Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin
Abstract
:1. Introduction
- Decrease the amount of sodium silicate solution used;
- Preserve the three-dimensional amorphous network built from TO4 (T = Si, Al) tetrahedral, joined at the corners with oxygen;
- Guarantee adequate mechanical properties.
2. Materials and Methods
2.1. Materials
2.1.1. Metakaolin, Sodium Silicate Solition and Sodium Hydroxide
2.1.2. Forest Biomass Ash
2.1.3. Aggregates
2.2. Methods
2.2.1. Mixture Proportioning and Mixing
2.2.2. Workability
2.2.3. Mechanical Strength
2.2.4. Capillary Water Absorption
2.2.5. Shrinkage Test
2.2.6. Thermal Cycles
3. Results and Discussion
4. Conclusions
- The inclusion of FBA as a partial replacement of metakaolin in geopolymer mortars results in a proportional increasing of workability. This behavior can be ascribed to both an increased rate in the dissolution of metakaolin due to both the higher alkalinity of the reaction mixes containing FBA their greater average particles size than metakaolin.
- Weight ratio (metakaolin + FBA)/ alkali silicate solution (dry basis) increases from 1.35/1 when only metakaolin is used to 1.7:1 when 30% wt of FBA is used.
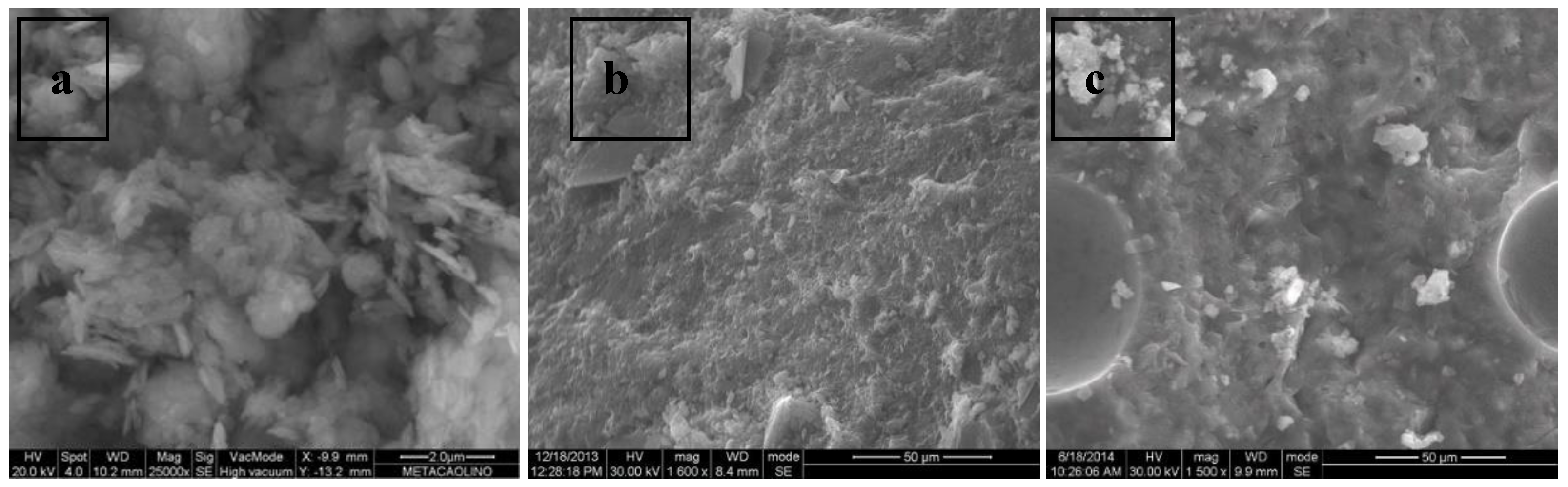
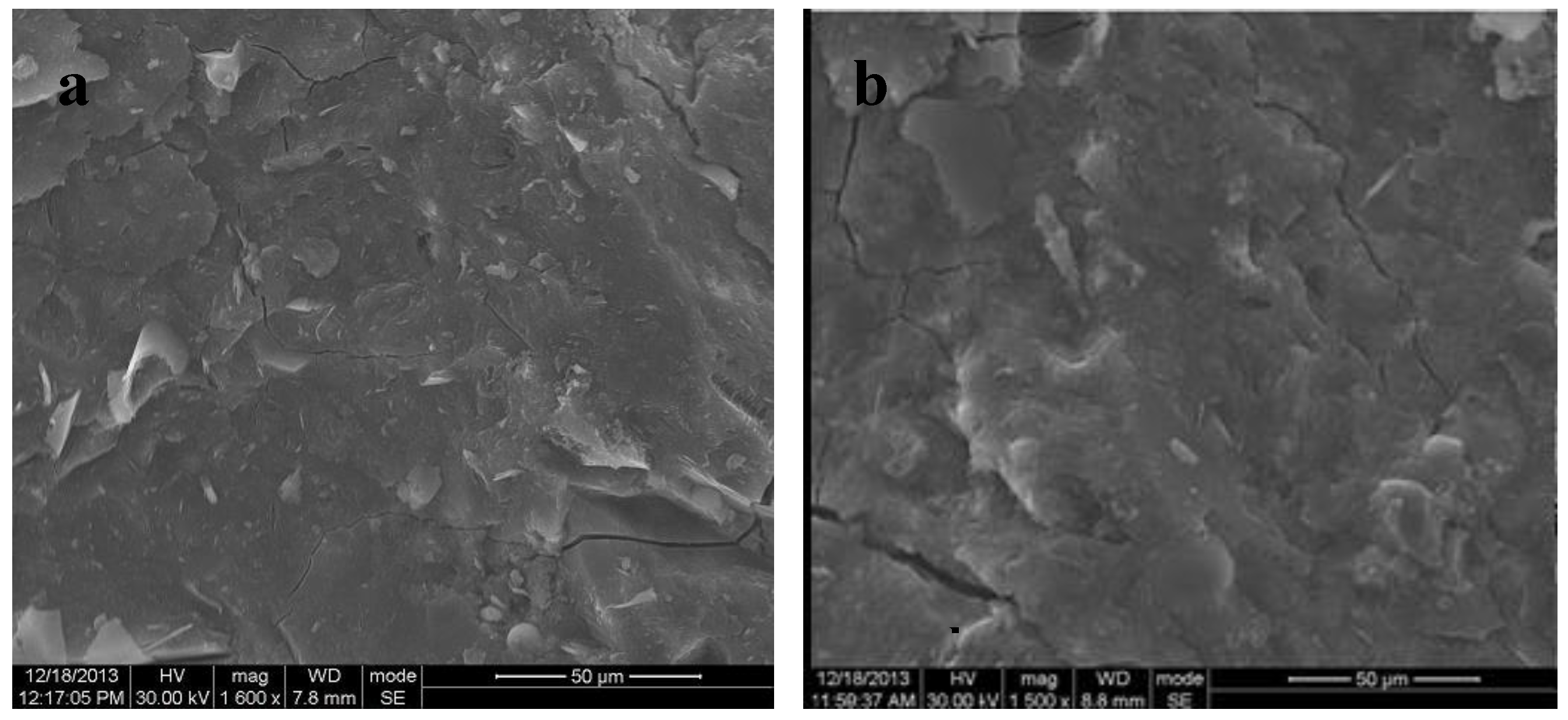
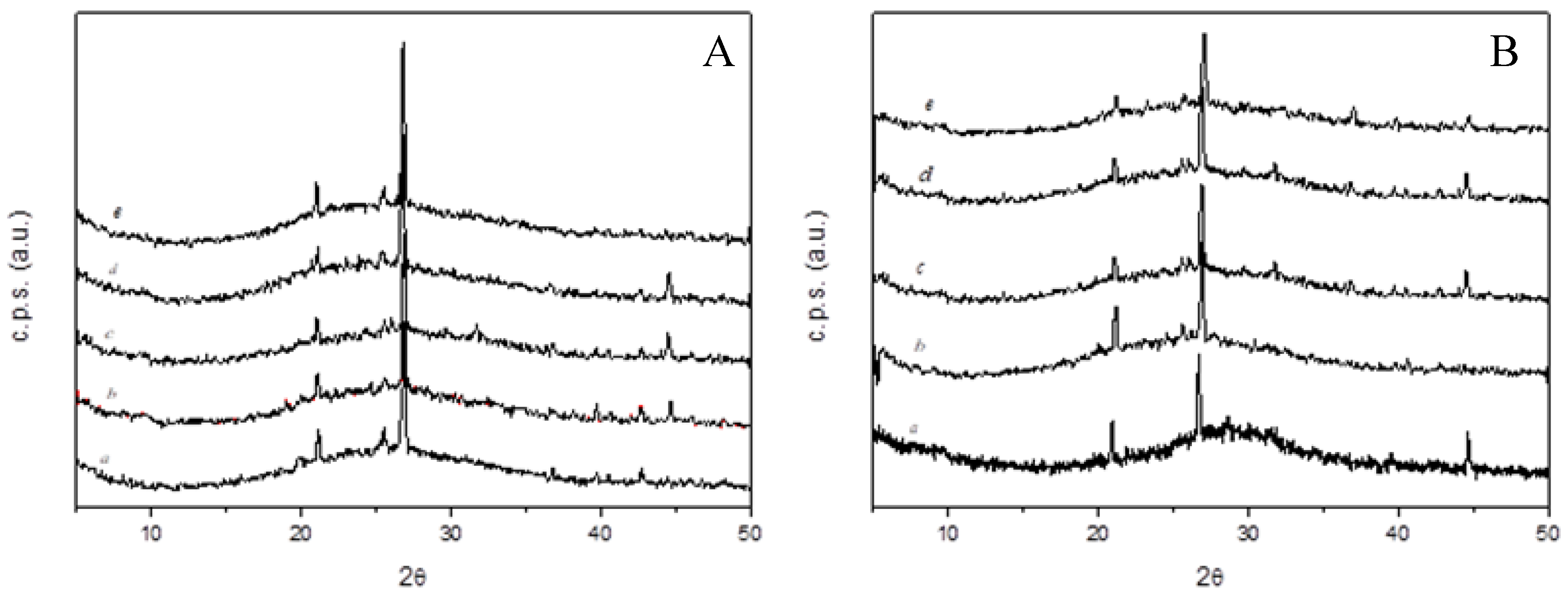
- All of the manufactured geopolymeric mortars show at XRD analysis the characteristic broad hump centered at approximately 28° 2θ, regardless of FBA content. SEM analysis reveals that geopolymers mortars with only metakaolin show a dense and continuous gel-like matrix with few clear particles or particle boundaries, while geopolymers mortars with FBA show a more porous and patchy matrix.
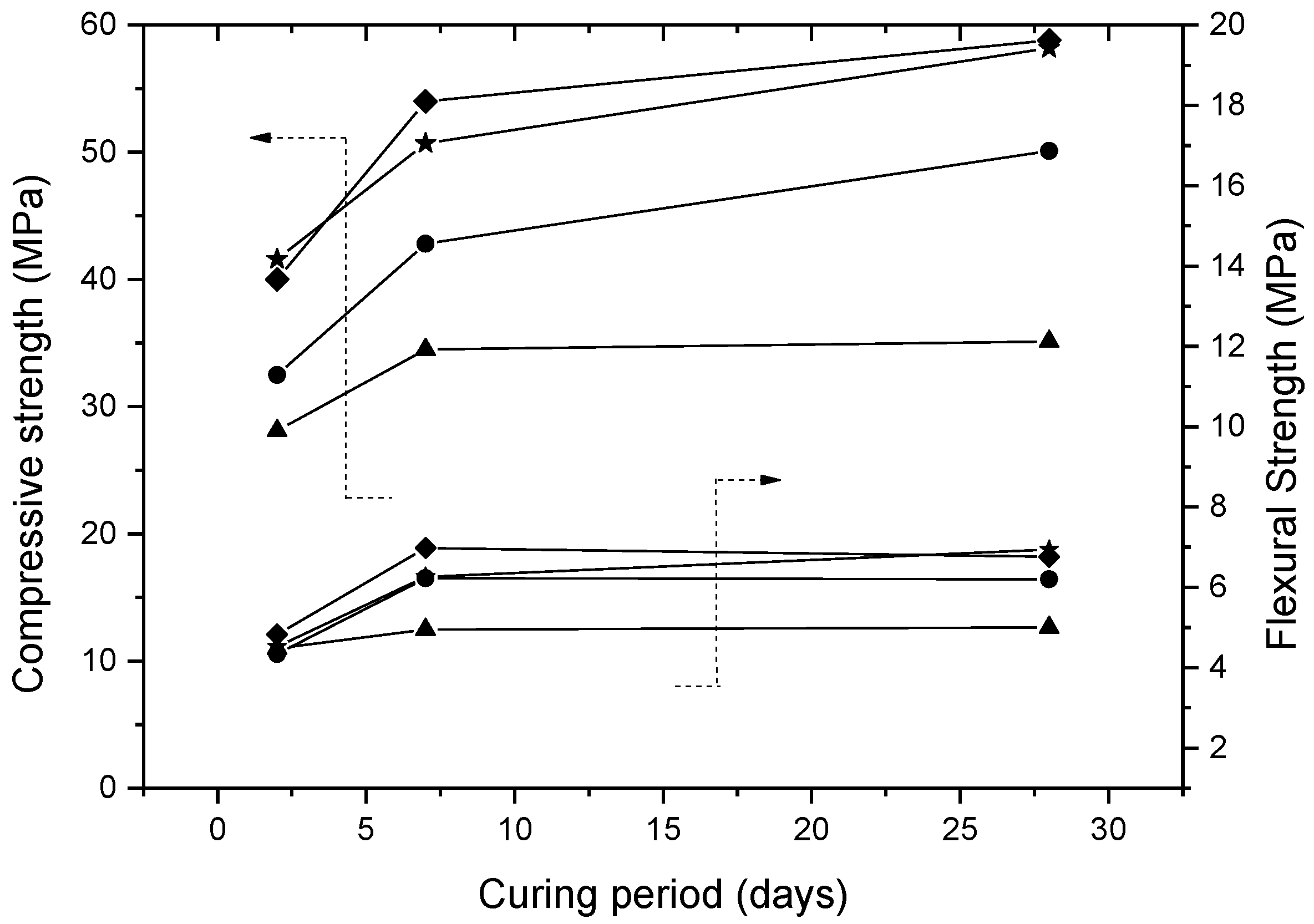
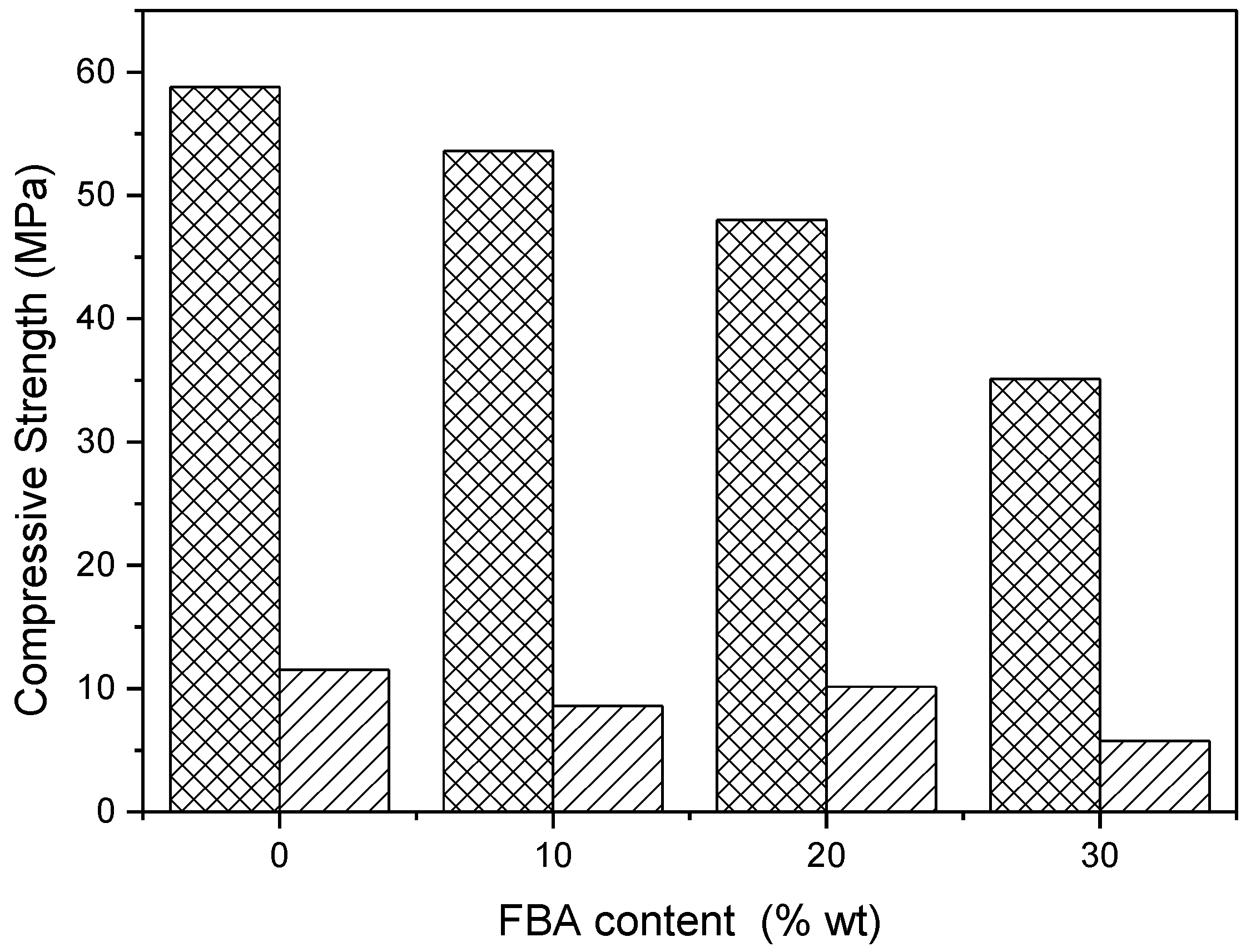
- The use of FBA at a level of replacement of higher than 10% wt affects the mechanical properties of the geopolymeric mortar, reducing proportionally the compressive and flexural strength of the mortars, for all curing times. Nevertheless, a compression strength of more than 35 MPa is still obtained with a replacement of 30% wt of metakaolin.
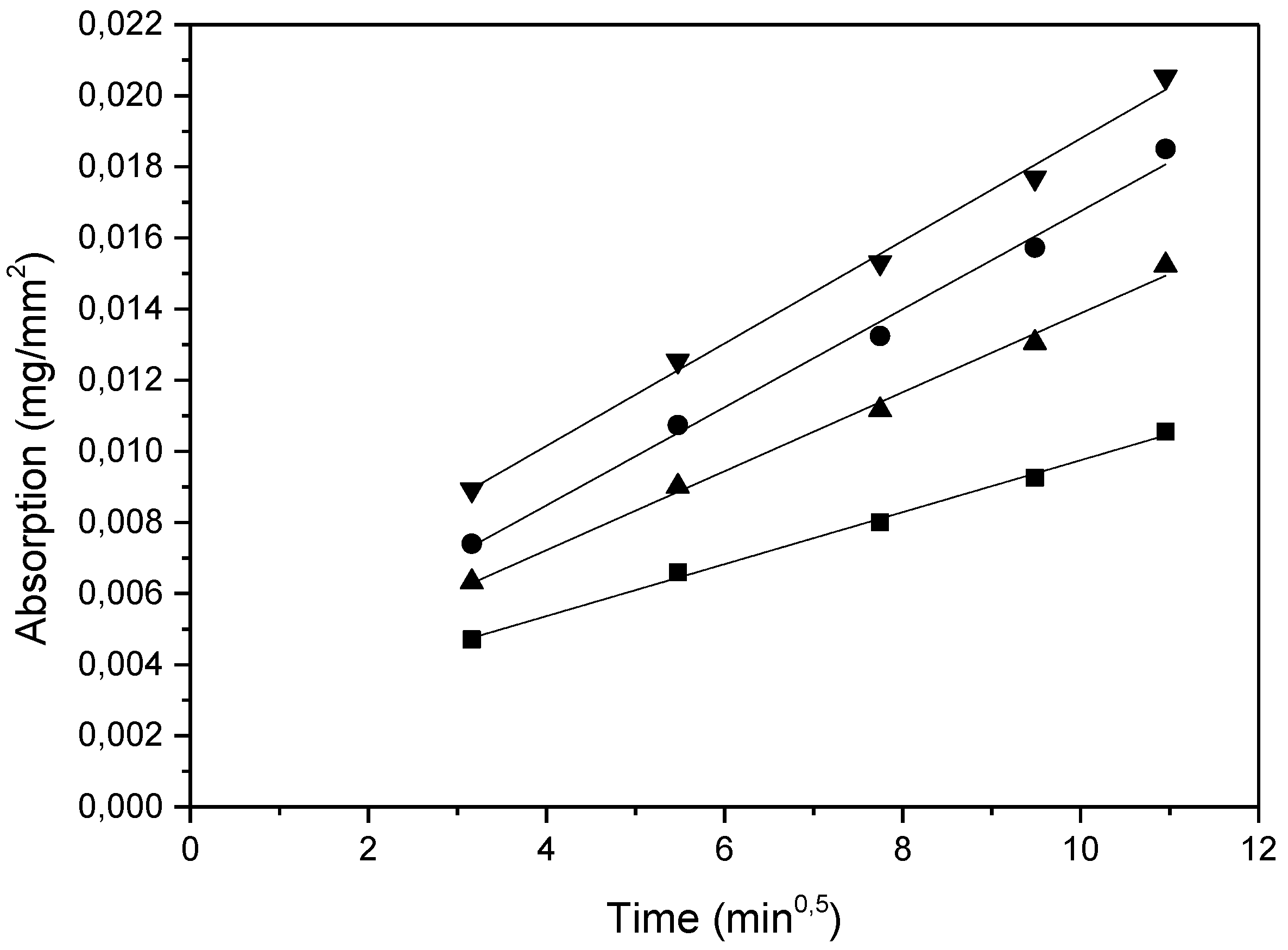
- Mortars containing FBA show higher sorptivities values. This suggests that FBA alters the structure of the pores.
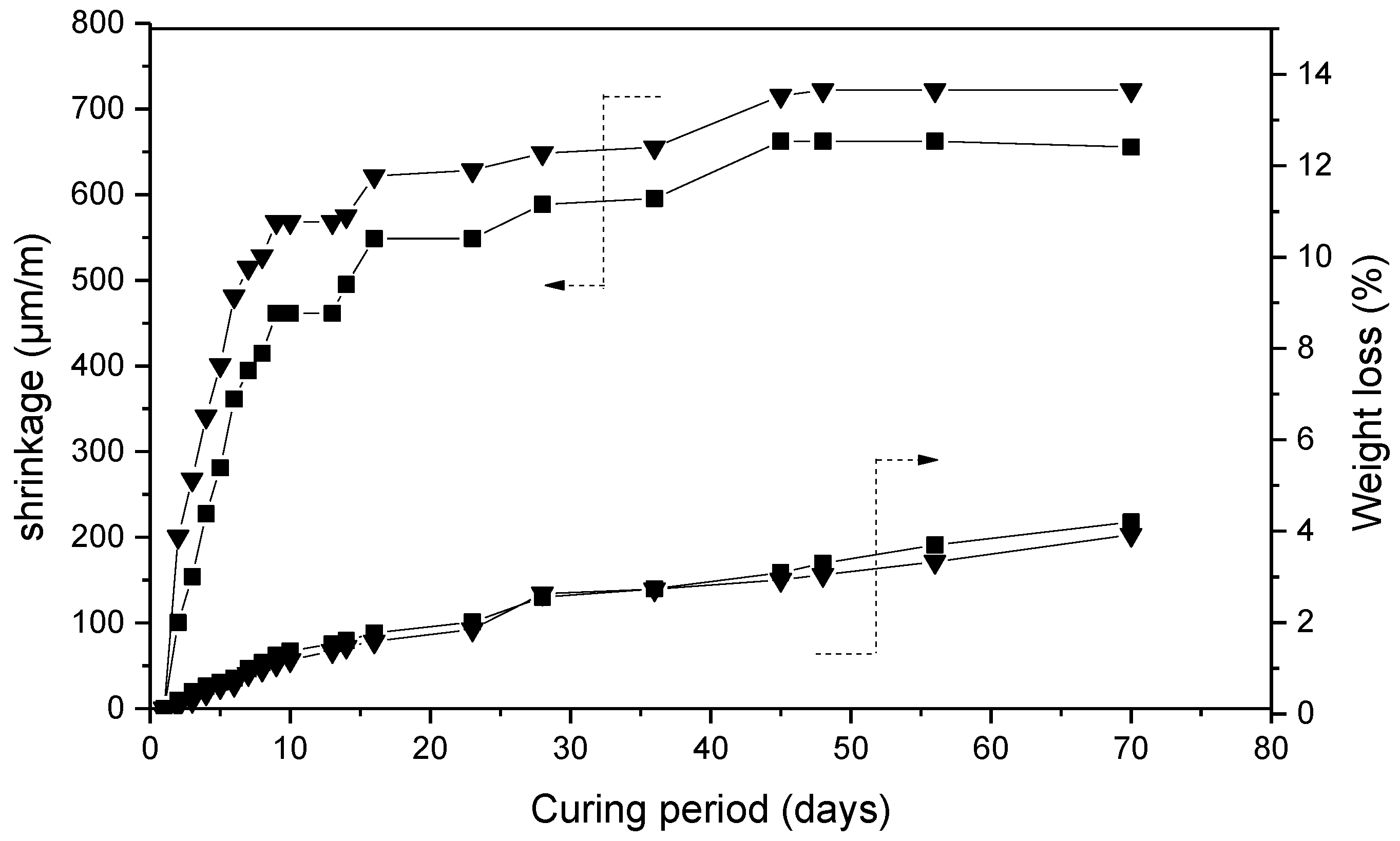
- The inclusion of FBA slightly increases both the drying shrinkage, due to a higher porosity.
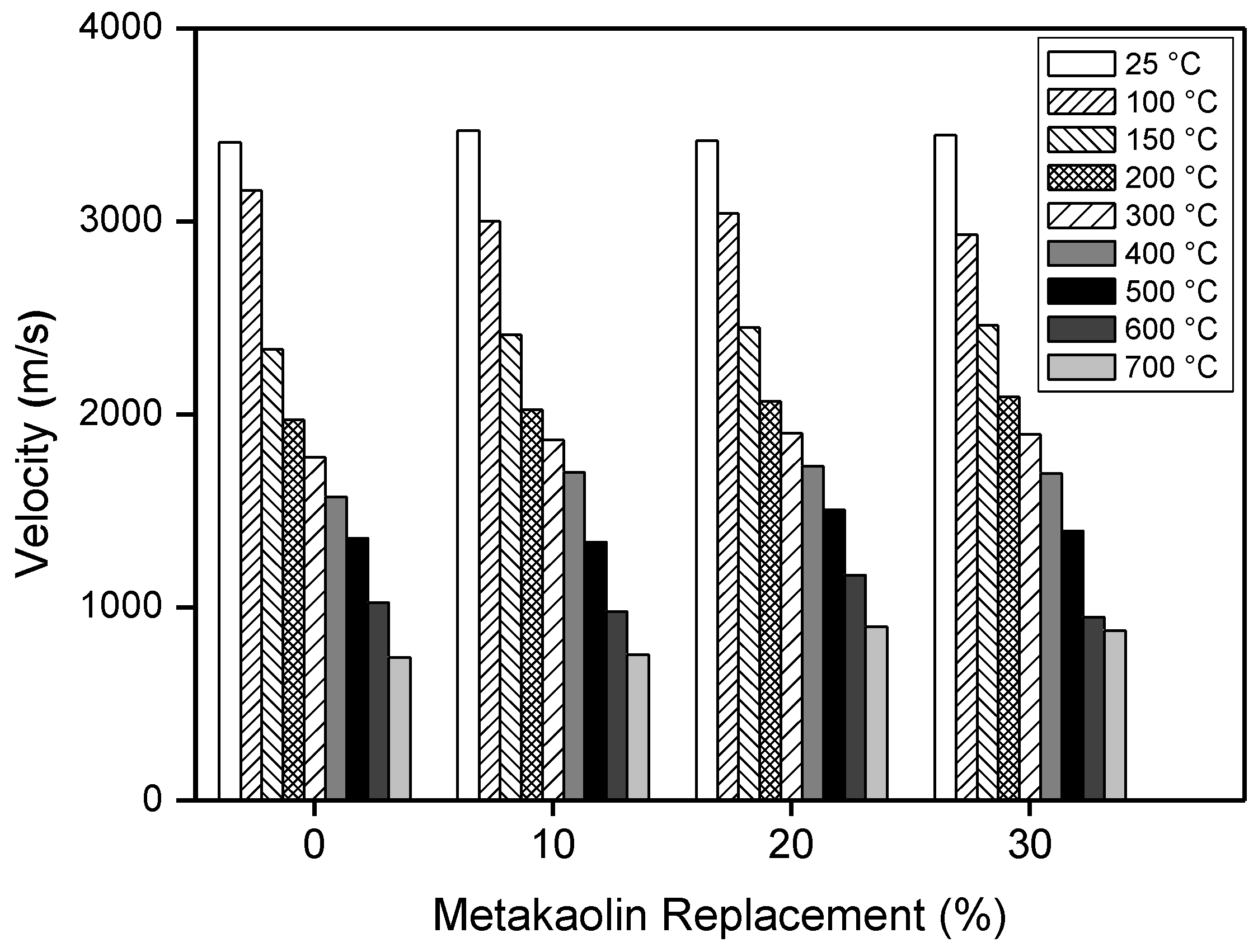
- All of the hardened mortars, after thermal treatments, show an overall loss of mechanical strength of about 80% of the initial value that can be attributed to the network strain and distortion due to dehydration and shrinkage, and to a degree of dissolution of quartz under the used temperatures and alkaline conditions.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siddique, R. Waste Materials and By-Products in Concrete, 1st ed.; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2008; ISBN 978-3-540-74294-4. [Google Scholar]
- Campbell, A.G. Recycling and disposing of wood ash. TAPPI J. 1990, 73, 141–143. [Google Scholar]
- Etiegni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Ochoa de Alda, J.A.G. Feasibility of recycling pulp and paper mill sludge in the paper and board industries. Resour. Conserv. Recycl. 2008, 52, 965–972. [Google Scholar] [CrossRef]
- Frontera, P.; Candamano, S.; Iacobini, I.; Crea, F. Eco-efficient self-compacting concrete with silica sand waste. Adv. Mat. Inf. Technol. Process. 2014, 87, 205–212. [Google Scholar]
- Candamano, S.; Crea, F.; Romano, D.; Iacobini, I. Workability, Strength and Drying Shrinkage of Structural Mortar Containing Forest Biomass Ash in Partial Replacement of Cement. Adv. Mat. Res. 2014, 1051, 737–742. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Construcc. 2014, 64, 022. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Development of greener alkali-activated cement: Utilisation of sodium carbonate for activating slag and fly ash mixtures. J. Clean. Prod. 2014, 113, 66–75. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials State-of-the-Art Report, RILEM TC 224-AAM; Springer Netherlands: Dordrecht, Netherlands, 2014; pp. 59–85. [Google Scholar]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing Series in Civil and Structural Engineering: Number 54; Woodhead Publishing: Cambridge, UK, 2015; pp. 19–43. [Google Scholar]
- O’Connor, S.J.; MacKenzie, K.J.D.; Smith, M.; Hanna, J. Ion exchange in the charge-balancing sites of aluminosilicate inorganic polymers. J. Mater. Chem. 2010, 20, 10234–10240. [Google Scholar] [CrossRef]
- Bortnovsky, O.; Dědeček, J.; Tvarůžková, Z.; Sobalík, Z. Metal ions as probes for characterization of geopolymer materials. J. Am. Ceram. Soc. 2008, 91, 3052–3057. [Google Scholar] [CrossRef]
- Sazama, P.; Bortnovsky, O.; Dědeček, J.; Tvarůžková, Z.; Sobalík, Z. Geopolymer based catalysts-new group of catalytic materials. Catal. Today 2011, 164, 92–99. [Google Scholar] [CrossRef]
- Miccio, F.; Natali, A.; Natali Murri, A.; Landi, E. Synthesis and characterization of geopolymer oxygen carriers for chemical looping combustion. Appl. Energy 2017, 194, 136–147. [Google Scholar] [CrossRef]
- Candamano, S.; Frontera, P.; Macario, A.; Aloise, A.; Crea, F. New material as Ni-support for hydrogen production by ethanol conversion. Adv. Mat. Inf. Technol. Process. 2014, 87, 115–121. [Google Scholar]
- Li, L.; Wang, S.; Zhu, Z. Geopolymeric adsorbents from fly ash for dye removal from aqueous solution. J. Colloid Interface Sci. 2006, 300, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Pan, D.; Liu, B.; Volinsky, A.A.; Fincan, M.; Du, J.; Zhang, S. Immobilization mechanism of Pb in fly ash-based geopolymer. Constr. Build. Mater. 2017, 34, 123–130. [Google Scholar] [CrossRef]
- Lamuta, C.; Candamano, S.; Crea, F.; Pagnotta, L. Direct piezoelectric effect in geopolymeric mortars. Mater. Des. 2016, 107, 57–64. [Google Scholar] [CrossRef]
- Candamano, S.; Frontera, P.; Macario, A.; Crea, F.; Nagy, J.B.; Antonucci, P.L. Preparation and characterization of active Ni-supported catalyst for syngas production. Chem. Eng. Res. Des. 2015, 96, 78–86. [Google Scholar] [CrossRef]
- UNI EN 1015–3:2007 Consistency of Wet Mortar. Available online: https://infostore.saiglobal.com/store/details.aspx?ProductID=610562 (accessed on 11 September 2017).
- UNI-EN 196–1:2005 Methods of Testing Cement-Part 1: Determination of Strength. Available online: https://infostore.saiglobal.com/en-au/Standards/UNI-EN-196-1-2005-583689/ (accessed on 11 September 2017).
- UNI EN 1015–18:2004 Methods of Test For Mortar For Masonry-Determination Of Water Absorption Coefficient Due To Capillary Action Of Hardened Mortar. Available online: https://infostore.saiglobal.com/store/details.aspx?ProductID=640959 (accessed on 11 September 2017).
- Sousa-Coutinho, J. The combined benefits of CPF and RHA in improving the durability of concrete structures. Cem. Concr. Compos. 2003, 25, 51–59. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.; Weng, L. Dissolution processes, Hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 2007, 42, 3007–3014. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; O’farrel, M.A. Water sorptivity test for mortar and concrete. Mater. Struct. 1998, 31, 568–574. [Google Scholar] [CrossRef]
- Ohdaira, E.; Masuzawa, N. Water content and its effect on ultrasound propagation in concrete—The possibility of NDE. Ultrasonics 2000, 38, 546–552. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. The effect of alkali metal type and silicate concentration on the thermal stability of geopolymers Geopolymer. Green Chemistry and Sustainable Development Solutions. In Proceedings of the World Congress Geopolymer, Perth, Australia, 28–29 September 2005; pp. 189–193, ISBN 9782951482005. [Google Scholar]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composite derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
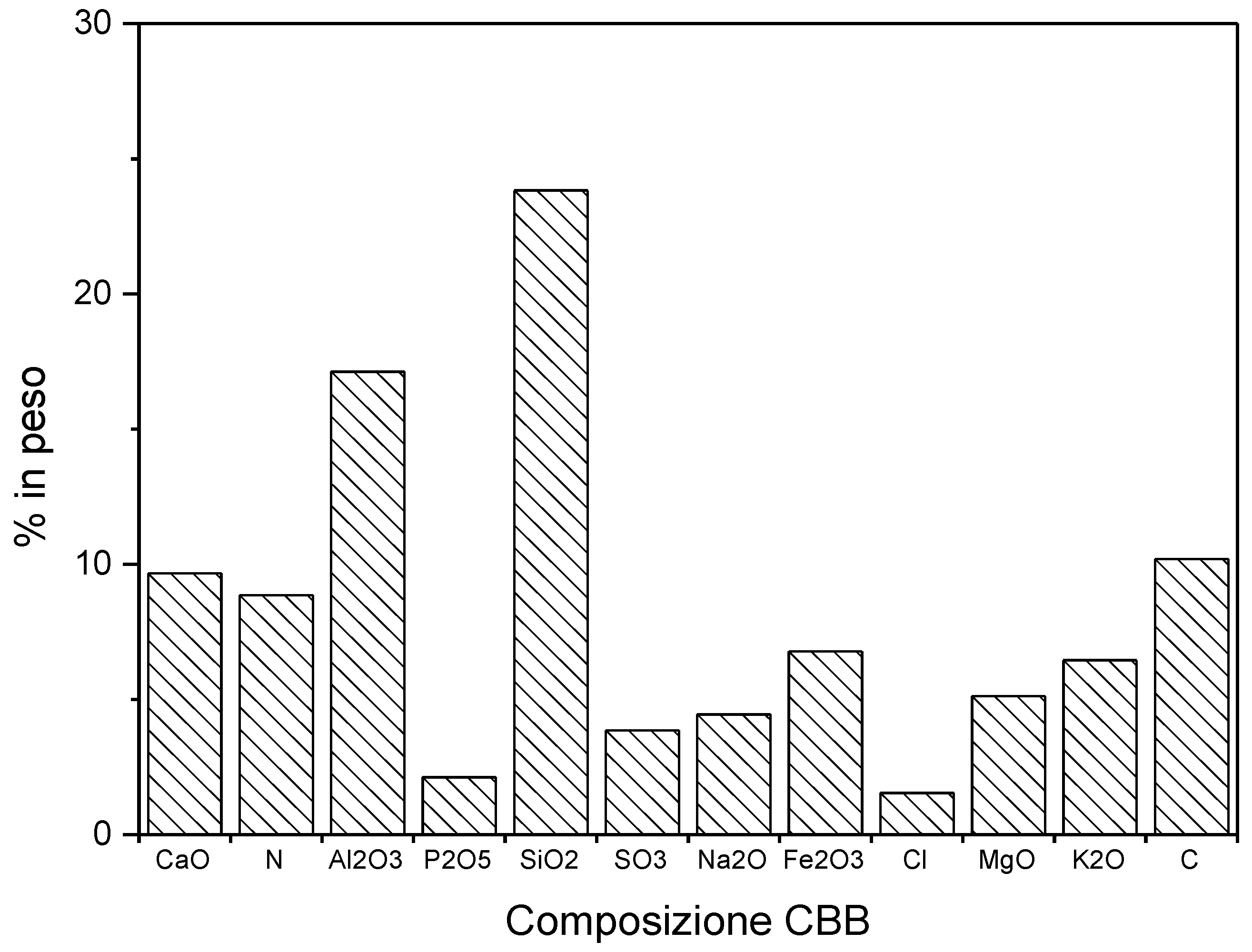
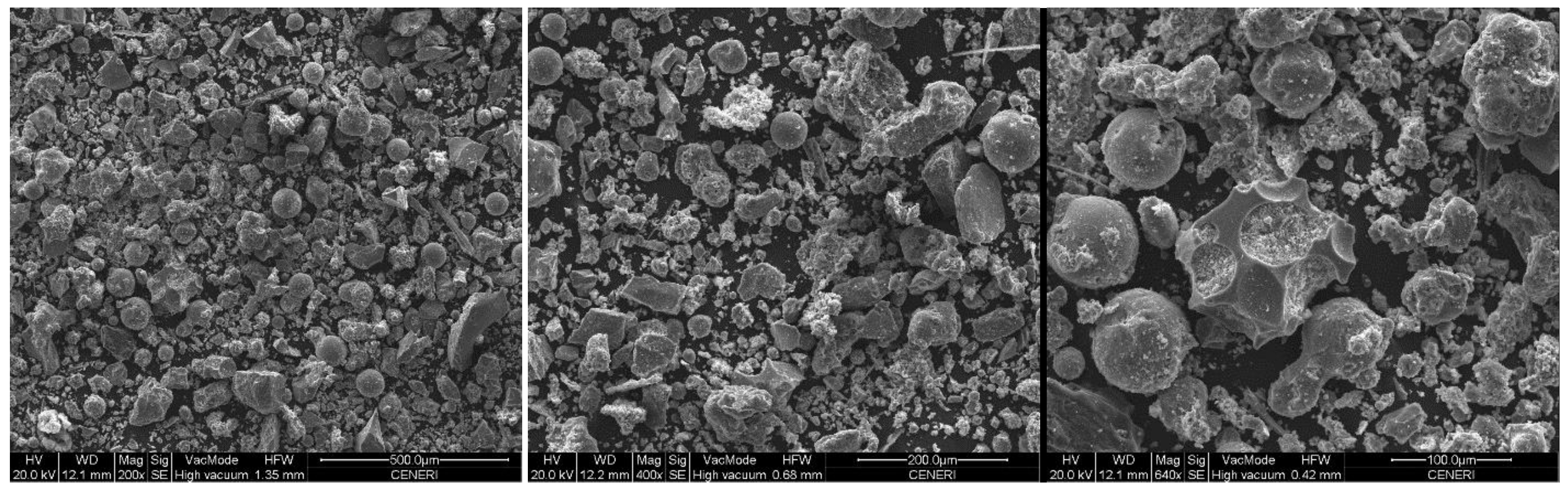
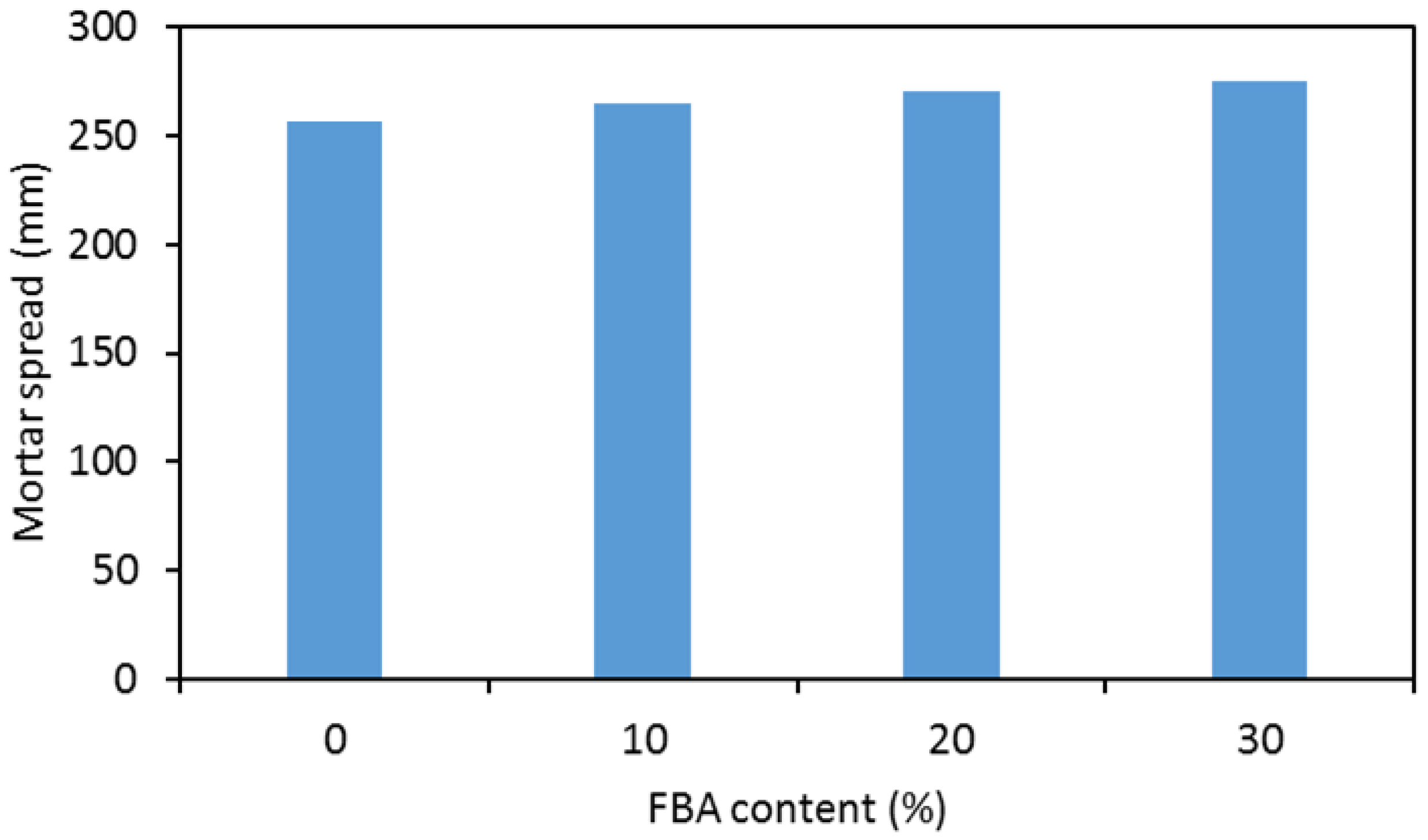
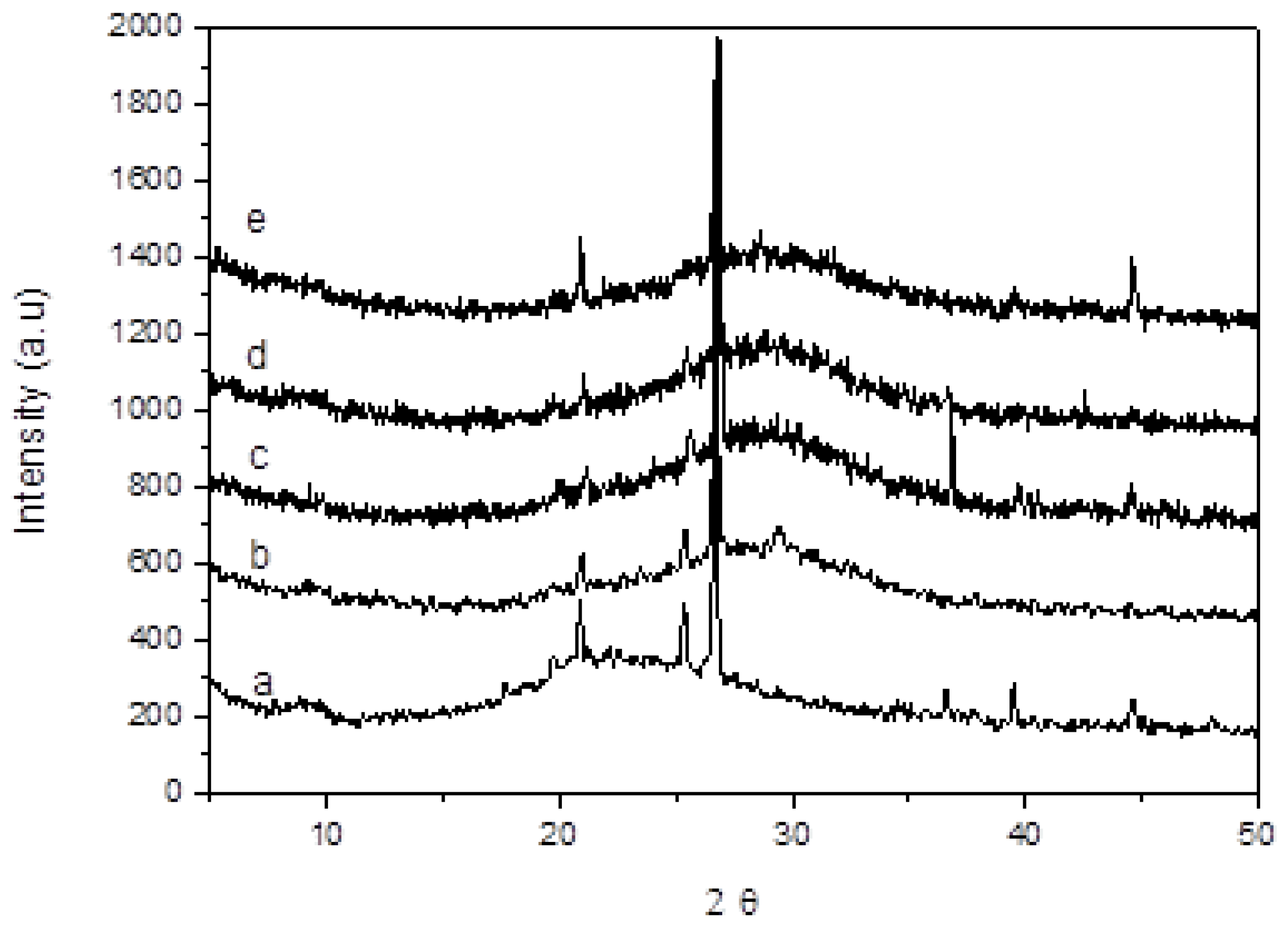

| Component (%wt) | Metakaolin | Sodium Silicate Solution |
|---|---|---|
| Al2O3 | 42.02 | - |
| SiO2 | 53.9 | 29.6 |
| K2O | 0.30 | - |
| Na2O | 0.09 | 13.76 |
| Fe2O3 | 1.52 | - |
| TiO2 | 1.90 | - |
| MgO | - | - |
| P2O5 | - | - |
| CaO | - | - |
| SO3 | - | - |
| MnO | - | - |
| LOI | 1.00 | - |
| H2O | - | 56.78 |
| Mix No. | W/B (wt) | SiO2/Al2O3 (molar) | Na2O/Al2O3 (molar) | FBA/ Binder (wt) | Sand/Binder (wt) | B: Alkali Silicate Solution (dry basis) (wt) |
|---|---|---|---|---|---|---|
| 0S | 0.53 | 3.8 | 1 | 0 | 2.8 | 1.35:1 |
| 10S | 0.53 | 3.8 | 1 | 0.1 | 2.8 | 1.45:1 |
| 20S | 0.53 | 3.8 | 1 | 0.2 | 2.8 | 1.55:1 |
| 30S | 0.53 | 3.8 | 1 | 0.3 | 2.8 | 1.7:1 |
| Time (min) | H2O Dis (g) | FBA (g) | Metakaolin (g) |
|---|---|---|---|
| Solution 1 | 50 | 5 | 0 |
| Solution 2 | 50 | 0 | 5 |
| Time (min) | pH Solution 1 | pH Solution 2 |
|---|---|---|
| 1 | 12.54 | 6.315 |
| 5 | 12.82 | 6.375 |
| 10 | 12.85 | 6.387 |
| Mix No. | Eq. y = Sx + a | S | a | R2 |
|---|---|---|---|---|
| 0S | 7.307E-4 | 0.00244 | 0.996 | |
| 10S | 0.00111 | 0.00278 | 0.994 | |
| 20S | 0.00138 | 0.00297 | 0.991 | |
| 30S | 0.00144 | 0.00439 | 0.994 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candamano, S.; De Luca, P.; Frontera, P.; Crea, F. Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin. Environments 2017, 4, 74. https://doi.org/10.3390/environments4040074
Candamano S, De Luca P, Frontera P, Crea F. Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin. Environments. 2017; 4(4):74. https://doi.org/10.3390/environments4040074
Chicago/Turabian StyleCandamano, Sebastiano, Pierantonio De Luca, Patrizia Frontera, and Fortunato Crea. 2017. "Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin" Environments 4, no. 4: 74. https://doi.org/10.3390/environments4040074
APA StyleCandamano, S., De Luca, P., Frontera, P., & Crea, F. (2017). Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin. Environments, 4(4), 74. https://doi.org/10.3390/environments4040074








