athabasca oil sands with a focus on analytically unresolved intermediate-volatility organic compounds. Atmos. Chem. Phys. 2018, 18, 17819–17841. [Google Scholar] [CrossRef]
- Moradi, M.; You, Y.; Hung, H.; Li, J.; Park, R.; Alexandrou, N.; Moussa, S.G.; Jantunen, L.; Robitaille, R.; Staebler, R.M. Fugitive emissions of polycyclic aromatic compounds from an oil sands tailings pond based on fugacity and inverse dispersion flux calculations. Environ. Pollut. 2021, 269, 116115. [Google Scholar] [CrossRef]
- Volik, O.; Elmes, M.; Petrone, R.; Kessel, E.; Green, A.; Cobbaert, D.; Price, J. Wetlands in the athabasca oil sands region: The nexus between wetland hydrological function and resource extraction. Environ. Rev. 2020, 28, 246–261. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Jariyasopit, N.; Schuster, J.K.; Harner, T. Insights into sources and occurrence of oxy- and nitro-PAHs in the alberta oil sands region using a network of passive air samplers. Environ. Pollut. 2021, 286, 117513. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Petroleum Refining; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781466591608. [Google Scholar]
- Bryers, R.W. Utilization of petroleum coke and petroleum coke/coal blends as a means of steam raising. Fuel Process. Technol. 1995, 44, 121–141. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, I.; Muir, D.; Charland, J.P. Scavenging ratios of polycyclic aromatic compounds in rain and snow in the Athabasca oil sands region. Atmos. Chem. Phys. 2015, 15, 1421–1434. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Ajwa, H.A. Evaluation of Wind Erosion Emissions Factors for Air Quality Modeling. Soil Sci. Soc. Am. J. 2011, 75, 1285–1294. [Google Scholar] [CrossRef]
- Barrie, L.A. The Fate of Particulate Emissions from an Isolated Power Plant in the Oil Sands Area of Western Canada. Ann. N. Y. Acad. Sci. 1980, 338, 434–452. [Google Scholar] [CrossRef]
- Wang, X.; Chow, J.C.; Kohl, S.D.; Yatavelli, L.N.R.; Percy, K.E.; Legge, A.H.; Watson, J.G. Wind erosion potential for fugitive dust sources in the Athabasca Oil Sands Region. Aeolian Res. 2015, 18, 121–134. [Google Scholar] [CrossRef]
- Wieder, R.K.; Vile, M.A.; Albright, C.M.; Scott, K.D.; Vitt, D.H.; Quinn, J.C.; Burke-Scoll, M. Effects of altered atmospheric nutrient deposition from Alberta oil sands development on Sphagnum fuscum growth and C, N and S accumulation in peat. Biogeochemistry 2016, 129, 1–19. [Google Scholar] [CrossRef]

| Variable | Variable Selection Counts | |||||
|---|---|---|---|---|---|---|
| Number of Models with Selected Variable | BMIIs | Number of Sites with Variable | ||||
| TA | TR | EPT | EQ | |||
| SBM-FPU | 25 | 8 | 10 | 6 | 1 | 13 |
| MSP * | 23 | 7 | 7 | 5 | 4 | 14 |
| MSWS * | 20 | 12 | 7 | 11 | 7 | 15 |
| MST * | 19 | 7 | 3 | 4 | 5 | 12 |
| SML-CBP | 14 | 5 | 2 | 4 | 3 | 10 |
| KM-CBP | 9 | 1 | 1 | 0 | 7 | 9 |
| MRM-CBP | 8 | 4 | 4 | 0 | 0 | 5 |
| SML-FPU | 8 | 2 | 2 | 3 | 1 | 7 |
| ALD | 7 | 2 | 3 | 1 | 1 | 6 |
| JPM-CBP | 7 | 0 | 1 | 1 | 5 | 7 |
| SBM-CBP | 7 | 1 | 2 | 1 | 3 | 7 |
| SML-PCP | 5 | 1 | 1 | 3 | 0 | 4 |
| SAN-CBP | 4 | 1 | 2 | 1 | 0 | 3 |
| SBM-PCP | 4 | 0 | 0 | 1 | 3 | 4 |
| SML-PCS | 4 | 0 | 0 | 1 | 3 | 3 |
| HM-CBP | 3 | 1 | 1 | 0 | 1 | 3 |
| HM-SCP | 3 | 1 | 1 | 0 | 1 | 3 |
| SMR-ST | 3 | 2 | 0 | 0 | 1 | 3 |
| HM-PCP | 2 | 0 | 1 | 0 | 1 | 2 |
| HM-PCS | 2 | 0 | 0 | 0 | 2 | 2 |
| SBM-PCS | 2 | 0 | 0 | 1 | 1 | 1 |
| SBM-SCP | 2 | 0 | 0 | 1 | 1 | 2 |
| CLD | 1 | 0 | 0 | 0 | 1 | 1 |
| SFB-B | 1 | 1 | 0 | 0 | 0 | 1 |
| BMII | STR-L | JP-U | SHL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DR | Variable | β | DR | Variable | β | DR | Variable | β | |
| TA | 0.69 | Intercept | 3.1 × 10−16 | 0.21 | Intercept | 5.5 × 10−17 | 0.12 | Intercept | 8.1 × 10−16 |
| MST | −0.218 | MSP | −0.064 | MST | 0.001 | ||||
| MSP | −0.696 | SBM-FPU | −0.209 | SML-CBP | −0.133 | ||||
| SBM-FPU | −0.420 | SBM-FPU | −0.033 | ||||||
| SBM-CBP | −0.123 | ||||||||
| SML-CBP | −0.026 | ||||||||
| KM-CBP | −0.049 | ||||||||
| TR | 0.81 | Intercept | 1.4 × 10−16 | 0.31 | Intercept | 4.5 × 10−16 | 0.13 | Intercept | 2.7 × 10−16 |
| MSP | −0.549 | SBM-FPU | −0.267 | SBM-FPU | −0.154 | ||||
| SBM-FPU | −0.396 | SML-CBP | −0.020 | ||||||
| SAN-CBP | −0.019 | ||||||||
| EPT | 0.00 | Intercept | −2.8 × 10−16 | 0.08 | Intercept | 7.1 × 10−16 | 0.07 | Intercept | −6.3 × 10−16 |
| MST | 0.019 | SAN-CBP | −0.067 | ||||||
| EQ | 0.65 | Intercept | 1.8 × 10−15 | 0.38 | Intercept | −1.9 × 10−16 | 0.41 | Intercept | −2.1 × 10−17 |
| MSWS | 0.112 | JPM-CBP | −0.249 | JPM-CBP | −0.390 | ||||
| MST | −0.034 | KM-CBP | −0.046 | SML-PCS | −0.009 | ||||
| MSP | 0.176 | SML-PCS | −0.042 | KM-CBP | −0.007 | ||||
| JPM-CBP | −0.415 | SFB-ST | −0.024 | ||||||
| SML-FPU | −0.211 | ||||||||
| SML-CBP | −0.128 | ||||||||
| HM-PCS | −0.113 | ||||||||
| SBM-FPU | −0.027 | ||||||||
| BMII | STR-U | JP-L | ||||
|---|---|---|---|---|---|---|
| DR | Variable | β | DR | Variable | β | |
| TA | 0 | Intercept | 1.8 × 10−16 | 0.65 | Intercept | 1.9 × 10−15 |
| MSWS | 0.280 | |||||
| MST | 0.139 | |||||
| MSP | −0.353 | |||||
| SBM-FPU | −0.379 | |||||
| MRM-CBP | −0.372 | |||||
| TR | 0.85 | Intercept | 1.7 × 10−16 | 0.15 | Intercept | 4.4 × 10−16 |
| MST | −0.016 | MSP | −0.158 | |||
| MSP | −0.376 | SBM-FPU | −0.023 | |||
| MRM-CBP | −0.556 | |||||
| ALD | −0.432 | |||||
| JPM-CBP | −0.367 | |||||
| SBM-FPU | −0.141 | |||||
| EPT | 0 | Intercept | 6.7 × 10−16 | 0.28 | Intercept | 2.3 × 10−16 |
| MSP | −0.051 | |||||
| SBM-FPU | −0.274 | |||||
| ALD | −0.075 | |||||
| SML-CBP | −0.003 | |||||
| EQ | 0 | Intercept | 9.7 × 10−16 | 0.59 | Intercept | −3.8 × 10−17 |
| SBM-PCP | −0.206 | |||||
| KM-CBP | −0.183 | |||||
| SBM-SCP | −0.159 | |||||
| SBM-CBP | −0.085 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciszewski, T.J. Exploring the Influence of Industrial and Climatic Variables on Communities of Benthic Macroinvertebrates Collected in Streams and Lakes in Canada’s Oil Sands Region. Environments 2021, 8, 123. https://doi.org/10.3390/environments8110123
Arciszewski TJ. Exploring the Influence of Industrial and Climatic Variables on Communities of Benthic Macroinvertebrates Collected in Streams and Lakes in Canada’s Oil Sands Region. Environments. 2021; 8(11):123. https://doi.org/10.3390/environments8110123
Chicago/Turabian StyleArciszewski, Tim J. 2021. "Exploring the Influence of Industrial and Climatic Variables on Communities of Benthic Macroinvertebrates Collected in Streams and Lakes in Canada’s Oil Sands Region" Environments 8, no. 11: 123. https://doi.org/10.3390/environments8110123
APA StyleArciszewski, T. J. (2021). Exploring the Influence of Industrial and Climatic Variables on Communities of Benthic Macroinvertebrates Collected in Streams and Lakes in Canada’s Oil Sands Region. Environments, 8(11), 123. https://doi.org/10.3390/environments8110123