Decomposition of Used Tyre Rubber by Pyrolysis: Enhancement of the Physical Properties of the Liquid Fraction Using a Hydrogen Stream
Abstract
1. Introduction
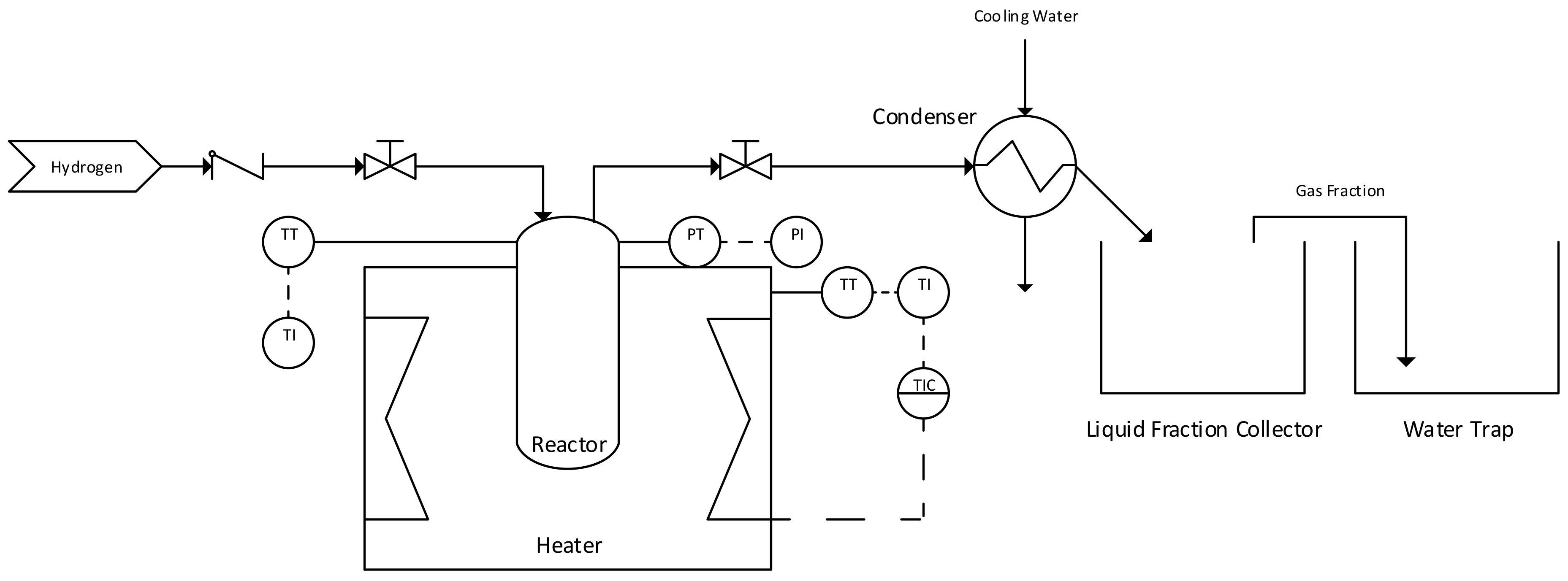
2. Materials and Methods
3. Results and Discussion
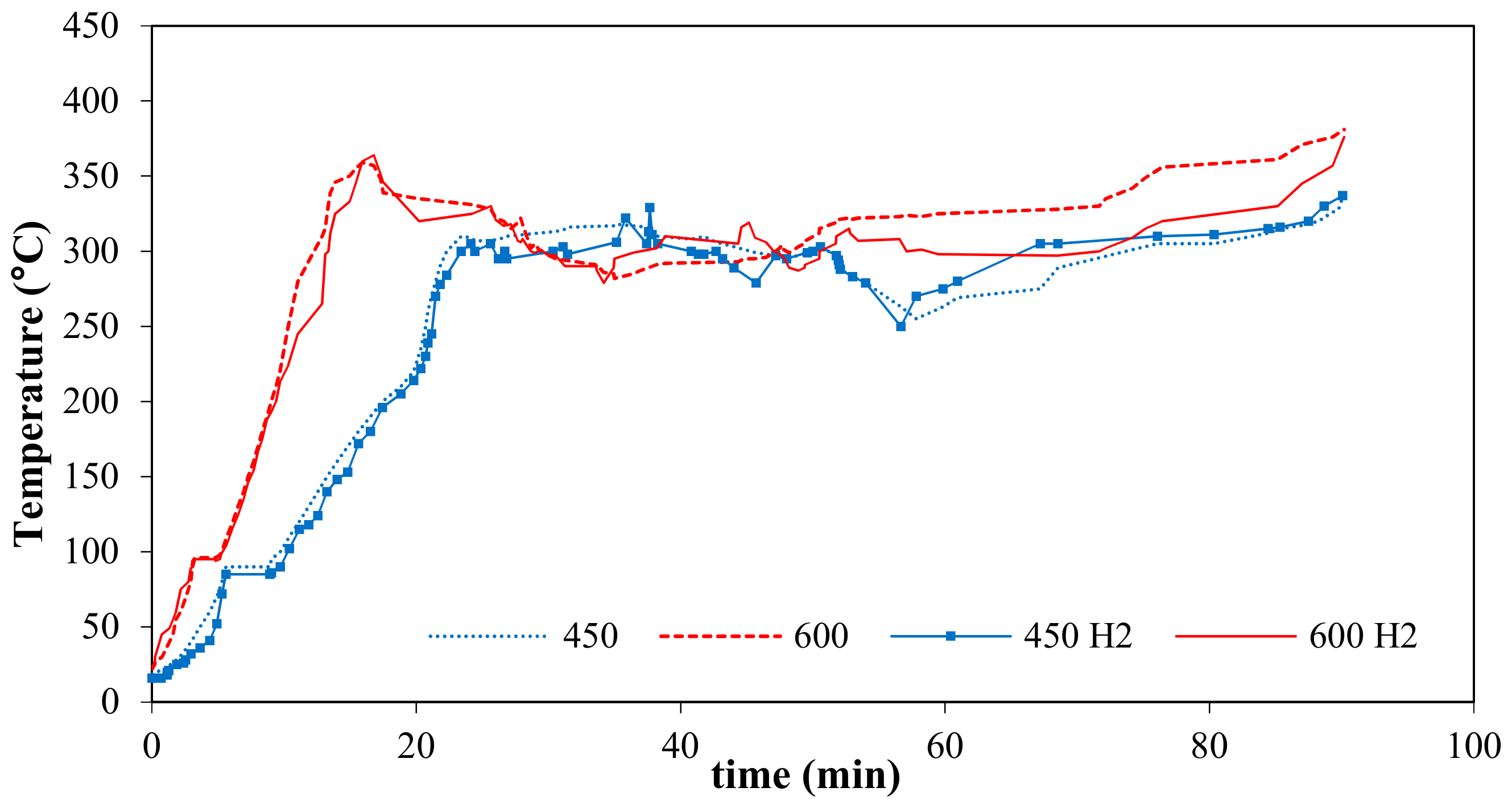
3.1. Thermographs
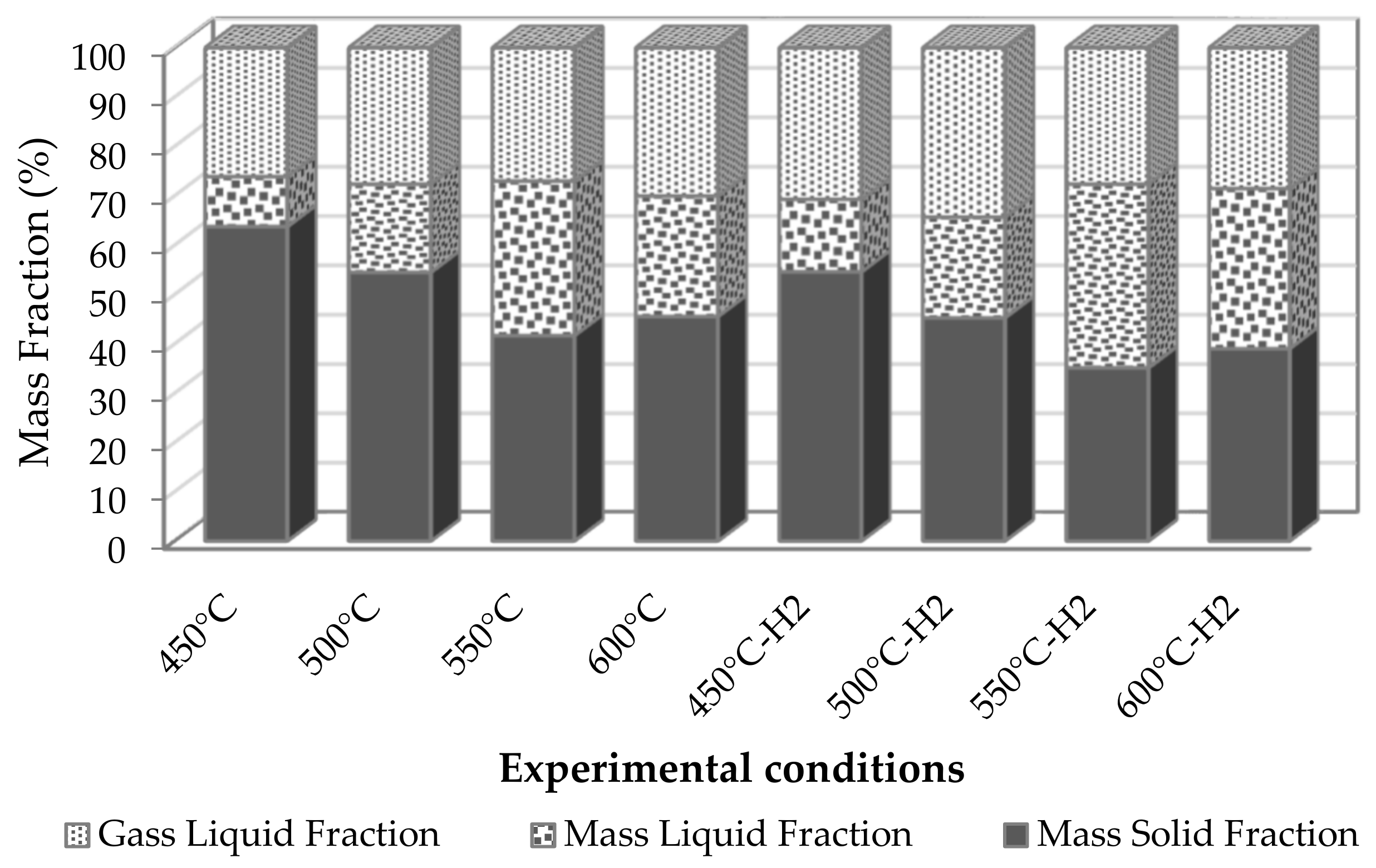
3.2. Production of Pyrolytic Products.
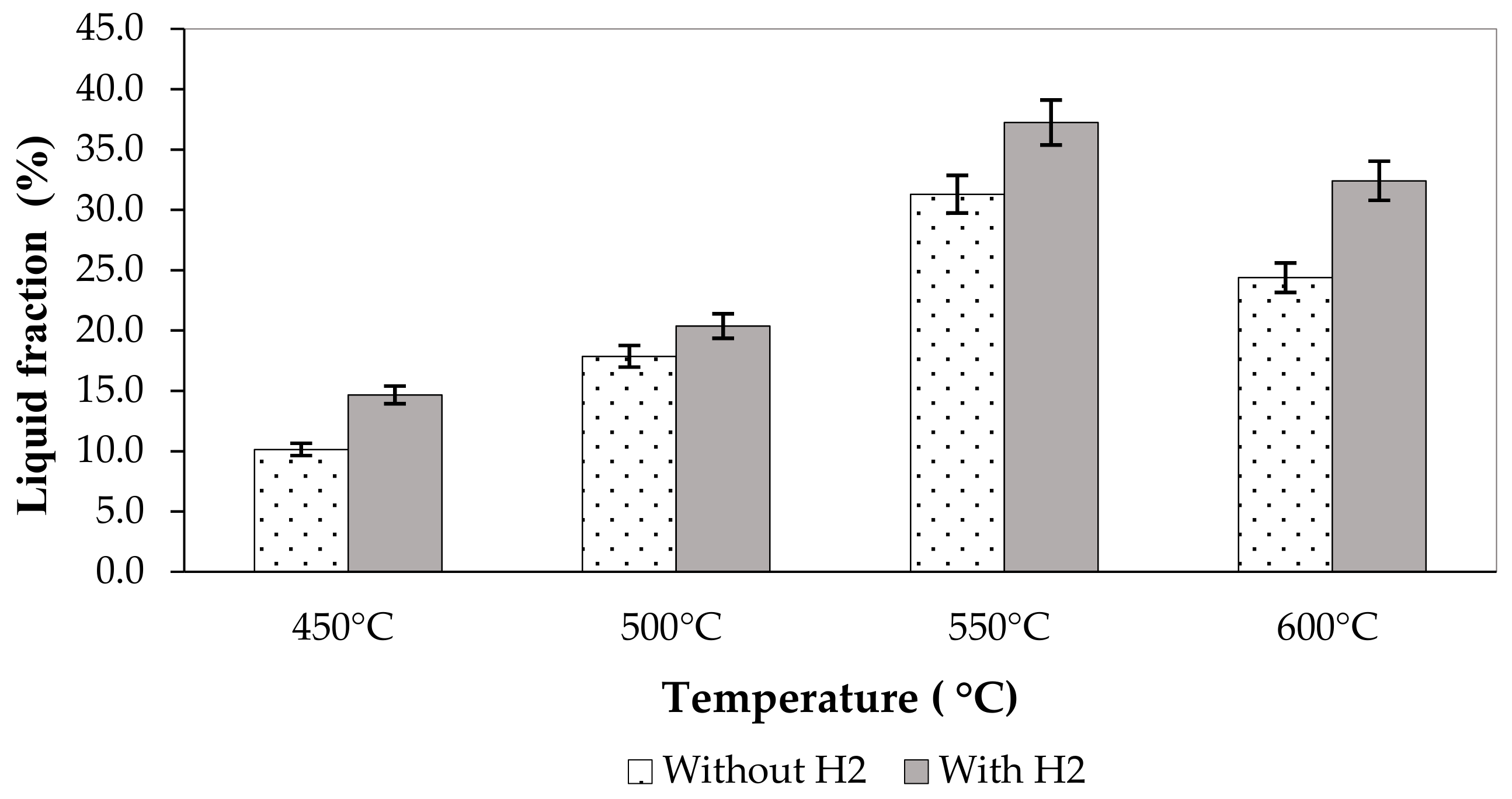
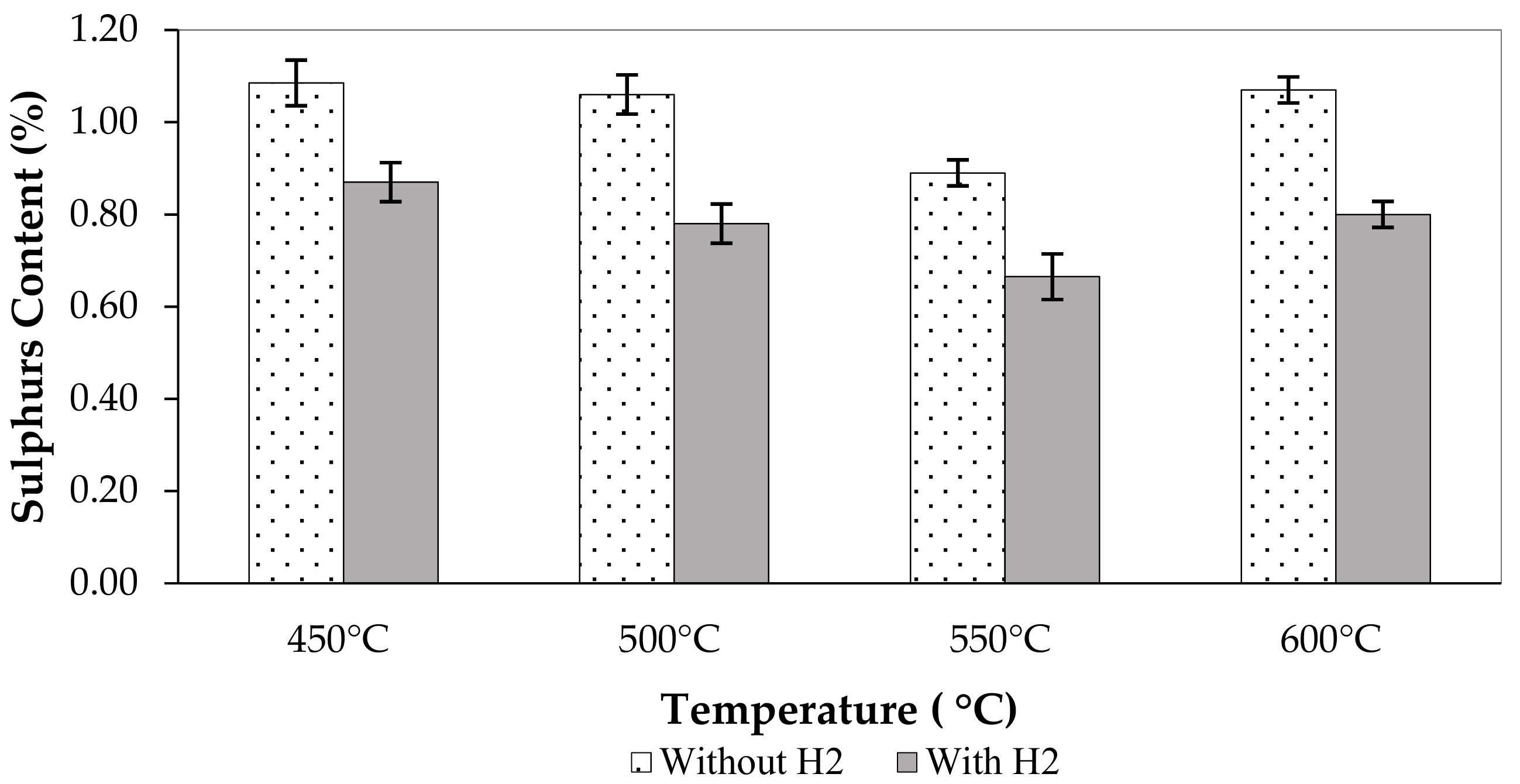
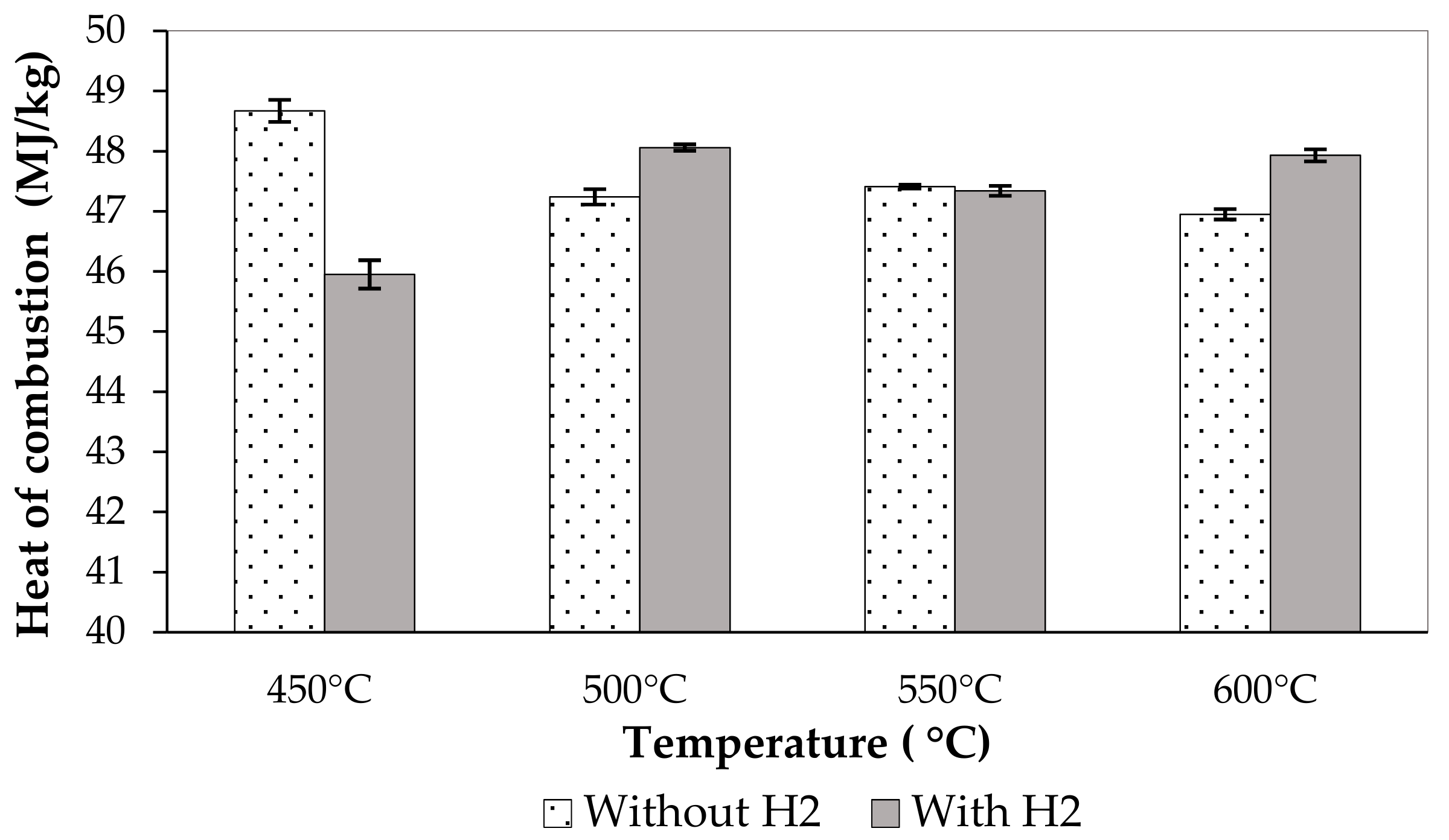
3.3. Effect of Pyrolysis Condition on the Pyrolytic Liquid Fuel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H.; Spencer, N. Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Evans, R.; Evans, A. The Composition of a Tyre: Typical Components; The Waste and Resources Action Programme: Banbury, UK, 2006. [Google Scholar]
- Sienkiewicz, M.; Janik, H.; Borzędowska-Labuda, K.; Kucińska-Lipka, J. Environmentally friendly polymer-rubber composites obtained from waste tyres: A review. J. Clean. Prod. 2017, 147, 560–571. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Fuel production from pyrolysis of natural and synthetic rubbers. Fuel 2017, 191, 403–410. [Google Scholar] [CrossRef]
- Nieuwenhuizen, P.J. Zinc accelerator complexes: Versatile homogeneous catalysts in sulfur vulcanization. Appl. Catal. A Gen. 2001, 207, 55–68. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Miguel, G.S.; Fowler, G.D.; Sollars, C.J. Pyrolysis of Tire Rubber: Porosity and Adsorption Characteristics of the Pyrolytic Chars. Ind. Eng. Chem. Res. 1998, 37, 2430–2435. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1,3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis. 2001, 58–59, 803–811. [Google Scholar] [CrossRef]
- Aydin, H.; Ilkiliç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Mkhizeb, N.M.; Danonb, B.; Van der Grypb, P.; Görgensb, J.F.; Bilbaoa, J.; Olazara, M. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor. Energy 2017, 128, 463–474. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; Caballero, B.M.; De Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Temperature selective condensation of tyre pyrolysis oils to maximise the recovery of single ring aromatic compounds. Fuel 2003, 82, 1023–1031. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis-Thermogravimetric Analysis of Tires and Tyre Components. Fuel Energy Abstr. 1995, 14, 1277–1283. [Google Scholar]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Encinar, J.M.; Canito, J.L.; Rodriguez, J.J. Pyrolysis of automobile tires wastes. Influence of operating variable and kinetic study. Int. Symp. Anal. Appl. Pyrolysis 2001, 59, 667–683. [Google Scholar] [CrossRef]
- Choi, G.G.; Oh, S.J.; Kim, J.S. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content. Appl. Energy 2016, 170, 140–147. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; González, J. Fixed-bed pyrolysis of Cynara cardunculus L. Product yields and compositions. Fuel Process. Technol. 2000, 68, 209–222. [Google Scholar] [CrossRef]
- Lah, B.; Klinar, D.; Likozar, B. Pyrolysis of natural, butadiene, styrene-butadiene rubber and tyre components: Modelling kinetics and transport phenomena at different heating rates and formulations. Chem. Eng. Sci. 2013, 87, 1–13. [Google Scholar] [CrossRef]
- Choi, G.G.; Jung, S.H.; Oh, S.J.; Kim, J.S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Kalargaris I, Tian G, Gu S. Experimental characterisation of a diesel engine running on polypropylene oils produced at different pyrolysis temperatures. Fuel 2018, 211, 797–803. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.; Cai, L.; Shi, S.Q. Quality improvement of pyrolysis oil from waste rubber by adding sawdust. Waste Manag. 2014, 34, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castano, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Murena, F. Kinetics of sulphur compounds in waste tyres pyrolysis. J. Anal. Appl. Pyrolysis 2000, 56, 195–205. [Google Scholar] [CrossRef]
- Jantaraksa, N.; Prasassarakich, P.; Reubroycharoen, P.; Hinchiranan, N. Cleaner alternative liquid fuels derived from the hydrodesulfurization of waste tire pyrolysis oil. Energy Convers. Manag. 2015, 95, 424–434. [Google Scholar] [CrossRef]
- Arpa, O.; Yumrutas, R.; Demirbas, A. Production of diesel-like fuel from waste engine oil by pyrolitic distillation. Appl. Energy 2010, 87, 122–127. [Google Scholar] [CrossRef]
| Physical Property | Value |
|---|---|
| Compressibility | 20.08% |
| Aerated bulk density | 0.386 g/mL |
| Apparent bulk density | 0.405 g/mL |
| Packed bulk density | 0.483 g/mL |
| Average density | 0.434 g/mL |
| Porosity | 6.58% |
| Property | Method |
|---|---|
| Mass Liquid Fraction | Mass Balance |
| pH | ANSI 1/ASTM E 70 |
| Specific Gravity | ANSI/ASTM D 287 |
| API 2 Gravity | ANSI/ASTM D 287 |
| Kinematic Viscosity | ANSI/ASTM D 446 |
| Saybolt Viscosity | ANSI/ASTM D 446 |
| Sulphur Content | ANSI/ASTM D 129 |
| Gross Heat of Combustion | ANSI/ASTM D 240 |
| Water and Sediments | ANSI/ASTM D 96 |
| Ash Content | ANSI/ASTM D 482 |
| Flash Point | ANSI/ASTM D 56,92 |
| Experimental | Liquid Fraction | pH | Density | API Gravity | Kinematic Viscosity | Saybolt Viscosity |
| Conditions | % mass | g/mL | °API | cSt | Seconds | |
| 450 °C without H2 | 10.14 | 8.81 | 0.83 | 38.7 | 1.59 | 31.4 |
| 500 °C without H2 | 17.91 | 9.01 | 0.85 | 35.6 | 1.88 | 32.3 |
| 550 °C without H2 | 31.09 | 8.84 | 0.88 | 30.2 | 2.77 | 35.2 |
| 600 °C without H2 | 24.57 | 9.12 | 0.86 | 33.6 | 2.65 | 34.8 |
| 450 °C with H2 | 14.59 | 8.74 | 0.86 | 33.1 | 2.35 | 33.9 |
| 500 °C with H2 | 20.47 | 8.50 | 0.85 | 34.4 | 2.41 | 34.1 |
| 550 °C with H2 | 37.25 | 8.26 | 0.87 | 31.4 | 2.48 | 34.3 |
| 600 °C with H2 | 32.56 | 8.10 | 0.88 | 29.6 | 3.07 | 36.2 |
| Experimental | Sulphur Content | Heat of Combustion | Heat of Combustion | Flash Point | Water and Sediments | Ash Content |
| Conditions | % mass | cal/g | MJ/kg | °C | % mass | % mass |
| 450 °C without H2 | 1.09 | 11,625.0 | 48.67 | 32.0 | 0.90 | 0.10 |
| 500 °C without H2 | 1.06 | 11,283.5 | 47.24 | 32.5 | 0.75 | 0.04 |
| 550 °C without H2 | 0.89 | 11,324.5 | 47.41 | 30.5 | 0.75 | 0.04 |
| 600 °C without H2 | 1.07 | 11,214.5 | 46.95 | 35.5 | 0.35 | 0.04 |
| 450 °C with H2 | 0.87 | 10,975.0 | 45.95 | 34.5 | 0.55 | 0.04 |
| 500 °C with H2 | 0.78 | 11,479.0 | 48.06 | 30.5 | 0.40 | 0.04 |
| 550 °C with H2 | 0.67 | 11,307.0 | 47.34 | 31.0 | 0.25 | 0.08 |
| 600 °C with H2 | 0.80 | 11,449.0 | 47.93 | 31.0 | 0.30 | 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Canon, A.; Muñoz-Camelo, Y.F.; Singh, P. Decomposition of Used Tyre Rubber by Pyrolysis: Enhancement of the Physical Properties of the Liquid Fraction Using a Hydrogen Stream. Environments 2018, 5, 72. https://doi.org/10.3390/environments5060072
Ramirez-Canon A, Muñoz-Camelo YF, Singh P. Decomposition of Used Tyre Rubber by Pyrolysis: Enhancement of the Physical Properties of the Liquid Fraction Using a Hydrogen Stream. Environments. 2018; 5(6):72. https://doi.org/10.3390/environments5060072
Chicago/Turabian StyleRamirez-Canon, Anyela, Yahir F. Muñoz-Camelo, and Paul Singh. 2018. "Decomposition of Used Tyre Rubber by Pyrolysis: Enhancement of the Physical Properties of the Liquid Fraction Using a Hydrogen Stream" Environments 5, no. 6: 72. https://doi.org/10.3390/environments5060072
APA StyleRamirez-Canon, A., Muñoz-Camelo, Y. F., & Singh, P. (2018). Decomposition of Used Tyre Rubber by Pyrolysis: Enhancement of the Physical Properties of the Liquid Fraction Using a Hydrogen Stream. Environments, 5(6), 72. https://doi.org/10.3390/environments5060072





