New Insights into Cellular Impacts of Metals in Aquatic Animals
Abstract
1. Introduction
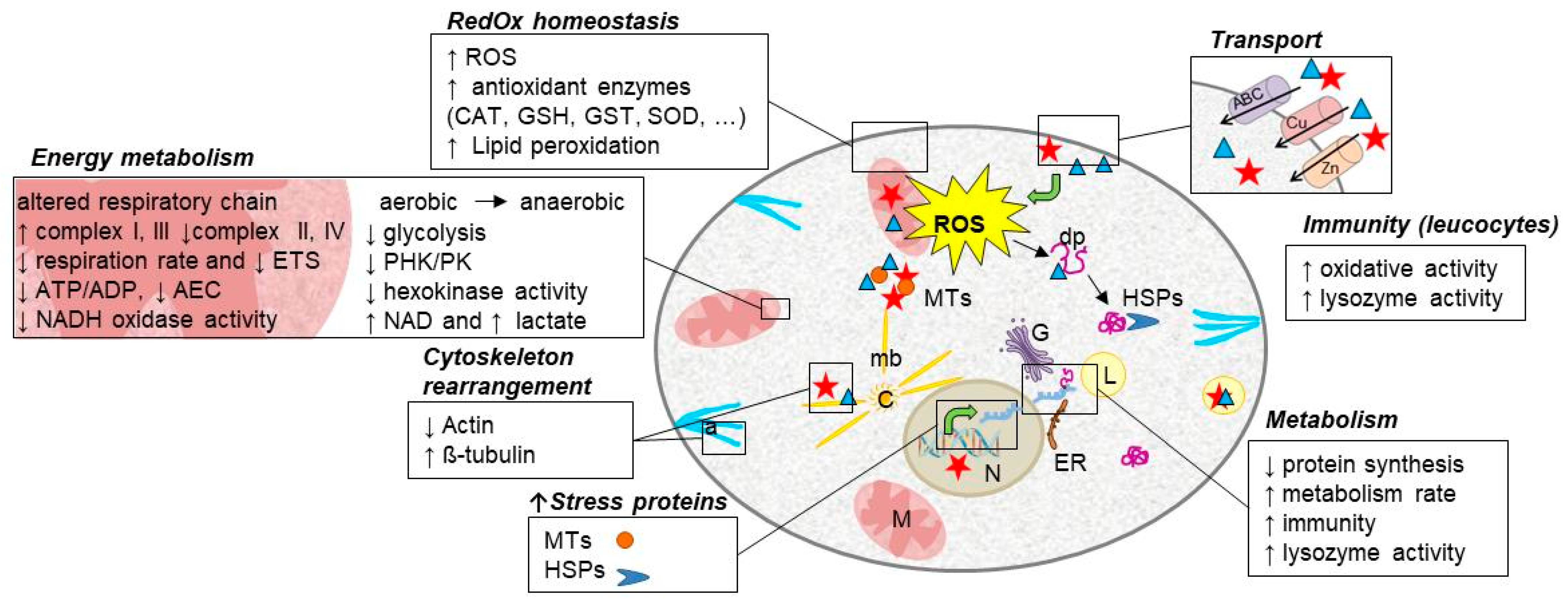
2. A Common Response to Toxic Metal Exposure
3. Overexpression of Stress Proteins
3.1. Metallothioneins
3.2. HSP
4. Redox Homeostasis Disturbance
5. Cytoskeleton Rearrangement
6. Metabolism Reshuffle
7. Impacts of Metals on Free Cellular Energy and Mitochondrial Metabolism
8. Impacts of Metals on Immunity
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Azimi, S.; Rocher, V. Influence of the water quality improvement on fish population in the Seine River (Paris, France) over the 1990–2013 period. Sci. Total Environ. 2016, 542, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Loizeau, J.; Edder, P.; De Alencastro, L.F.; Corvi, C.; Ramseier Gentile, S. La contamination du léman par les micropolluants—Revue de 40 ans d’études. Arch. Sci. 2013, 66, 117–136. [Google Scholar]
- Castruita, M.; Casero, D.; Karpowicz, S.J.; Kropat, J.; Vieler, A.; Hsieh, S.I.; Yan, W.; Cokus, S.; Loo, J.A.; Benning, C.; et al. Systems Biology Approach in Chlamydomonas Reveals Connections between Copper Nutrition and Multiple Metabolic Steps. Plant Cell 2011, 23, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Colás, N.; Sancenón, V.; Rodriguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Peñarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Page, M.D.; Kropat, J.; Hamel, P.P.; Merchant, S. Two Chlamydomonas CTR Copper Transporters with a Novel Cys-Met Motif Are Localized to the Plasma Membrane and Function in Copper Assimilation. Plant Cell 2009, 21, 928–943. [Google Scholar] [CrossRef]
- Monferran, M.V.; Agudo, J.A.S.; Pignata, M.L.; Wunderlin, D.A. Copper-induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environ. Pollut. 2009, 157, 2570–2576. [Google Scholar] [CrossRef]
- Razinger, J.; Drinovec, L.; Zrimec, A. Real-time visualization of oxidative stress in a floating macrophyte Lemna minor L. exposed to cadmium, copper, menadione, and AAPH. Environ. Toxicol. 2010, 25, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Sharma, G.D.; Panda, S. Responses of antioxidant metabolism and defense mechanism of aquatic macrophyte, Pistia stratiotes L. to zinc treatment under copper stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2011, 81, 422–427. [Google Scholar]
- Quintá, H.R.; Galigniana, N.M.; Erlejman, A.G.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. Management of cytoskeleton architecture by molecular chaperones and immunophilins. Cell Signal. 2011, 23, 1907–1920. [Google Scholar] [CrossRef]
- Doig, L.E.; Liber, K. Influence of dissolved organic matter on nickel bioavailability and toxicity to Hyalella azteca in water-only exposures. Aquat. Toxicol. 2006, 76, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K.M. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mebane, C.A.; Chowdhury, M.J.; De Schamphelaere, K.; Lofts, S.; Paquin, P.R.; Santore, R.C.; Wood, C.M. Metal Bioavailability Models: Current Status, Lessons Learned, Considerations for Regulatory Use, and the Path Forward. Environ. Toxicol. Chem. 2020, 39, 60–84. [Google Scholar] [CrossRef] [PubMed]
- RajeshKumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef]
- Mahino, F.; Nazura, U.; Mobarak, H. Heavy metal in aquatic ecosystem emphasizing its effect on tissue bioaccumulation and histopathology: A review. J. Environ. Sci. Technol. 2014, 7, 1–15. [Google Scholar]
- Banfalvi, G. Heavy Metals, Trace Elements and Their Cellular Effects. Cell. Eff. Heavy Met. 2011, 3–28. [Google Scholar] [CrossRef]
- Kim, B.-M.; Rhee, J.-S.; Jeong, C.-B.; Seo, J.S.; Park, G.S.; Lee, Y.-M.; Lee, J.-S. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 65–74. [Google Scholar] [CrossRef]
- Barjhoux, I.; Baudrimont, M.; Morin, B.; Landi, L.; Gonzalez, P.; Cachot, J. Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2012, 79, 272–282. [Google Scholar] [CrossRef]
- Risso-de-Faverney, C.; Devaux, A.; Lafaurie, M.; Girard, J.; Bailly-Maitre, P.G.A.B.; Delescluse, C. Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygene species. Aquat. Toxicol. 2001, 53, 65–76. [Google Scholar] [CrossRef]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Barjhoux, I.; Gonzalez, P.; Baudrimont, M.; Cachot, J. Molecular and phenotypic responses of Japanese medaka (Oryzias latipes) early life stages to environmental concentrations of cadmium in sediment. Environ. Sci. Pollut. Res. 2016, 23, 17969–17981. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Baudrimont, M.; Gonzalez, P.; Cuenot, Y.; Bourdineaud, J.; Boudou, A.; Gauthier, L. Genotoxic and stress inductive potential of cadmium in Xenopus laevis larvae. Aquat. Toxicol. 2006, 78, 157–166. [Google Scholar] [CrossRef]
- Giaginis, C.; Gatzidou, E.; Theocharis, S. DNA repair systems as targets of cadmium toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The Mismetallation of Enzymes during Oxidative Stress. J. Boil. Chem. 2014, 289, 28121–28128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J. Synergistic cellular responses to heavy metal exposure: A minireview. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.P.-L.; Au, C.Y.-M.; Chan, W.W.-L.; Chan, K.M. Characterization and localization of metal-responsive-element-binding transcription factors from tilapia. Aquat. Toxicol. 2010, 99, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, G.; Li, L.; Li, C.; Wang, T.; Zhang, G. Transcription factor CgMTF-1 regulates CgZnT1 and CgMT expression in Pacific oyster (Crassostrea gigas) under zinc stress. Aquat. Toxicol. 2015, 165, 179–188. [Google Scholar] [CrossRef]
- Ale, A.; Liberatori, G.; Vannuccini, M.L.; Bergami, E.; Ancora, S.; Mariotti, G.; Bianchi, N.; Corsi, I.; DeSimone, M.F.; Cazenave, J.; et al. Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2019, 211, 46–56. [Google Scholar] [CrossRef]
- Roesijadi, G. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 1992, 22, 81–113. [Google Scholar] [CrossRef]
- Bertrand, L.; Monferrán, M.V.; Mouneyrac, C.; Amé, M.V. Native crustacean species as a bioindicator of freshwater ecosystem pollution: A multivariate and integrative study of multi-biomarker response in active river monitoring. Chemosphere 2018, 206, 265–277. [Google Scholar] [CrossRef]
- Mijošek, T.; Marijić, V.F.; Dragun, Z.; Ivanković, D.; Krasnići, N.; Erk, M.; Gottstein, S.; Lajtner, J.; Perić, M.S.; Kepčija, R.M. Comparison of electrochemically determined metallothionein concentrations in wild freshwater salmon fish and gammarids and their relation to total and cytosolic metal levels. Ecol. Indic. 2019, 105, 188–198. [Google Scholar] [CrossRef]
- Bourdineaud, J.-P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.-L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Piano, A.; Valbonesi, P.; Fabbri, E. Expression of cytoprotective proteins, heat shock protein 70and metallothioneins, in tissues ofOstrea edulis exposed to heat andheavy metals. Cell Stress Chaperones 2004, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Geffard, A.; Amiard-Triquet, C.; Amiard, J.-C. Do seasonal changes affect metallothionein induction by metals in mussels, Mytilus edulis? Ecotoxicol. Environ. Saf. 2005, 61, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, W.-X. Facilitated Bioaccumulation of Cadmium and Copper in the OysterCrassostrea hongkongensisSolely Exposed to Zinc. Environ. Sci. Technol. 2013, 47, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Hanana, H.; Kleinert, C.; André, C.; Gagné, F. Influence of cadmium on oxidative stress and NADH oscillations in mussel mitochondria. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 216, 60–66. [Google Scholar] [CrossRef]
- Amiard, J.; Amiardtriquet, C.; Barka, S.; Pellerin, J.; Rainbow, P. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Boil. 2014, 2, 323–332. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Franzellitti, S. HSP expression in bivalves. Inf. Syst. J. 2008, 5, 135–161. [Google Scholar]
- Pestana, J.L.; Novais, S.C.; Norouzitallab, P.; Vandegehuchte, M.B.; Bossier, P.; De Schamphelaere, K. Non-lethal heat shock increases tolerance to metal exposure in brine shrimp. Environ. Res. 2016, 151, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Martin, L.; Howe, S.; Nelson, W.; Hegre, E.; Phelps, D. Tissue-Specific Differences in Accumulation of Stress Proteins in Mytilus edulis Exposed to a Range of Copper Concentrations. Toxicol. Appl. Pharmacol. 1994, 125, 206–213. [Google Scholar] [CrossRef]
- Santos, S.G.G.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Varela, J.L.; Mancera, J.; Fontaínhas-Fernandes, A.; Wilson, J.M. Metabolic and osmoregulatory changes and cell proliferation in gilthead sea bream (Sparus aurata) exposed to cadmium. Ecotoxicol. Environ. Saf. 2011, 74, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tedengren, M.; Olsson, B.; Bradley, B.; Zhou, L. Heavy metal uptake, physiological response and survival of the blue mussel (Mytilus edulis) from marine and brackish waters in relation to the induction of heat-shock protein. Hydrobiologia 1999, 393, 261–269. [Google Scholar] [CrossRef]
- Minghetti, M.; Leaver, M.; Taggart, J.B.; Casadei, E.; Auslander, M.; Tom, M.; George, S.G. Copper induces Cu-ATPase ATP7A mRNA in a fish cell line, SAF1. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 93–99. [Google Scholar] [CrossRef]
- Manduzio, H.; Monsinjon, T.; Rocher, B.; Leboulenger, F.; Galap, C. Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis. Aquat. Toxicol. 2003, 64, 73–83. [Google Scholar] [CrossRef]
- Nuran, E.; Hande, G.-O.; Nukhet, A.-B. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Me-tal induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Viarengo, A.; Canesi, L.; Pertica, M.; POli, G.; Moore, M.N.; Orunesu, M. Heavy metal effects on lipid peroxidation in the tissues of Mytilus galloprovincialis lam. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 1990, 97, 37–42. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Cherkasov, A.S.; Sokolova, I.M. Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J. Exp. Boil. 2008, 211, 577–586. [Google Scholar] [CrossRef]
- Morcillo, P.; Cordero, H.; Meseguer, J.; Esteban, M.A.; Cuesta, A. Toxicological in vitro effects of heavy metals on gilthead seabream (Sparus aurata L.) head-kidney leucocytes. Toxicol. Vitr. 2015, 30, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Pellegrini, D.; Winston, G.W.; Gorbi, S.; Giuliani, S.; Virno-Lamberti, C.; Bompadre, S. Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: The relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar. Pollut. Bull. 2002, 44, 912–922. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Dassenakis, M.; Scoullos, M.J.; Valavanidis, A. Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar. Pollut. Bull. 2007, 54, 1361–1371. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Cajaraville, M. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol. Vitr. 2003, 17, 539–546. [Google Scholar] [CrossRef]
- Eyckmans, M.; Benoot, D.; Van Raemdonck, G.A.; Zegels, G.; Van Ostade, X.; Witters, E.; Blust, R.; De Boeck, G. Comparative proteomics of copper exposure and toxicity in rainbow trout, common carp and gibel carp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Chora, S.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Proteomic response of mussels Mytilus galloprovincialis exposed to CuO NPs and Cu2+: An exploratory biomarker discovery. Aquat. Toxicol. 2014, 155, 327–336. [Google Scholar] [CrossRef]
- Thompson, E.; Taylor, D.A.; Nair, S.V.; Birch, G.; Haynes, P.A.; Raftos, D.A. Proteomic discovery of biomarkers of metal contamination in Sydney Rock oysters (Saccostrea glomerata). Aquat. Toxicol. 2012, 109, 202–212. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Habinck, E.; Sokolova, I.M. Differential sensitivity to cadmium of key mitochondrial enzymes in the eastern oyster, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 72–79. [Google Scholar] [CrossRef]
- Cicik, B.; Engin, K. The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turkish J. Vet. Anim. Sci. 2005, 29, 113–117. [Google Scholar]
- Meléndez-Morales, D.; Lugo, P.D.P.; Meléndez-Hevia, E.; Paz-Lugo, P. Glycolysis activity in flight muscles of birds according to their physiological function. An experimental model in vitro to study aerobic and anaerobic glycolysis activity separately. Mol. Cell. Biochem. 2009, 328, 127–135. [Google Scholar] [CrossRef]
- Maes, V. Le Métabolisme Énergétique Chez un Cyprinidé D’eau Douce, le Gardon Rutilus Rutilus: Vers le Développement de Nouveaux Biomarqueurs en Lien Avec la Contamination Par des Produits Phytosanitaires. Ph.D. Thesis, Université de Reims Champagne-Ardenne, Reims, France, December 2014. [Google Scholar]
- Maes, V.; Betoulle, S.; Jaffal, A.; Dedourge-Geffard, O.; Delahaut, L.; Geffard, A.; Palluel, O.; Sanchez, W.; Paris-Palacios, S.; Vettier, A.; et al. Juvenile roach (Rutilus rutilus) increase their anaerobic metabolism in response to copper exposure in laboratory conditions. Ecotoxicology 2016, 25, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Maes, V.; Vettier, A.; Jaffal, A.; Dedourge-Geffard, O.; Delahaut, L.; Geffard, A.; Betoulle, S.; David, E. Energy metabolism and pesticides: Biochemical and molecular responses to copper in roach Rutilus rutilus. J. Xenobiotics 2013, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Shi, W.; Han, Y.; Guan, X.; Rong, J.; Du, X.; Zha, S.; Tang, Y.; Liu, G. Anthropogenic Noise Aggravates the Toxicity of Cadmium on Some Physiological Characteristics of the Blood Clam Tegillarca granosa. Front. Physiol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Pertica, M.; Mancinelli, G.; Capelli, R.; Orunesu, M. Effects of copper on the uptake of amino acids, on protein synthesis and on ATP content in different tissues of Mytilus galloprovincialis Lam. Mar. Environ. Res. 1980, 4, 145–152. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.-X. NMR-based metabolomic studies on the toxicological effects of cadmium and copper on green mussels Perna viridis. Aquat. Toxicol. 2010, 100, 339–345. [Google Scholar] [CrossRef]
- Giacomin, M.; Jorge, M.B.; Bianchini, A. Effects of copper exposure on the energy metabolism in juveniles of the marine clam Mesodesma mactroides. Aquat. Toxicol. 2014, 152, 30–37. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Piccoli, G.; Stocchi, V.; Viarengo, A.; Gallo, G. In vitro and in vivo effects of heavy metals on mussel digestive gland hexokinase activity: The role of glutathione. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 261–268. [Google Scholar] [CrossRef]
- Liliom, K.; Wágner, G.; Pácz, A.; Cascante, M.; Kovács, J.; Ovádi, J. Organization-dependent effects of toxic bivalent ions. J. Boil. Inorg. Chem. 2000, 267, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Calow, P.; Forbes, V.E. How do physiological responses to stress translate into ecological and evolutionary processes? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 11–16. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Le Gal, Y.; Lagadic, L.; Lebras, S.; Caquet, T. Charge énergétique en adénylates (CEA) et autres biomarqueurs associés au métabolisme énergétique. In Biomarqueurs en Ecotoxicologie: Aspects Fondamentaux, 1st ed.; Lagadic, L., Caquet, B., Amiard, J.C., Ramade, F., Eds.; Masson: Paris, France, 1997; pp. 241–285. [Google Scholar]
- Mouneyrac, C.; Péry, A. Conséquences des perturbations du métabolisme énergétique. In Biomarqueurs en Écotoxicologie Aquatique, 2nd ed.; Amiard, J.C., Claude, A.-T., Eds.; Lavoisier Tec & Doc: Paris, France, 2017; pp. 270–297. [Google Scholar]
- Louis, F.; Devin, S.; Giambérini, L.; Potet, M.; David, E.; Pain-Devin, S. Energy allocation in two dreissenid species under metal stress. Environ. Pollut. 2019, 245, 889–897. [Google Scholar] [CrossRef]
- Sappal, R.; MacDougald, M.; Fast, M.; Stevens, D.; Kibenge, F.; Siah, A.; Kamunde, C. Alterations in mitochondrial electron transport system activity in response to warm acclimation, hypoxia-reoxygenation and copper in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2015, 165, 51–63. [Google Scholar] [CrossRef]
- Jorge, M.B.; Lauer, M.M.; Martins, C.D.M.G.; Bianchini, A. Impaired regulation of divalent cations with acute copper exposure in the marine clam Mesodesma mactroides. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 79–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sokolova, I.M.; Sokolov, E.P.; Ponnappa, K.M. Cadmium exposure affects mitochondrial bioenergetics and gene expression of key mitochondrial proteins in the eastern oyster Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Aquat. Toxicol. 2005, 73, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.; Ivanina, A.; Kurochkin, I. Effects of temperature and cadmium exposure on the mitochondria of oysters (Crassostrea virginica) exposed to hypoxia and subsequent reoxygenation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, S8. [Google Scholar] [CrossRef][Green Version]
- Sokolova, I.M.; Evans, S.; Hughes, F.M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J. Exp. Boil. 2004, 207, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). J. Exp. Boil. 2004, 207, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Achard-Joris, M.; Gonzalez, P.; Marie, V.; Baudrimont, M.; Bourdineaud, J.-P. Cytochrome c Oxydase Subunit I Gene is Up-regulated by Cadmium in Freshwater and Marine Bivalves. BioMetals 2006, 19, 237–244. [Google Scholar] [CrossRef]
- Navarro, A.; Faria, M.; Barata, C.; Piña, B. Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults. Environ. Pollut. 2011, 159, 100–107. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Betoulle, S.; Paris-Palacios, S.; Vernet, G. Immunology-Related Perturbations Induced by Copper and Chitosan in Carp (Cyprinus carpio L.). Arch. Environ. Contam. Toxicol. 2004, 47, 370–378. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Betoulle, S.; Paris-Palacios, S.; Vernet, G. Humoral immune factors modulated by copper and chitosan in healthy or parasitised carp (Cyprinus carpio L.) by Ptychobothrium sp. (Cestoda). Aquat. Toxicol. 2004, 68, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.; Jaffal, A.; Delahaut, L.; Palluel, O.; Porcher, J.-M.; Geffard, A.; Sanchez, W.; Betoulle, S. Effects of aluminium and bacterial lipopolysaccharide on oxidative stress and immune parameters in roach, Rutilus rutilus L. Environ. Sci. Pollut. Res. 2014, 21, 13103–13117. [Google Scholar] [CrossRef] [PubMed]
- Le Guernic, A.; Sanchez, W.; Palluel, O.; Bado-Nilles, A.; Floriani, M.; Turies, C.; Chadili, E.; Della Vedova, C.; Cavalié, I.; Adam-Guillermin, C.; et al. Acclimation capacity of the three-spined stickleback (Gasterosteus aculeatus, L.) to a sudden biological stress following a polymetallic exposure. Ecotoxicology 2016, 25, 1478–1499. [Google Scholar] [CrossRef][Green Version]
- Petitjean, Q. Response variability to multiple stressors exposure in wild gudgeons (Gobio occitaniae). Ph.D. Thesis, Université Toulouse III Paul Sabatier, Toulouse, France, 2019. [Google Scholar]
- Ciliberti, A.; Chaumot, A.; Recoura-Massaquant, R.; Chandesris, A.; François, A.; Coquery, M.; Ferréol, M.; Geffard, O. Caged Gammarus as biomonitors identifying thresholds of toxic metal bioavailability that affect gammarid densities at the French national scale. Water Res. 2017, 118, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, D.; Chaumot, A.; Charnot, A.; Almunia, C.; François, A.; Navarro, L.; Armengaud, J.; Salvador, A.; Geffard, O. Ecotoxico-Proteomics for Aquatic Environmental Monitoring: First in Situ Application of a New Proteomics-Based Multibiomarker Assay Using Caged Amphipods. Environ. Sci. Technol. 2017, 51, 13417–13426. [Google Scholar] [CrossRef]
- McDonagh, B.; Tyther, R.; Sheehan, D. Carbonylation and glutathionylation of proteins in the blue mussel Mytilus edulis detected by proteomic analysis and Western blotting: Actin as a target for oxidative stress. Aquat. Toxicol. 2005, 73, 315–326. [Google Scholar] [CrossRef]
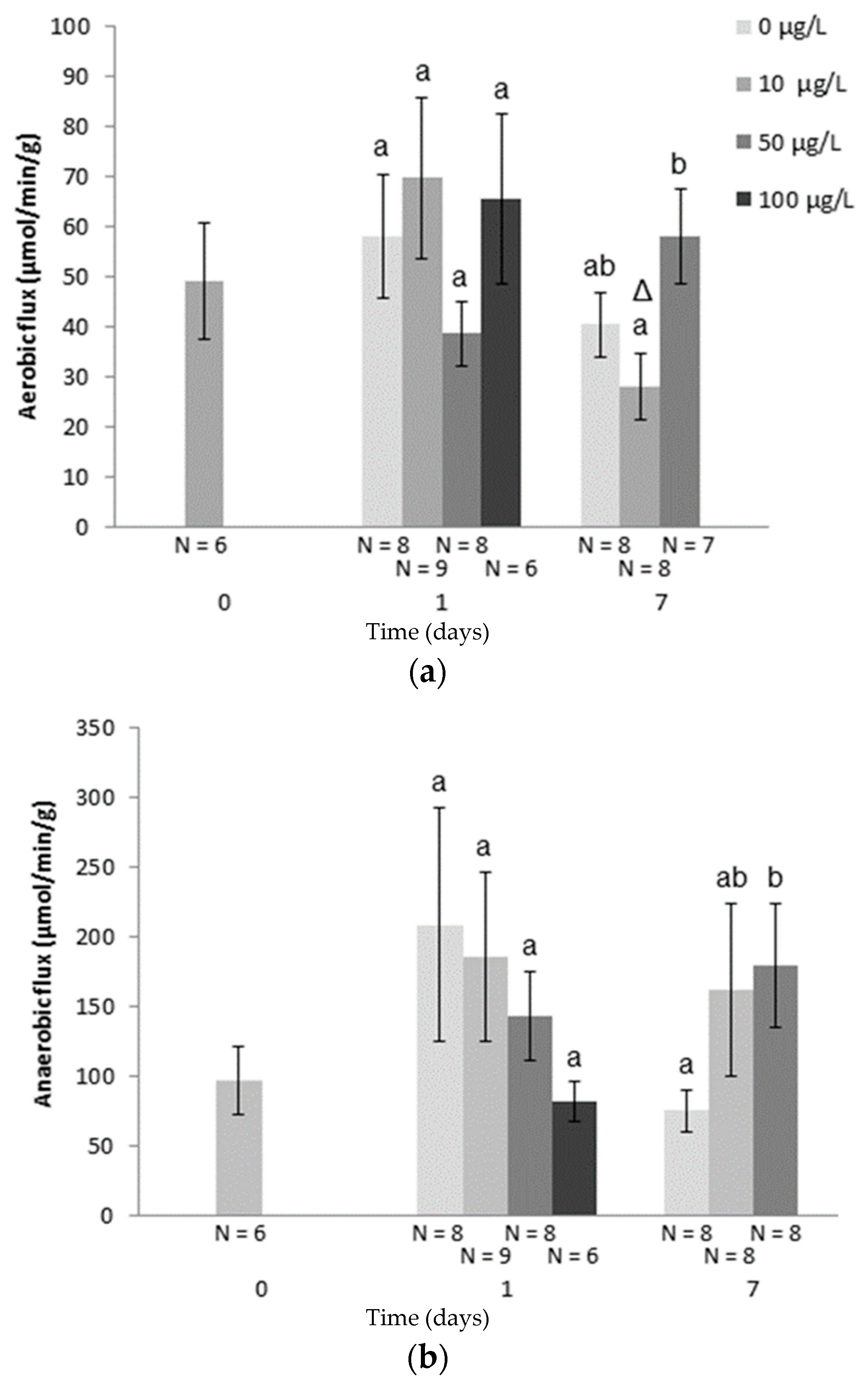
| Species | Metal | Concentration | Duration | Effect | |
|---|---|---|---|---|---|
| Crassostrea virginica (cell primary culture) | Cd | 5.6 to 22.4 µg/mL | 4 h | Gill cells: ↑HSP60 and HSP70. HSP70 shows a strong correlation with Cd concentrations. Hepatopancreas cells: higher uptake, but HSP70 was unchanged | [50] |
| Crassostrea hongkongensis | Zn, Cd, Cu (field) | n.a. | 2 months (caging) | Zn exposure: ↑Cu and Cd uptake and ↑MT | [36] |
| Echinogammarus acarinatu, Gammarus balcanicus, Salmo trutta | Cd, Cu (field) | n.a. | lifetime | Contaminated sites: ↑MT levels Contaminated sites: ↑MT levels Intestine: ↓metal content linked to detoxication abilities | [32] |
| Mytilus edulis | Cd, Cu, Zn (field) | n.a. | 8 months (caging) | In digestive gland MT abundance is linearly correlated to metal concentration, but not in gills; Seasonal modulation of MT abundance but metal-induction remains measurable | [35] |
| Cu, Cd | 10 µg/L, 100 µg/mussel | 7 days | Resistant populations induce more HSP70 than sensitive ones | [45] | |
| Cu | 100 µg/L | 7 days | ↑HSP70 and ↑HSP60 | [43] | |
| Mytilus galloprovincialis | Ag (NP) | 10 µg/L | 96 h | ↑MTs and ↑MT10 gene; no effect on MT20 gene | [29] |
| Ostrea edulis | Cd Zn Zn, Cd | 500 µg/L 500 µg/L 500 µg/L each | 7 days | Gills and digestive gland: ↑MT and HSP70 No effect Gills and digestive gland: ↑MT and ↑HSP70 | [34] |
| Palaemonetes argentinus | complex metal mixture (field) | n.a. | 96 h (caging) | Cephalothorax: ↑MT correlated significantly to Cd | [31] |
| Tigriopus japonicus | Cd, Cu | 5 to 100 μg/L | 96 h | hsp genes HSP20 and HSP70 are upregulated | [17] |
| Sparus aurata | Cd | 1.25 mg Cd/kg body mass (injection) | 7 days | Strong ↑HSP70 in the kidney; ↑HSP90 in liver | [44] |
| Species | Metal | Concentration | Duration | Effect | |
|---|---|---|---|---|---|
| Mytilus edulis | Cu, Cd, Zn | 40 µg/L | 6 days | ↓GSH in gill and hepatopancreas with Cu or Cd, but not with Zn | [49] |
| Cu | 25 µg/L | 7 days | ↑SOD activity for the three identified isoforms | [47] | |
| Mytilus galloprovincialis | Cd (field) | n.a. | lifetime | Gills: ↑CAT activity and high lipoperoxidation at polluted sites | [53] |
| Sparus aurata Leukocytes | Cd | 5.6 mg/L | 2 h | ↑CAT gene; no SOD gene expression modification | [51] |
| Fibroblast cell line SAF1 | Cu, Cd | 15.9 mg/L, 1.12 mg/L | 24 h | No SOD gene expression modification | [46] |
| Tigriopus japonicus | Cd, Cu | 100 μg/L | 96 h | ↑GST and SOD activities; ↑GSH with Cu treatment but not with Cd | [17] |
| Species | Metal | Concentration | Duration | Effect | |
|---|---|---|---|---|---|
| Carassius auratus gibelio, Cyprinus carpio, Oncorhynchus mykiss | Cu | 50 μg/L | 3 days | ↓Actin in common carp and rainbow trout ↑F- actin capping protein subunit β in common carp only | [55] |
| Mytilus edulis | Cd, Cu, Pb, Zn, PAHs (field) | n.a. | lifetime | ↑actin carbonylation (gills) and ↑glutathionylation (digestive gland) in polluted sites | [90] |
| Mytilus galloprovincialis (cell primary culture) | Cu, Cd | 5.6 to 112 mg Cd/L; 3.18 to 12.72 mg Cu /L | 24 h | Hemocytes: actin cytoskeleton alteration | [54] |
| Cu | 10 µg/L | 15 days | Gills: ↓actin; Digestive gland: ↓actin, ↑tubulin | [56] | |
| Saccostrea glomerata | Cd, Cu, Pb, Zn | 5, 50, 100 µg/L | 4 days | ↓Actin (Cu and Zn), ↑actin (Pb) or nor modified (Cd) | [57] |
| Species | Metal | Concentration | Duration | Effect | |
|---|---|---|---|---|---|
| Crassostrea virginica | Cd | 22 mg/L | 4 h | ↑Metabolism rate | [58] |
| Cyprinus carpio | Cd | 1 mg/L | 10 days | ↓Glycogen levels | [59] |
| Dreissena bugensis | Cd | 100 µg/L | 7 days | NADH oxidase activity is negatively correlated with the accumulation | [37] |
| Mytilus galloprovincialis | Cu | 80 µg/L | 7 days | ↓Protein synthesis | [65] |
| Cd, Cu | 40 mg/L | 3 days | ↓hexokinase activity by 35% (Cu) | [68] | |
| Perna viridis | Cd, Cu | 50 µg/L, 20 µg/L respectively | 7 days | Both metals alone or in combination ↑glycogen, ↑NAD, ↑lactate and ↓ATP/ADP | [66] |
| Sparus aurata | Cd | 1.25 mg Cd/kg body mass (injection) | 7 days | ↓activity and expression of Na+/K+-ATPase in the major osmoregulatory organs, gill, and kidney | [44] |
| Tegillarca granosa | Cd | 50 µg/L | 10 days | ↓PHK and ↓PK activities to 70% of the control | [64] |
| Species | Metal | Concentrations | Duration | Effect | ||
|---|---|---|---|---|---|---|
| Crassostrea virginica | Cd | 45 mg/L | Assay duration | Activity of mitochondrial complexes I to IV: ↓ or ↑ depending on the complex (gills and hepatopancreas) | [58] | |
| Crassostrea virginica | Cd | 50 µg/L | 30 days | Gills mitochondria: ↓Mitochondria ADP-stimulated respiration rate | [78] | |
| Crassostrea virginica | Cd | 25 µg/L | 21 days | Gills mitochondria: ↓Mitochondria ADP-stimulated respiration rate Gills: ↓ATP/ADP; ↓AEC | [77] | |
| Crassostrea virginica In vitro exposed mitochondria | Cd | 5,62 mg/L | Assay duration | ↓Mitochondrial respiration rates (gills mitochondria) | [80] | |
| Crassostrea virginica In vitro exposed hemocytes | Cd | 22.5 mg/L | 72 h | ↓ATP | [79] | |
| Dreissena polymorpha | Cd | 10 µg/L | 7 days | ↓SFG after 90 min ↑SFG after 7 days | [74] | |
| Mesodesma mactroides In vitro exposed mitochondria | Cu | 5 mg/L | 1 h | ↓Succinate dehydrogenase activity (gills and digestive gland mitochondria) | [76] | |
| Oncorhynchus mykiss In vitro exposed mitochondria | Cu | 0.32, 0.64, 1.27 mg/L | Assay duration | Liver mitochondria respiratory chain complex: ↑Complex I and III basal respiration rates ↓Complex II and IV basal respiration rates | [75] | |
| Rutilus rutilus | Cu | 100 µg/L | 7 days | ↓ETS after 24h (white muscle) | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Saux, A.; David, E.; Betoulle, S.; Bultelle, F.; Rocher, B.; Barjhoux, I.; Cosio, C. New Insights into Cellular Impacts of Metals in Aquatic Animals. Environments 2020, 7, 46. https://doi.org/10.3390/environments7060046
Le Saux A, David E, Betoulle S, Bultelle F, Rocher B, Barjhoux I, Cosio C. New Insights into Cellular Impacts of Metals in Aquatic Animals. Environments. 2020; 7(6):46. https://doi.org/10.3390/environments7060046
Chicago/Turabian StyleLe Saux, Aimie, Elise David, Stéphane Betoulle, Florence Bultelle, Béatrice Rocher, Iris Barjhoux, and Claudia Cosio. 2020. "New Insights into Cellular Impacts of Metals in Aquatic Animals" Environments 7, no. 6: 46. https://doi.org/10.3390/environments7060046
APA StyleLe Saux, A., David, E., Betoulle, S., Bultelle, F., Rocher, B., Barjhoux, I., & Cosio, C. (2020). New Insights into Cellular Impacts of Metals in Aquatic Animals. Environments, 7(6), 46. https://doi.org/10.3390/environments7060046








