Abstract
The reuse of treated wastewaters could contribute to reducing water stress. In this research, ultrasound application on bacterial inactivation in municipal wastewater (MWW) was evaluated. Total and fecal coliforms were used as standard fecal indicators; volatile suspended solids (VSS) were analyzed too. Samples were taken from the effluent of secondary clarifiers. In addition, inactivation tests were carried out on pure cultures of E. coli (EC) and B. subtilis (BS). Sonication was performed at 20 kHz, 35% amplitude and 600 W/L for 15, 30 and 45 min. After 15 min of sonication, bacterial density was reduced by 1.85 Log10 MPN/100 mL for EC and 3.16 Log10 CFU/mL for BS. After 30 min, no CFU/mL of BS were observed in MWW and, after 45 min, the reduction of total and fecal coliforms was practically 6.45 Log10 MPN/100mL. Inactivation mechanism was made by cavitation, which causes irreversible damage to the cell wall. Although high bacterial densities were employed, percentages of inactivation >99% were reached at 45 min. This research contributes to the implementation of ultrasound as a disinfection technique with high potential due to its high efficiency without producing byproducts. In fact, the water meets the guidelines for reuse in direct human contact services.
1. Introduction
Population and industrial growth demands an intensive use of natural resources. One of them is water supply due to the great magnitude in which it is required. In particular, the use of water resources implies higher levels of pollution due to the generation of wastewaters [1,2]. Nevertheless, treated wastewaters could be used to reduce the water stress generated by the water supply in industrial and/or municipal activities. This is achieved by treating wastewater to meet quality parameters adequate at human and consumption levels [3].
Conventional treatment of municipal wastewater consists of pre-treatment, primary, secondary and tertiary treatment [4]. The last one refers to the disinfection process, where chemical oxidation is the most commonly used through liquid chlorine or gas. However, its major disadvantage is byproducts formation such as trihalomethanes (40–50%), haloacetic acid (28–35%), haloacetonitriles (9–15%) and others (<23%) [5], which are considered toxic and bioaccumulative through the trophic chain. In addition, these byproducts present in wastewater could have negative effects such as acute toxicity, mutagenicity or carcinogenesis in organisms found in water bodies. In addition, chlorine dioxide gas is the most used, which entails risks to occupational health, since a 1000 ppm concentration causes death within a few minutes of exposure [6].
On the other hand, advanced oxidation processes (AOPs) have demonstrated high removal efficiencies, but with a focus on oxidation of dissolved organic matter, especially persistent organic pollutants [6,7]. One of the most viable is heterogeneous photocatalysis using titanium dioxide or zinc oxides as catalysts. The mechanism of action of this photocatalysis suggests a high technical efficiency in viral and bacterial inactivation. Nonetheless, current designs are laboratory scale and have a great deal of heterogeneity in design, so it is necessary to investigate, in detail, the engineering design, modeling and actinometry to consider large scale operations [8,9,10].
A viable alternative for disinfection is ultrasound, which is proving to be able to inactivate microorganisms through a series of physical, chemical and mechanical effects derived from cavitation. An advantage of ultrasound disinfection is that bacteria do not develop a resistance against it, or produce toxic byproducts [11,12,13]. Indeed [11], evaluated the effect of ultrasound on Escherichia coli and Staphylococcus aureus concentrations in wastewater samples, obtaining a reduction of 2.6 logarithmic units.
The most important operating parameters of the ultrasound application in wastewater disinfection are frequency, amplitude, and sonication time. High values of cell disintegration at frequencies lower than 100 kHz have been shown [13,14,15]. Amplitude has been found to have a certain proportional correlation with thermal energy in the aqueous medium, causing a greater inactivation of microorganisms. Finally, it is known that sonication time is important in treatment; the longer the sonication time, the greater the microorganisms’ inactivation [11,14,15].
Although several authors have focused on the study of one or more ultrasound operating parameters in the disinfection process [16], the ideal sonication time has not yet been evaluated considering optimal amplitude and frequency conditions and feasibility of being scalable for a municipal wastewater treatment plant [16,17].
Therefore, the objective of this research was to evaluate the ideal sonication time under optimal conditions of frequency and amplitude in samples of conventional municipal wastewater containing total and fecal coliforms. The research was carried out in order to comply with the national and international standards for the reuse of treated wastewaters [18].
2. Materials and Methods
2.1. Samples Selection and Characterization
Ultrasonic disinfection tests were performed on municipal wastewater samples and pure cultures of EC and BS. Municipal wastewater was taken from effluent of secondary settlers of four wastewater treatment plants, which have an activated sludge system as secondary treatment, located in Morelos, México. A total of 39 composite samples were taken every 4 days during six months of sampling following the [19,20,21] methodology. Fecal and total coliforms, total suspended solids and volatile suspended solids were analyzed in all samples.
Pure cultures tests were performed with Escherichia coli ATCC11229, due to it being a bioindicator of fecal contamination in water [21] and with Bacillus subtilis ATCC6633, for their differentiation in cell wall. Both microorganisms were evaluated in exponential phase stages, as described in [22,23].
2.2. Microbial Growth Curve
In order to evaluate the application of ultrasound in samples with the highest possible concentration of bacteria, microbial growth curves were determined for reference bacteria: Escherichia coli ATCC 11229 and Bacillus subtilis ATCC 6633. Those curves were carried out in two different stages, pre-inoculum and subsequently in a kinetic study. The pre-inoculum was obtained by inoculating an isolated colony in 100 mL of nutrient broth and was incubated at 37 °C at 120 rpm during 14 h. The second stage consisted of a kinetic study, through an optical density recorder of 1% of the pre-inoculum in 250 mL of nutritive broth, every 60 min in a spectrophotometer HACH DR5000, Loveland, Co, USA, ƛ = 600. The method, and their main characteristics used to quantify EC, BS and coliforms are described in Table 1. Finally, the specific growth rate (μ) obtained, was calculated relating the biomass amount generated with time. The formula used was indicated in Equation (1).
where,

Table 1.
Main characteristics of the methodologies used for quantifying the microorganisms evaluated during sonication tests.
- X2 = Absorbance corresponding to biomass concentration at time two, nm
- X1 = Absorbance corresponding to initial biomass concentration, nm
- dt = Difference between final and initial time, h.
2.3. Sonication Parameters
Ultrasound equipment used was brand Cole-Parmer model CP505, Vernon Hills, IL, USA, of 200 ± 1.2 W. Optimal frequency and amplitude preliminary tests were performed, considering the operating parameters established in previous studies [26,27,28]. For that reason, 26 kHz frequency, 20 W/L ultrasonic density and 35% amplitude were set as operating parameters in this investigation. Both parameters have been found in 15, 30, 45 and 60 min of sonication times. It should be noted that all combinations made between amplitude, frequency and sonication time are feasible to scale in a wastewater treatment plant, considering installation, maintenance and operation cost (including energy consumption) [13,29].
2.4. Bacteria Viability during Ultrasound Tests
E. coli (EC) viability was determined according to the most probable number method (MPN). Besides, B subtilis (BS) inactivation was determined by plating serial dilution of the culture. Both methods were carried out in accordance with the standard described in [30,31,32].
The bacterial suspensions were sonicated using different intensities and frequencies. Samples were taken after 1, 2, 5, 10, and 15 min and analyzed by two methods; counting of colony forming units (CFUs) via plate counting and determination of the fluorescence of fluorescein diacetate absorbed into the bacteria cells (Food Drug Administration analysis). Each determination is the mean of at least three experiments and for the graphical representation the readings were expressed into logarithmic units.
On the other hand, volatile and total solids suspended were determined in the wastewater samples [27,29] during ultrasound application, since both are important for the reuse of treated water, and in particular volatiles refer to the amount of suspended organic matter, in which the microorganisms are the majority content.
2.5. Experimental Design and Statistical Analysis
During experimentation, amplitude and frequency were fixed variables. Thus, statistical treatments were based on two factors: (1) Microorganism type was evaluated with two levels EC and BS; (2) Sonication time was evaluated with four levels 15, 30, 45 and 60 min. All combinations were performed by triplicate with temperature monitoring. In this regard, experimental design corresponds to a randomized block design, so results were compared through a one-way analysis of variance (One-Way ANOVA), α = 0.05. Before ANOVA was made, a normality and homoscedasticity were verified through the Kolmogórov-Smirnov test. All analyses were made in the MINITAB 15 Inc. software, State College, PA, USA.
3. Results and Discussion
3.1. Pure Cultures Standardization of E. coli and B. subtilis
Growth curve of EC as a time function is shown in Figure 1, and linearized tendency to obtain growth rate is represented in Figure 1b; it is observed that Lag phase was reached approximately 1 h after starting the culture, at this time exponential phase began and lasted 4 h, ending 5 h after starting the bacterial culture. Consequently, stationary phase began at 5 h after the start of culture. Based on these results, aliquots for ultrasonic disinfection tests were taken at 4 h after culture started, to guarantee that EC cells were in their exponential growth phase. Growth kinetic values match with those reported by [27,32], which indicate a doubling concentration of EC every 20 min. In addition, four hours of exponential phase is due because optimal conditions of growing were used for their development (temperature control, oxygenation and medium composition).
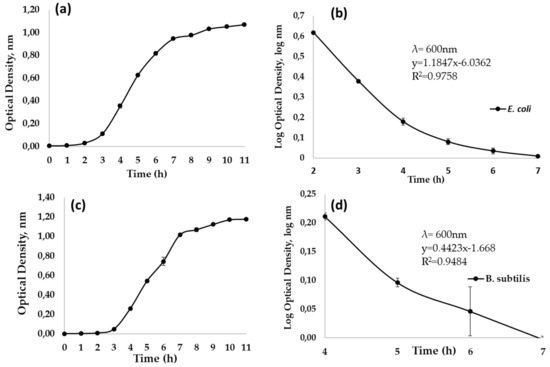
Figure 1.
Microbial growth curves of bacteria grown at 37 °C, 120 rpm for 11 h. (a) E. coli optical density obtained in time function; (b) E. coli linearized tendency in time function for obtaining growth rate (c) B. subtilis optical density obtained in time function; (d) B. subtilis linearized tendency in time function to obtain growth rate.
Similarly, in Figure 1 the growth of BS culture is observed, in which Lag phase is observed during the first two hours, after that exponential phase began and lasted 5 h, which took 7 h of total duration. In this regard, the aliquot evaluated was taken at five hours of process, which is shorter than times reported by [33] in other species of Bacillus and Clostridium genus. The above is due to the fact that BS metabolizes a wide variety of sugars including xylose, arabinose, malaise, sucrose and glucose, and a wide variety of polymers (proteins and carbohydrates) [34].
3.2. Sampling and Quantification of Coliforms and Suspended Solids
Table 2 shows distribution time of total and fecal coliforms in effluent of secondary clarifier of treatment plant. Daily average was 1.0 × 106 ± 1.8 × 105 MPN/100 mL for total coliforms and 9.72 × 105 ± 1.02 × 104 MPN/100 mL for fecal coliforms. Samples were obtained at 7:00 a.m. in which maximum values were recorded with 4.06 × 106 MPN/100 mL for both types of coliforms. This guarantees that obtained results with ultrasound treatment are scalable to the highest microbiological contamination load, values that also coincide with the maximums reported for municipal wastewater [25,35].

Table 2.
Total and fecal coliforms and suspended matter characterization of secondary effluents of municipal wastewater treatment plants.
On the other hand, Total Suspended Solids (TSS) and Volatile Solids (VSS) average values, in different times (sampling events) are shown in Table 2. The first column refers to the different sampling hours on the same day, established according to the procedures indicated for sampling in wastewater treatment plants [3,4,24].
In all cases, 80% of the TSS was obtained as VSS concentration, which corresponds to conventional municipal wastewater values [3,4]. Therefore, the remaining 20% was considered as inorganic suspended matter, such as clays. In addition, increase in the concentration of coliforms is proportional to the concentration of suspended solids (Table 2). This suggests that suspended solid could be a monitoring parameter during the assessment of ultrasound application in wastewater. Ultrasound application tests were performed on the samples at 7:00 a.m., since they had the highest concentrations of bacteria and suspended solids (Table 2). The results of this assessment are presented in Section 3.3.2.
3.3. Ultrasound Treatment
3.3.1. Bacterial Inactivation
Table 3 and Figure 2 show general results of ultrasound application to different samples of microorganisms evaluated. After 15 min of sonication, bacterial removal in residual water was 0.11 Log10 MPN/100 mL for total coliforms and 0.89 Log10 MPN/100 mL for EC with significant differences between them (p < 0.05). For pure cultures, the removal efficiency in BS was significantly higher than in EC (p < 0.05), since EC was inactivated in 1.85 Log10 MPN/100 mL and BS in 3.16 Log10 CFU/mL. Each of these presented significantly greater inactivation than that obtained for total coliforms and fecal coliforms.

Table 3.
Microorganism average concentration according to ultrasound application at different times.
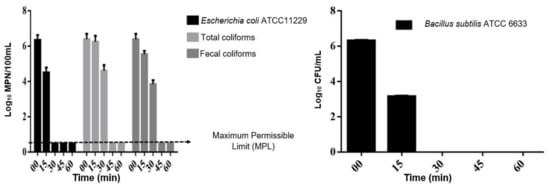
Figure 2.
Treatment with ultrasound at a frequency: 20 kHz, amplitude: 35% and sonication time: 15, 30, 45, 60 min. on (a) total and fecal coliforms, and E. coli and (b) Bacillus subtilis.
For pure cultures (EC and BS) in growth media, a complete inactivation (5.92 Log10 MPN/100 mL and 6.32 Log10 CFU/mL) was observed at 30 min of sonication, while for coliforms (total and fecal) present in the wastewater, 45 min of the physicochemical treatment was required for a complete bacterial inactivation.
The highest time in wastewater treatment is due to the coexistence of around 100 bacterial species, which are more resistant together to sonication treatment [33]. At least four bacteria correspond to coliform genus (Escherichia, Kleibsella, Enterobacter and Citrobacter), and as well other unicellular and pluricellular microorganisms are present, which form a consortium of microorganisms. This consortium in population dynamics causes each microorganism to be more resistant to stress conditions, than if there were only one specie [34,35,36] as it was observed in pure cultures with EC and BS. In addition, wastewater from secondary clarifier still contains dissolved organic compounds and floccules/aggregates that are also attacked by ultrasound [18]; for that reason it requires higher sonication time to eliminate microorganisms.
Total and fecal coliforms removal at 30 min of sonication were 1.77 Log10 MPN/100 mL and 2.57 Log10 MPN/100 mL respectively with a significance difference between them (p < 0.05) (Figure 2). The higher inactivation of fecal coliforms may be related to the fact that total coliforms also include genera Kleibsiella, Enterobacter and Citrobacter [37,38], which are less susceptible to various disinfecting agents than Escherichia [24,38,39].
Considering that all the genera mentioned above are gram negative bacteria, its cell wall is formed by an outer membrane which is composed of phospholipids that are affected by the increase in temperature modifying its permeability, causing alterations in cellular components and input and output nutrients. It is also important to not forget that at temperatures higher than 60 °C proteins, enzymes and nucleic acids begin to denature [40]. In addition to this, hydroxyl ions and free radicals are generated during the treatment with ultrasound, which act by oxidizing essential components of bacteria (lipids, proteins and genetic material), generating H2O2 as a product which is not considered a carcinogen, unlike chlorine disinfection byproducts [14,41,42].
In contrast, gram-positive microorganisms, such as BS, do not have an outer membrane. However, they have a thick layer of peptidoglycan and inside it has a membrane. In the middle of these two walls there is a periplasmic space, in which a greater cavitation is generated by the differences in pressure [42,43,44,45], which explains the fact that it presented the highest degree of inactivation compared to EC and both types of coliforms (Figure 2 and Table 3).
Independently of bacterial inactivation percentage, disinfection objective in wastewater treatment is to guarantee public health through complying with established maximum permissible limits (MPL). The main indicators of pathogenic contamination are fecal and total coliforms. In this sense, in Figure 2 it is observed that at 45 min bacterial concentration is below the MPL which are 1000 MPN/100 mL for indirect contact and 240 MPN/100 mL for direct contact [46]. The reduction of microorganisms at 45 min is so effective, that it is microbiologically with the MPL of 2 MPN/100 mL established by [46,47] that refers to water use and human consumption, as well as the guidelines of the World Health Organization for quality of drinking water.
The above indicates that the rupture of bacteria cell wall is caused by cavitation which is related with temperature increasing of the medium, giving rise to a greater permeability of the outer membrane. In fact, in the first 15 min of treatment a temperature of 50 °C was reached, eliminating genus Enterobacter that does not subsist above 40 °C [47]. Subsequently, at 30 min, a temperature of 60 °C was recorded, reaching a maximum of 65 °C from 45 min, which suggests the generation of numerous microbubbles that give rise to repeated implosions, due to the pressure gradient [7,47], in addition to generation of hydroxyl radicals, which have affinity for the compounds with fatty acids that characterize the cell wall [46,47].
Results of bacterial inactivation by ultrasound were similar to those reported by [44], at the same frequency but greater amplitude (35% instead 20%); the same removal percentage was obtained in a shorter time. The above match with the fact that the inactivation of EC is proportional to the increase in amplitude, due to the heat energy generated benefiting the process of bacteria inactivation [13].
Joyce et al., 2003 [14], reported that at 15 min of treatment a complete elimination of gram-positive bacteria was reached, a similar situation to BS in this research. It is important to indicate that operation parameters of frequency at 20 kHz and amplitude at 35% during 45 min were used in this research, considering in a continuous flow system these parameters are feasible to scale due to the energy consumption it represents [16].
3.3.2. Suspended Solid Removal
TSS and VSS removal as a function of time sonication is observed in Figure 3. Before sonication treatment (t = 0) VSS proportion was approximately 80% of the TSS. However, this proportion is decreasing as a function of sonication time, which could be related to VSS composition, which are almost entirely microorganisms [3,17]. As explained in the present investigation, sonication causes irreversible damage to the cellular structures of bacteria, in addition to viruses and protozoa, which are precisely those that are quantified together as VSS. In this sense, results indicate the elimination of microorganisms, expressed as SSV, in 52.2, 70.4, 82.7 and 87.6% at 15, 30, 45 and 60 min of sonication respectively (Figure 3).
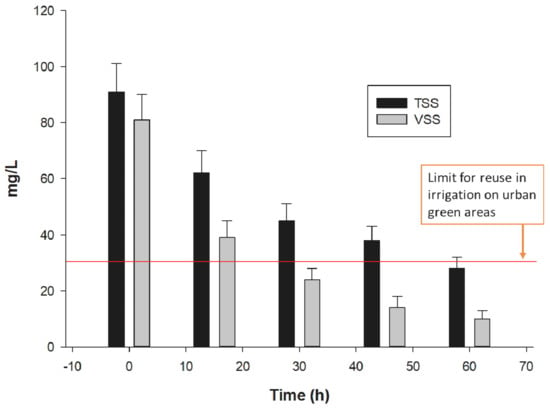
Figure 3.
Solid suspended removal by treatment with ultrasound at a frequency: 20 kHz, amplitude: 35% and sonication time: 15, 30, 45, 60 min. TSS = Total suspended solids and VSS = Volatile suspended solid.
Despite the fact that residual water samples had been treated by secondary settlers, the ultrasonic treatment allowed to reduce the concentrations until 18% of secondary treatment effluent concentration, complying with the established international values for direct human contact, including irrigation on urban green areas [2,21,25,44]. The high removal efficiencies of suspended solids, despite the low concentration of organic pollutants, is due to the diversity of mechanisms generated by ultrasound in aqueous solutions such as cavitation, shear forces and oxidation by generated hydroxyl radicals [22,46], with the advantage that microorganisms cannot adapt to these mechanisms unlike UV irradiation, and byproducts are not generated as in ozone and chlorination treatment [28,29,45].
3.3.3. Energy Consumption
In addition to the technical feasibility of the ultrasound for bacterial inactivation, it is important to consider the energy consumption of such technology on a large scale. In this sense, and based on the results of the previous sections, a sonication time of 45 min and an applied power of 20 W is considered, which is equivalent to an energy consumption of 12.5 kWh/m3 with the bacteriological characteristics and suspended solids evaluated in the present investigation. For UV disinfection (UVD), values between 0.5 and 10 kWh/m3 have been reported [48,49] and for ozonation (OZD) between 20 and 100 kWh/m3 [49,50].
The economic cost of using the ultrasound could be from 1.25 to 25 times that of UVD. However, ultrasound does not have the disadvantages of UVD related to the presence of suspended material, which hinders the penetration of light into the water column [48,50,51]. In contrast, the results of the present investigation demonstrated the reduction of suspended solids at the same time as disinfection. Another point to consider is the costs associated with lamp replacement at the UVD, which have a maximum duration of 0.7 years [49], while ultrasound only requires preventive maintenance every six months.
4. Conclusions
The present work contributes to establishing the design and operation parameters necessary for scaling ultrasound technology as a disinfection stage in municipal wastewater treatment trains. In effect, sonication results obtained suggest a cell wall affectation causing its lysis, reaching reductions of up to 6.45 logarithmic units of bacterial density. Sonication treatment reach sufficient water quality values parameters to comply with the maximum permissible limit of 240 MPN/100 mL indicated in the Official Mexican Standard [46], and established by WHO and EPA [2,40], resulting in high quality water suitable for reuse in activities related to direct public contact. In addition, these results were obtained considering high microbiological loads compared to previous studies.
Total inactivation of fecal coliform bacteria was obtained by the conjunction of 30 min of sonication time, 20 kHz frequency and 35% amplitude. Fecal coliform absence in treated wastewater is an indicator of safety for use in activities such as green irrigation areas. Ultrasound application would considerably reduce the demand for drinking water for this activity, and in addition could avoid the use of chemical agents that may be harmful to the health of ecosystems such as chlorine.
Besides, results of suspended solids removal indicate that the ultrasound treatment implementation could be technically feasible due to a significant decrease in the concentration of a wide range of pollutants after secondary treatment [20,21,32], so it is also suggested to evaluate, based on the results of this research, the effect of ultrasound on treated municipal wastewater, considering nitrogen, phosphorus, dissolved organic matter and other more resistant pathogens, such as helminths eggs and in general, physical-chemical monitoring parameters. With the analysis of these results and the analysis of the energy consumption carried out in this work, the technical and economic feasibility of the application of ultrasound on a large scale can be established.
Acknowledgments
The authors thank the National Council of Science and Technology of Mexico for the financial support given to the second author for the research.
Author Contributions
Adriana Roé-Sosa and Gabriela E. Moeller-Chávez conceived and designed the experiments; Monserrat Vázquez-Lópezper formed the experiments and analyzed the data; Juan L. García Rojas contributed reagents/materials/analysis tools; Adriana Roé-Sosa wrote the paper and analyzed the statistic section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP.241; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2015; Available online: https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf (accessed on 2 February 2018).
- UNESCO, U. N. United Nations World Report on Development of Water Resources. Wastewater, the Unused Resource. United Nations Educational Scientific; United Nations Educational Organization: Paris, France, 2015; pp. 1–7. [Google Scholar]
- Eddy, M.A.; Burton, F.L.; Tchobanoglous, G.; Tsuchihashi, R. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2013; p. 2048, ISSN-13 978-0073401188. [Google Scholar]
- Vera, I.K. Performance of 14 full-scale sewage treatment plants: Comparison between four aerobic technologies regarding effluent quality, sludge production and energy consumption. Environ. Technol. 2013, 34, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wanhong, G.; Wontae, L. Formation of disinfection byproducts upon chlorine dioxide preoxidation followed by chlorination or chloramination of natural organic matter. Chemosphere 2013, 91, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances & Disease Registry. Available online: https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=1079&tid=36 (accessed on 5 February 2018).
- Zhang, W.; Jia, B.; Wang, Q.; Dionysois, D. Visible-light sensitization of TiO2 photocatalysts via wet chemical N-doping for the degradation of dissolved organic compounds in wastewater treatment: A review. J. Nanopart. Res. 2015, 17, 221. [Google Scholar] [CrossRef]
- Ban, Y.; Wang, X. Features and Application of titanium Dioxide Thin Films in Water Treatment. Procedia Eng. 2011, 24, 663–666. [Google Scholar]
- Lazar, M.A.; Varghese, S.; Santhosh, S.N. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Shahid, M.; McDonagh, A.; Kim, J.H.; Shon, H.K. Magnetised titanium dioxide (TiO2) for water purification: Preparation, characterisation and application. Desalin. Water Treat. 2014, 54, 979–1002. [Google Scholar] [CrossRef]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl. Envrion. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.; Poulios, I.; Nikolakaki, E.; Mantzavinos, D. Sonochemical disinfection of municipal wastewater. J. Hazard. Mater. 2007, 146, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, A.; Joris, K.; Lambert, N.; Rediers, H.; Declerck, P.; Delaedt, Y.; Liers, S. Evaluation of process parameters of ultrasonic treatment of bacterial suspensions in a pilot scale water disinfection system. Ultrasound Sonochem. 2010, 17, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication tieme on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Furuta, M.; Yamaguchi, M.; Tsukamoto, T.; Yim, B.; Stavarache, C.; Hasiba, K.; Maeda, Y. Inactivation of Escherichia coli by Ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 57–60. [Google Scholar] [CrossRef]
- Carrére, H.; Dumas, C.; Battimelli, A.; Bastone, D.; Delgenés, J.; Steyer, J.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yadav, N.; Rawat, L.; Goyal, M. Effect of two waves of ultrasonic on waste treatment. Chem. Eng. Process Technol. 2014, 5, 3–6. [Google Scholar] [CrossRef]
- European Commission, Environment, Water. Available online: http://ec.europa.eu/environment/water/reuse-actions.htm (accessed on 4 February 2018).
- Wilson, N. Soil, Water and Groundwater Sampling, 1st ed.Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 145–197. ISBN 1-56670-073-6. [Google Scholar]
- Pahazri, N.F.G. Production and harvesting of microalgae biomass from wastewater: A critical review. Environ. Technol. Rev. 2016, 5, 39–56. [Google Scholar] [CrossRef]
- Europe Commission and Its Priorities. Available online: https://ec.europa.eu/info/law/better-regulation/initiatives/com-2017-753/feedback_en (accessed on 5 February 2018).
- Blume, T.; Neis, U. Improving clorine disinfection of wastewater by ultrasound application. Water Sci. Technol. 2005, 52, 139–144. [Google Scholar] [PubMed]
- Madigan, T.; Martinko, M.; Bender, S.; Buckley, H.; Stahl, A.; Brock, T. Brock Biology of Microorganisms, 14th ed.; Pearson: England, UK, 2015; pp. 176–210. ISBN 9788490352793. [Google Scholar]
- Watson, S.K. Carbon dioxide capture using Escherichia coli expressing carbonic anhydrase in a foam bioreactor. Environ. Technol. 2016, 37, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Lebotte, M.J.; Pierce, B.E. Microbiology Laboratory Theory and Application, 3rd ed.; Morton Publishing: Englewood, CO, USA, 2016; pp. 326–389. ISBN 978-1617314773. [Google Scholar]
- Bsoul, A.A.; Magnina, J.P.; Commenges-Bernolea, N.; Gondrexona, N.; Willison, J.; Petriera, C. Effectiveness of ultrasound for the destruction of Mycobacterium sp. strain (6PY1). Ultrason. Sonochem. 2010, 17, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, H.; Li, Z.; Zhao, J.; Yun, Y. Experimental study on the disinfection efficiencies of a continuous-flow ultrasound/ultraviolet baffled reactor. Ultrason. Sonochem. 2015, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ohrdes, H.; Ille, I.; Twiefel, J.; Wallaschek, J.; Noqueira, R.; Rosenwinkel, K. A control system for ultrasound devices utilized for inactivating E. coli in wastewater. Ultrason. Sonochem. 2018, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, N.; Landi, M.; Belgiorno, V.; Napoli, R. Wastewater disinfection by combination of ultrasound and ultraviolet irradiation. J. Hazard. Mater. 2009, 168, 925–992. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, G.; Willemse, M.; Hoefs, S.; Cremers, G.; Van den Heuvel, E. The Most Probable Limit of Detection (MPL) for rapid microbiological methods. J. Microbiol. Methods 2010, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.; Green, L.H. Practical Handbook of Microbiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 208–236. ISBN 1466587393. [Google Scholar]
- Novak, M.; Pfeiffer, T.; Lenski, R.E.; Sauer, U.; Bonhoeffer, S. Experimental tests for an evolutionary trade-off between growth rate and yield in E. coli. Am. Nat. 2006, 168, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Lalman, J.A.; Biswas, N. Biodegradation of Red B dye Bacillys sp. OY1-2. Environ. Technol. 2004, 25, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Glymph, T. Wastewater Microbiology a Handbook for Operators, 2nd ed.; American Water Works Association: Denver, CO, USA, 2011; pp. 25–36, ISSN-11 161300012X; ISSN-09 9781613000120. [Google Scholar]
- Ramalho, R.S. Introduction to Wastewater Treatment Processes, 2nd ed.; Academic Press: Quebec, QC, Canada, 2012; pp. 20–61. ISBN 0-12-576550-9. [Google Scholar]
- Master, M.; Ela, P. Introduction to Environmental Engineering and Science, 3th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2008; pp. 256–279. ISBN 0131481932. [Google Scholar]
- Saxena, G.; Bharagava, R.; Kaithwas, G.; Raj, A. Microbial indicators, pathogens and methods for their monitoring in water environment. J. Water Health 2015, 13, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Water Environments Federation; American Society of Civil Engineers; Envrionmental and Water Resources Institute. Design of Municpal Wastewater Treatment Plants, 5th ed.; McGraw-Hill: New York, NY, USA, 2009; pp. 502–526. ISBN 0071663584. [Google Scholar]
- Hadjianghelou, A.; Darakas, E. Survival of faecal coliforms in diluted sewage. Int. J. Environ. Stud. 2000, 58, 71–83. [Google Scholar] [CrossRef]
- Koivunen, J.; Siitonen, A.; Heinonen-Tanski, H. Elimination of enteric bacteria in biological-chemical wastewater treatment and tertiary filtration units. Water Res. 2003, 37, 690–698. [Google Scholar] [CrossRef]
- Payment, P.; Plante, R.; Cejka, P. Removal of indicator bacteria, human enteric viruses, Giardia Cysts, and Cryptosporidium oocysts at a large wastewater primary treatment facility. Can. J. Microbiol. 2001, 47, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mezule, L.T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination 2009, 248, 152–159. [Google Scholar] [CrossRef]
- Jyoti, K.; Pandit, A. Effect of cavitation on chemical disinfection efficiency. Water Res. 2004, 38, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- United States Envrionmental Protection Agency. Laws & Regulations. Available online: https://www.epa.gov/regulatory-information-topic/regulatory-information-topic-water#drinking (accessed on 5 February 2018).
- Ashokkumar, M. The characterization of acoustic cavitation bubbles–An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V.; Belgiorno, V.; Landi, M.; Zarra, T.; Napoli, R.M.A. Effect of sonolysis on waste activated sludge solubilisation and anaerobic biodegradability. Desalination 2009, 249, 762–767. [Google Scholar] [CrossRef]
- Neis, U.; Blume, T. Ultrasonic disinfection of wastewater effluents for high-quality reuse. Water Sci. Technol. Water Supply 2003, 3, 261–267. [Google Scholar]
- Macauley, J.J.; Qiang, Z.; Adams, C.D.; Surampalli, R.; Mormile, M.R. Disinfection of swine wastewater using chlorine, ultraviolet light and ozone. Water Res. 2006, 40, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Oriya Electricity and Lighting Ltd. Available online: http://www.oria.co.il/?categoryId=85627&itemId=189434 (accessed on 15 March 2018).
- Mizuta, K.; Shimda, M. Benchmarking energy consumption in municipal wastewater treatment plants in Japan. Water Sci. Technol. 2010, 62, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).