Short Term Elevated CO2 Interacts with Iron Deficiency, Further Repressing Growth, Photosynthesis and Mineral Accumulation in Soybean (Glycine max L.) and Common Bean (Phaseolus vulgaris L.)
Abstract
1. Introduction
2. Materials and methods
2.1. Plant Material and Growth Conditions
2.2. Morphometric Parameters
2.3. Photosynthesis
2.4. Ferric Chelate Reductase (FCR) Assay
2.5. Organic Acids
2.6. Minerals
2.7. Statistical Analysis
3. Results
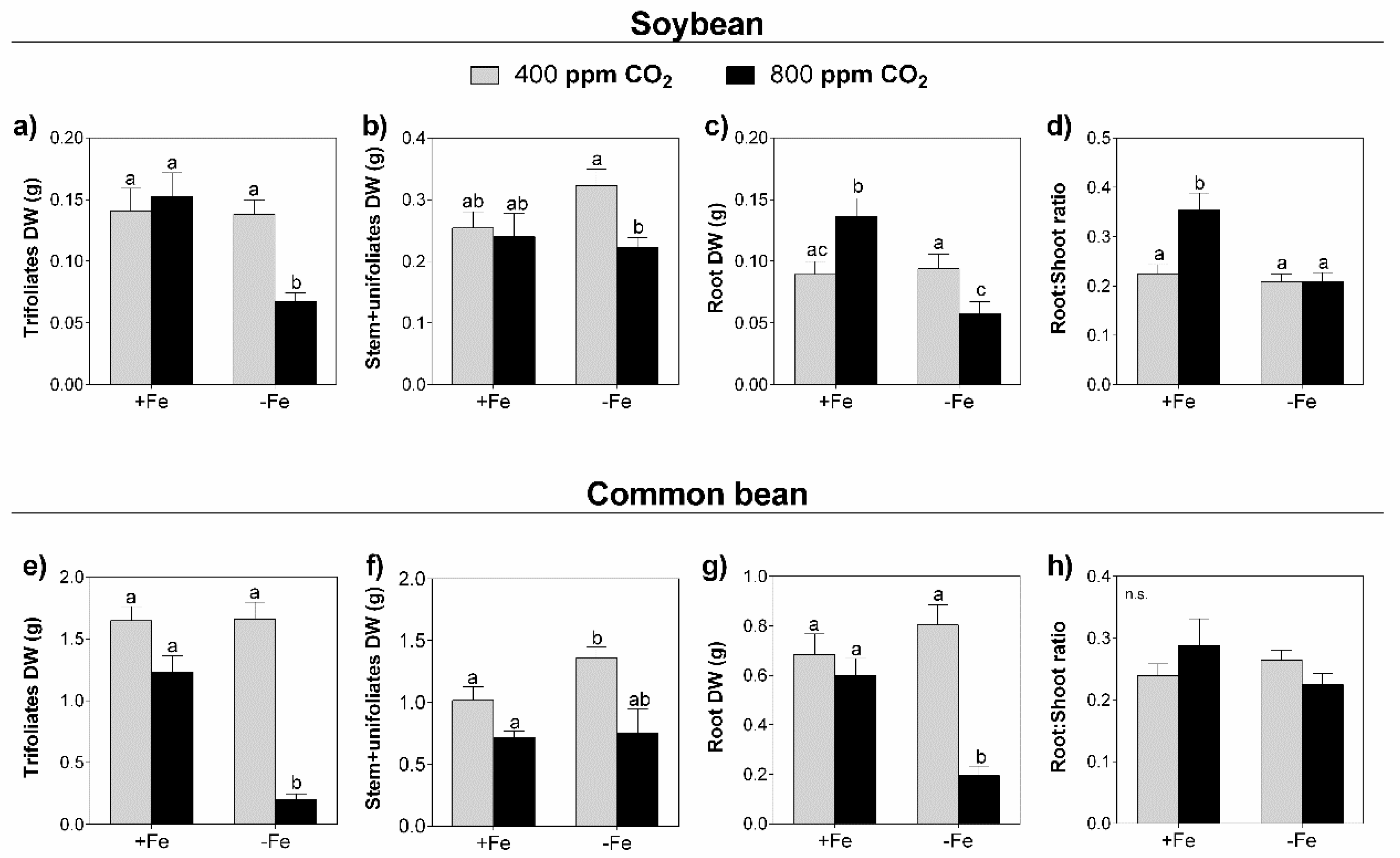
3.1. Biomass Accumulation
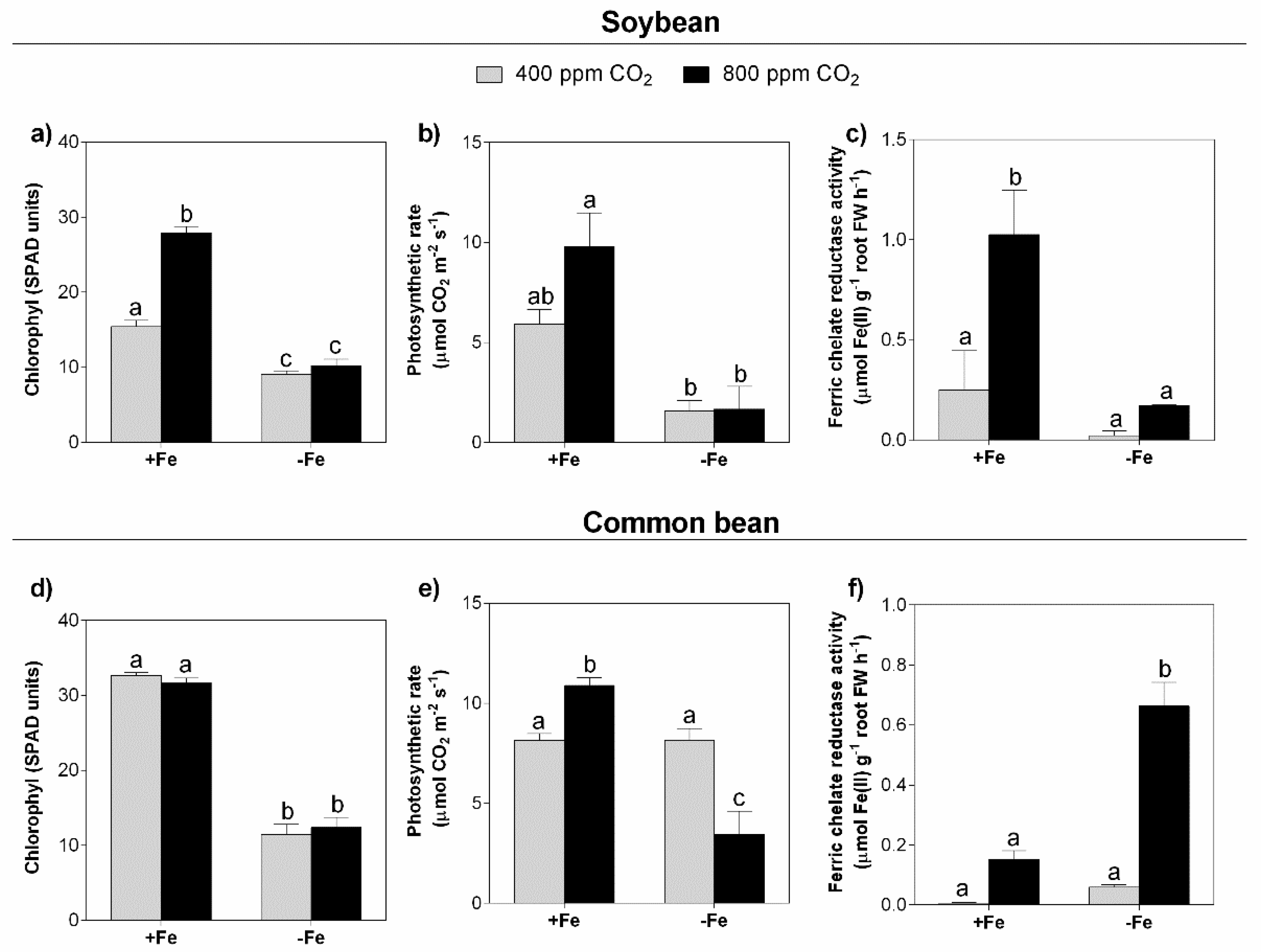
3.2. Photosynthesis and Photosynthetic Pigments
3.3. Ferric Chelate Reductase (FCR) Activity
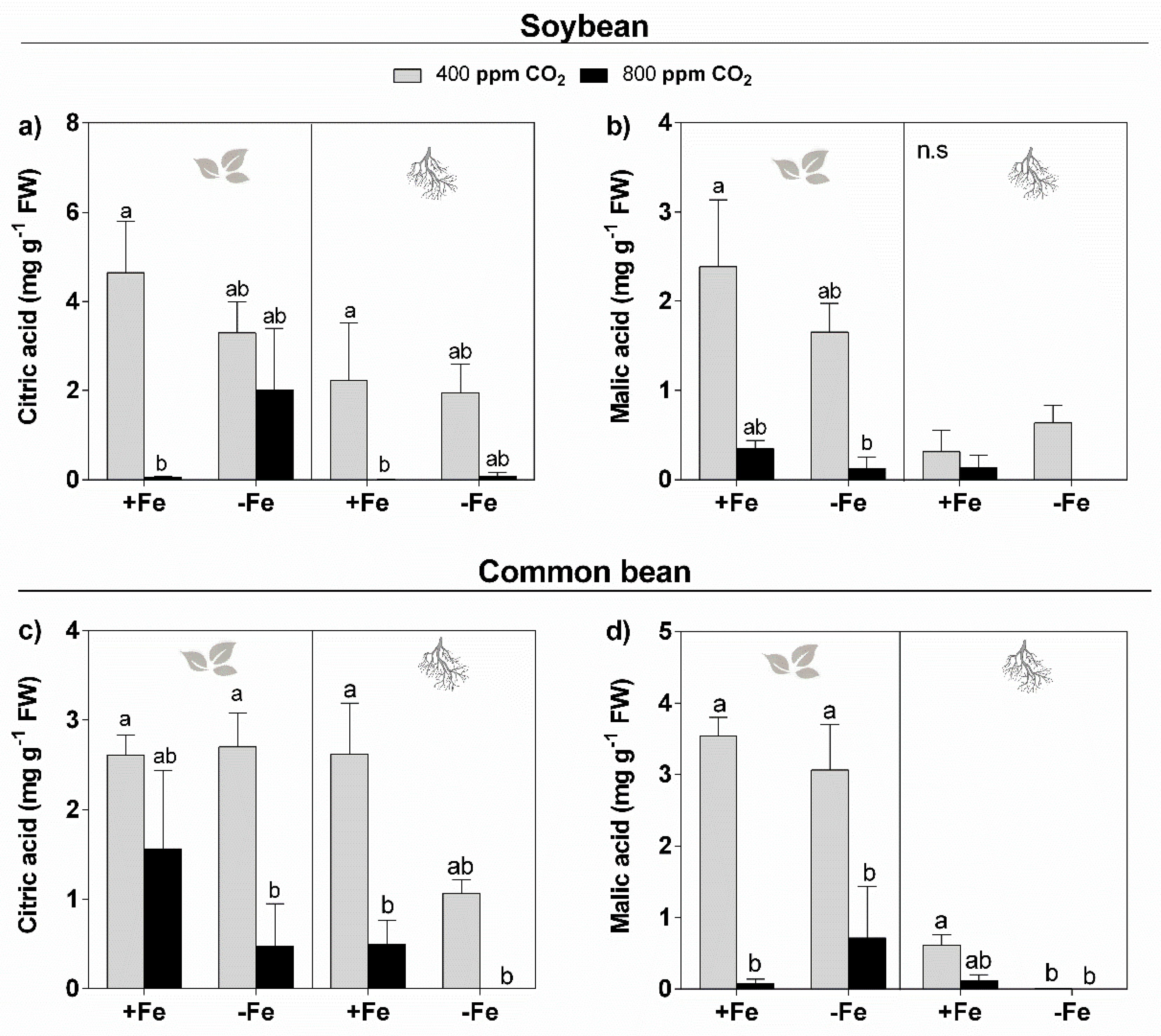
3.4. Organic Acids
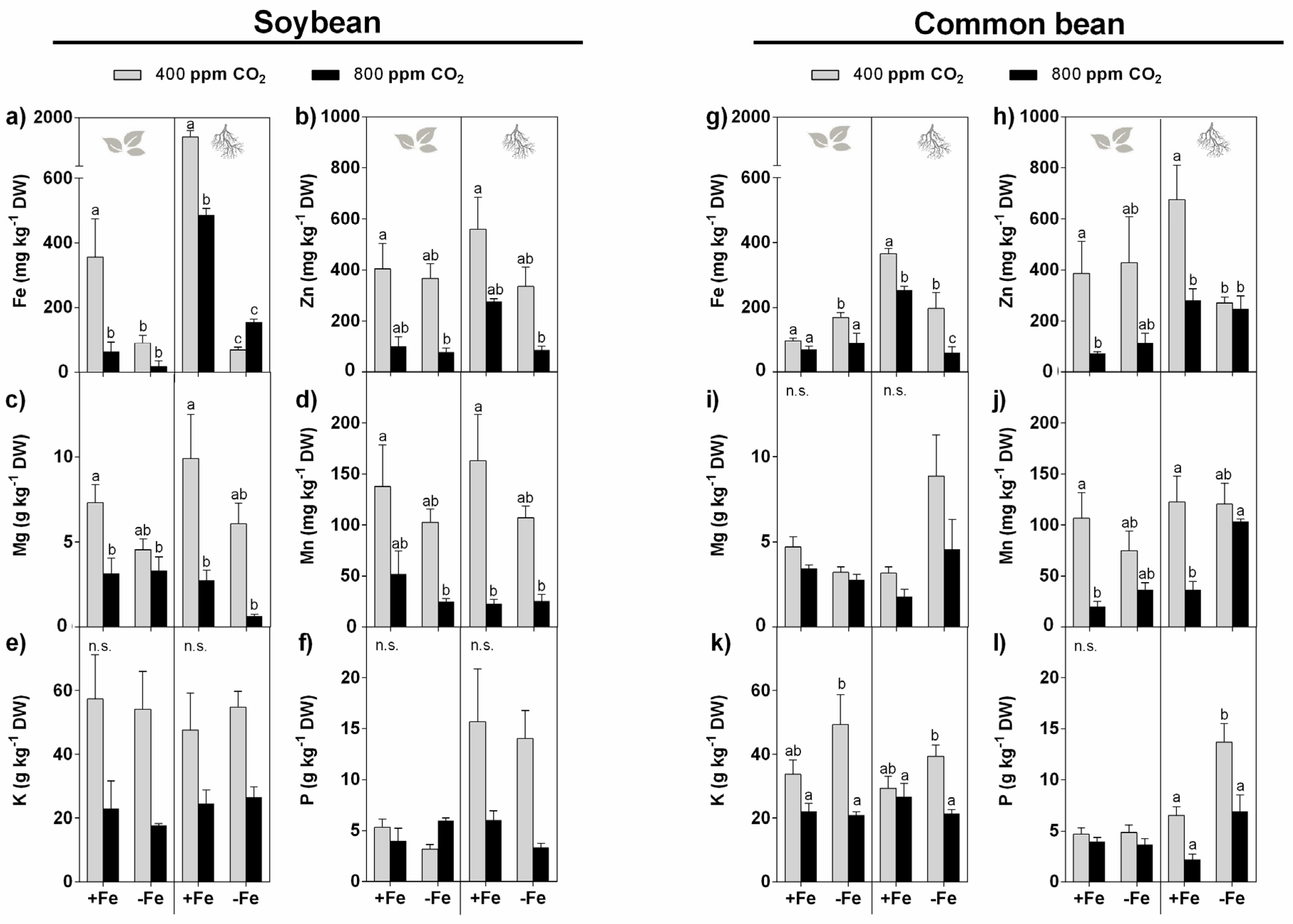
3.5. Mineral Accumulation
3.5.1. Effect of eCO2
3.5.2. Effect of Fe Restriction
3.5.3. Interactive Effect of eCO2 and Restricted Fe Supply
4. Discussion
4.1. Elevated CO2 and Fe Restriction Led to General Biomass Decrease in Both Legume Species
4.2. Depending on the Legume Species, eCO2 Stimulates Photosynthesis, but in Combination with Fe Deficiency, This Effect Is Lost
4.3. Ferric Chelate Reductase Activity Is Stimulated under eCO2
4.4. Elevated CO2 Reduces the Organic Acid Levels in the Leaves and Roots of Soybean and Common Bean
4.5. The Mineral Levels of Both Soybean and Common Bean Plants Are Highly Affected by eCO2 and Fe Deficiency on Their Own and in Combination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Keeling, R.F.; Walker, S.J.; Piper, S.C.; Bollenbacher, A.F. Scripps UCSD Atmospheric CO2 Concentrations (ppm) Derived from in Situ Air Measurements at Mauna Loa, Observatory, Hawaii: Latitude 19.5°N Longitude 155.6°W Elevation 3397m. Available online: http://scrippsco2.ucsd.edu/assets/data/atmospheric/stations/in_situ_co2/monthly/monthly_in_situ_co2_mlo.csv (accessed on 23 August 2021).
- Franks, P.J. Tansley review Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef]
- Haque, M.S.; Karim, M.A.; Haque, M.M.; Hamid, A.; Nawata, E. Effect of elevated CO2 concentration on growth, chlorophyll content and yield of mungbean (Vigna radiata L. Wilczek) genotypes. Jpn. J. Trop. Agr. 2005, 49, 189–196. [Google Scholar]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat: Results from a three-year FACE experiment. Plant Biol. 2009. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M.; Shah, D.; Maurya, S.; Jagadish, K.S.V. Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crop. Res. 2017, 206, 149–157. [Google Scholar] [CrossRef]
- Rao, N.K.S.; Mamatha, H.; Laxman, R.H. Effect of elevated CO2 on growth and yield of French bean (Phaseolus vulgaris L.) genotypes. Legum. Res. Int. J. 2015, 38, 72. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Bell, R.W. Potassium starvation limits soybean growth more than the photosynthetic processes across CO2 levels. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, M.; Brand, J.; Tausz-Posch, S.; Armstrong, R.D.; O’Leary, G.L.; Fitzgerald, G.J.; Tausz, M. Yield, growth and grain nitrogen response to elevated CO2 in six lentil (Lens culinaris) cultivars grown under Free Air CO2 Enrichment (FACE) in a semi-arid environment. Eur. J. Agron. 2017, 87, 50–58. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Jablonski, L.M.; Wang, X.; Curtis, P.S. Plant reproduction under elevated CO2 conditions: A meta-analysis of reports on 79 crop and wild species. New Phytol. 2002, 156, 9–26. [Google Scholar] [CrossRef]
- Medek, D.E.; Schwartz, J.; Myers, S.S. Estimated effects of future atmospheric CO2 concentrations on protein intake and the risk of protein deficiency by country and region. Environ. Health Perspect. 2017, 125, 087002. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- He, J.; Zhang, R.X.; Peng, K.; Tagliavia, C.; Li, S.; Xue, S.; Liu, A.; Hu, H.; Zhang, J.; Hubbard, K.E.; et al. The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytol. 2018, 218, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, I.; Aparicio-Tejo, P.M.; Avila, C.; Cañas, R.; Sakalauskiene, S.; Aranjuelo, I. Root-shoot interactions explain the reduction of leaf mineral content in Arabidopsis plants grown under elevated [CO2] conditions. Physiol. Plant. 2016, 65–79. [Google Scholar] [CrossRef]
- Jauregui, I.; Aparicio-Tejo, P.M.; Avila, C.; Rueda-López, M.; Aranjuelo, I. Root and shoot performance of Arabidopsis thaliana exposed to elevated CO2: A physiologic, metabolic and transcriptomic response. J. Plant Physiol. 2015, 189, 65–76. [Google Scholar] [CrossRef]
- Noguchi, K.; Watanabe, C.K.; Terashima, I. Effects of elevated atmospheric CO2 on primary metabolite levels in Arabidopsis thaliana Col-0 leaves: An examination of metabolome data. Plant Cell Physiol. 2015, 56, 2069–2078. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Clemente, T.E.; Grusak, M.A. Evaluation of constitutive iron reductase (AtFRO2) expression on mineral accumulation and distribution in soybean (Glycine max. L.). Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Covarrubias, J.I.; Rombolà, A.D. Physiological and biochemical responses of the iron chlorosis tolerant grapevine rootstock 140 Ruggeri to iron deficiency and bicarbonate. Plant Soil 2013, 370, 305–315. [Google Scholar] [CrossRef]
- Abadía, J.; López-Millán, A.-F.; Rombolà, A.; Abadía, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2018, 163, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Houshmandfar, A.; Fitzgerald, G.J.; Tausz, M. Elevated CO2 decreases both transpiration flow and concentrations of Ca and Mg in the xylem sap of wheat. J. Plant Physiol. 2015, 174, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, P. The understanding of the plant iron deficiency responses in strategy I plants and the role of ethylene in this process by omic approaches. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; González-Guerrero, M.; Grusak, M.A.; Valdés-López, O. Common bean: A legume model on the rise for unraveling responses and adaptations to iron, zinc, and phosphate deficiencies. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa-Harand, A.; Almeida, C.C.S.; Mosiolek, M.; Blair, M.W.; Schweizer, D.; Guerra, M. Extensive ribosomal DNA amplification during Andean common bean (Phaseolus vulgaris L.) evolution. Theor. Appl. Genet. 2006, 112, 924–933. [Google Scholar] [CrossRef]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, F.M.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Feng, Z.; Rütting, T.; Pleijel, H.; Wallin, G.; Reich, P.B.; Kammann, C.I.; Newton, P.C.D.; Kobayashi, K.; Luo, Y.; Uddling, J. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob. Chang. Biol. 2015, 21, 3152–3168. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; De Souza, A.P.; Long, S.P.; Ort, D.R. The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Tausz, M.; Bilela, S.; Bahrami, H.; Armstrong, R.; Fitzgerald, G.; O’Leary, G.; Simon, J.; Tausz-Posch, S.; Rennenberg, H. Nitrogen nutrition and aspects of root growth and function of two wheat cultivars under elevated [CO2]. Environ. Exp. Bot. 2017, 140, 1–7. [Google Scholar] [CrossRef]
- Dong, J.; Xu, Q.; Gruda, N.; Chu, W.; Li, X.; Duan, Z. Elevated and super-elevated CO2 differ in their interactive effects with nitrogen availability on fruit yield and quality of cucumber. J. Sci. Food Agric. 2018, 98, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Fangmeier, A.; De Temmerman, L.; Mortensen, L.; Kemp, K.; Burke, J.; Mitchell, R.; Van Oijen, M.; Weigel, H.J. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment “ESPACE-wheat”. Eur. J. Agron. 1999, 10, 215–229. [Google Scholar] [CrossRef]
- Ma, Z.; Flynn, J.; Libra, G.; Shi, Z. Elevated CO2 accelerates phosphorus depletion by common bean (Phaseolus vulgaris) in association with altered leaf biochemical properties. Pedosphere 2018, 28, 422–429. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Sale, P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann. Bot. 2015, 116, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, I.; Smith, S.E.; Smith, F.A.; Watts-Williams, S.J.; Clausen, S.S.; Grønlund, M. Plant growth responses to elevated atmospheric CO2 are increased by phosphorus sufficiency but not by arbuscular mycorrhizas. J. Exp. Bot. 2016, 67, 6173–6186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandey, R.; Zinta, G.; AbdElgawad, H.; Ahmad, A.; Jain, V.; Janssens, I.A. Physiological and molecular alterations in plants exposed to high [CO2] under phosphorus stress. Biotechnol. Adv. 2015, 33, 303–316. [Google Scholar] [CrossRef]
- Yilmaz, O.; Kahraman, K.; Ozturk, L. Elevated carbon dioxide exacerbates adverse effects of Mg deficiency in durum wheat. Plant Soil 2017, 410, 41–50. [Google Scholar] [CrossRef]
- Niu, Y.; Ahammed, G.J.; Tang, C.; Guo, L.; Yu, J. Physiological and transcriptome responses to combinations of elevated CO2 and magnesium in Arabidopsis thaliana. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef]
- Reddy, K.R.; Zhao, D. Interactive effects of elevated CO2 and potassium deficiency on photosynthesis, growth, and biomass partitioning of cotton. F. Crop. Res. 2005, 94, 201–213. [Google Scholar] [CrossRef]
- Jin, C.W.; Du, S.T.; Chen, W.W.; Li, G.X.; Zhang, Y.S.; Zheng, S.J. Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol. 2009, 150, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999, 141, 1–26. [Google Scholar] [CrossRef]
- Heinemann, A.B.; de H.N. Maia, A.; Dourado-neto, D.; Ingram, K.T.; Hoogenboom, G. Soybean (Glycine max (L.) Merr.) growth and development response to CO2 enrichment under different temperature regimes. Eur. J. Agron. 2006, 24, 52–61. [Google Scholar] [CrossRef]
- Rogers, H.H.; Peterson, C.M.; McCrimmon, J.M.; Cure, J.D. Response of soybean roots to elevated atmospheric carbon dioxide. Plant Cell Environ. 1992, 15, 749–752. [Google Scholar] [CrossRef]
- Bunce, J.A. Contrasting responses of seed yield to elevated carbon dioxide under field conditions within Phaseolus vulgaris. Agric. Ecosyst. Environ. 2008, 128, 219–224. [Google Scholar] [CrossRef]
- Salsman, K.J.; Jordan, D.N.; Smith, S.D.; Neuman, D.S. Effect of atmospheric CO2 enrichment on root growth and carbohydrate alloca- tion of Phaseolus spp. Int. J. Plant Sci. 1999, 160, 1075–1081. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Kumar, U.; Quick, W.P.; Barrios, M.; Sta Cruz, P.C.; Dingkuhn, M.; Hunsaker, D.; Adamsen, F.; Lamorte, R.; Leavitt, S.; Thompson, T.; et al. Atmospheric CO2 concentration effects on rice water use and biomass production. PLoS ONE 2017, 12, e0169706. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef]
- Kant, S.; Seneweera, S.; Rodin, J.; Materne, M.; Burch, D.; Rothstein, S.J.; Spangenberg, G. Improving yield potential in crops under elevated CO2: Integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Schaedler, T.A. Iron cofactor assembly in plants. Annu. Rev. Plant Biol. 2014, 65, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Abadìa, J. Leaf responses to Fe deficiency: A review. J. Plant Nutr. 1992, 15, 1699–1713. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Faralli, M.; Grove, I.G.; Hare, M.C.; Kettlewell, P.S.; Fiorani, F. Rising CO2 from historical concentrations enhances the physiological performance of Brassica napus seedlings under optimal water supply but not under reduced water availability. Plant Cell Environ. 2017, 40, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N., and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
- Lambreva, M.; Stoyanova-Koleva, D.; Baldjiev, G.; Tsonev, T. Early acclimation changes in the photosynthetic apparatus of bean plants during short-term exposure to elevated CO2 concentration under high temperature and light intensity. Agric. Ecosyst. Environ. 2005, 106, 219–232. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; De Souza, A.P.; Ament, M.R.; Gleadow, R.M.; Ort, D.R. High sink strength prevents photosynthetic down-regulation in cassava grown at elevated CO2 concentration. J. Exp. Bot. 2021, 72, 542–560. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M. Lou Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef]
- Krouma, A.; Gharsalli, M.; Abdelly, C. Differences in response to iron deficiency among some lines of common bean. J. Plant Nutr. 2003, 26, 2295–2305. [Google Scholar] [CrossRef]
- Slatni, T.; Krouma, A.; Gouia, H.; Abdelly, C. Importance of ferric chelate reductase activity and acidification capacity in root nodules of N2-fixing common bean (Phaseolus vulgaris L.) subjected to iron deficiency. Symbiosis 2009, 47, 35–42. [Google Scholar] [CrossRef]
- Slatni, T.; Ben Salah, I.; Kouas, S.; Abdelly, C. The role of nodules in the tolerance of common bean to iron deficiency. J. Plant Res. 2014, 127, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Knewtson, S.J.B.J.J.B.; Astudillo, C.; Li, C.-M.M.; Fernandez, A.C.; Grusak, M.A. Variation and inheritance of iron reductase activity in the roots of common bean (Phaseolus vulgaris L.) and association with seed iron accumulation QTL. BMC Plant Biol. 2010, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.J.; Waters, B.M. Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: Implications for iron deficiency chlorosis. J. Exp. Bot. 2016, 67, 5671–5685. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Morales, F.; Gogorcena, Y.; Abadía, A.; Abadía, J. Metabolic responses in iron deficient tomato plants. J. Plant Physiol. 2009, 166, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Tomasi, N.; Rizzardo, C.; Gottardi, S.; Terzano, R.; Alfeld, M.; Janssens, K.; De Nobili, M.; Mimmo, T.; Cesco, S. Iron allocation in leaves of Fe-deficient cucumber plants fed with natural Fe complexes. Physiol. Plant. 2015, 154, 82–94. [Google Scholar] [CrossRef]
- Yi, Y.; Guerinot, M.L. Genetic evidence that induction of root Fe (III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant, J. 1996, 10, 835–844. [Google Scholar] [CrossRef]
- Santos, C.S.; Roriz, M.; Carvalho, S.M.P.; Vasconcelos, M.W. Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.). Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.S.; Silva, A.I.; Serrão, I.; Carvalho, A.L.; Vasconcelos, M.W. Transcriptomic analysis of iron deficiency related genes in the legumes. Food Res. Int. 2013, 54, 1162–1171. [Google Scholar] [CrossRef]
- Gama, F.; Saavedra, T.; da Silva, J.P.; Miguel, M.G.; de Varennes, A.; Correia, P.J.; Pestana, M. The memory of iron stress in strawberry plants. Plant Physiol. Biochem. 2016, 104, 36–44. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Sanz-sáez, Á.; Jáuregui, I.; Irigoyen, J.J.; Araus, J.L.; Sánchez-díaz, M. Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J. Exp. Bot. 2013, 64, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.J.; Gama, F.; Saavedra, T.; Miguel, M.G.; Da Silva, J.P.; Abadía, A.; de Varennes, A.; Pestana, M. Changes in the concentration of organic acids in roots and leaves of carob-tree under Fe deficiency. Funct. Plant Biology 2014, 41, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, J.I.; Rombolà, A.D. Organic acids metabolism in roots of grapevine rootstocks under severe iron deficiency. Plant Soil 2015, 165–175. [Google Scholar] [CrossRef]
- Bencke-Malato, M.; De Souza, A.P.; Ribeiro-Alves, M.; Schmitz, J.F.; Buckeridge, M.S.; Alves-Ferreira, M. Short-term responses of soybean roots to individual and combinatorial effects of elevated [CO2] and water deficit. Plant Sci. 2019, 280, 283–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deuchande, T.; Soares, J.; Nunes, F.; Pinto, E.; Vasconcelos, M.W. Short Term Elevated CO2 Interacts with Iron Deficiency, Further Repressing Growth, Photosynthesis and Mineral Accumulation in Soybean (Glycine max L.) and Common Bean (Phaseolus vulgaris L.). Environments 2021, 8, 122. https://doi.org/10.3390/environments8110122
Deuchande T, Soares J, Nunes F, Pinto E, Vasconcelos MW. Short Term Elevated CO2 Interacts with Iron Deficiency, Further Repressing Growth, Photosynthesis and Mineral Accumulation in Soybean (Glycine max L.) and Common Bean (Phaseolus vulgaris L.). Environments. 2021; 8(11):122. https://doi.org/10.3390/environments8110122
Chicago/Turabian StyleDeuchande, Teresa, José Soares, Fábio Nunes, Elisabete Pinto, and Marta W. Vasconcelos. 2021. "Short Term Elevated CO2 Interacts with Iron Deficiency, Further Repressing Growth, Photosynthesis and Mineral Accumulation in Soybean (Glycine max L.) and Common Bean (Phaseolus vulgaris L.)" Environments 8, no. 11: 122. https://doi.org/10.3390/environments8110122
APA StyleDeuchande, T., Soares, J., Nunes, F., Pinto, E., & Vasconcelos, M. W. (2021). Short Term Elevated CO2 Interacts with Iron Deficiency, Further Repressing Growth, Photosynthesis and Mineral Accumulation in Soybean (Glycine max L.) and Common Bean (Phaseolus vulgaris L.). Environments, 8(11), 122. https://doi.org/10.3390/environments8110122





