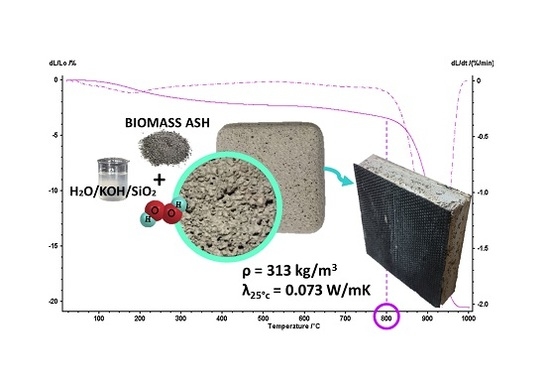
Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Mineralogical Characterization
3.2. Macro and Microstructural Characterization
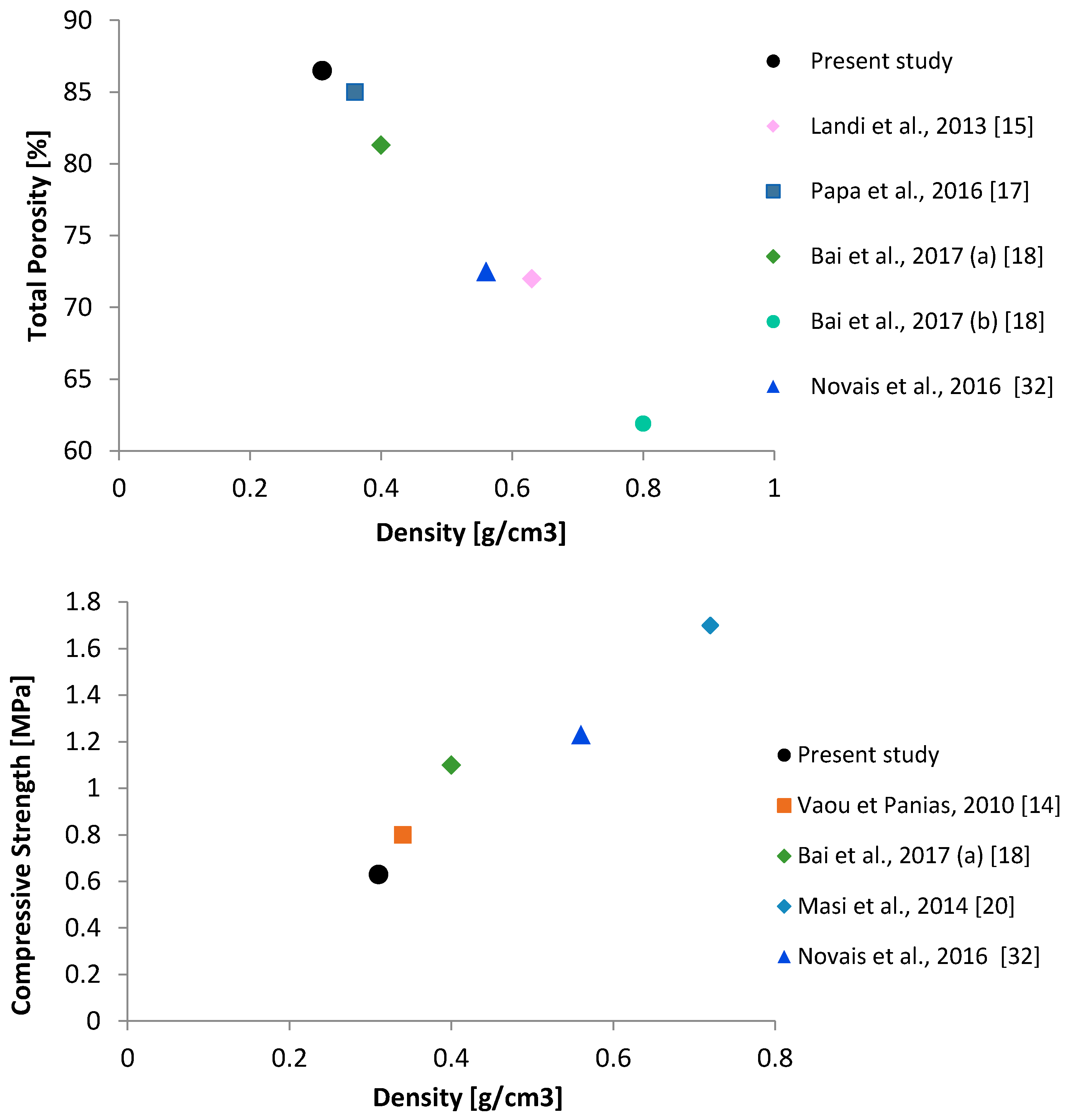
3.3. Density, Pore Size Distribution and Compressive Strength
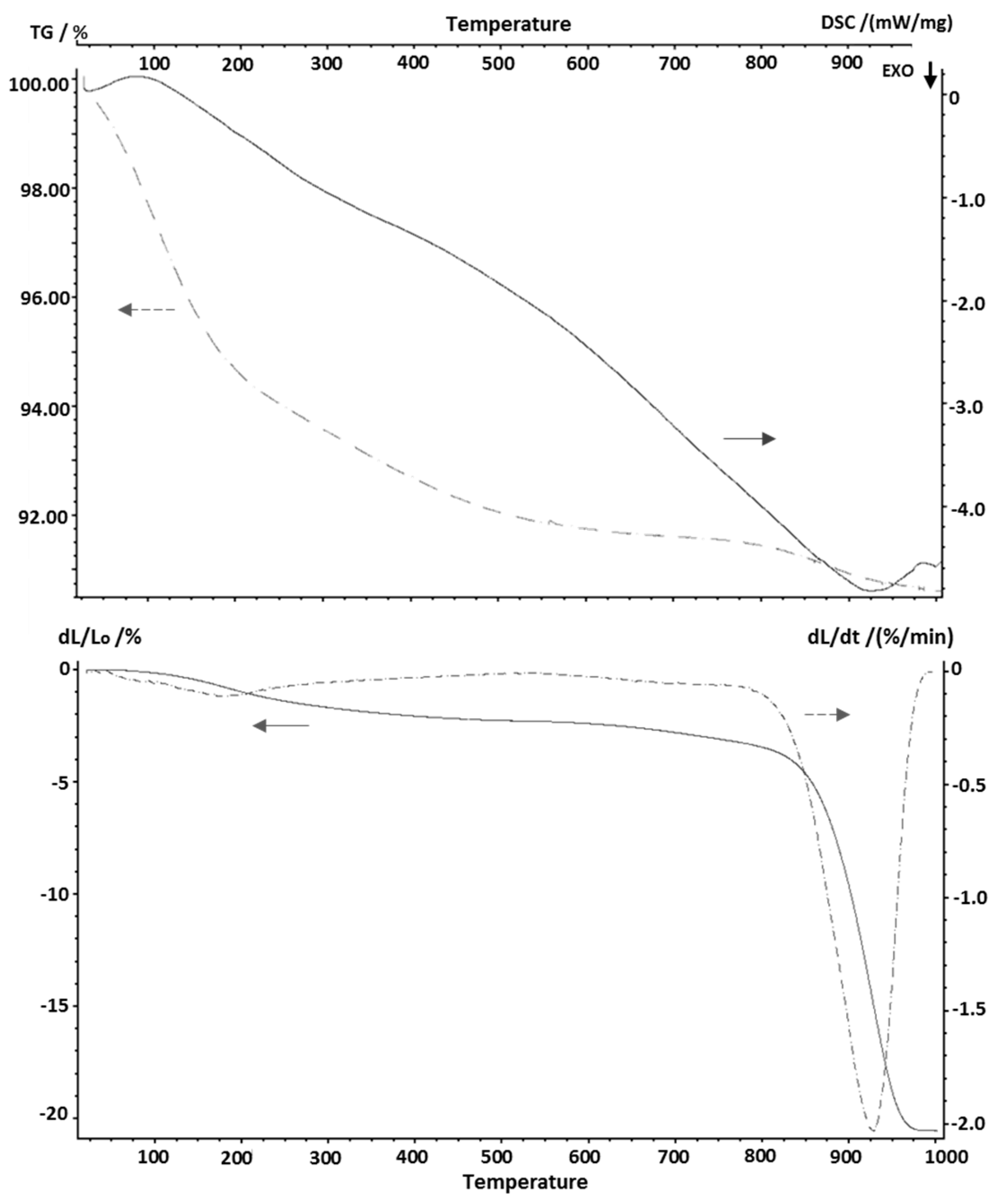
3.4. Thermal Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Schiavoni, S.; D’Alessandro, F.; Bianchi, F.; Asdrubali, F. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Sudareva, N.G.; Smyslova, L.A. Domestic heat-insulation materials for shipbuilding. Russ. J. Gen. Chem. 2010, 80, 2134–2142. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mazzocchi, M.; Laghi, L.; Morganti, M.; Francisconi, J.; Landi, E. Production and characterization of lightweight vermiculite/geopolymer-based panels. Mater. Des. 2015, 85, 266–274. [Google Scholar] [CrossRef]
- Peacock, R.D.; Reneke, P.A.; Jones, W.W.; Bukowski, R.W.; Babrauskas, V. Concepts for fire protection of passenger rail transportation vehicles: Past, present and future. Fire Mater. 1995, 19, 71–87. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Thue, J.V.; Tenpierik, M.J.; Grynning, S.; Uvsløkk, S.; Gustavsen, A. Vacuum insulation panels for building applications: A review and beyond. Energy Build. 2010, 42, 147–172. [Google Scholar] [CrossRef]
- Fongang, R.T.T.; Pemndje, J.; Lemougna, P.N.; Chinje Melo, U.; Nanseu, C.P.; Nait-Ali, B.; Kamseu, E.; Leonelli, C. Cleaner production of the lightweight insulating composites: Microstructure, pore network and thermal conductivity. Energy Build. 2015, 107, 113–122. [Google Scholar] [CrossRef]
- Natali Murri, A.; Medri, V.; Landi, E. Production and thermomechanical characterization of wool–geopolymer composites. J. Am. Ceram. Soc. 2017, 100, 2822–2831. [Google Scholar] [CrossRef]
- Kamseu, E.; NGouloure, Z.N.M.; Nait-Ali, B.; Zekeng, S.; Melo, U.C.; Rossignol, S.; Leonelli, C. Cumulative pore volume, pore size distribution and phases percolation in porous inorganic polymer composites: Relation microstructure and effective thermal conductivity. Energy Build. 2015, 88, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Lyon, R.E.; Balaguru, P.N.; Foden, A.; Sorathia, U.; Davidovits, J.; Davidovics, M. Fire-resistant aluminosilicate composites. Fire Mater. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 2nd ed.; Geopolymer Institute: Saint Quentin, France, 2008; ISBN 9782951482098. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Kriven, W.M.; Bell, J.L.; Gordon, M. Microstructure and microchemistry of fully-reacted geopolymers and geopolymer matrix composites. Ceram. Trans. 2003, 153, 227–250. [Google Scholar] [CrossRef]
- Vaou, V.; Panias, D. Thermal insulating foamy geopolymers from perlite. Miner. Eng. 2010, 23, 1146–1151. [Google Scholar] [CrossRef]
- Landi, E.; Medri, V.; Papa, E.; Dedecek, J.; Klein, P.; Benito, P.; Vaccari, A. Alkali-bonded ceramics with hierarchical tailored porosity. Appl. Clay Sci. 2013, 73, 56–64. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Dedecek, J.; Jirglova, H.; Benito, P.; Vaccari, A.; Landi, E. Effect of metallic Si addition on polymerization degree of foamed alkali-aluminosilicates. Ceram. Int. 2013, 39, 7657–7668. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and insulating properties of silica-fume based foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram. Int. 2017, 43, 2267–2273. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Vickers, L.; Bignozzi, M.C.; van Riessen, A. A comparison between different foaming methods for the synthesis of light weight geopolymers. Ceram. Int. 2014, 40, 13891–13902. [Google Scholar] [CrossRef]
- Kamseu, E.; Nait-Ali, B.; Bignozzi, M.C.; Leonelli, C.; Rossignol, S.; Smith, D.S. Bulk composition and microstructure dependence of effective thermal conductivity of porous inorganic polymer cements. J. Eur. Ceram. Soc. 2012, 32, 1593–1603. [Google Scholar] [CrossRef]
- Komnitsas, K.A. Potential of geopolymer technology towards green buildings and sustainable cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- Natali Murri, A.; Medri, V.; Ruffini, A.; Papa, E.; Landi, E. Study of the chemical activation of hydroxyapatite rich ashes as raw materials for geopolymers. Ceram. Int. 2015, 41, 9734–9744. [Google Scholar] [CrossRef]
- Natali Murri, A.; Medri, V.; Piancastelli, A.; Vaccari, A.; Landi, E. Production and characterization of geopolymer blocks based on hydroxyapatite rich biomass ashes. Ceram. Int. 2015, 41, 12811–12822. [Google Scholar] [CrossRef]
- Mingazzini, C.; Scafè, M.; Mazzanti, F.; Bezzi, F.; Giorgini, L.; Zattini, G.; D’Angelo, E.; Laghi, L.; De Aloysio, G.; Bandini, S. Flame resistant composite panels processed from Preceramic Prepregs. In Proceedings of the 15th Conference & Exhibition of the European Ceramic Society ECERS 2017, Budapest, Hungary, 9–13 July 2017. [Google Scholar]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Smith, M.E.; Wong, A. A multinuclear MAS NMR study of calcium-containing aluminosilicate inorganic polymers. J. Mater. Chem. 2007, 17, 5090–5096. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram NaO–CaO–AlO–SiO–HO. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.A.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Roels, S.; Elsen, J.; Carmeliet, J.; Hens, H. Characterization of pore structure by combining mercury porosimetry and micrography. Mater. Struct. 2001, 34, 76–82. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of ceramics from metakaolin-based geopolymers. Part II: K-based geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Natali Murri, A.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. High temperature behavior of ambient cured alkali-activated materials based on ladle slag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Potassium geopolymer foams made with silica fume pore forming agent for thermal insulation. J. Porous Mater. 2013, 20, 37–46. [Google Scholar] [CrossRef]
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Compos. 2000, 22, 321–329. [Google Scholar] [CrossRef]
- Hamad, A.J. Materials, production, properties and application of aerated lightweight concrete: Review. Int. J. Mater. Sci. Eng. 2014, 2, 152–157. [Google Scholar] [CrossRef]

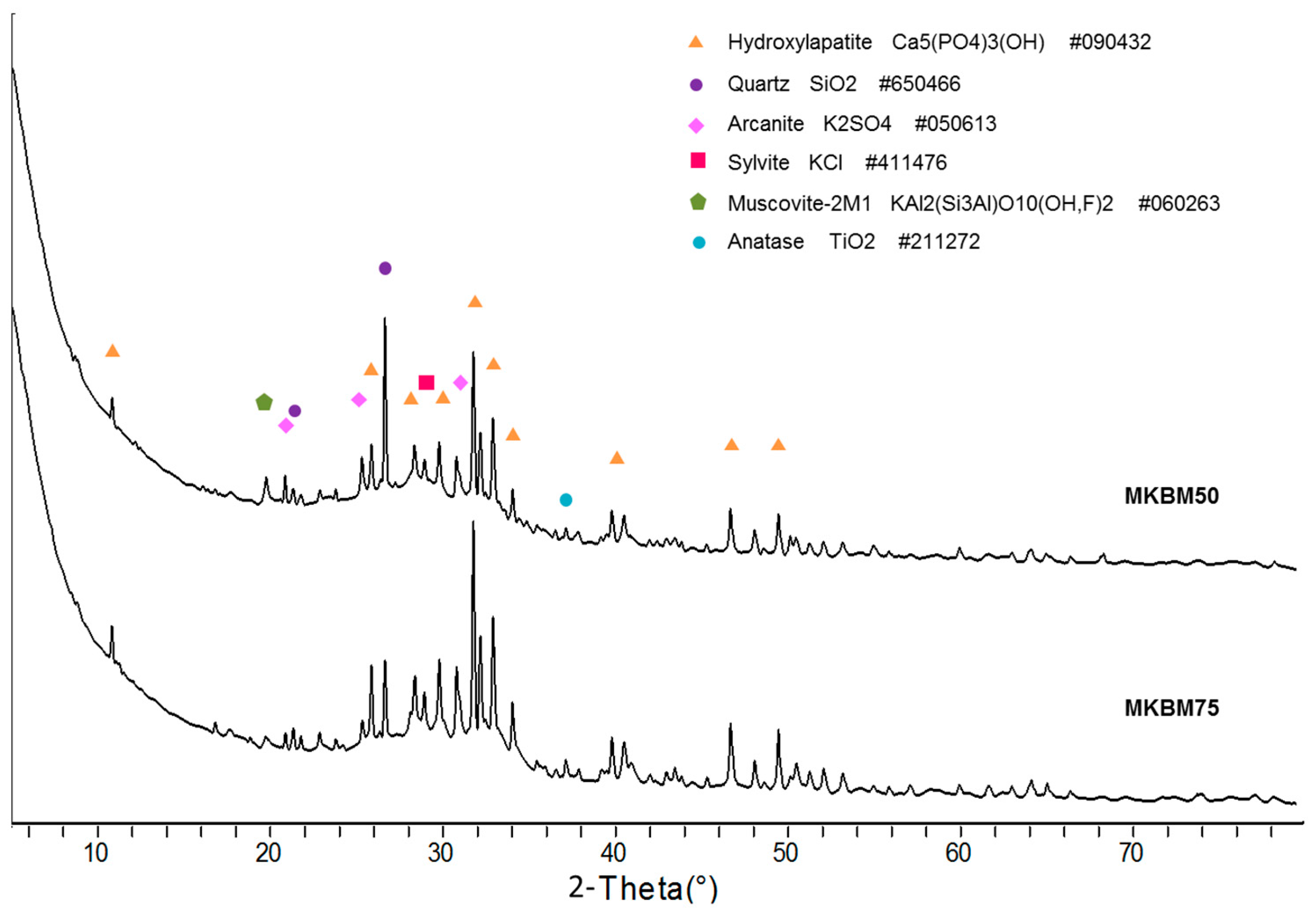
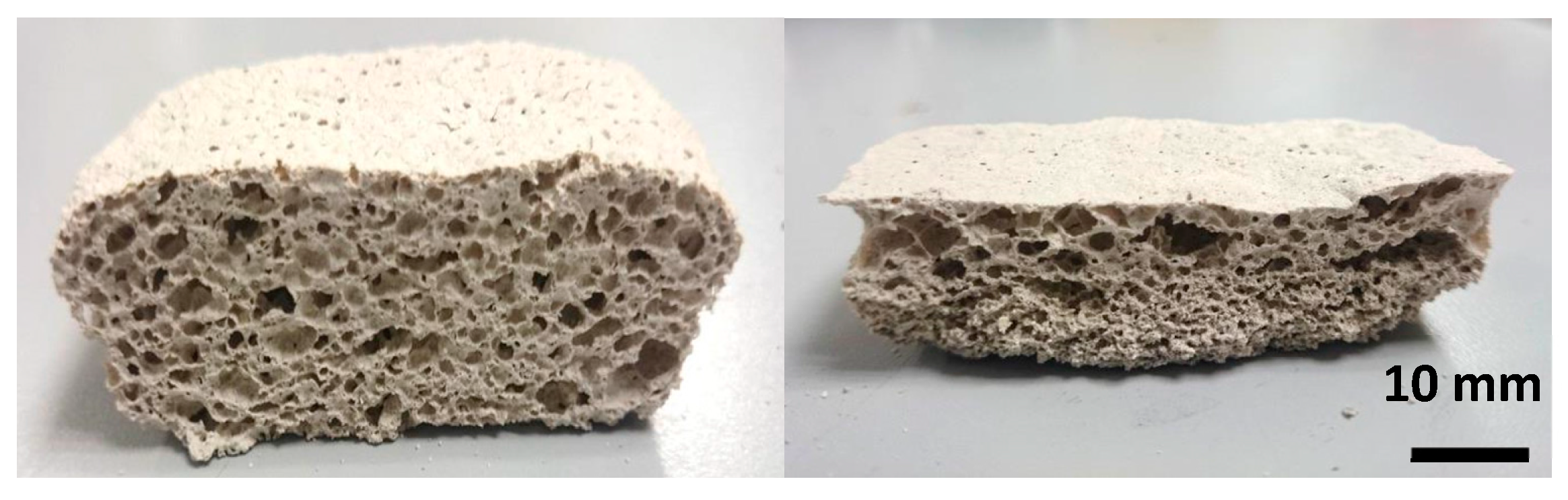
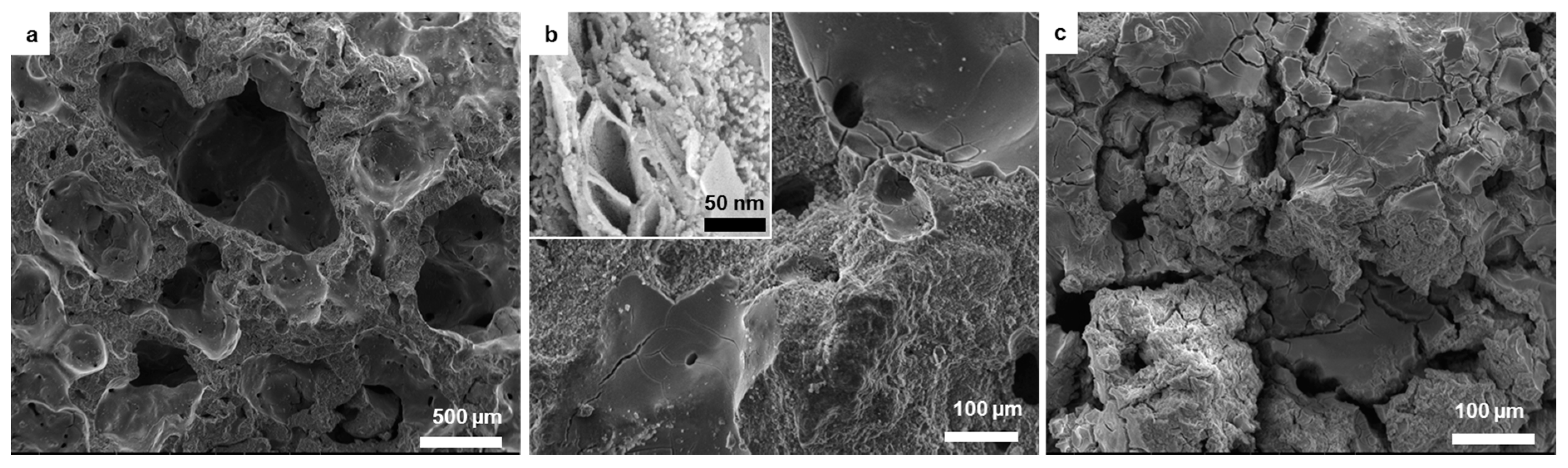

| Element | O | Ca | P | K | Cl | Si | Mg | Na | Fe | S | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [wt. %] | 51.3 | 22.3 | 5.2 | 6.8 | 3.1 | 2.8 | 2.1 | 2.0 | 2.0 | 1.8 | 0.7 |
| Mixture | MK [wt. %] | BM [wt. %] | KS23 [wt. %] | H2O2 [wt. % on the Whole Mixture] |
|---|---|---|---|---|
| MKBM50 | 25.0 | 25.0 | 50.0 | 5 |
| MKBM75 | 12.5 | 37.5 | 50.0 | 5 |
| Average Elemental Ratios [At. %] | MKBM50 | MKBM75 | (Ca, K)ASH Garcia-Lodeiro et al. [28] |
|---|---|---|---|
| Ca/Si | 0.1 | 0.2 | 0 < Ca/Si < 0.3 |
| Si/Al | 2.1 | 2.8 | 1.2 < Si/Al < 10 |
| K/Al | 0.5 | 3.5 | 0 < K/Al < 1.9 |
| Sample | ρb [kg/m3] | ρt [kg/m3] | Po [vol. %] | Pc-u [vol. %] | Pt [vol. %] | Median Pore Diameter (0.01–1000 µm) [µm] |
|---|---|---|---|---|---|---|
| MKBM50 | 313 ± 3 | 2288 ± 1 | 54.4 | 32.1 | 86.5 | 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natali Murri, A.; Medri, V.; Papa, E.; Laghi, L.; Mingazzini, C.; Landi, E. Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite. Environments 2017, 4, 86. https://doi.org/10.3390/environments4040086
Natali Murri A, Medri V, Papa E, Laghi L, Mingazzini C, Landi E. Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite. Environments. 2017; 4(4):86. https://doi.org/10.3390/environments4040086
Chicago/Turabian StyleNatali Murri, Annalisa, Valentina Medri, Elettra Papa, Luca Laghi, Claudio Mingazzini, and Elena Landi. 2017. "Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite" Environments 4, no. 4: 86. https://doi.org/10.3390/environments4040086
APA StyleNatali Murri, A., Medri, V., Papa, E., Laghi, L., Mingazzini, C., & Landi, E. (2017). Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite. Environments, 4(4), 86. https://doi.org/10.3390/environments4040086