UV Sensitization of Nitrate and Sulfite: A Powerful Tool for Groundwater Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
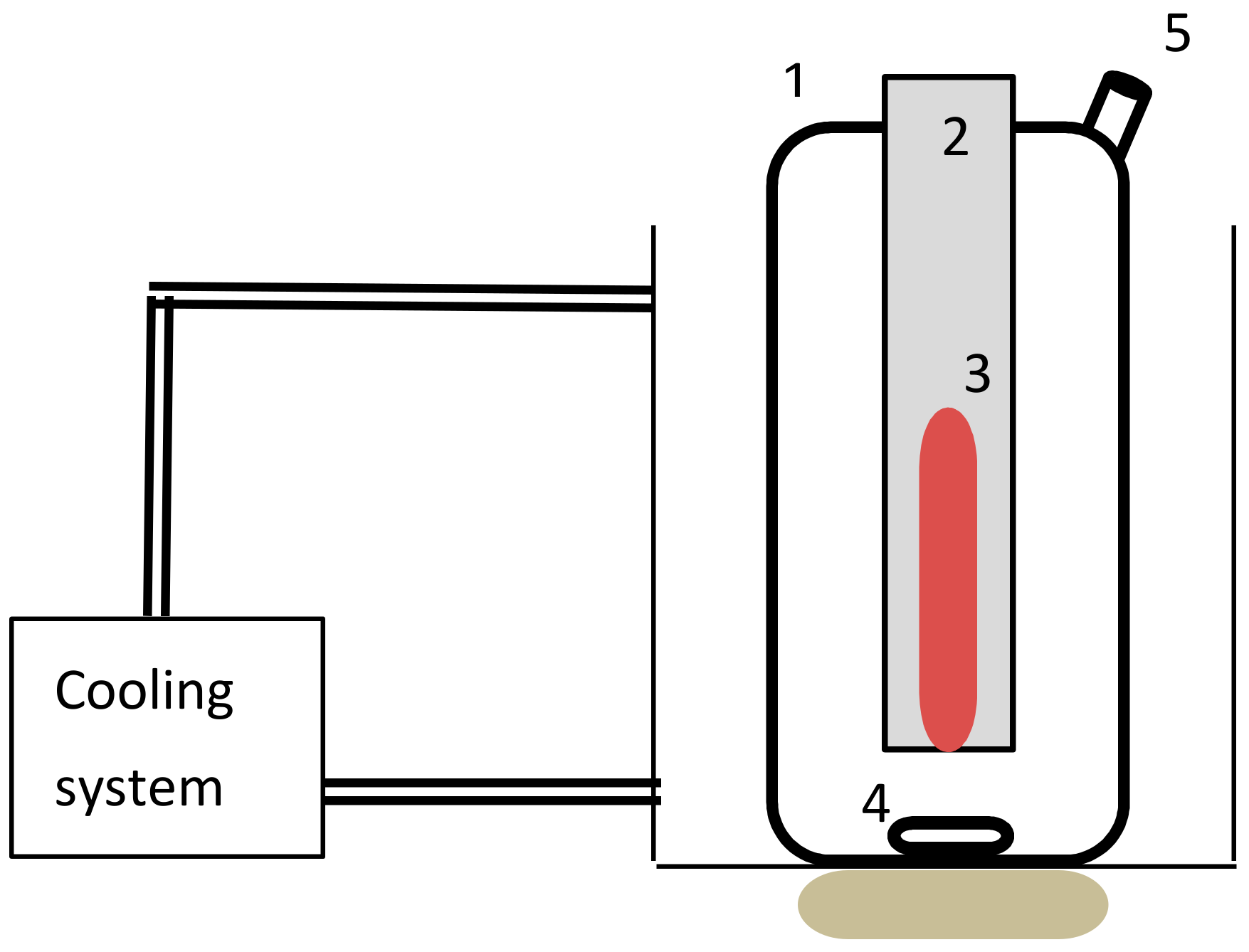
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
3. Results and Discussion
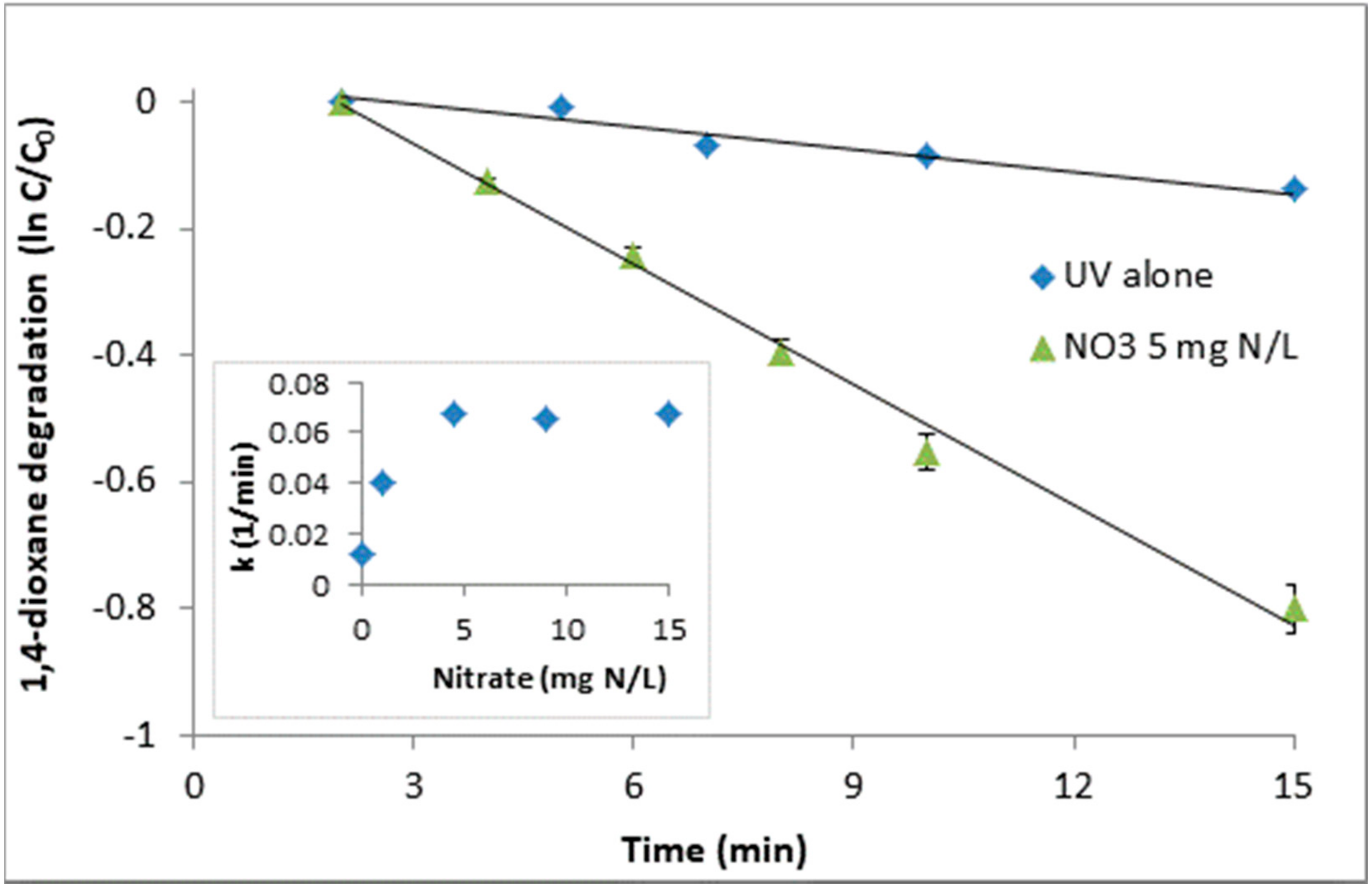
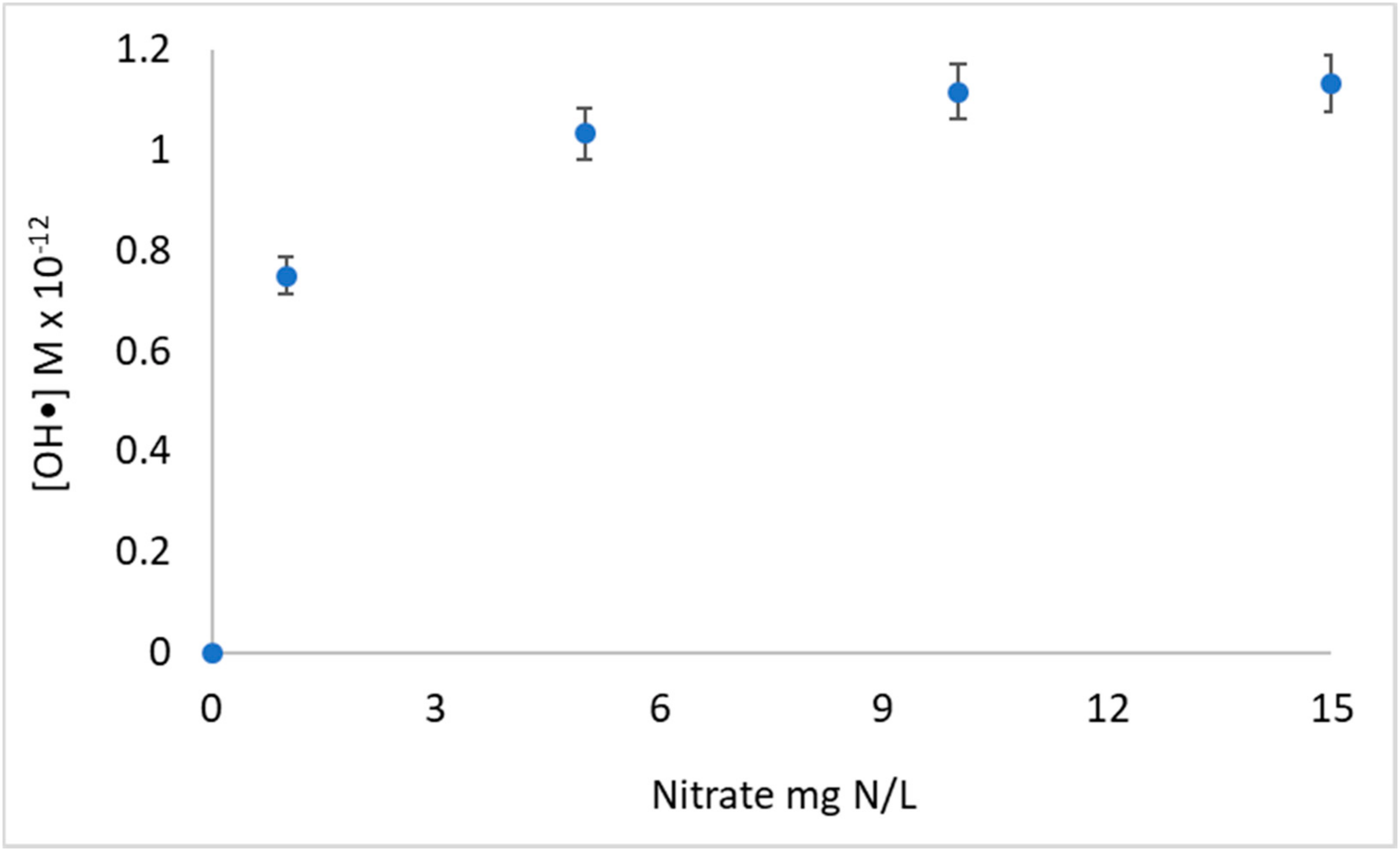
3.1. UV/NO3 Degradation of 1,4-dioxane: The Impact of Initial Nitrate Concentration
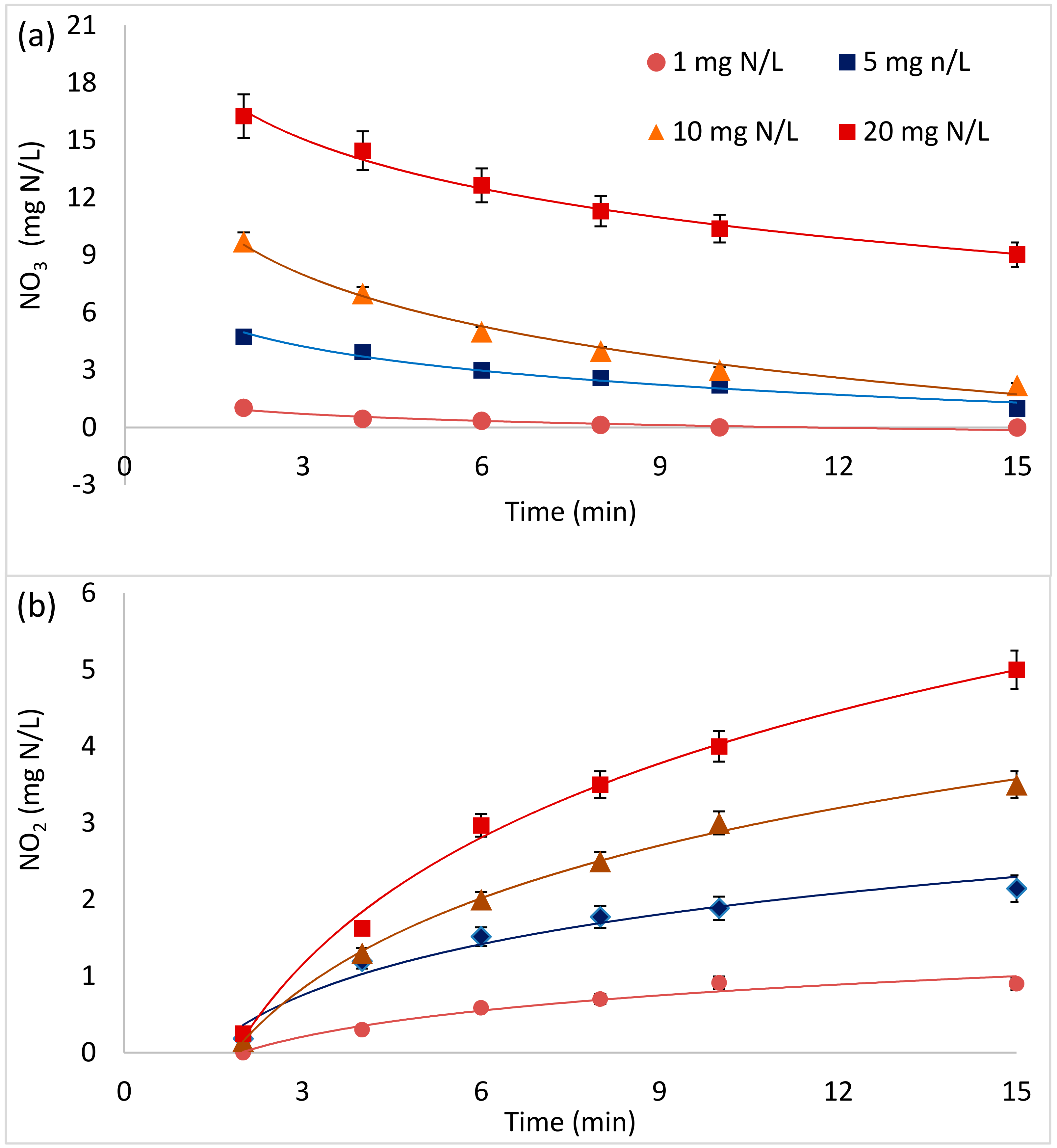
3.2. Nitrate Decay and Nitrite Formation
3.3. Effect of Bicarbonate on the UV/NO3 Process
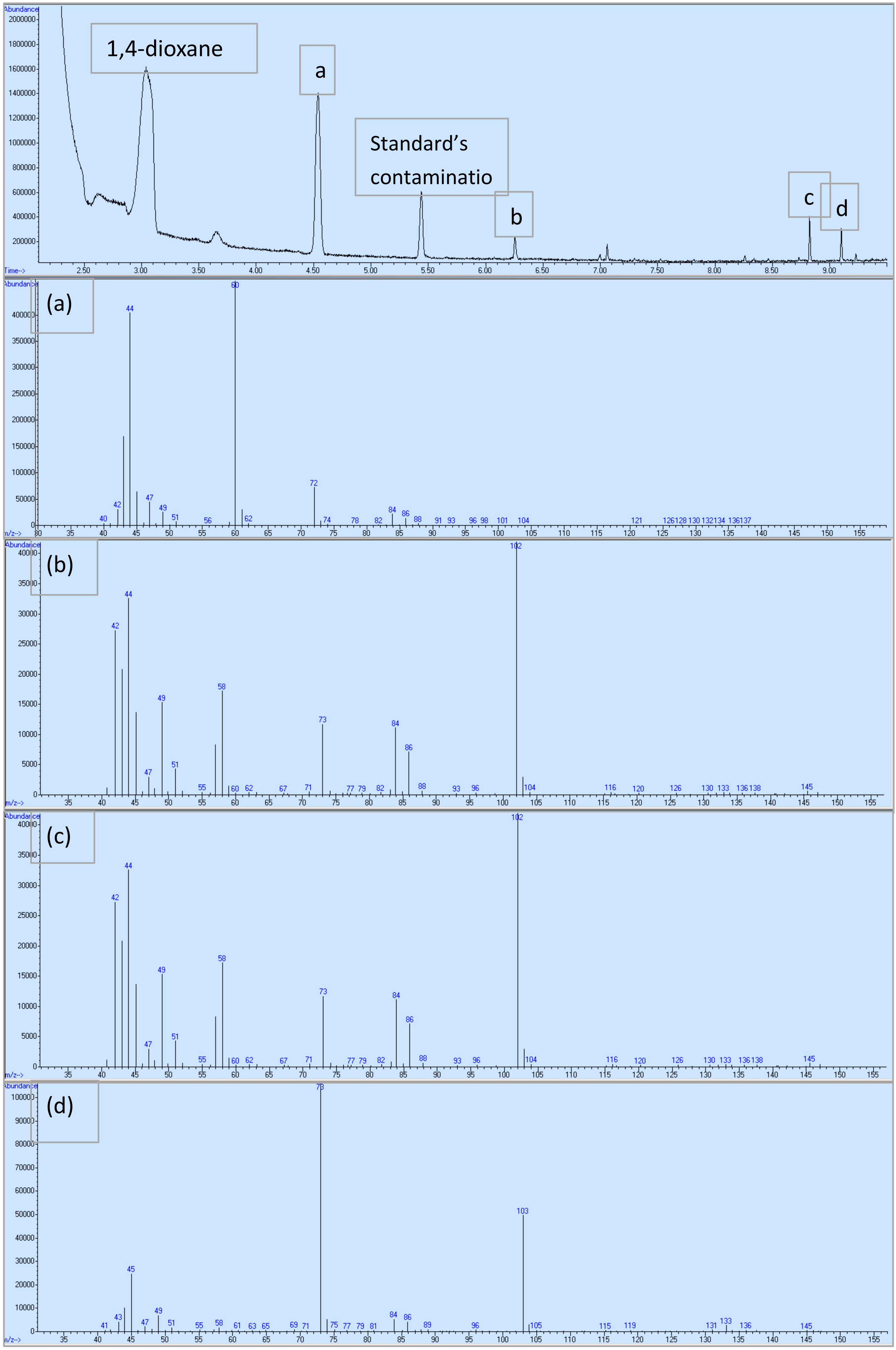
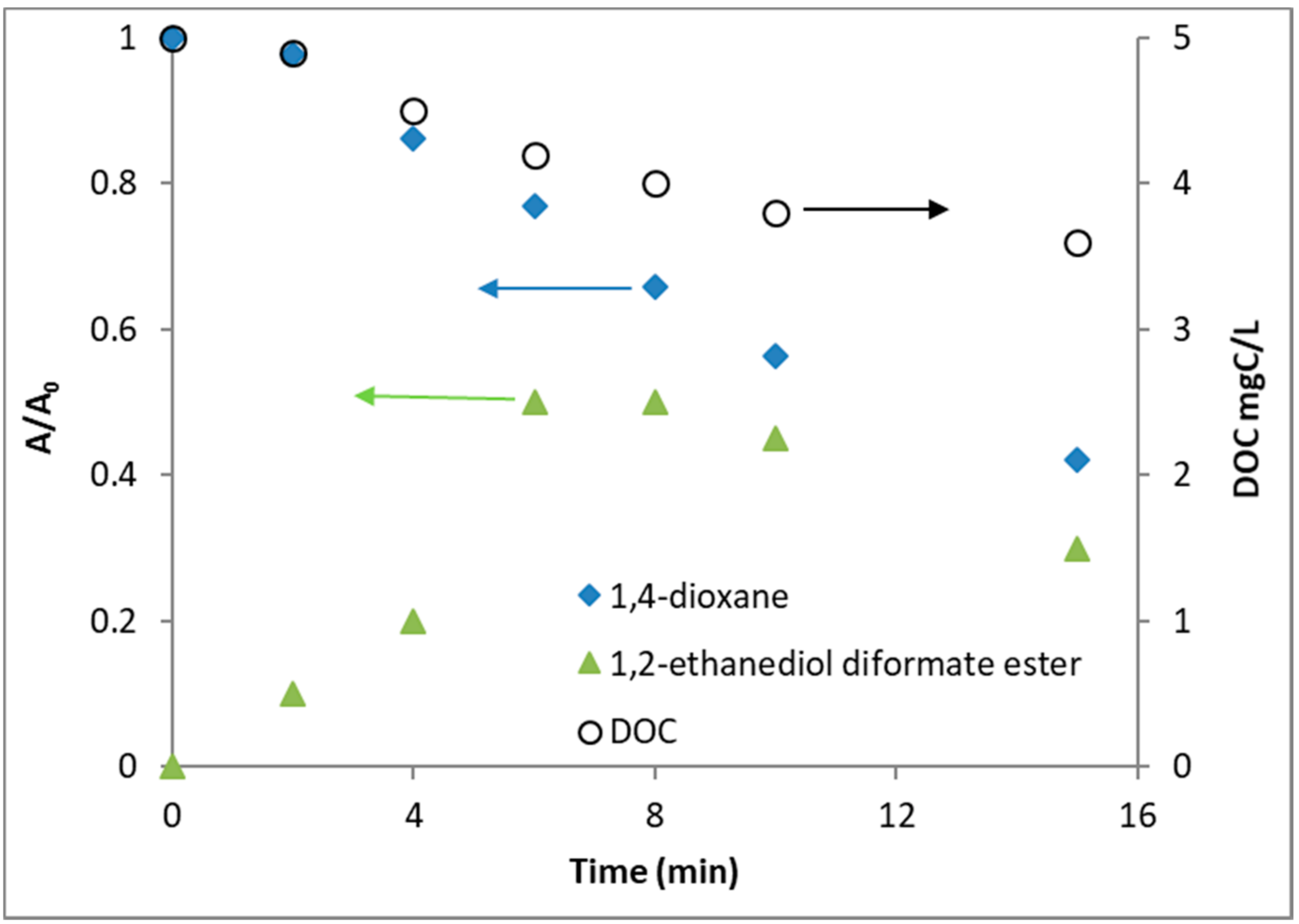
3.4. 1,4-Dioxane Oxidation Intermediates
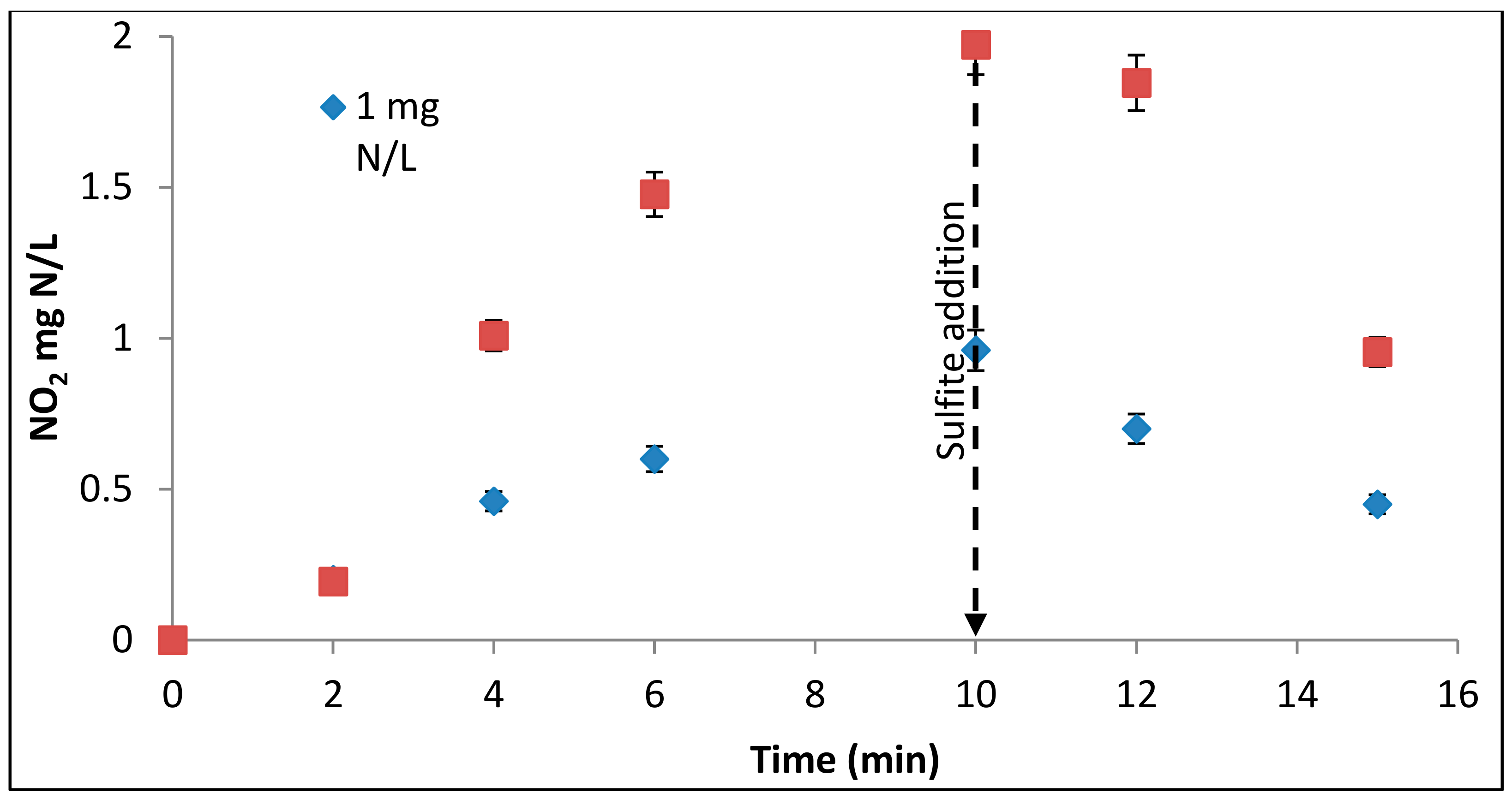
3.5. Nitrite Reduction by UV/SO3
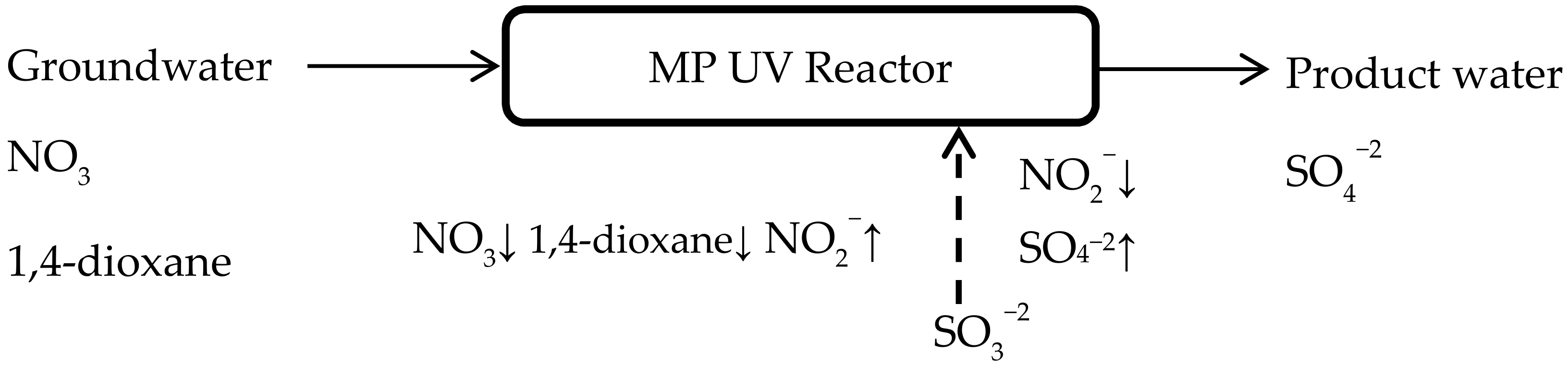
3.6. Implications for Groundwater Remediation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zafiriou, O.C.; Bonneau, R. Wavelength-dependent quantum yield of OH radical formation from photolysis of nitrite ion in water. Photochem. Photobiol. 1987, 45, 723–727. [Google Scholar] [CrossRef]
- Zepp, R.G.; Holgne, J.; Bader, H. Nitrate-induced photooxidation of trace organic chemicals in water. Environ. Sci. Technol. 1987, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Marotta, R.; Paxéus, N. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Daniels, M.; Meyers, R.V.; Belardo, EV. Photochemistry of the aqueous nitrate system. I. Excitation in the 300-m.mu. band. J. Phys. Chem. 1968, 72, 389–399. [Google Scholar] [CrossRef]
- Shuali, U.; Ottolenghi, M.; Rabani, J.; Yelin, Z. On the photochemistry of aqueous nitrate solutions excited in the 195-nm band. J. Phys. Chem. 1969, 73, 3445–3451. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Advanced reduction processes: A new class of treatment processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Love, N.G.; Linden, K.G. The role of effluent nitrate in trace organic chemical oxidation during UV disinfection. Water Res. 2012, 46, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Lester, Y.; Ferrer, I.; Thurman, E.M.; Linden, K.G. Demonstrating sucralose as a monitor of full-scale UV/AOP treatment of trace organic compounds. J. Hazard Mater. 2014, 280, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ge, Y.; Chang, S.X.; Luo, W.; Chang, J. Nitrate in groundwater of China: Sources and driving forces. Glob. Environ. Chang. 2013, 23, 1112–1121. [Google Scholar] [CrossRef]
- Haran, M.; Samuels, R.; Uri Mingelgrin, S.G. Quality indicators of the state of chemical pollution in Israel. Isr. J. Chem. 2002, 42, 119–132. [Google Scholar] [CrossRef]
- Zhou, Z. A Global Assessment of Nitrate Contamination in Groundwater-Internship report. International Groundwater Resources Assessment Center 2015. Available online: https://www.un-igrac.org/resource/global-assessment-nitrate-contamination-groundwater (accessed on 20 January 2015).
- Shalev, N.; Burg, A.; Gavrieli, I.; Lazar, B. Nitrate contamination sources in aquifers underlying cultivated fields in an arid region-The Arava Valley, Israel. Appl. Geochem. 2015, 63, 322–332. [Google Scholar] [CrossRef]
- Nolan, B.T.; Ruddy, B.C.; Hitt, K.J.; Helsel, D.R. Risk of nitrate in groundwaters of the United States—A national perspective. Environ. Sci. Technol. 1997, 31, 2229–2236. [Google Scholar] [CrossRef]
- Spalding, R.F.; Exner, M.E. Occurrence of Nitrate in Groundwater—A Review. J. Environ. Qual. 1993, 22, 392. [Google Scholar] [CrossRef]
- Adamson, D.T.; Mahendra, S.; Walker, K.L.; Rauch, S.R.; Sengupta, S.; Newell, C.J. A Multisite Survey to Identify the Scale of the 1,4-Dioxane Problem at Contaminated Groundwater Sites. Environ. Sci. Technol. Lett. 2014, 1, 254–258. [Google Scholar] [CrossRef]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Pye, V.I.; Patrick, R. Ground Water Contamination in the United States. Science 1983, 221, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.K.G. Environmental Investigation and Remediation: 1,4-Dioxane and other Solvent Stabilizers; CRC Press: Boca Roca, FL, USA, 2010. [Google Scholar]
- Abe, A. Distribution of 1,4-dioxane in relation to possible sources in the water environment. Sci. Total Environ. 1999, 227, 41–47. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency; Integrated Risk Information System (IRIS). 1,4-Dioxane; National Center for Environmental Assessment: Washington, DC, USA, 1999.
- 1,4-Dioxane in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/14dioxane0505.pdf (accessed on 12 August 2005).
- Otto, M.; Nagaraja, S. Treatment technologies for 1,4-Dioxane: Fundamentals and field applications. Remediation 2007, 17, 81–88. [Google Scholar] [CrossRef]
- Stefan, M.I.; Bolton, J.R. Mechanism of the degradation of 1,4-dioxane in dilute aqueous solution using the UV/hydrogen peroxide process. Environ. Sci. Technol. 1998, 32, 1588–1595. [Google Scholar] [CrossRef]
- Vescovi, T.; Coleman, H.M.; Amal, R. The effect of pH on UV-based advanced oxidation technologies-1,4-Dioxane degradation. J. Hazard Mater. 2010, 182, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Corena, J.R.; Bergendahl, J.A.; Hart, F.L. Advanced oxidation of five contaminants in water by UV/TiO2: Reaction kinetics and byproducts identification. J. Environ. Manag. 2016, 181, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, E.J.; Linden, K.G. The ROH, UV concept to characterize and the model UV/H2O2 process in natural waters. Environ. Sci. Technol. 2007, 41, 2548–2553. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Linden, K.G. UV photolysis of nitrate: Effects of natural organic matter and dissolved inorganic carbon and implications for UV water disinfection. Environ. Sci. Technol. 2001, 35, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Batchelor, B. Impacts of natural organic matter on perchlorate removal by an advanced reduction process. J. Environ. Sci. Health Part A Toxic Hazardous Subst. Environ. Eng. 2014, 49, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, B.P.; Batchelor, B. Perchlorate reduction by the sulfite/ultraviolet light advanced reduction process. J. Hazard Mater. 2013, 262, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Degradation of 1,2-dichloroethane with advanced reduction processes (ARPs): Effects of process variables and mechanisms. Chem. Eng. J. 2014, 237, 300–307. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, S.; Li, L.; Wang, T.; Liao, X.; Ye, Y. An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, C.; Zheng, W.; Wang, G. Degradation of Polybrominated Aiphenyl Ethers in a UV Advanced Reduction Process with Different Reducing Agents. IOP Conf. Ser. Earth Environ. Sci. 2018, 113. [Google Scholar] [CrossRef]
- Bensalah, N.; Nicola, R.; Abdel-Wahab, A. Nitrate removal from water using UV-M/S2O42-advanced reduction process. Int. J. Environ. Sci. Technol. 2014, 11, 1733–1742. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. Actinometers Based on NO3− and H2O2 Excitation: Applications for Industrial Photoreactors. Environ. Sci. Technol. 2008, 42, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Draper, W.M.; Dhoot, J.S.; Remoy, J.W.; Perera, S.K. Trace-level determination of 1,4-dioxane in water by isotopic dilution GC and GC-MS. Analyst 2000, 125, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O)−in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Peldszus, S.; Andrews, S.A.; Souza, R.; Smith, F.; Douglas, I.; Bolton, J.; Huck, P.M. Effect of medium-pressure UV irradiation on bromate concentrations in drinking water, a pilot-scale study. Water Res. 2004, 38, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Elvis, X.; Schlenk, D.; Haizhou, L. Cyto- and geno-toxicity of 1,4-dioxane and its transformation products during ultraviolet-driven advanced oxidation processes. Environ. Sci. Water Res. Technol. 2018, 4, 1213. [Google Scholar] [CrossRef]
- Maurino, V.; Calza, P.; Minero, C.; Pelizzetti, E.; Vincenti, M. Light-assisted 1,4-dioxane degradation. Chemosphere 1997, 35, 2675–2688. [Google Scholar] [CrossRef]
- Gonzalez, M.G.; Oliveros, E.; Wörner, M.; Braun, A.M. Vacuum-ultraviolet photolysis of aqueous reaction systems. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 225–246. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, C.; Zhao, X.; Chen, J.; Zhou, Q. Efficient photoreductive decomposition of N-nitrosodimethylamine by UV/iodide process. J. Hazard Mater. 2017, 329, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Curry, M.A. 1,4 dioxane removal from groundwater using point-of-entry water treatment techniques. Master’s Theses, University of New Hampshire, Durham, NH, USA, September 2012. [Google Scholar]
- Dzengel, J.; Theurich, J.; Bahnemann, D.W. Formation of nitroaromatic compounds in advanced oxidation processes: Photolysis versus photocatalysis. Environ. Sci. Technol. 1999, 33, 294–300. [Google Scholar] [CrossRef]

| Initial NO3 | Sulfite (mg/L) | Sulfate (mg/L) | ||
|---|---|---|---|---|
| 12 min | 15 min | 12 min | 15 min | |
| 1 mg N/L | 35 (±4) | 12 (±2) | 11 (±3) | 23 (±4) |
| 5 mg N/L | 61 (±7) | 42 (±5) | 15 (±2) | 28 (±5) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lester, Y.; Dabash, A.; Eghbareya, D. UV Sensitization of Nitrate and Sulfite: A Powerful Tool for Groundwater Remediation. Environments 2018, 5, 117. https://doi.org/10.3390/environments5110117
Lester Y, Dabash A, Eghbareya D. UV Sensitization of Nitrate and Sulfite: A Powerful Tool for Groundwater Remediation. Environments. 2018; 5(11):117. https://doi.org/10.3390/environments5110117
Chicago/Turabian StyleLester, Yaal, Asmaa Dabash, and Darine Eghbareya. 2018. "UV Sensitization of Nitrate and Sulfite: A Powerful Tool for Groundwater Remediation" Environments 5, no. 11: 117. https://doi.org/10.3390/environments5110117
APA StyleLester, Y., Dabash, A., & Eghbareya, D. (2018). UV Sensitization of Nitrate and Sulfite: A Powerful Tool for Groundwater Remediation. Environments, 5(11), 117. https://doi.org/10.3390/environments5110117




