Multi-Scale Modeling and Study of Aerosol Growth in an Amine-based CO2 Capture Absorber
Abstract
1. Introduction
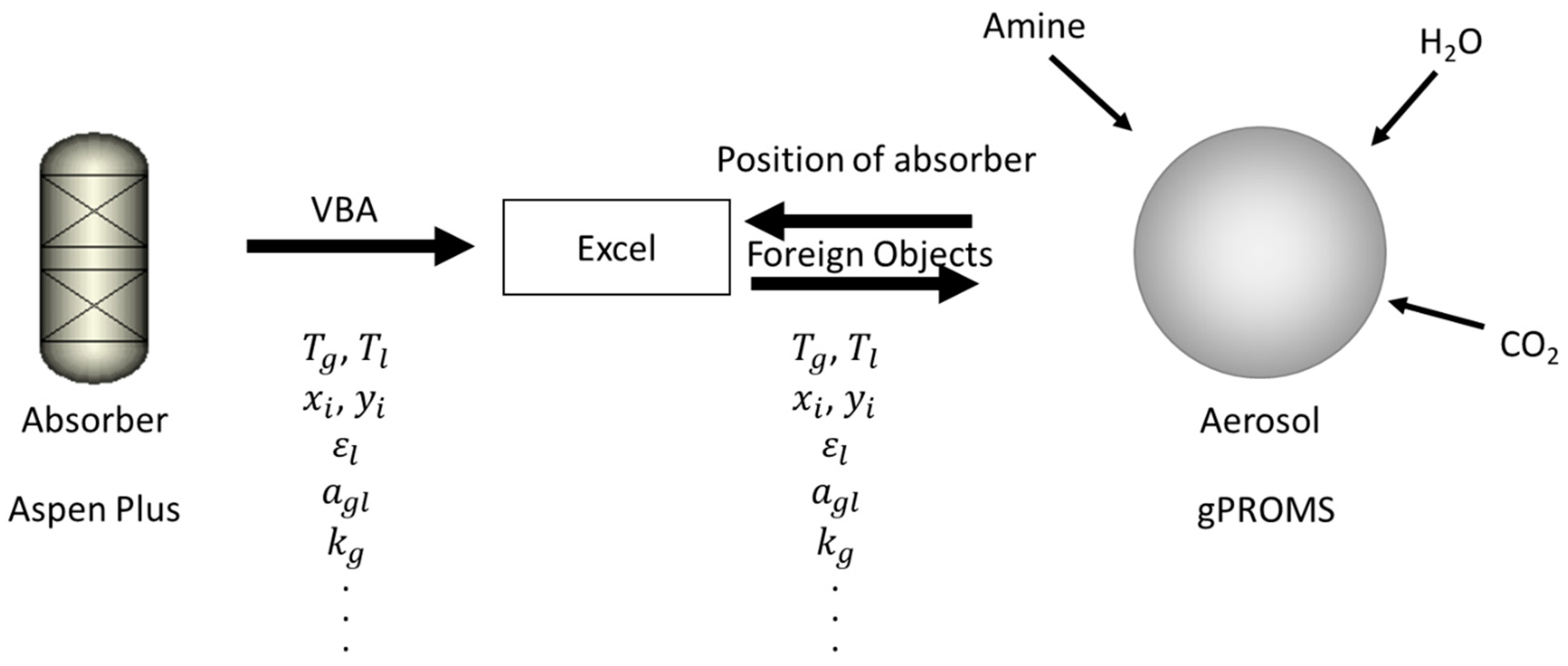
2. Modeling Approach
2.1. Data Extraction from Absorber Model
2.2. Amine Aerosol Growth Modeling
2.3. Physical Properties
2.4. Amine Absorber Modeling Using Aspen Plus
2.5. Experiments
3. Results and Discussion
3.1. Investigation of the Experiment
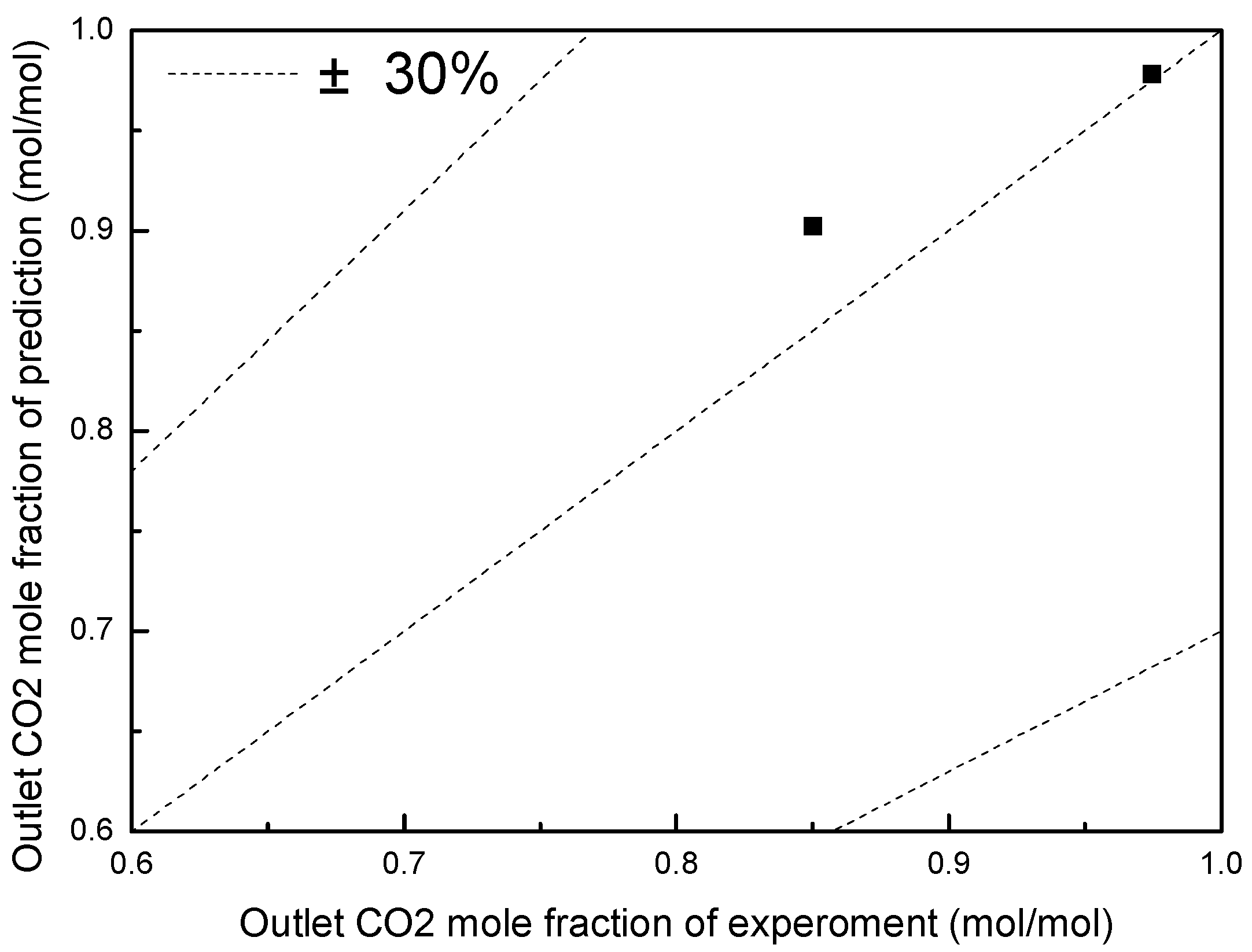
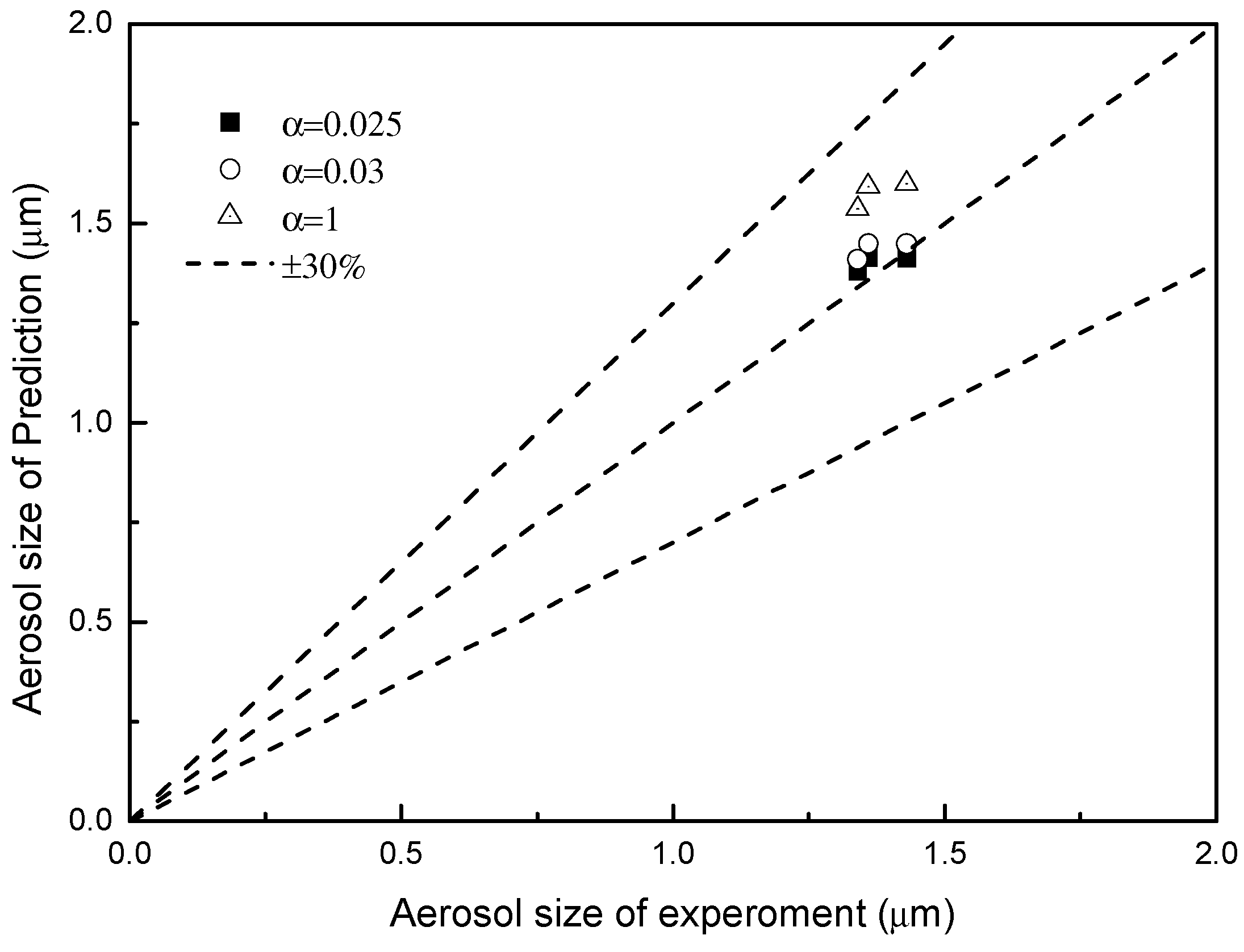
3.2. Validation of Absorber and Aerosol Growth Model
3.3. Effect of PNC on Aerosol Growth
3.4. Effect of PNC on MEA Emissions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-J.; Chang, C.-C.; Wong, D.S.-H.; Jang, S.-S.; Ou, J.-J. Control Strategies for Flexible Operation of Power Plant Integrated with CO2 Capture Plant, Computer Aided Chemical Engineering. AIChE J. 2012, 58, 2697–2704. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Environmental impacts of absorption-based CO 2 capture unit for post-combustion treatment of flue gas from coal-fired power plant. Int. J. Greenh. Gas Control 2007, 1, 318–342. [Google Scholar] [CrossRef]
- Dave, N.; Do, T.; Jackson, P.; Feron, P.; Azzi, M.; Attalla, M. CO2 Capture Mongstad-Project B Theoretical Evaluation of the Potential to Form and Emit Harmful Compounds, Task 1: Process Chemistry; The Commonwealth Scientific and Industrial Research Organisation (CSIRO) Report; CSIRO: Canberra, Australia, 2010.
- Fine, N.A.; Goldman, M.J.; Nielsen, P.T.; Rochelle, G.T. Managing n-nitrosopiperazine and dinitrosopiperazine. Energy Procedia 2013, 37, 273–284. [Google Scholar] [CrossRef]
- Kamijo, T.; Kajiya, Y.; Endo, T.; Nagayasu, H.; Tanaka, H.; Hirata, T.; Yonekawa, T.; Tsujiuchi, T. SO3 impact on amine emission and emission reduction technology. Energy Procedia 2013, 37, 1793–1796. [Google Scholar] [CrossRef]
- Khakharia, P.; Brachert, L.; Mertens, J.; Anderlohr, C.; Huizinga, A.; Fernandez, E.S.; Schallert, B.; Schaber, K.; Vlugt, T.J.; Goetheer, E. Understanding aerosol based emissions in a Post Combustion CO2 Capture process: Parameter testing and mechanisms. Int. J. Greenh. Gas Control 2015, 34, 63–74. [Google Scholar] [CrossRef]
- Mertens, J.; Brachert, L.; Desagher, D.; Thielens, M.; Khakharia, P.; Goetheer, E.; Schaber, K. ELPI+ measurements of aerosol growth in an amine absorption column. Int. J. Greenh. Gas Control 2014, 23, 44–50. [Google Scholar] [CrossRef]
- Onda, K.; Takeuchi, H.; Okumoto, Y. Mass transfer coefficients between gas and liquid phases in packed columns. J. Chem. Eng. Jpn. 1968, 1, 56–62. [Google Scholar] [CrossRef]
- Brachert, L.; Mertens, J.; Khakharia, P.; Schaber, K. The challenge of measuring sulfuric acid aerosols: Number concentration and size evaluation using a condensation particle counter (CPC) and an electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 67, 21–27. [Google Scholar] [CrossRef]
- Bradie, J.; Dickson, A. Removal of Entrained Liquid Droplets by Wire-Mesh Demisters. In Proceedings of the Institution of Mechanical Engineers, Conference Proceedings; SAGE Publications Sage: London, UK, 1969; pp. 195–203. [Google Scholar]
- El-Dessouky, H.T.; Alatiqi, I.M.; Ettouney, H.M.; Al-Deffeeri, N.S. Performance of wire mesh mist eliminator. Chem. Eng. Process. Process Intensif. 2000, 39, 129–139. [Google Scholar] [CrossRef]
- Fulk, S.M.; Chen, E.; Rochelle, G.T. Aerosol Mitigation in Amine-Based CO2 Capture. In Proceedings of the 2nd Post Combustion Capture Conference (PCCC2), Bergen, Norway, 17–20 September 2013. [Google Scholar]
- Kang, J.-L.; Zhang, Y.; Fulk, S.; Rochelle, G.T. Modeling amine aerosol growth in the absorber and water wash. Energy Procedia 2017, 114, 959–976. [Google Scholar] [CrossRef]
- Majeed, H.; Knuutila, H.; Hillestad, M.; Svendsen, H.F. Gas phase amine depletion created by aerosol formation and growth. Int. J. Greenh. Gas Control 2017, 64, 212–222. [Google Scholar] [CrossRef]
- Majeed, H.; Hillestad, M.; Knuutila, H.; Svendsen, H.F. Predicting aerosol size distribution development in absorption columns. Chem. Eng. Sci. 2018, 192, 25–33. [Google Scholar] [CrossRef]
- Majeed, H.; Svendsen, H.F. Characterization of aerosol emissions from CO2 capture plants treating various power plant and industrial flue gases. Int. J. Greenh. Gas Control 2018, 74, 282–295. [Google Scholar] [CrossRef]
- Xu, Q.; Rochelle, G. Total pressure and CO2 solubility at high temperature in aqueous amines. Energy Procedia 2011, 4, 117–124. [Google Scholar] [CrossRef]
- Kang, J.-L.; Sun, K.; Wong, D.S.-H.; Jang, S.-S.; Tan, C.-S. Modeling studies on absorption of CO2 by monoethanolamine in rotating packed bed. Int. J. Greenh. Gas Control 2014, 25, 141–150. [Google Scholar] [CrossRef]
- AspenTech. Rate-Based Model of the CO2 Capture Process by MEA Using Aspen Plus; AspenTech: Bedford, MA, USA, 2013. [Google Scholar]
- Stichlmair, J.; Bravo, J.; Fair, J. General model for prediction of pressure drop and capacity of countercurrent gas/liquid packed columns. Gas Sep. Purif. 1989, 3, 19–28. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.-C.; Peng, C.-C.; Wong, D.S.-H.; Jang, S.-S.; Shen, J.-F. Aspen Plus rate-based modeling for reconciling laboratory scale and pilot scale CO2 absorption using aqueous ammonia. Int. J. Greenh. Gas Control 2015, 34, 117–128. [Google Scholar] [CrossRef]
- Kaufman, S.L. Electrospray diagnostics performed by using sucrose and proteins in the gas-phase electrophoretic mobility molecular analyzer (GEMMA). Anal. Chim. Acta 2000, 406, 3–10. [Google Scholar] [CrossRef]
- Lee, F.-C.; Lu, Y.-F.; Chou, F.-C.; Cheng, C.-F.; Ho, R.-M.; Tsai, D.-H. Mechanistic study of gas-phase controlled synthesis of copper oxide-based hybrid nanoparticle for CO oxidation. J. Phys. Chem. C 2016, 120, 13638–13648. [Google Scholar] [CrossRef]







| From Absorber | |
|---|---|
| From Thermodynamics | ,, , |
| From Empirical equations | |
| [18] | |
| [19] | |
| [19] | |
| [17] | |
| Absorber | |
|---|---|
| Packing height (cm) | 30 |
| Diameter (cm) | 2.54 |
| Packing type | Plastic Raschig Rings |
| Packing surface area (m2/m3) | 610 |
| Lean solvent T (°C) | 30 |
| Liquid flow rate(L/min) | 0.2 |
| MEA concentration (wt%) | 30 |
| Gas inlet T (°C) | 30 |
| Gas flow rate(L/min) | 1.5/3 |
| Inlet CO2 | 20% |
| Inlet N2 | 80% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-L.; Liu, K.-T.; Wong, D.S.-H.; Jang, S.-S.; Tsai, D.-H. Multi-Scale Modeling and Study of Aerosol Growth in an Amine-based CO2 Capture Absorber. Environments 2020, 7, 58. https://doi.org/10.3390/environments7080058
Kang J-L, Liu K-T, Wong DS-H, Jang S-S, Tsai D-H. Multi-Scale Modeling and Study of Aerosol Growth in an Amine-based CO2 Capture Absorber. Environments. 2020; 7(8):58. https://doi.org/10.3390/environments7080058
Chicago/Turabian StyleKang, Jia-Lin, Kuan-Ting Liu, David Shan-Hill Wong, Shi-Shang Jang, and De-Hao Tsai. 2020. "Multi-Scale Modeling and Study of Aerosol Growth in an Amine-based CO2 Capture Absorber" Environments 7, no. 8: 58. https://doi.org/10.3390/environments7080058
APA StyleKang, J.-L., Liu, K.-T., Wong, D. S.-H., Jang, S.-S., & Tsai, D.-H. (2020). Multi-Scale Modeling and Study of Aerosol Growth in an Amine-based CO2 Capture Absorber. Environments, 7(8), 58. https://doi.org/10.3390/environments7080058







