1. Introduction
Environmental pollution, including sources of water supply, represents a severe environmental problem that adversely affects human health. Soluble organic substances (SOS), in particular phenol, formaldehyde, oxalic acid, etc., are among the main hazardous pollutants of wastewaters Their presence in waters contributes to the behavior of various chemical chain reactions, leading to a decrease of oxygen in water sources, water pollution, and the death of living organisms. Among the most dangerous water pollutants are fat-soluble and water-soluble dyes (the sources of which are textile), waste from the paint and varnish industry, and production waste of finishing materials, cellulose and detergents. Many dyes are toxic, as they have carcinogenic and mutagenic properties. The dyes are introduced into natural environments via the wastewaters system, where they harm the life activity of ecosystems and adversely influence the processes of self-purification in bodies of water. Due to this risk, chromaticity is one of the key normative parameters of wastewaters. For dyes, a sufficiently low level of permissible concentration in water are from 10 to 0.0025 mg/L.
Various methods are used for removing soluble organic dyes from aqueous media. An effective methods is to facilitate the destruction of organic substances up to CO
2 and H
2O. Photooxidative destruction, through the use of ozone [
1,
2], or UV-irradiation (UVI) [
3], can be used to achieve this. At the same time, (in homogeneous catalysis) the Ruff-Fenton system (Fe
3+/Fe
2+ + H
2O
2 + UVI) is successfully applied. This course of action results in a continuous photo-reduction of salts Fe
3+ to Fe
2+, and the generation of •OH—radicals to occur [
4]. For this system to successfully function, sufficiently hard and expensive UV irradiation within 200–280 nm is necessary. The disadvantage of using this system is the required application of large quantities of iron ions in a dissolved form, and low pH values to eliminate their hydrolysis. The latter disadvantage can be eliminated when using a ferrioxalate system consisting of a soluble iron complex in subacid and neutral solutions [
5,
6]. In this case, it is possible to use softer UVI. Literature on use of the Fenton-like system, in which ions of polyvalent metals and metals with zero charge Me
0 (Al, Fe, etc.) act as a source of radicals, are known [
7]. In conditions of heterogeneous photocatalysis, high activity in the oxidation processes of SOS is manifested by catalysts based on oxides of titanium, copper, cobalt, precious metals, etc., as well as their compositions [
8,
9,
10,
11]. Composites, obtained by alloying TiO
2 with different contents of carbon-containing components, are widely applied in catalysis during photodegradation of organic pollutants under conditions of visible radiation [
12,
13]. At the same time, the decolorization degree of dyes reaches more than 90%. A combination of processes of homogeneous and heterogeneous oxidation during photocatalytic purification of effluents from organic pollutants is rarely used. In Reference [
14], a combined action of ferrioxalate/H
2O
2 andTiO
2 was studied. However, expensive and requiring regeneration catalysts are used in the majority of the mentioned variants of oxidative destruction of SOS.
In this regard, the problem of the integrated purification of wastewaters with all kinds of organic pollutants is relevant, and development of new cheap sorbents and technologies is of great scientific and practical importance. Cheap and available sorbents often include a hydrophobic polypropylene (PP) fiber, obtained from polypropylene production wastes and their derivatives, which can be used in the purification of industrial effluents, such as oil, petroleum products, etc. [
15,
16]. In addition, various methods of modifying the PP fiber are described in the literature, in order to bestow hydrophilicity properties to it, and to use it in the future to purify industrial effluents [
17,
18,
19,
20].
In our previous work [
21,
22,
23,
24,
25,
26], we showed that the composites, obtained by modifying natural sorbents (zeolites and peat) by metal ions (M
n+), exhibited high catalytic activity in conditions of UVI and the Ruff-Fenton system during degradation of organic pollutants in aqueous media.
In continuing our earlier work, we have chosen to devote this paper to investigating of the possibility of using composites, based on the PP fiber, in the process of photo-oxidative destruction of various organic pollutants using the Fenton-like system, and under visible radiation conditions.
2. Materials and Methods
The polypropylene fiber was modified using electrophysical, through the methods of ion implantation (II) [
27,
28] and super high frequency (SHF)-irradiation [
29]. Using the mentioned methods, it is possible to obtain composite materials with nanoparticles of various metals, fixed on the surface of a polymer fiber, which allows controlling purposefully the composites’ properties.
The description of the used ion implanter is given in [
27,
28]. The conditions for conducting ion implantation are presented in
Table 1.
In case of using the SHF-irradiation method, iron nanoparticles were introduced into the volume of fibers with subsequent treatment of the mixture by an electromagnetic irradiator with a 400 W power for 30 min [
29]. At the same time, iron particles were heated, and polypropylene was partially melted, and (after cooling-down) were mechanically fixed on the fiber surface.
After obtaining composites based on the PP fiber using II and SHF-irradiation methods, their characterization was carried out. This included the determination of porosity, specific surface size, elemental composition and distribution of the metal-modifier (Fe0) on the surface of polymer fibers. The mentioned characteristics are used to assess photocatalytic activity of the obtained composite materials during the oxidative degradation of organic pollutants in aqueous and aqueous-organic media, using the Fenton-like system (Fe0 → Fe3+ + H2O2 + UVI) and visible radiation.
The porosity and the specific surface of the composites were determined by low-temperature adsorption of nitrogen vapors, according to the BET method [
30] using an automated sorption plant “3Flex” produced by Micromeritics (Norcross, GA, USA).
Elemental analysis, and the nature of the metal distribution over the fiber surface, were established using an energy dispersive microanalyzer “Quantax 70” (Bruker, Germany), and a scanning electron microscope “HITACHITM-3000” (Tokyo, Hitachi, Japan).
It is important to note the formation of a surface, with a necessary power of the acid-base centers of Lewis and Bronsted, allows for the prediction of reactivity and sorption properties of the material. When studying acid-base properties of the surface, a pH-measurement method (kinetic variant) and an indicator method, as described in Reference [
31], were used. The kinetic variant allows assessing the change of the average acidity of the surface as a manifestation of the total effect of interaction of two sets of centers—acids and bases (of both Lewis and Bronsted)—with water. The indicator method gives more reliable information about the state of the composites’ surface, and allowed us to assess the distribution of active centers over a wide range of
pK.
The acid-base water-soluble dyes (rhodamine C, eosin, brilliant green) and fat-soluble azo dyes (blue, red, Sudan yellow) were studied as contaminating organic pollutants.
The photochemical reaction was carried out in static conditions, with constant stirring in a quartz reactor, where the composite sample and the standard test dye solution were placed. A mercury-quartz lamp of DRL type (240–1100 nm)—with a power of 250 W (λmax = 540 nm)—and a source with a visible luminous range—a LED lamp “DIORA 30” (OOO, MBR-groups, Sankt-Peterburg, Russia) with a power consumption of 30 W and a light flux of 2500 lm—were applied as a radiation source.
Oxidative destruction of dyes from aqueous and aqueous-organic media were carried out as follows: The sample of the iron-containing composite weighing 0.050 g was placed in a quartz cup with a capacity of 50–20.0 mL of the dye solution and 0.2 mL of 0.10 MH2O2 were added. pH = 2 was created in the solution by adding HCl, to avoid hydrolysis of iron ions (III). The cup with the mixture was placed on the magnetic stirrer for stirring, and was subjected to UVI or visible radiation. The irradiation time was 20 min. After that, the solution was separated from the catalyst by centrifugation, and the amount of the pollutant remaining in the solution was determined spectrophotometrically (using a spectrophotometer SF-56 device).
To assess the adsorption value of water-soluble and fat-soluble dyes on the surface of iron-containing composites, similar experiments were carried out. However, this time in glass cups without external influences, and without addition of hydrogen peroxide. The contact time of the composites with the dye solutions was 6 h.
Since fat-soluble dyes are insoluble in water, the solvent was selected for them. The following ratios of organic solvents to water were chosen: (1) dimethylformamide (DMFM): isoamyl alcohol: water = (3:2:2); (2) isoamyl alcohol: ethyl alcohol: water (3:2:2); and (3) ethyl alcohol: water = 1:10.
To reveal the role of the catalyst in the Fenton-like system, experiments were conducted using a similar procedure, but without participation of composites. Irradiation was carried out in quartz cups for 20 min.
These studies were not conducted under visible irradiation.
The initial concentration of dyes in all experiments was constant and amounted to 10 mg/L.
3. Results and Discussion
In the paper, the characterization of the composite materials was carried out. However, when determining the porosity and the specific surface of the composites and the raw PP fiber, it was established that, during degassing of the samples in vacuum for 4 h at 120 °C, the specific area of the raw PP fiber and the composites was less than 0.01 m2/g, and the pore size was less than 1 nm, respectively. It is difficult to assume how the absence of porosity and a small value of the specific surface will influence the photocatalytic activity of the composites during oxidative destruction of organic pollutants.
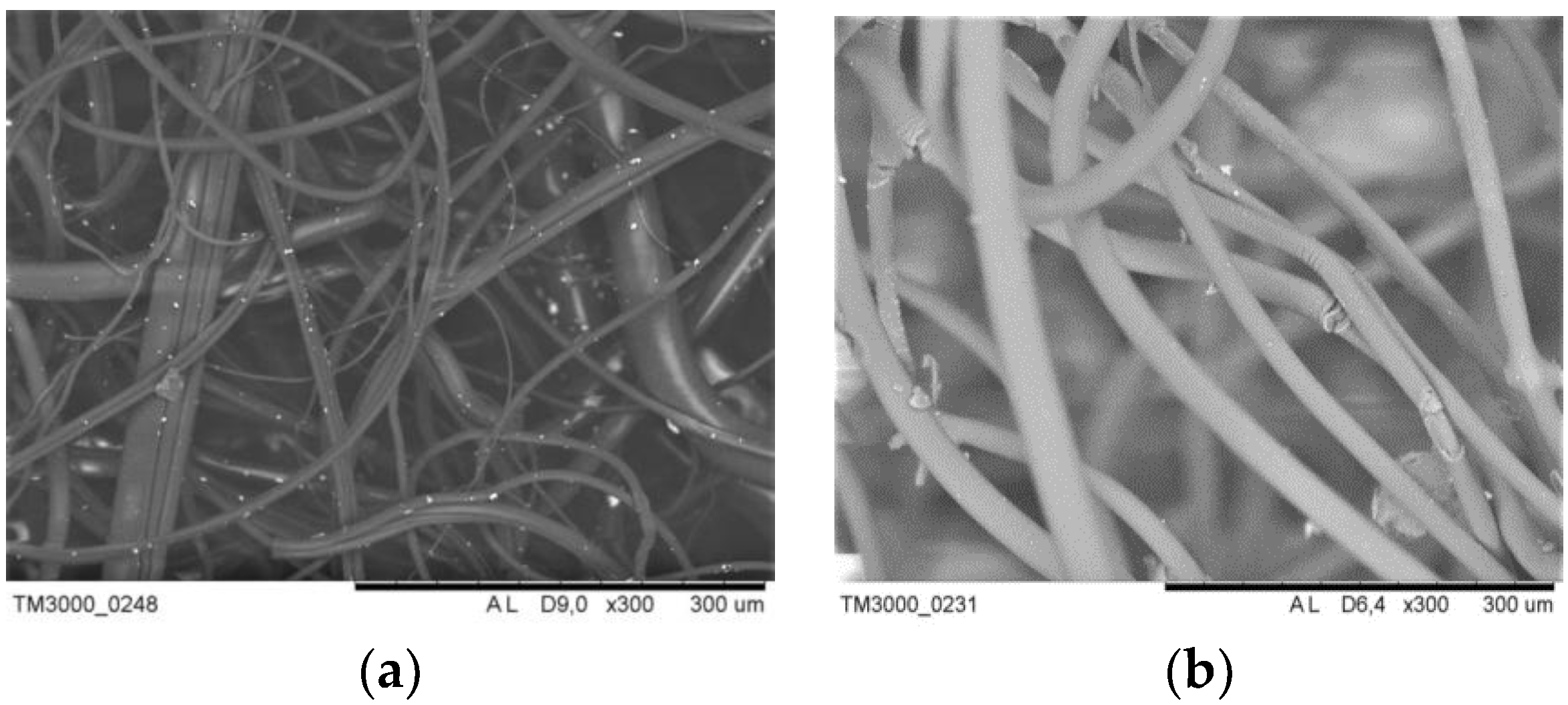
Below there are the experimental results on the distribution of a metal-modifier on the surface of the composites (
Figure 1).
Figure 1 shows that the distribution of the metal-modifier on the surface of the PP fiber is not uniform enough for all cases of modification. However, it is possible that a more uniform distribution over the surface is observed for iron nanoparticles in the composite obtained using SHF-irradiation.
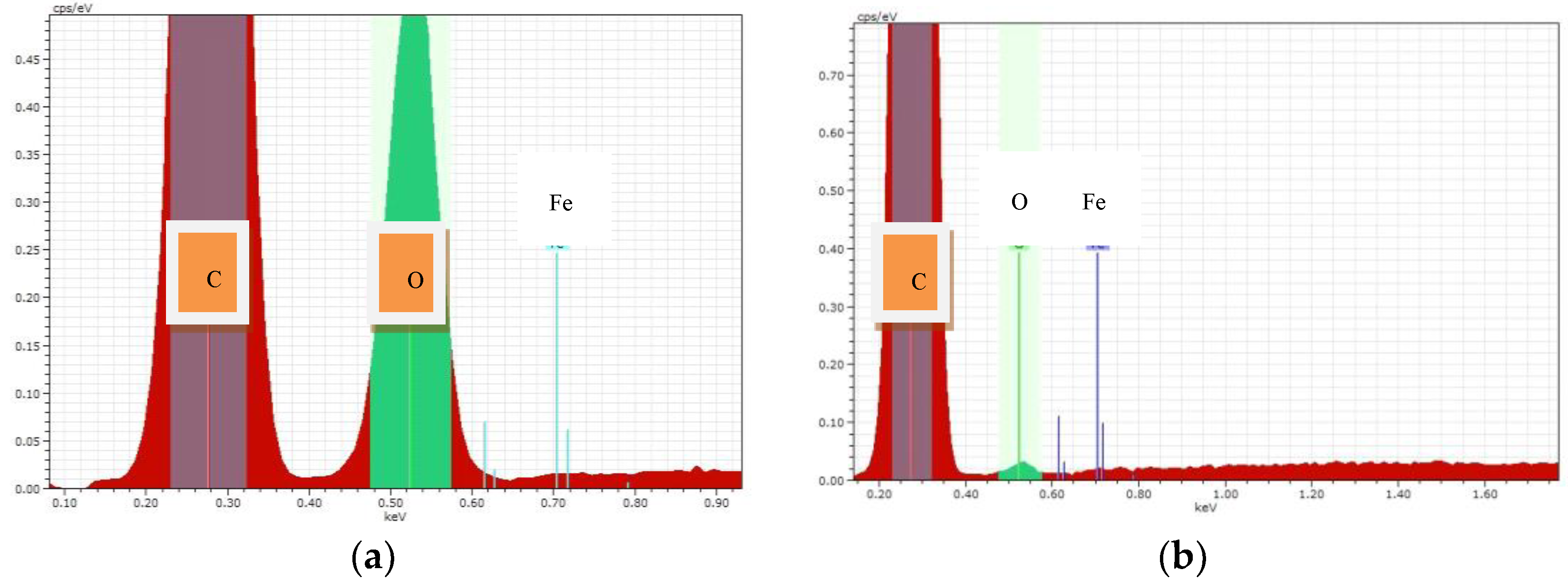
Elemental analysis showed the content (%, mass.) of the metal-modifier in the composites. These results are in
Table 2 and
Figure 2.
Table 2 shows that the content of iron nanoparticles in the sample treated with SHF-irradiation is almost 3.5 times greater than in the samples treated with the II method. This may affect the photocatalytic activity of the test samples. It can be assumed that a sample treated with SHF will be more photoactive during degradation of organic pollutants.
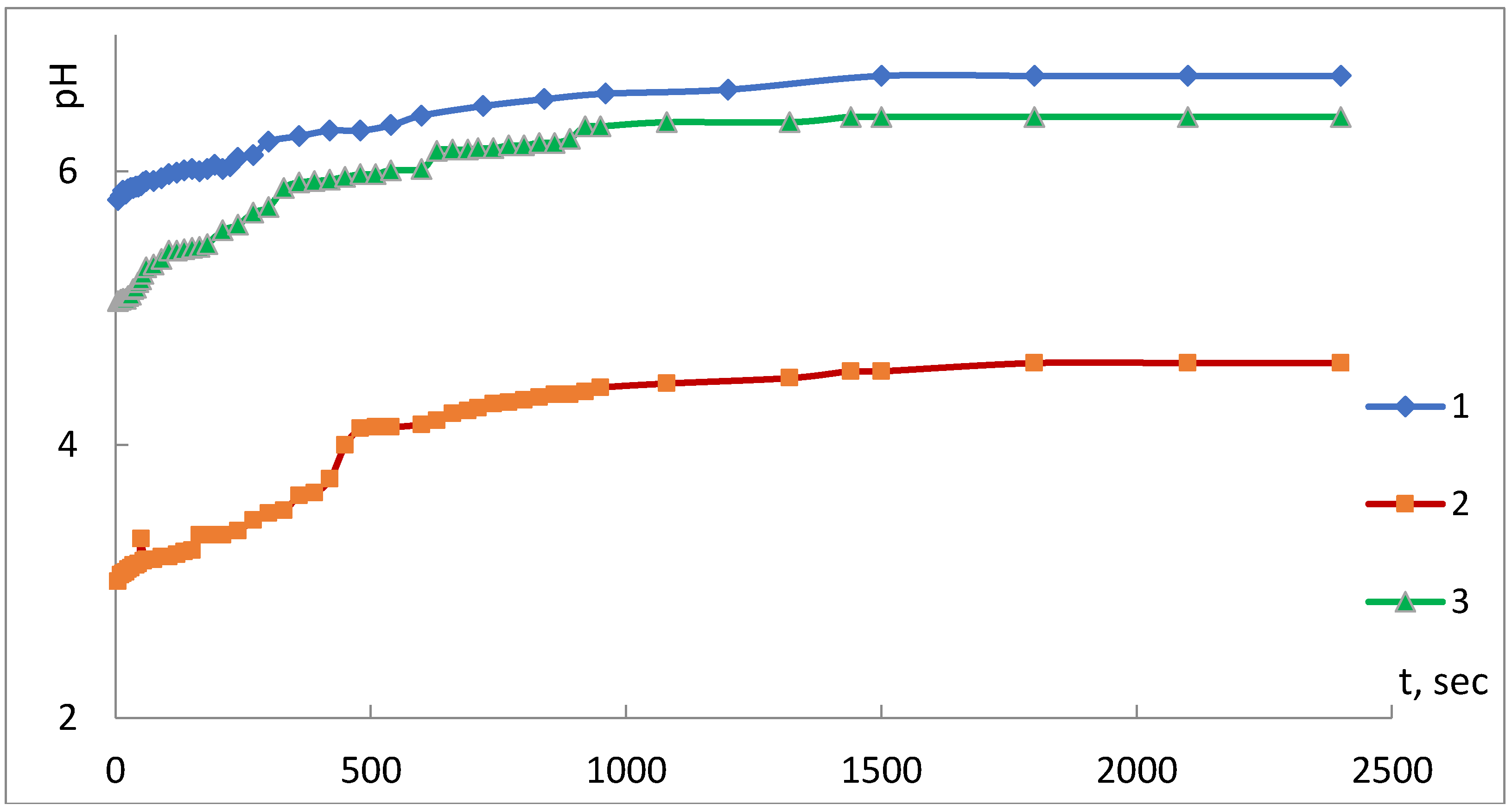
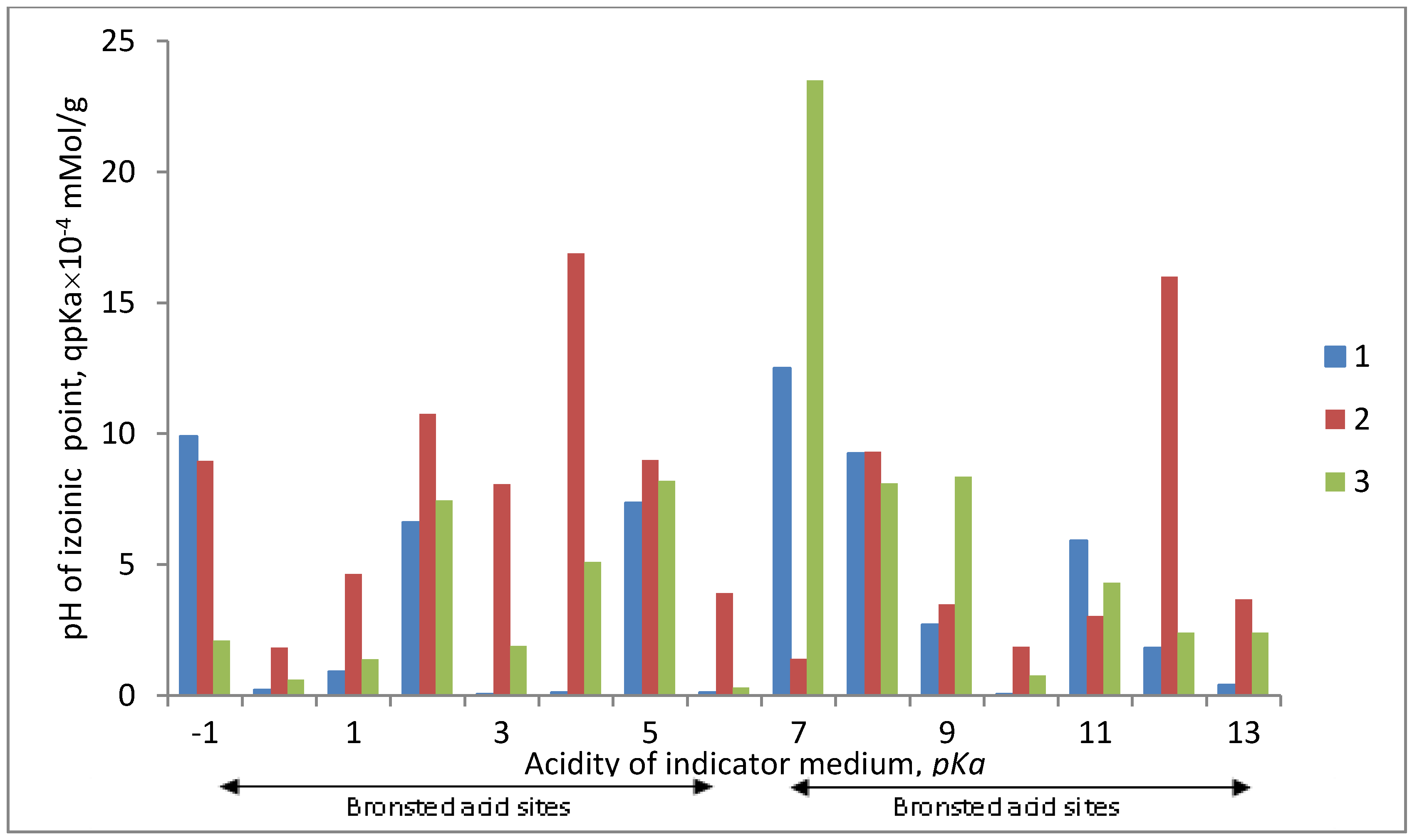
The results of the assessment of acid-base properties of the surface of the iron-containing composites under study are presented in
Figure 3 and
Figure 4.
Figure 3 presents the results of the study of a kinetic version of the pH-measurement method.
Analysis of the kinetic curves of pH variation of the composite in water indicates that the time taken to achieve the adsorption-electrochemical equilibrium, and establishment of a constant value of the pH parameter of the isoionic state (pH
iis), is from 1500 to 2400 s. The shape of the kinetic curves allows concluding about an increase in acidity of the composites’ surface state in comparison with the raw PP fiber. It is especially noticeable that the composite obtained using the SHF method is conditioned, in the authors’ opinion, by the large quantity of the metal in this sample (
Table 2).
Figure 4 shows the distribution spectra of adsorption centers of indicators on the surface of the samples under study, representing a series of bands with maxima of different intensities.
Modification of the PP fiber surface by the Fe0 metal, using the II method, leads to an insignificant decrease of the total number of centers on the surface. The figure shows that the region of Lewis basic centers decreases (pKa from −1 to 0) and the region of acid sites (pKa from 1 to 5) and basic Bronsted centers (pKa from 9 to 12) decreases. At the same time, the region of neutral nature increases (pKa = 7) at the expense of the embedded metal. The general state of the surface is sub acid.
When modifying the PP fiber by Fe0 using the SHF method, as well as in the previous case, the total number of centers on the surface decreases, the region of basic centers (pKa from 9 to 12) and Bronsted acid sites (pKa from 1 to 5.5) decreases compared to the original sample. At that, the band in the neutral region of pKa (pKa = 7) increases. The general state of the surface is also subacid. It is necessary to note that in this case the main Lewis centers (pKa from −1 to 0) on the composite surface remain practically unchanged in the intensity as compared to the original one.
These results, on the distribution of adsorption centers on the surface of composites, fit very well with the data of pH-measurement studies.
Based on the kinetic curves, pH medium variations in aqueous media were calculated:
- -
concentration of active centers of this force, equivalent to the amount of the adsorbed dye (
qpKa), is
where
Cind—indicator concentration (1.5 × 10
−4 moL/L);
Vind—indicator volume taken for the analysis, mL; a
1 and a
2—sample weights, correspondingly, in the “working” and “blank” experiments, g.
The “
“ sign corresponds to a unidirectional change of
D1 and D
2 relatively
D0, i.e.,
D1 and
D2 >
D0 or
D1 <
D0,
D2 <
D0. The “
” sign corresponds to a multidirectional change of D
1 and D
2 relatively
D0, i.e.,
D1 <
D0 and
D2 >
D0 or
D1 >
D0,
D2 <
D0 [
26].
- -
Hammett acidity function is
where
H0—Hammett acidity function;
pKa—acidity of the indicator medium;
—pH of the isoionic point (pH
iip).
This is a value that indicates the general acidity or basicity of the solid surface:
where
pKa and
pKb—acidity and basicity of the indicator medium, respectively;
pKw—water dissociation constant.
The results of the calculated data are presented in
Table 3.
As we expected, the results presented in
Table 3 are evidence of the fact that modification of the PP fiber leads to a significant decrease in the content of active sites (
qpKa), as compared to the raw fiber owing to embedding of the metal. The calculated values of the Hammet acidity function (
H0) allowed comparing the tested samples by acid-base properties. Thus, for the composite, obtained using the SHF method, the Hammett acidity function was 5.29, which points to the presence of stronger Lewis and Bronsted acid sites in this sample as compared to the original sample and the composite obtained using the II method.
Thus, based on the obtained data, it is possible to conclude that embedding of the metal did not change significantly the distribution of the coordination centers on the composites’ surface in comparison with the original sample, but only reduced their concentration. Therefore, it can be assumed that the composites can exhibit both sorption, owing to adsorption of indicators on acid-base centers of the surface, and catalytic activities in reactions of oxidative destruction of organic pollutants under conditions of UVI and visible radiation.
The results of the research of sorption activity of composites with respect to water-soluble and fat-soluble dyes are presented in
Table 4 and
Table 5.
As
Table 4 and
Table 5 show, water-soluble and fat-soluble dyes are adsorbed weakly on the surface of iron-containing composites in normal conditions despite the fact that on the fiber surface there are acid-base centers of Lewis and Bronsted, and the composition of acidic and main groups of dyes includes donor atoms (O,N). At the same time, as the experimental data shows an increase in the contact time of the composites with the dye solutions, from 6 to 24 h, does not influence the value of their absorption.
The research of photocatalytic activity of composites with respect to organic dyes in UVI conditions was conducted using the procedure the authors described earlier. At the same time, the authors supposed that under conditions of the Fenton-like system, according to literature sources, appearance of Fe3+ ions would result in intensification of the process of organic pollutants oxidation at the expense of formation of hydroxyl radicals.
In addition, as the authors of References [
32,
33,
34,
35,
36] state that in the Fenton-like system in the dye, vibrational motions of the chromophores induce oscillations of energy of the photoexcited state. This transfers it to the catalyst, and the means the resulting •OH radicals can also interact with unsaturated dye molecules, and decolorize it according to the scheme:
- (1)
RH + •OH → R• + H2O
- (2)
R• + Fe3+ → Fe2+ + R+
- (3)
H2O + R+ → ROH + H+,
where RH—unsaturated dye molecules.
Table 6 and
Table 7 present the results of decolorization of water-soluble and fat-soluble dyes in conditions of the Fenton-like system and visible radiation involving composites, obtained using SHF and II methods. The decolorization degree was calculated by the formula
where
C0,
Cx—initial and residual concentrations of the dye solution, respectively.
Table 6 and
Table 7 show that the obtained composites, as compared to the original sample, manifest a high photocatalytic activity in the Fenton-like system, and in the visible radiation range with respect to water-soluble and fat-soluble dyes. This is regardless of the modification method and the amount of the embedded metal. The decolorization degree of dyes amounts to 80–100% on average. The use of a visible source of radiation during oxidative destruction of organic dyes was the most promising and energetically advantageous alternative when purifying waters from organic pollutants.
In order to reveal the role of iron-containing composites in the reaction of oxidative destruction of dyes in conditions of the Fenton-like system (Fe0/Fe3+ + H2O2 + UVI), the authors carried out experiments using the previously described procedure, but without the catalyst: A standard test solution of the dye + H2O2 + UVI. The results of the research showed that under these conditions, the dyes undergo weak destruction. Their decolorization degree was for fat-soluble dyes: red—16%, blue—23%, and yellow—17%; for water-soluble dyes: rhodamine C—21%, brilliant green—23%, and eosin—22%.
The conducted studies showed that an essential role in conditions of the Fenton-like system belongs to the iron-containing catalyst, which is a source of iron ions in the solution and ensures the generation of •OH radicals for oxidative destruction of organic pollutants. The research of the raw PP fiber, the iron-containing composite obtained by the II method, as well as the composites after oxidative destruction of water-soluble dyes, such as brilliant green and fat-soluble Sudan blue by IR spectroscopy, confirmed the authors’ assumption. The IR spectra of all mentioned samples are identical (spectra are not given). This points to the absence of any interactions on the composites’ surface, and gives reasons to believe that in the authors’ studies (in conditions of the Fenton-like system), a dominating role in degradation of organic pollutants belongs to homogeneous catalysis.
Since no studies on dyes degradation using a visible radiation source (without a catalyst) have been carried out, it is possible to assume that complete decolorization in the solution is due to adsorption on the composites surface, and an ability of dyes to absorb visible radiation. The role of iron (III) ions as a catalyst is not excluded.
Thus, based on the evidence stated above, it is possible to conclude that the dye removal mechanism using composites based of the PP fiber is of complicated nature. This depends on three factors. Firstly, the sorption activity of the composite surface. Secondly, on the participation of catalysts in a homogeneous-heterogeneous system of the following type: Composite (Fe
0) + H
+ medium → Fe
3+/UVI/H
2O
2). Thirdly, on the ability to absorb light by the dyes themselves (“blank” experience). At the same time, the main role in decolorization of dyes, in the authors’ opinion, belongs to the process of their photo oxidation according to the scheme proposed by authors of References [
4,
7,
32,
33,
34,
35,
36], which the authors of this paper presented earlier.