Water Contaminated by Industrial Textile Dye: Study on Decolorization Process
Abstract
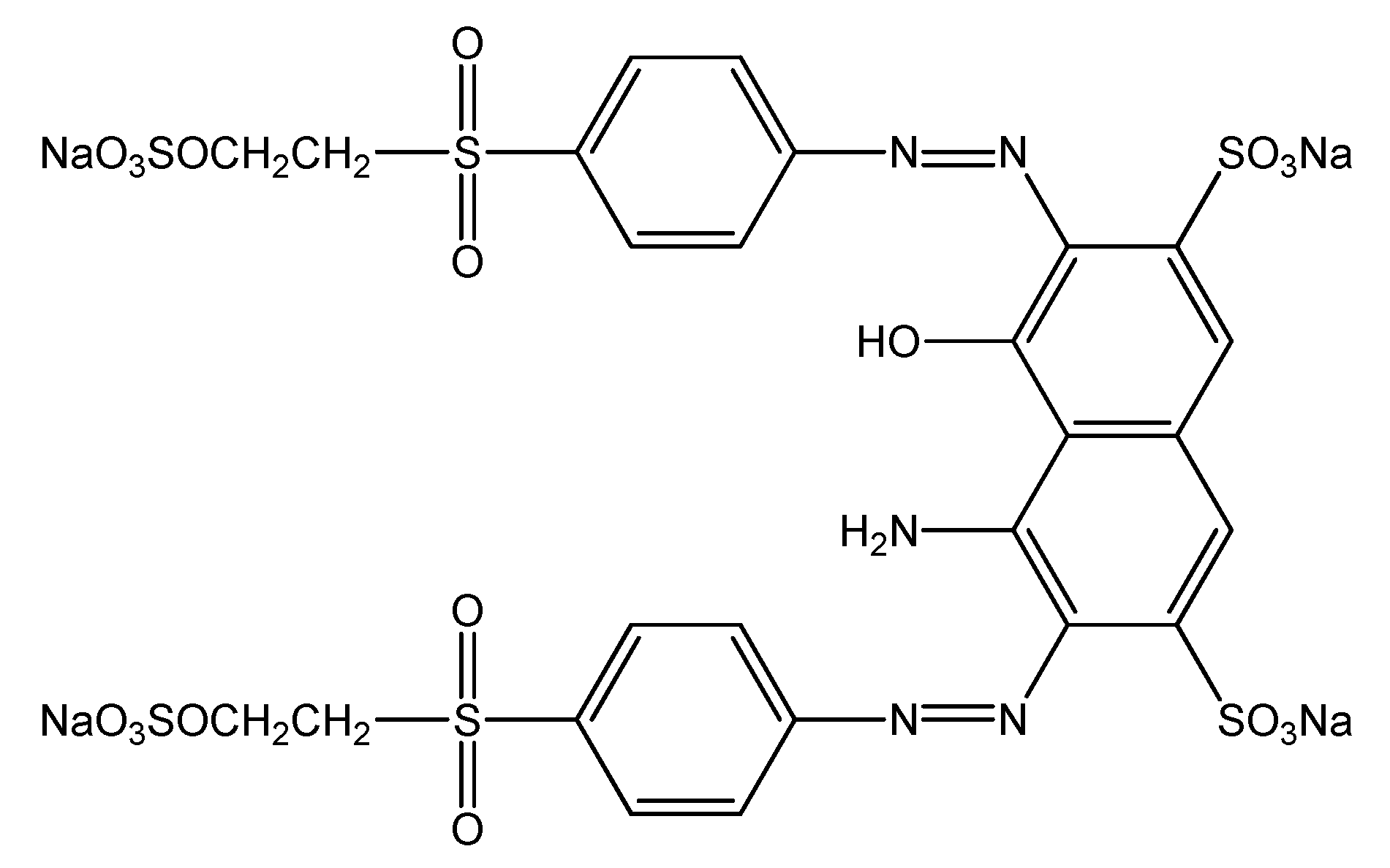
1. Introduction
2. Materials and Methods
3. Results and Discussion
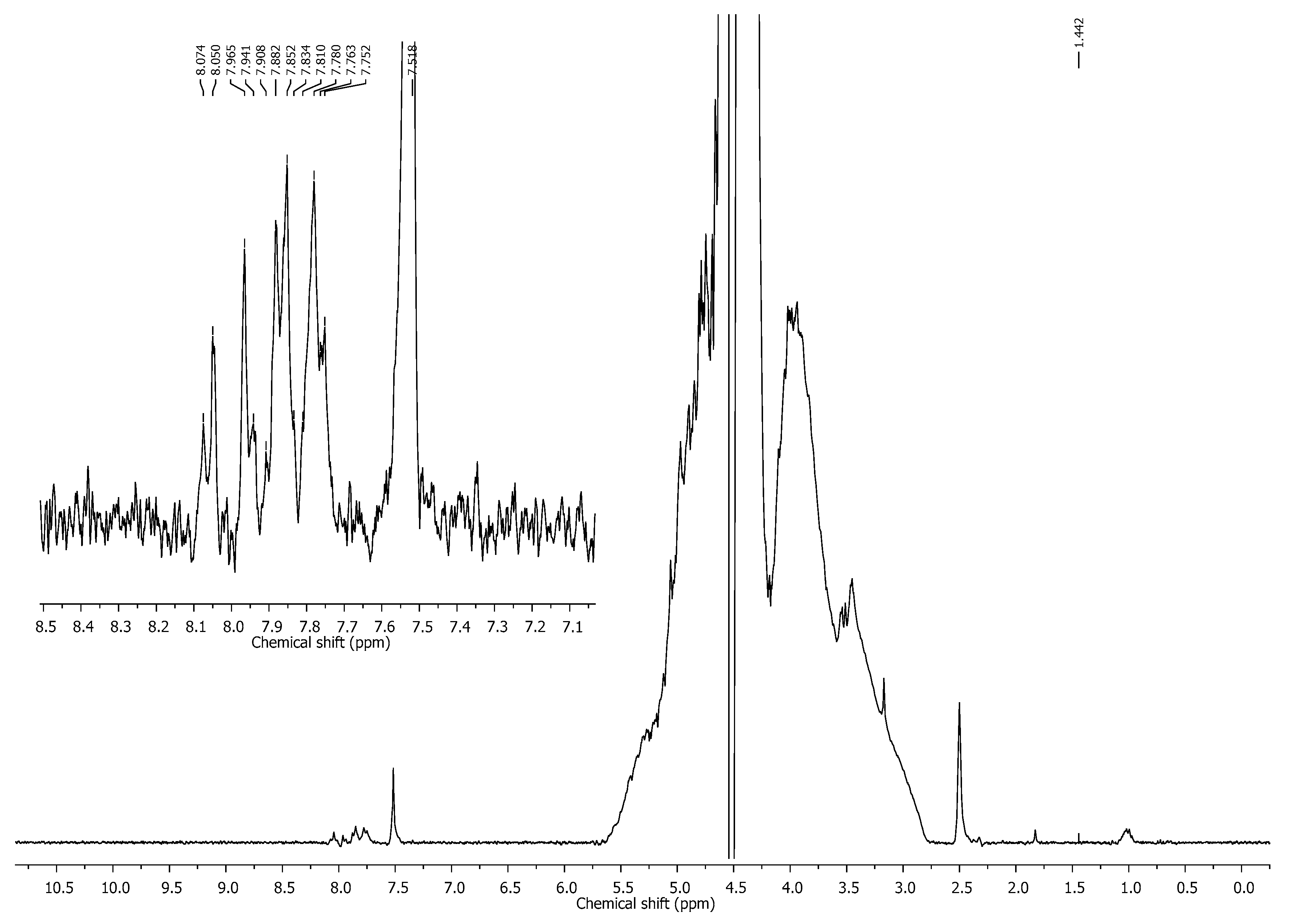
3.1. UV Analysis
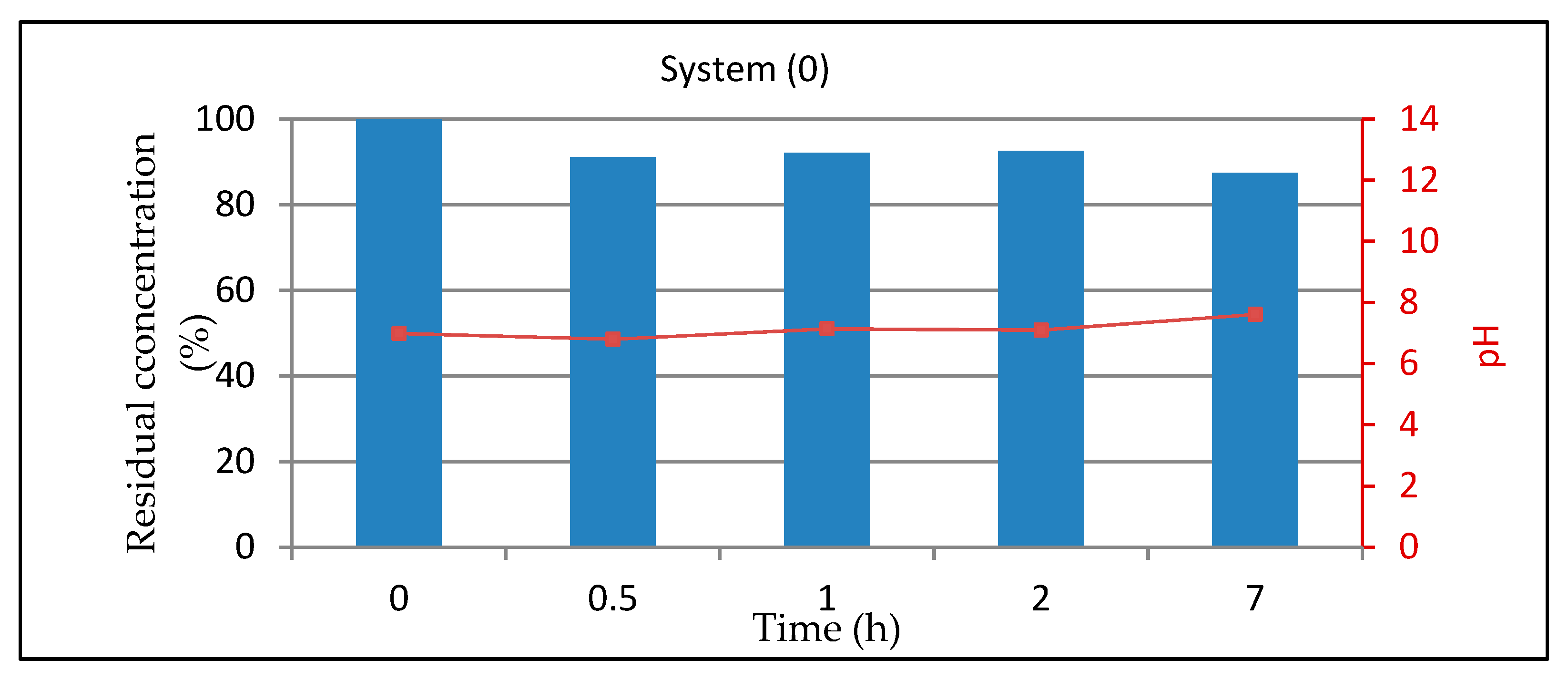
3.1.1. System 0 (10 mL of Dye Solution)
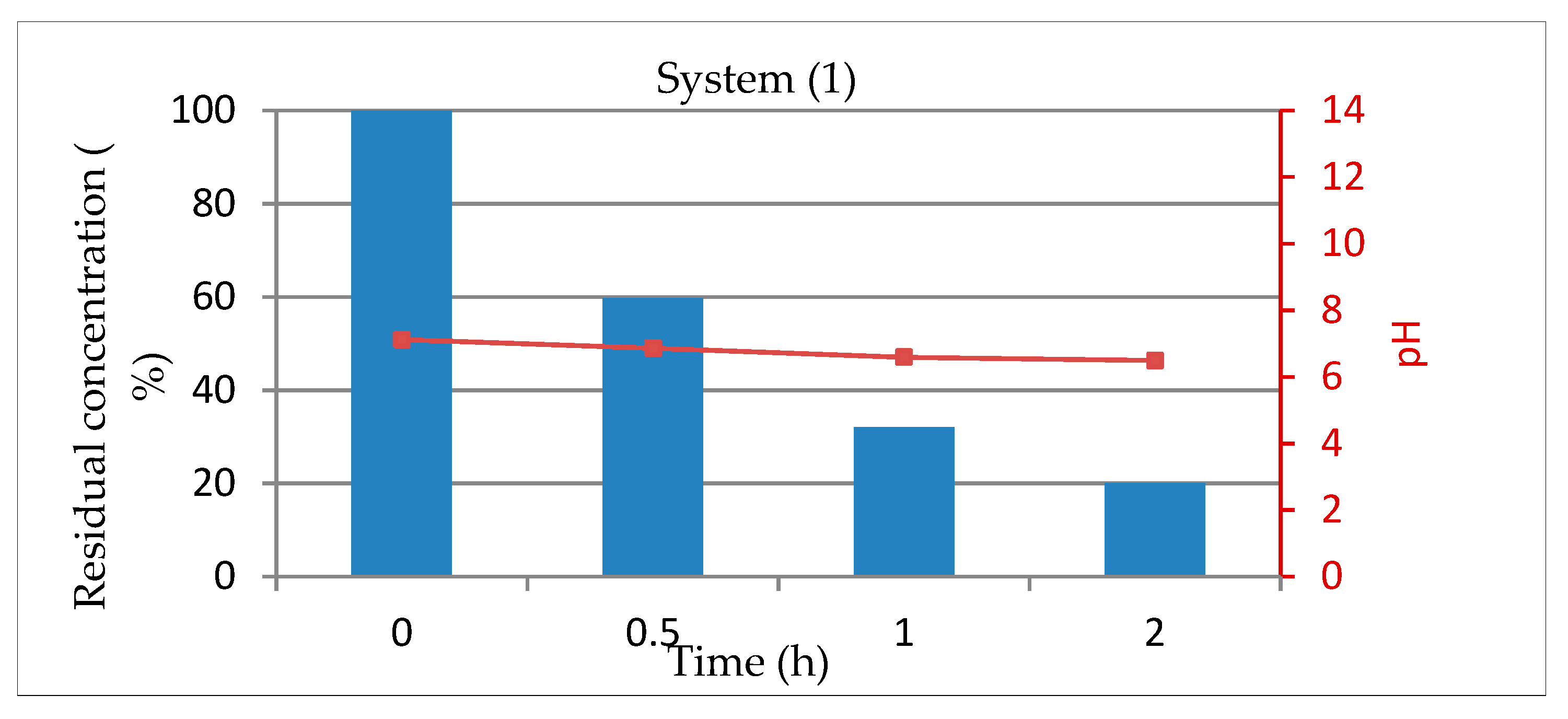
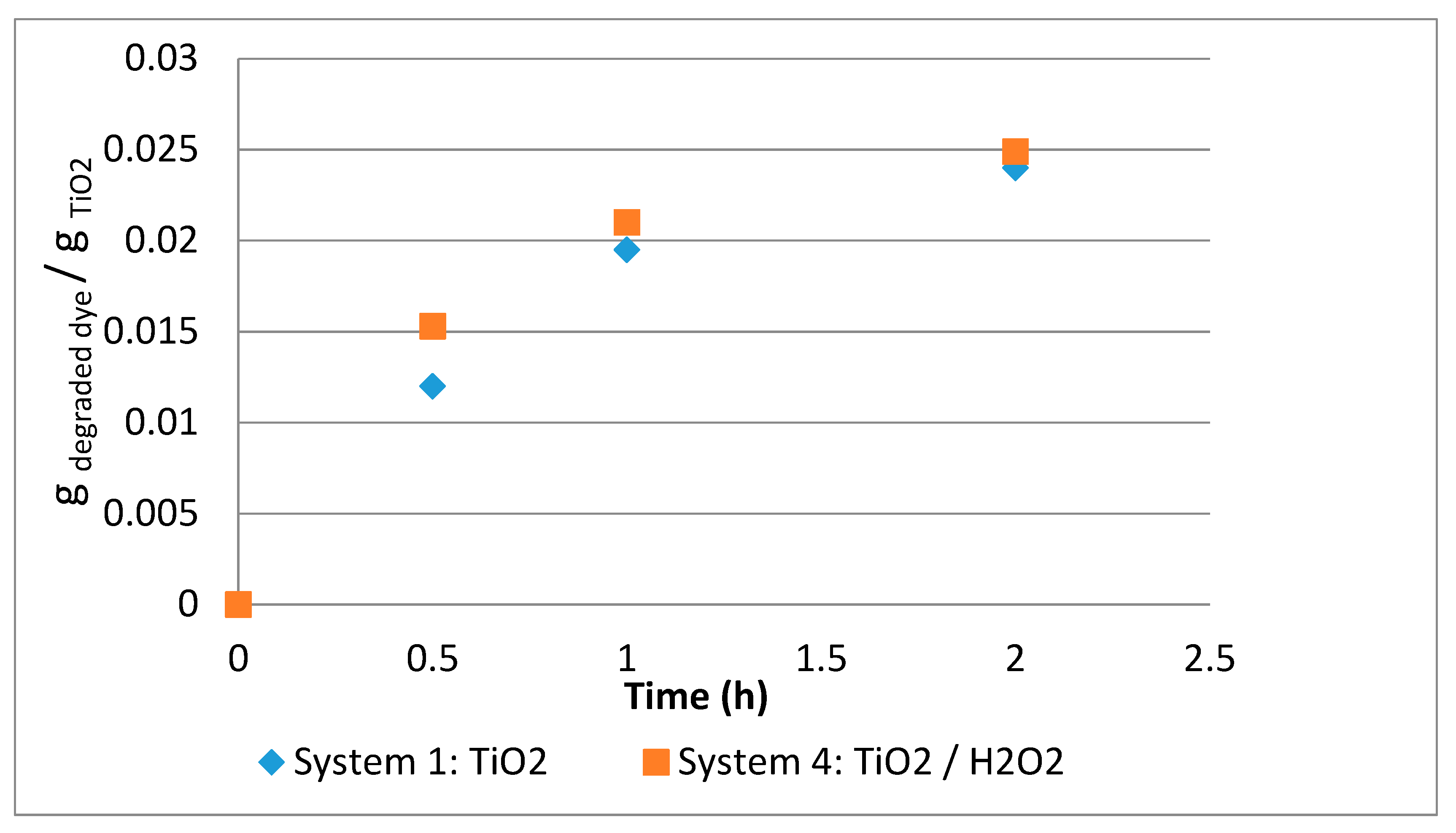
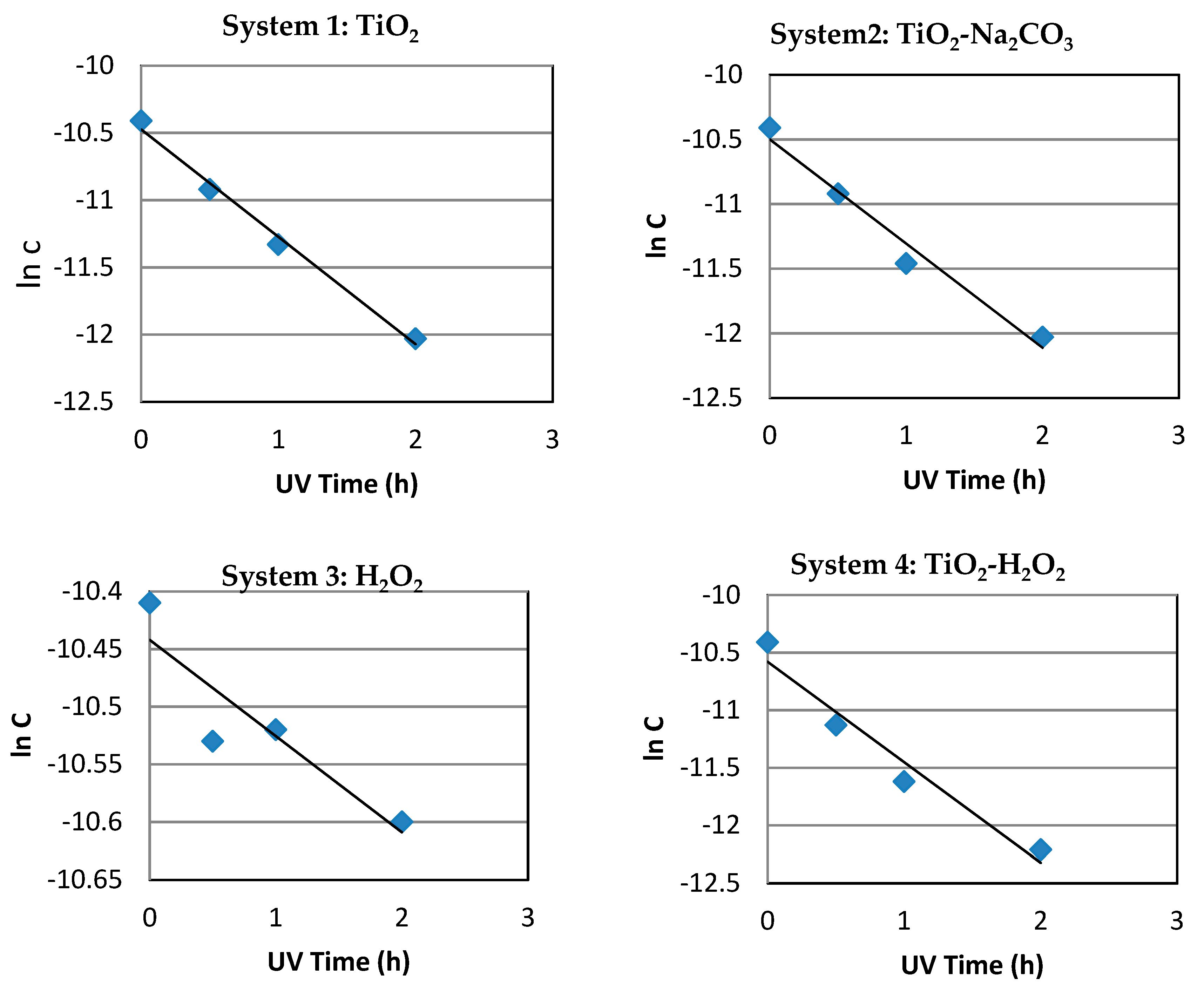
3.1.2. System 1 (10 mL of Dye Solution/0.01 g TiO2)
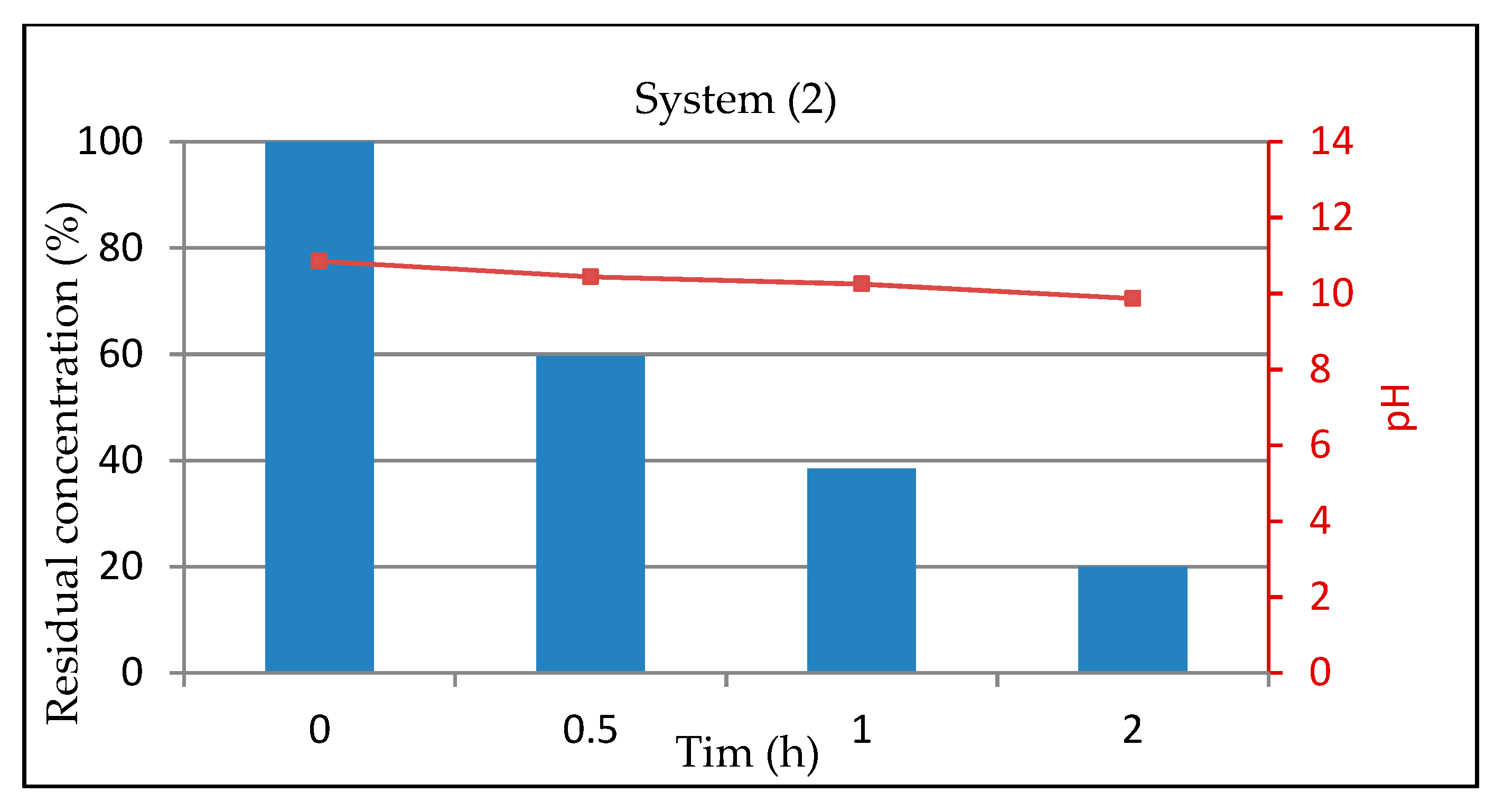
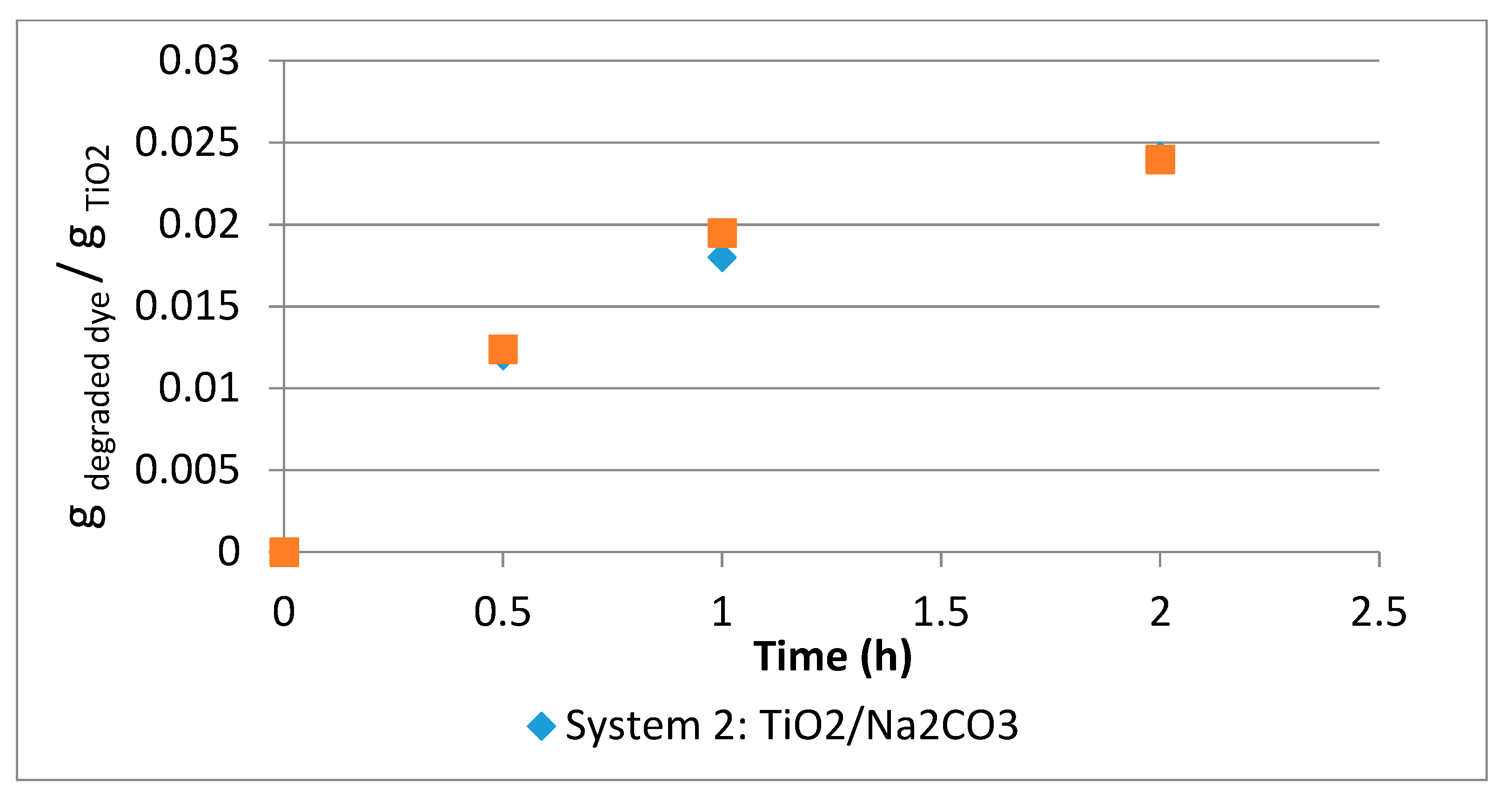
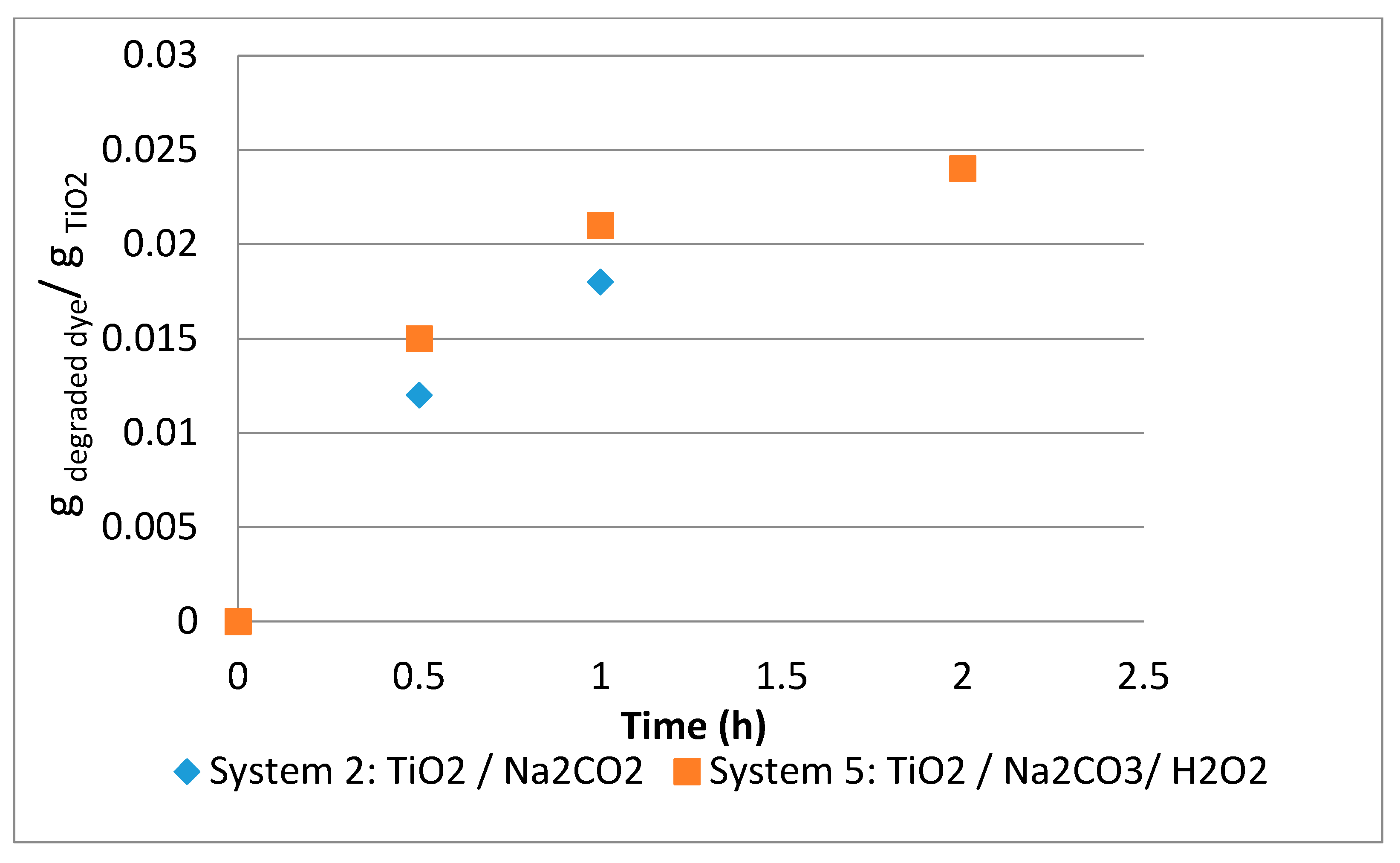
3.1.3. System 2 (10 mL Dye Solution + (0.01 g TiO2 + 0.01 g Na2CO3)
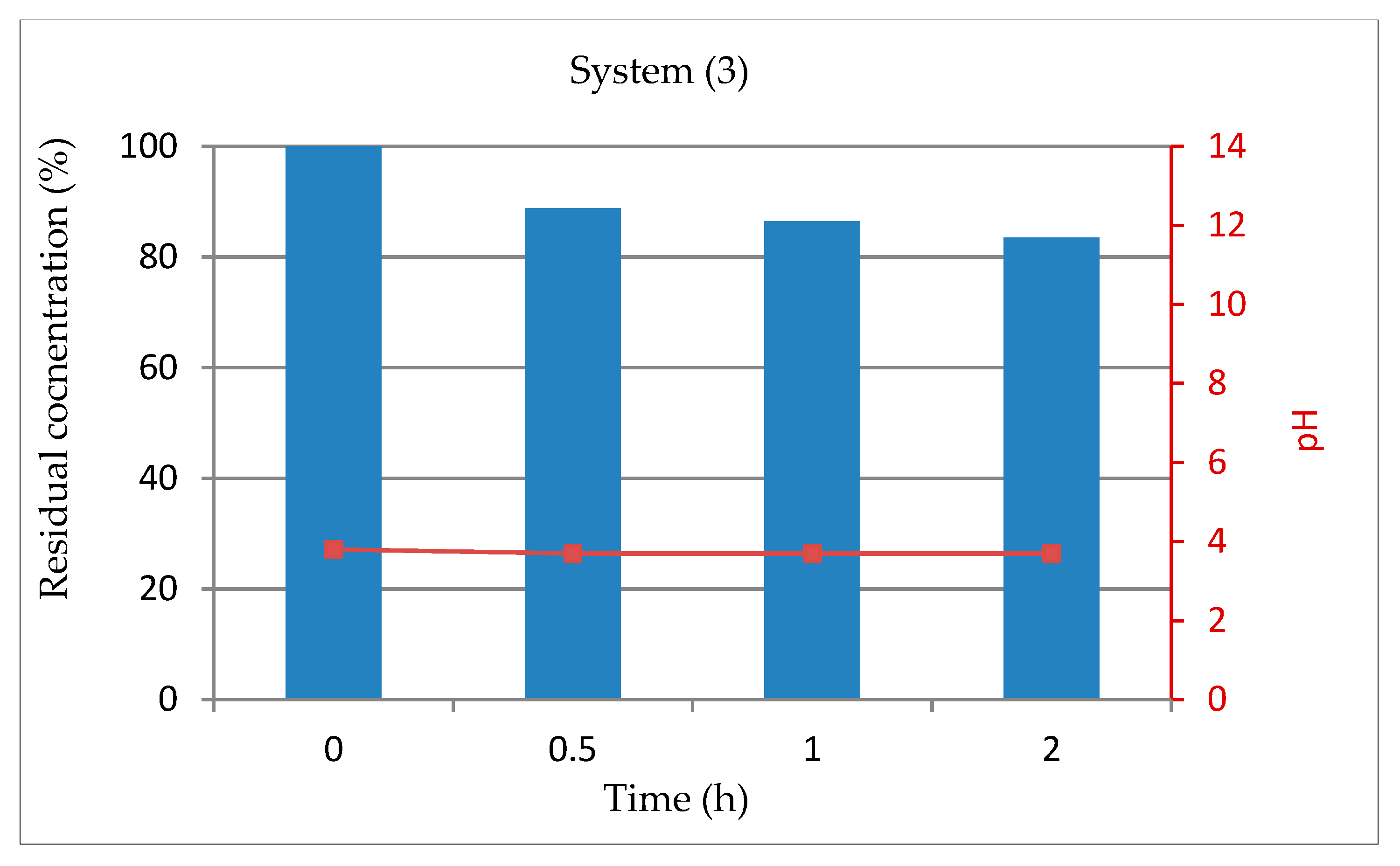
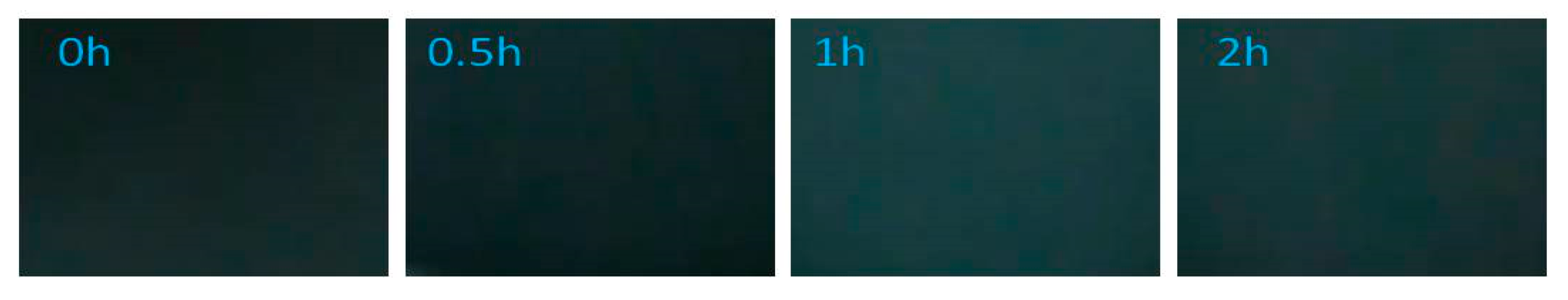
3.1.4. System 3 (10 mL Dye Solution + 0.5mL H2O2)
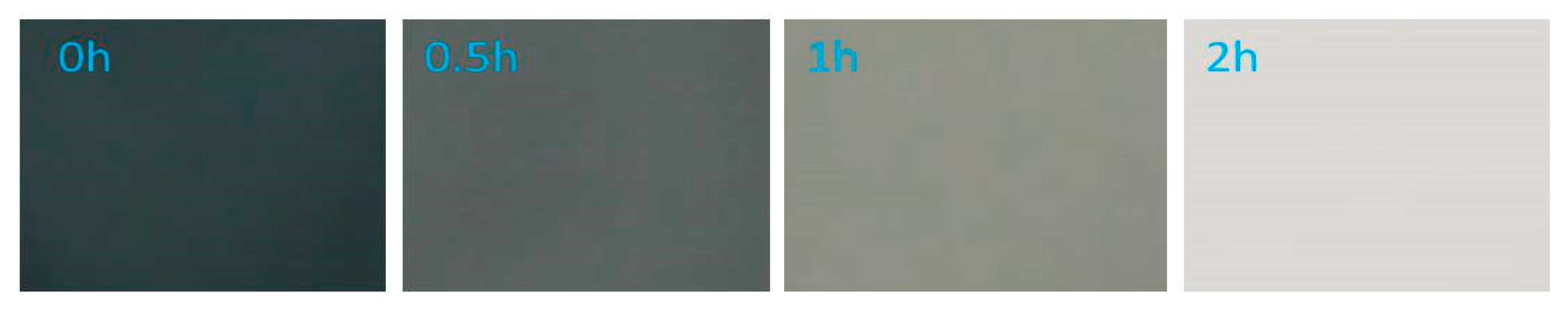
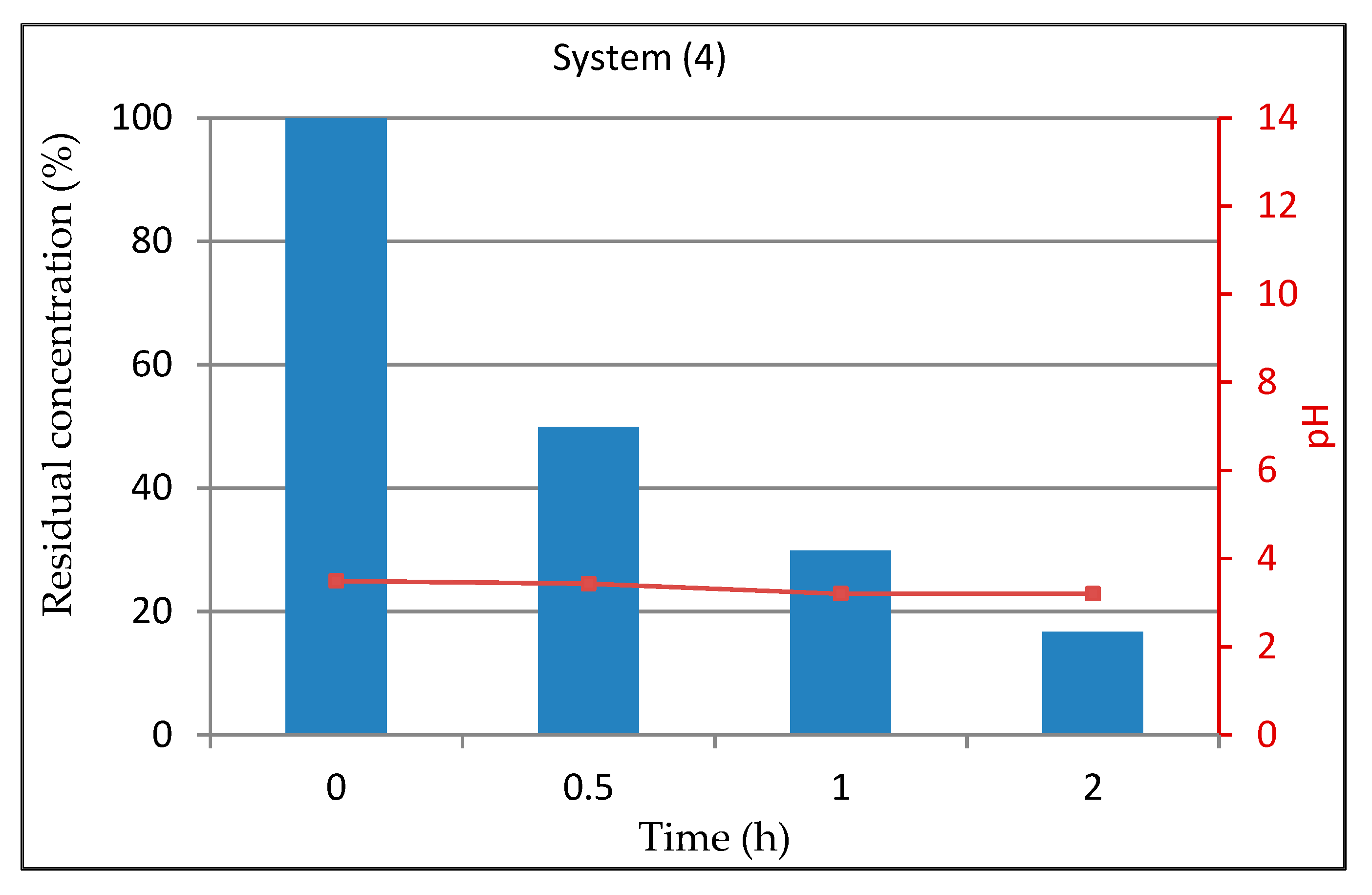
3.1.5. System 4 (10 mL Dye Solution + (0.01 g TiO2 + 0.5mL H2O2)
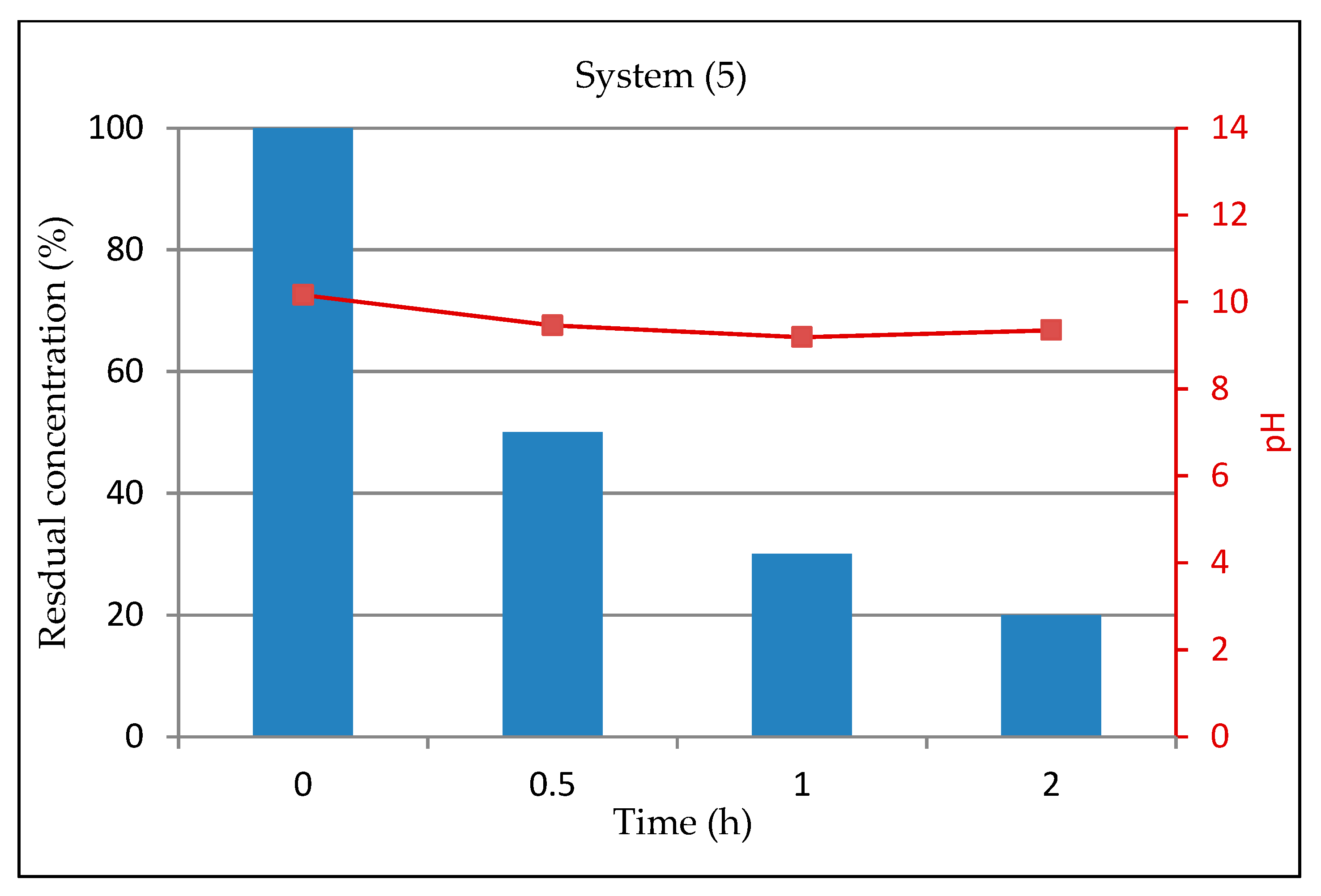
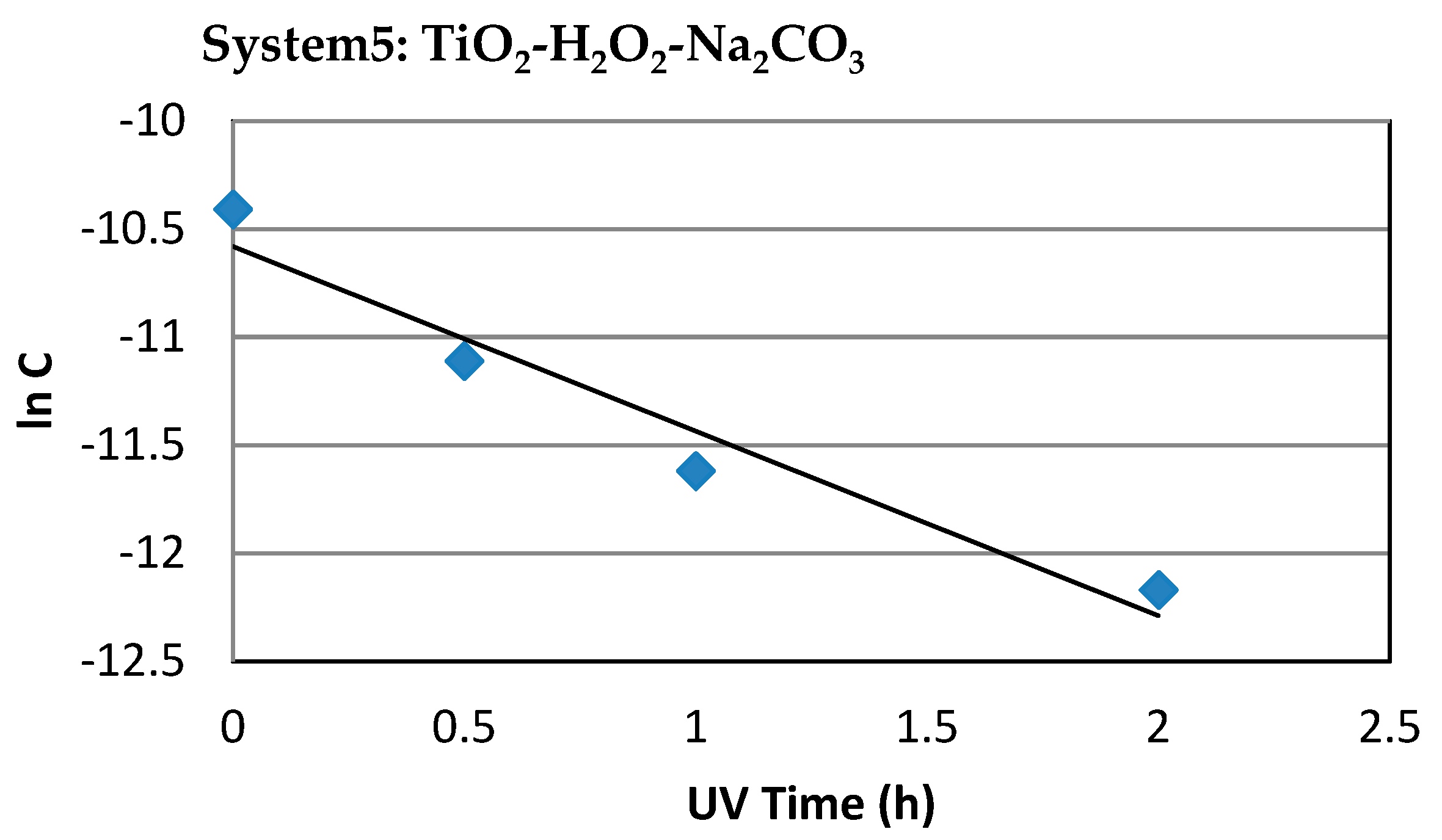
3.1.6. System 5 (10 mL Dye Solution + (0.01 g TiO2 + 0.5mL H2O2 + 0.01 g Na2CO3)
3.2. Comparison between Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Gallo, V. Oxygenated polycyclic aromatic hydrocarbons in size-segregated urban aerosol. J. Aerosol. Sci. 2015, 87, 126–134. [Google Scholar] [CrossRef]
- Zhuo, S.; Du, W.; Shen, G.; Li, B.; Liu, J.; Cheng, H.; Xing, B.; Tao, S. Estimating relative contributions of primary and secondary sources of ambient nitrated and oxygenated polycyclic aromatichydrocarbons. Atmos. Environ. 2017, 159, 126–134. [Google Scholar] [CrossRef]
- Filice, M.; De Luca, P.; Guido, G.P. Particular matter pollution in university area: Traffic flow analysis. Environ. Eng. Manag. J. 2009, 8, 1407–1412. [Google Scholar] [CrossRef]
- Livesley, S.J.; McPherson, E.G.; Calfapietra, C. The Urban Forest and Ecosystem Services: Impacts on Urban Water, Heat, and Pollution Cycles at the Tree, Street, and City Scale. J. Environ. Qual. 2016, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Squillace, P.J.; Scott, J.C.; Moran, M.J.; Nolan, B.T.; Kolpin, D.W. VOCs, Pesticides, Nitrate, and Their Mixtures in Groundwater Used for Drinking Water in the United States. Environ. Sci. Technol. 2002, 36, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Venkatarama Reddy, B.V. Sustainable materials for low carbon buildings. Int. J. Low Carbon Technol. 2009, 4, 175–181. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
- De Luca, P.; Roberto, B.; Vuono, D.; Siciliano, C.; Nagy, J.B. Preparation and optimization of natural glues based on larice pine resin. Iop Conf. Ser. Mater. Sci. Eng. 2018, 374, 012071. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- De Luca, P.; Carbone, I.; Nagy, J.B. Green building materials: A review of state of the art studies of innovative materials. J. Green Build. 2017, 12, 141–161. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Jiang, Y.; Chen, K.; Yan, X.; Zhang, D.; Li, R.; Tang, H. Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria. Appl. Catal. B Environ. 2015, 166–167, 231–240. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic Cementitious Materials: Influence of the Microstructure of Cement Paste on Photocatalytic Pollution Degradation. Environ. Sci. Technol. 2009, 43, 8948–8952. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Serpione, N.; Pellizzatti, E. Photocatalysis Fundamental and Applications; Sons, J.W., Ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- De Luca, P.; De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; Nagy, J.B. Preparation and characterization of plasters with photodegradative action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef]
- Chiing-Chang, C.; Fu-Der, M.; Kung-Tung, C.; Chia-Wei, W.; Chung-Shin, L. Photocatalyzed N-de-methylation and degradation of crystal violet in titania dispersions under UV irradiation. Dye. Pigm. 2007, 75, 434–442. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Macounová, K.; Urban, J.; Krýsová, H.; Krýsa, J.; Jirkovský, J.; Ludvík, J. Photodegradation of metamitron(4-amino-6-phenyl-3-methyl-1,2,4-triazin-5(4H)-one) on TiO2. J. Photochem. Photobiol. A Chem. 2001, 140, 93–98. [Google Scholar]
- D’Auria, M.; Emanuele, L.; Racioppi, R.; Velluzzi, V. Photochemical degradation of crude oil: Comparison between direct irradiation, photocatalysis, and photocatalysis on zeolite. J. Hazard. Mater. 2009, 164, 32–38. [Google Scholar] [CrossRef]
- Manouchehr, N.; Khodayar, G.; Kazem, M. Photocatalytic degradation of azo dye Acid Red 114 in water with TiO2 supported on clinoptilolite as a catalyst. Desalination 2008, 219, 293–300. [Google Scholar]
- Uma, S.; Rodrigues, S.; Martyanov, I.N.; Klabunde, K.J. Exploration of photocatalytic activities of titanosilicate ETS-10 and transition metal incorporated ETS-10. Micropor. Mesopor. Mat. 2004, 67, 181–187. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Nagy, J.B. Activated ceramic materials with deposition of photocatalytic titano-silicate micro-crystal. Sustain. Chem. 2011, 154, 155–165. [Google Scholar]
- Nash, M.; Lobo, R.F.; Doren, D.J. Photocatalytic oxidation of ethylene by ammonium exchanged ETS-10 and AM-6. Appl. Catal. B-Environ. 2009, 88, 232–239. [Google Scholar] [CrossRef]
- Vuono, D.; Guzzo, M.; De Luca, P.; Nagy, J.B. Physico-chemical characterization of zirconium-based self-bonded ETS-4 pellets. J. Therm. Anal. Calorim. 2014, 116, 169–182. [Google Scholar] [CrossRef]
- Guoqing, G.; Tetuya, K.; Katsuki, K.; Kunio, K.; Eiichi, A.; Akira, Y. Photocatalytic reactivity of noble metal-loaded ETS-4 zeolites. Inorg. Chem. Commun. 2004, 7, 618–620. [Google Scholar]
- Turta, N.A.; De Luca, P.; Bilba, N.; Nagy, J.B. Synthesis of titanosilicate ETS-10 in presence of cetyltrimethylammonium bromide. Microporous Mesoporous Mater. 2008, 112, 425–431. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; Nagy, J.B. Synthesis of self-bonded pellets of ETS-4 phase by new methodology of preparation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012003. [Google Scholar] [CrossRef]
- De Luca, P.; Vuono, D.; Filice, M. Self-bonded ETS-10 pellets containing iron. Environ. Eng. Manag. J. 2009, 8, 1009–1015. [Google Scholar] [CrossRef]
- De Luca, P.; Poulsen, T.G.; Salituro, A.; Tedeschi, A.; Vuono, D.; Kònya, Z.; Madaràsz, D.; Nagy, J.B. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 1257–1267. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; Nagy, J.B. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 400. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; Nagy, J.B.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef] [PubMed]
- Policicchio, A.; Vuono, D.; Rugiero, T.; De Luca, P.; Nagy, J.B. Study of MWCNTs adsorption perfomances in gas processes. J. CO2 Util 2015, 10, 30–39. [Google Scholar] [CrossRef]
- De Luca, P.; Nappo, G.; Siciliano, C.; Nagy, J.B. The role of carbon nanotubes and cobalt in the synthesis of pellets of titanium silicates. J. Porous Mater. 2018, 25, 283–296. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How farhave we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. Stem imaging to characterize nanoparticle emissions and help to design nanosafer paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Bressot, C.; Aubry, A.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Assessment of functional nanomaterials in medical applications: Can time mend public and occupational health risks related to the products’ fate? J. Toxicol. Environ. Healthpart A 2018, 81, 957–973. [Google Scholar] [CrossRef]
- Bressot, C.; Manier, N.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Environmental release of engineered nanomaterials from commercial tiles under standardized abrasion conditions. J. Hazard. Mater. 2017, 322, 276–283. [Google Scholar] [CrossRef]
- Bressot, C.; Shandilya, N.; Jayabalan, T.; Fayet, G.; Voetz, M.; Meunier, L.; Le Bihan, O.; Aguerre-Chariol, O.; Morgeneyer, M. Exposure assessment of nanomaterials at production sites by a short time sampling (sts) approach strategy and first results of measurement campaigns. Process Saf. Environ. Prot. 2018, 116, 324–332. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in europe and the world. J. Nanopart. Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2013, 1, 65–70. [Google Scholar] [CrossRef]
- Keller, A.A.; Vosti, W.; Wang, H.; Lazareva, A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanopart. Res. 2014, 16, 2489. [Google Scholar] [CrossRef]
- Göhler, D.; Stintz, M. Granulometric characterization of airborne particulate release during spray application of nanoparticle-doped coatings. J. Nanopart. Res. 2014, 16, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgeneyer, M.; Ramirez, A.; Smith, S.M.; Tweedie, R.; Heng, J.; Maass, S.; Bressot, C. Particle technology as a uniform discipline? Towards a holistic approach to particles, their creation, characterisation, handling and processing! Chem. Eng. Res. Des. 2019, 146, 162–165. [Google Scholar] [CrossRef]
- Hildenbrand, S.; Schmahl, F.W.; Wodarz, R.; Dartsch, P.C. Azo Dyes and Carcinogenic Aromatic Amines in Cell Cultures. Int. Arch. Occup. Environ. Health. 1999, 72, 11. [Google Scholar] [CrossRef]
- Neumann, H.G. Aromatic Amines in Experimental Cancer Research: Tissue-Specific Effects, an Old Problem and New Solutions. J. Crit. Rev. Toxicol. 2007, 37, 211–236. [Google Scholar] [CrossRef]
- Eren, Z. Ultrasound as a basic and auxiliary process for dye remediation: A review. J. Environ. Manag. 2012, 104, 127–141. [Google Scholar] [CrossRef]
- Benkli, Y.E.; Can, M.F.; Turan, M.; Çelik, M.S. Modification of organo-zeolite surface for the removal of reactive azo dyes in fixed-bed reactors. Water Res. 2005, 39, 487–493. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.U. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies. Chem. Eng. J. 2012, 200–202, 59–67. [Google Scholar] [CrossRef]
- Armağan, B. Factors affecting the performances of sepiolite and zeolite for the treatment of textile wastewater. J. Environ. Sci. Health A 2003, 38, 883–896. [Google Scholar] [CrossRef]
- Armağan, B.; Ozdemir, O.; Turan, M.; Celik, M.S. The removal of reactive azo dyes by natural and modified zeolites. J. Chem. Technol. Biotechnol. 2003, 78, 725–732. [Google Scholar] [CrossRef]
- Mirzaei, N.; Hadi, M.; Gholami, M.; Fard, R.F.; Aminabad, M.S. Sorption of acid dye by surfactant modificated natural zeolites. J. Taiwan Inst. Chem. E. 2016, 59, 186–194. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Drioli, E.; Candamano, S.; Poerio, T. Crystallization and assembling of FAU nanozeolites on porous ceramic supports for zeolite membrane synthesis. Microporous Mesoporous Mater. 2016, 228, 141–146. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Solangi, I.B.; Sherazi, S.T.H.; Memon, S. Synthesis and application of p-tert-butylcalix[8]arene immobilized material for the removal of azo dyes. J. Hazard. Mater. 2011, 186, 651–658. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Solangi, I.B.; Sherazi, S.T.H.; Memon, S. A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 2011, 268, 83–89. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Bhatti, A.A.; Solangi, I.B.; Sherazi, S.T.H.; Memon, S. Adsorption of direct black-38 azo dye on p-tert-butylcalix[6]arene immobilized material. Arab. J. Chem. 2014, 7, 125–131. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Solangi, I.B.; Sherazi, S.T.H.; Memon, S. Synthesis and application of calix[4]arene based resin for the removal of azo dyes. J. Hazard. Mater. 2009, 172, 234–239. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Xu, X.; Xing, L.; Han, S.; Duan, P.; Song, W.; Jia, R. Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour. Technol. 2016, 210, 123–130. [Google Scholar] [CrossRef]
- Jin, L.; Sun, Q.; Xu, Q.; Xu, Y. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O. Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes sunset yellow and Congo red. J. Clean. Prod. 2018, 179, 235–245. [Google Scholar] [CrossRef]
- You, S.J.; Damodar, R.A.; Hou, S.C. Degradation of Reactive Black 5 dye using anaerobic/aerobic membrane bioreactor (MBR) and photochemical membrane reactor. J. Hazard. Mater. 2010, 177, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Du, W.N.; Chen, S.T. Photo and chemocatalytic oxidation of dyes in water. J. Environ. Manag. 2018, 206, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Photo-electrochemical treatment of reactive dyes in wastewater and reuse of the effluent: Method optimization. Materials 2014, 7, 7349–7365. [Google Scholar] [CrossRef] [PubMed]
- Sengil, I.A.; Ozacar, M. The decolorization of C.I. Reactive Black 5 in aqueous solution by electrocoagulation using sacrificial iron electrodes. J. Hazard. Mater. 2009, 161, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Won, S.W.; Yun, Y.S. Treatment of complex Remazol dye effluent using sawdust and coal based activated carbons. J. Hazard. Mater. 2009, 167, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube r546464-k-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, Z.; Wu, Y.; Yu, J.; Sun, L.; Lin, C. Acid Orange II degradation through a heterogeneous Fenton-like reaction using Fe–TiO2 nanotube arrays as a photocatalyst. J. Mater. Chem. A 2015, 3, 8537–8544. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit Assess. 2011, 183, 151–195. [Google Scholar] [CrossRef]
- Lv, J.L.; Zhai, S.R.; Fan, Y.; Lei, Z.M.; An, Q.D. Preparation of β-CD and Fe3O4 integrated multifunctional bioadsorbent for highly efficient dye removal from water. J. Taiwan Inst. Chem. Eng. 2016, 62, 209–218. [Google Scholar] [CrossRef]
- Du, K.; Ghorai, U.K.; Chattopadhyay, K.K.; Banerjee, D. Removal of textile dyes by carbon nanotubes: A comparison between adsorption and UV assisted photocatalysis. Phys. E Low Dimens. Syst. Nanostruct. 2018, 99, 6–15. [Google Scholar]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F. Photocatalytic degradation of a model textile dye using carbon-doped titanium dioxide and visible light. J. Water Process Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Hickman, R.; Walker, E.; Chowdhury, S. TiO2-PDMS composite sponge for adsorption and solar mediated photodegradation of dye pollutants. J. Water Process Eng. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Copete-Pertuz, L.S.; Pérez-Grisales, M.S.; Castrillón-Tobón, M.; Londoño, G.A.C.; García, G.T.; Martínez, A.L.M. Decolorization of Reactive Black 5 Dye by Heterogeneous Photocatalysis with TiO2/UV. Rev. Colomb. Quim. 2018, 47, 36–44. [Google Scholar]
- Qiu, M.; Shou, J.; Ren, P.; Jiang, K. A comparative study of the azo dye reactive black 5 degradation by UV/TiO2 and photo-fenton processes. J. Chem. Pharm. Res. 2014, 6, 2046–2051. [Google Scholar]
- Salem, I.A.; El-Ghamry, H.A.; El-Ghobashy, M.A. Catalytic decolorization of Acid blue 29 dye by H2O2 and a heterogeneous catalyst. Beni-Suef. Univ. J. Basic Appl. Sci. 2014, 3, 186–192. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Xiao, H.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Accelerated TiO2 photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J. 2012, 193–194, 290–295. [Google Scholar] [CrossRef]
- Khan, S.R.; Khalid, M.U.; Jamil, S.; Li, S.; Mujahid, A.; Janjua, M.R.S.A. Photocatalytic degradation of reactive black 5 on the surface of tin oxide microrods. J. Water Health 2018, 16, 773–781. [Google Scholar] [CrossRef]
- Yu, C.H.; Wu, C.H.; Ho, T.H.; Andy Hong, P.K. Decolorization of C.I. Reactive Black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: A comparative study. Chem. Eng. J. 2010, 158, 578–583. [Google Scholar] [CrossRef]
- Egerton, T.A.; Purnama, H. Does hydrogen peroxide really accelerate TiO2 UV-C photocatalyzed decolouration of azo-dyes such as Reactive Orange 16? Dye. Pigm. 2014, 101, 280–285. [Google Scholar] [CrossRef]
- Neamtu, M.; Siminiceanu, I.; Yediler, A.; Kettrup, A. Kinetics of decolorization and mineralization of reactive azo dyes in aqueous solution by the UV/H2O2 oxidation. Dye. Pigm. 2002, 53, 93–99. [Google Scholar] [CrossRef]
- Peralta-Zamora, P.; Gouvêa, C.A.K.; Wypych, F.; Durán, N. Effect of Na2CO3 on the photocatalytic degradation of remazol brilliant blue R. Toxicol. Environ. Chem. 2001, 80, 83–93. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A. Effect of common reactive dye auxiliaries on the ozonation of dyehouse effluents containing vinylsulphone and aminochlorotriazine dyes. Desalination 2000, 130, 61–71. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, R.; Guo, Y.; Xu., L.; Liu, J. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: Kinetic analysis. J. Hazard. Mater. 2011, 190, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P.; Bellardita, M.; Palmisano, L. Photocatalytic Processes in Membrane Reactors. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3101–3138. [Google Scholar]
- Antoniou, A.I.; Pepe, D.A.; Aiello, D.; Siciliano, C.; Athanassopoulos, C.M. Chemoselective protection of glutathione in the preparation of bioconjugates: The case of trypanothione disulfide. J. Org. Chem. 2016, 81, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Furia, E.; Siciliano, C.; Bongiorno, D.; Napoli, A. Study of the coordination of ortho-tyrosine and trans-4-hydroxyproline with aluminum(III) and iron(III). J. Mol. Liq. 2018, 269, 387–397. [Google Scholar] [CrossRef]
- Temperini, A.; Piazzolla, F.; Minuti, L.; Curini, M.; Siciliano, C. General, mild, and metal-free synthesis of phenyl selenoesters from anhydrides and their use in peptide synthesis. J. Org. Chem. 2017, 82, 4588–4603. [Google Scholar] [CrossRef] [PubMed]
- Minuti, L.; Ballerini, E.; Barattucci, A.; Bonaccorsi, P.M.; Di Gioia, M.L.; Leggio, A.; Siciliano, C.; Temperini, A. A unified strategy for the synthesis of three conicol marine natural products. Tetrahedron 2015, 71, 3253–3262. [Google Scholar] [CrossRef]
- Aiello, D.; Materazzi, S.; Risoluti, R.; Thangavel, H.; Di Donna, L.; Mazzotti, F.; Casadonte, F.; Siciliano, C.; Sindona, G.; Napoli, A. A major allergen in rainbow trout (Oncorhynchus mykiss): Complete sequences of parvalbumin by MALDI tandem mass spectrometry. Mol. Biosyst. 2015, 11, 2373–2382. [Google Scholar] [CrossRef]
- Siciliano, C.; De Marco, R.; Guidi, L.E.; Spinella, M.; Liguori, A. A one-pot procedure for the preparation of N-9-fluorenylmethyloxycarbonyl- α-amino diazoketones from α-amino acids. J. Org. Chem. 2012, 77, 10575–10582. [Google Scholar] [CrossRef]
- Di Donna, L.; Napoli, A.; Sindona, G.; Athanassopoulos, C. A comprehensive evaluation of the kinetic method applied in the determination of the proton affinity of the nucleic acid molecules. J. Am. Soc. Mass Spectrom. 2004, 15, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, F.; Di Donna, L.; Napoli, A.; Aiello, D.; Siciliano, C.; Athanassopoulos, C.M.; Sindona, G. N-hydroxysuccinimidyl p-methoxybenzoate as suitable derivative reagent for isotopic dilution assay of biogenic amines in food. J. Mass Spectrom. 2014, 49, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. Rsc Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. A review. Appl. Catal. B-Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Sousa Oliveira, A.; Pavesi, T.; Maia, C.G.; Vieira Ferreira, L.F.; Costa Moreira, J. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef] [PubMed]





| System | Composition |
|---|---|
| 0 | 10 mL dye solution |
| 1 | 10 mL dye solution + 0.01 g TiO2 |
| 2 | 10 mL dye solution + (0.01 g TiO2 + 0.01 g Na2CO3) |
| 3 | 10 mL dye solution + 0.5 mL H2O2 |
| 4 | 10 mL dye solution + (0.01 g TiO2 + 0.5 mL H2O2) |
| 5 | 10 mL dye solution + (0.01 g TiO2 + 0.5 mL H2O2 + 0.01 g Na2CO3) |
| Additives | K (h−1) | ln C | R2 | |
|---|---|---|---|---|
| System 1 | TiO2 | 0.80 | 10.5 | 0.992 |
| System 2 | TiO2–Na2CO3 | 0.81 | 10.5 | 0.973 |
| System 3 | H2O2 | 0.08 | 10.4 | 0.823 |
| System 4 | TiO2–H2O2 | 0.87 | 10.6 | 0.952 |
| System 5 | TiO2–H2O2–Na2CO3 | 0.85 | 10.6 | 0.948 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Foglia, P.; Siciliano, C.; Nagy, J.B.; Macario, A. Water Contaminated by Industrial Textile Dye: Study on Decolorization Process. Environments 2019, 6, 101. https://doi.org/10.3390/environments6090101
De Luca P, Foglia P, Siciliano C, Nagy JB, Macario A. Water Contaminated by Industrial Textile Dye: Study on Decolorization Process. Environments. 2019; 6(9):101. https://doi.org/10.3390/environments6090101
Chicago/Turabian StyleDe Luca, Pierantonio, Paola Foglia, Carlo Siciliano, Jànos B. Nagy, and Anastasia Macario. 2019. "Water Contaminated by Industrial Textile Dye: Study on Decolorization Process" Environments 6, no. 9: 101. https://doi.org/10.3390/environments6090101
APA StyleDe Luca, P., Foglia, P., Siciliano, C., Nagy, J. B., & Macario, A. (2019). Water Contaminated by Industrial Textile Dye: Study on Decolorization Process. Environments, 6(9), 101. https://doi.org/10.3390/environments6090101







