Bioremoval of Phenol from Aqueous Solutions Using Native Caribbean Seaweed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Biomass
2.2. Reagents and Solutions
2.3. Characterization of Adsorbents
2.4. Adsorption Studies
2.5. Data Analysis
3. Results and Discussion
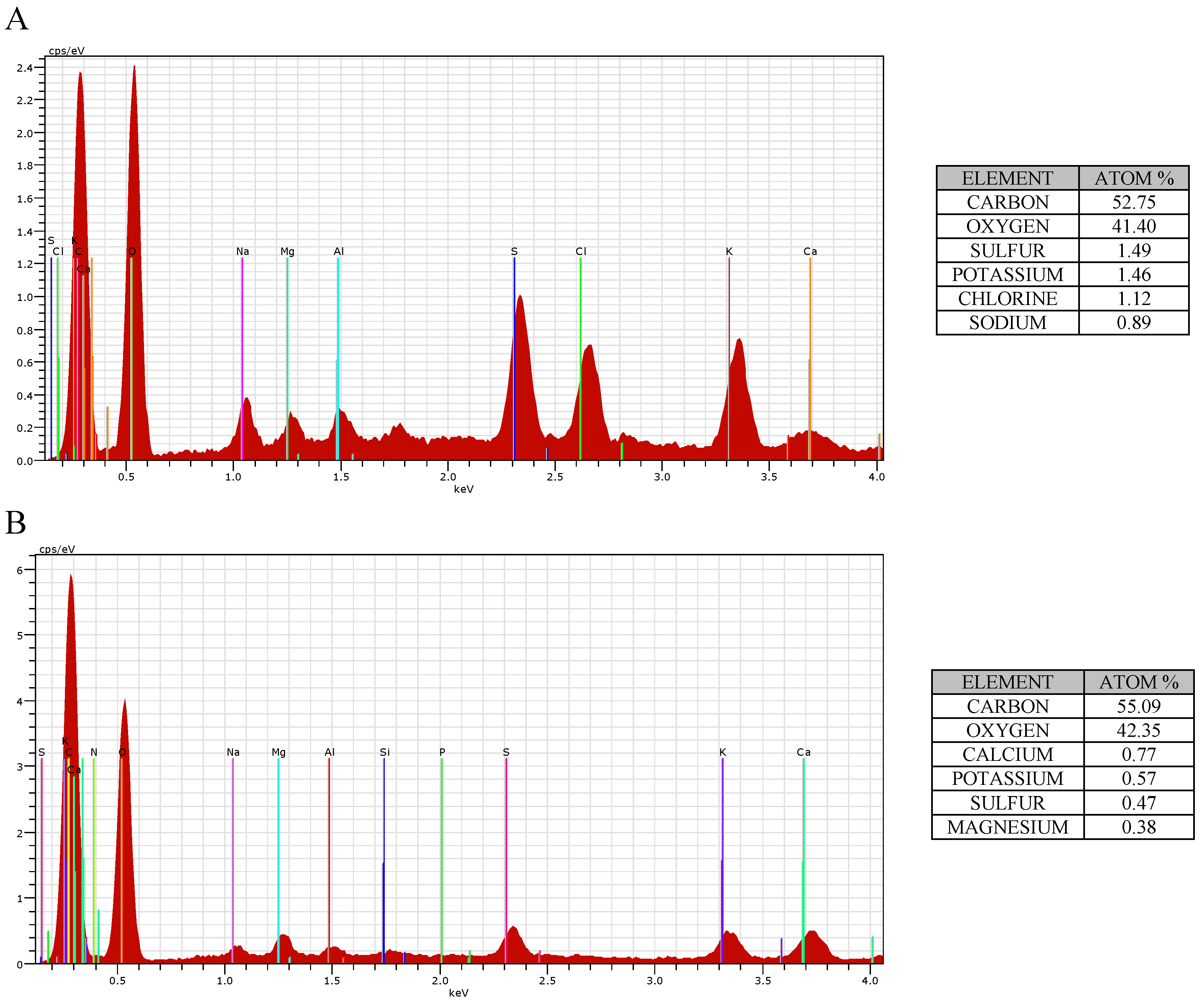
3.1. Characterization of the Adsorbents
3.2. Adsorption Tests
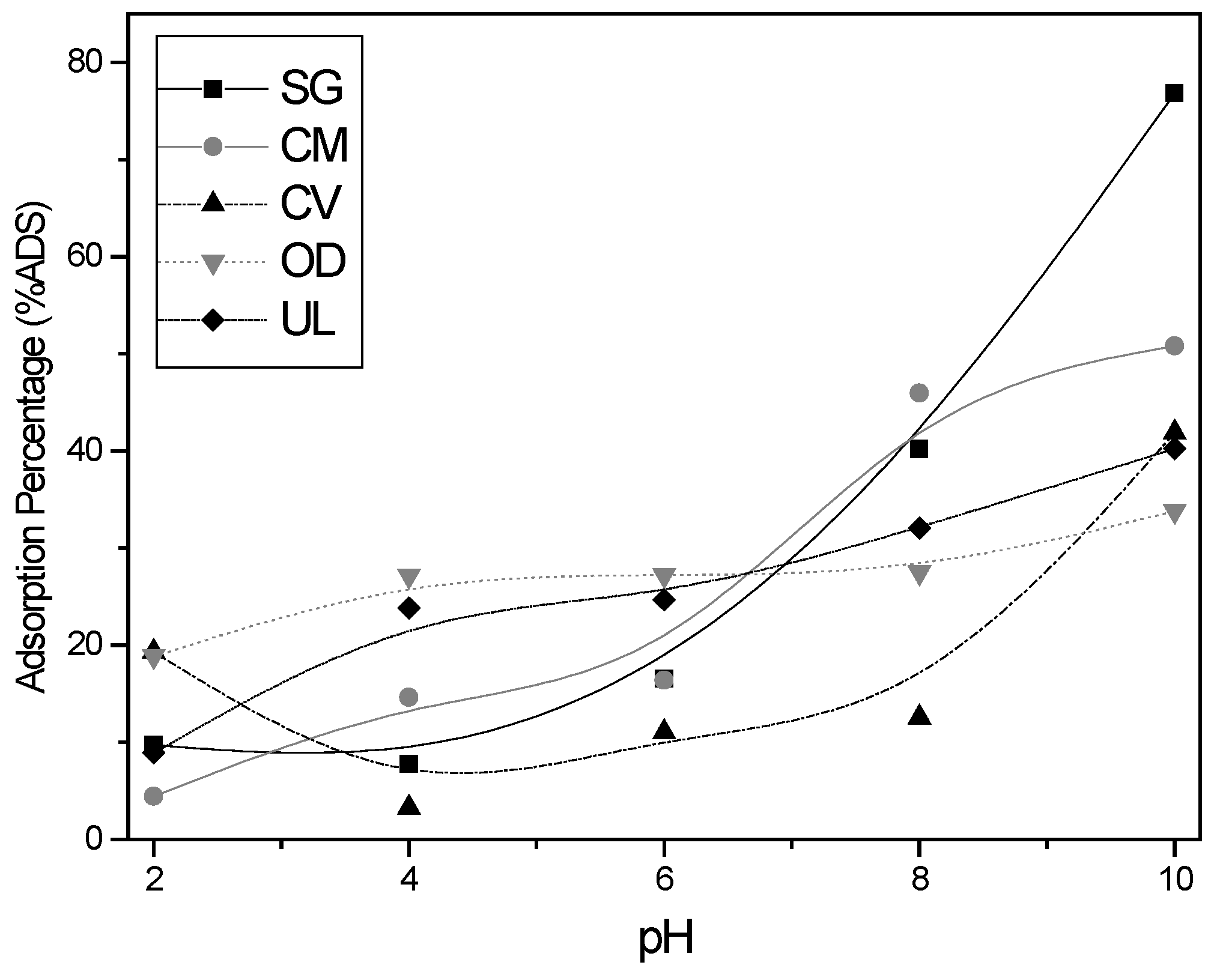
3.2.1. Effect of Initial pH Value
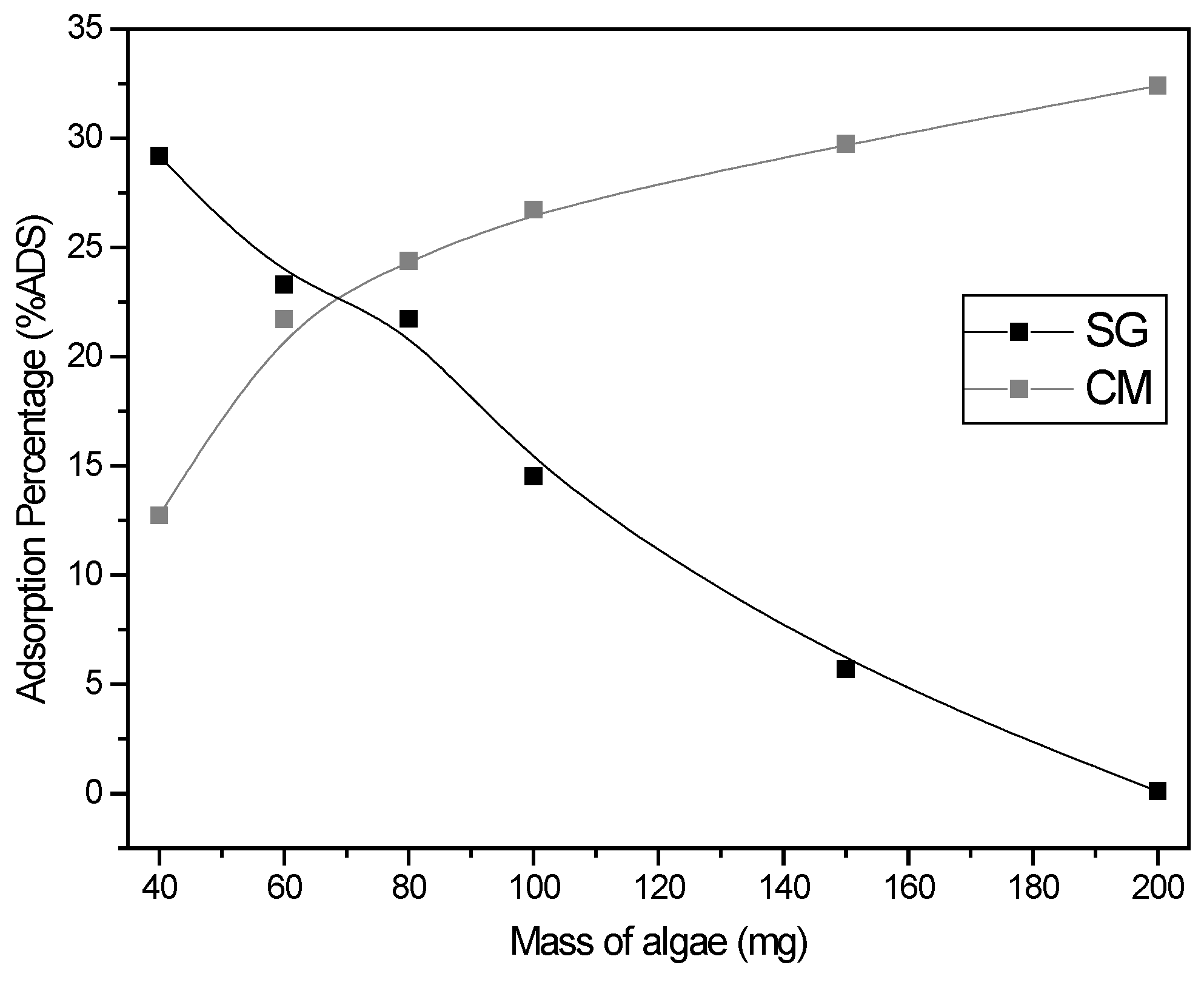
3.2.2. Effect of Adsorbent Dose
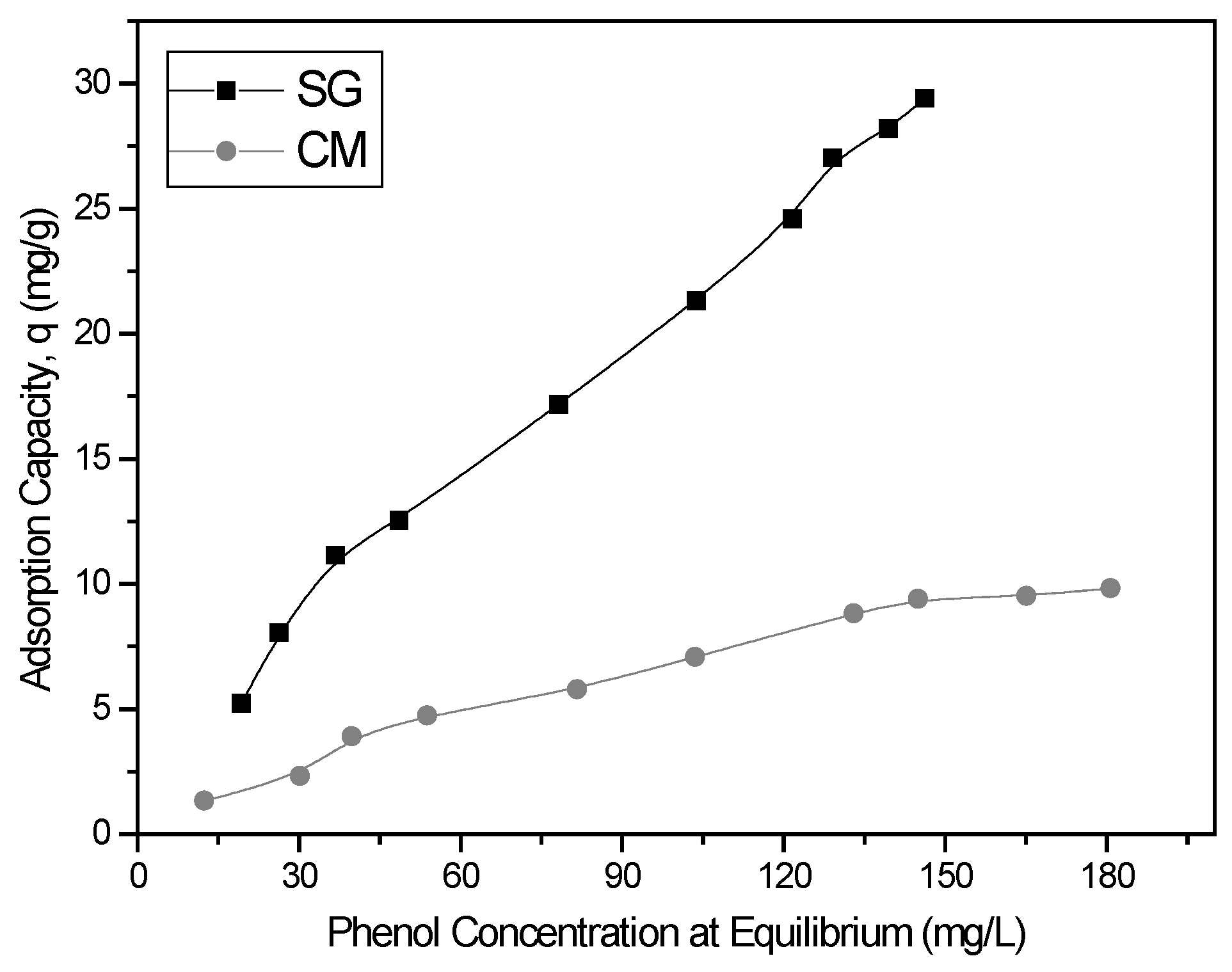
3.2.3. Equilibrium Modelling
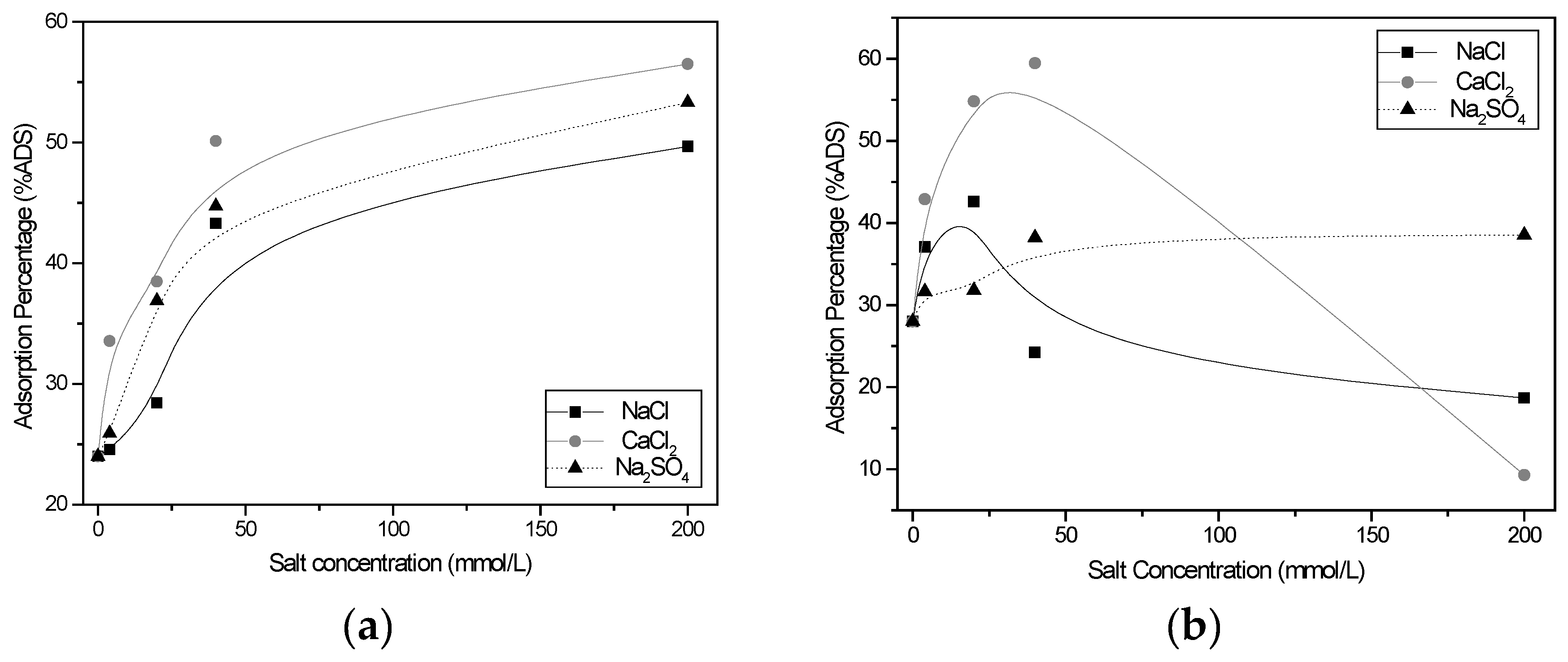
3.2.4. Effect of Salinity
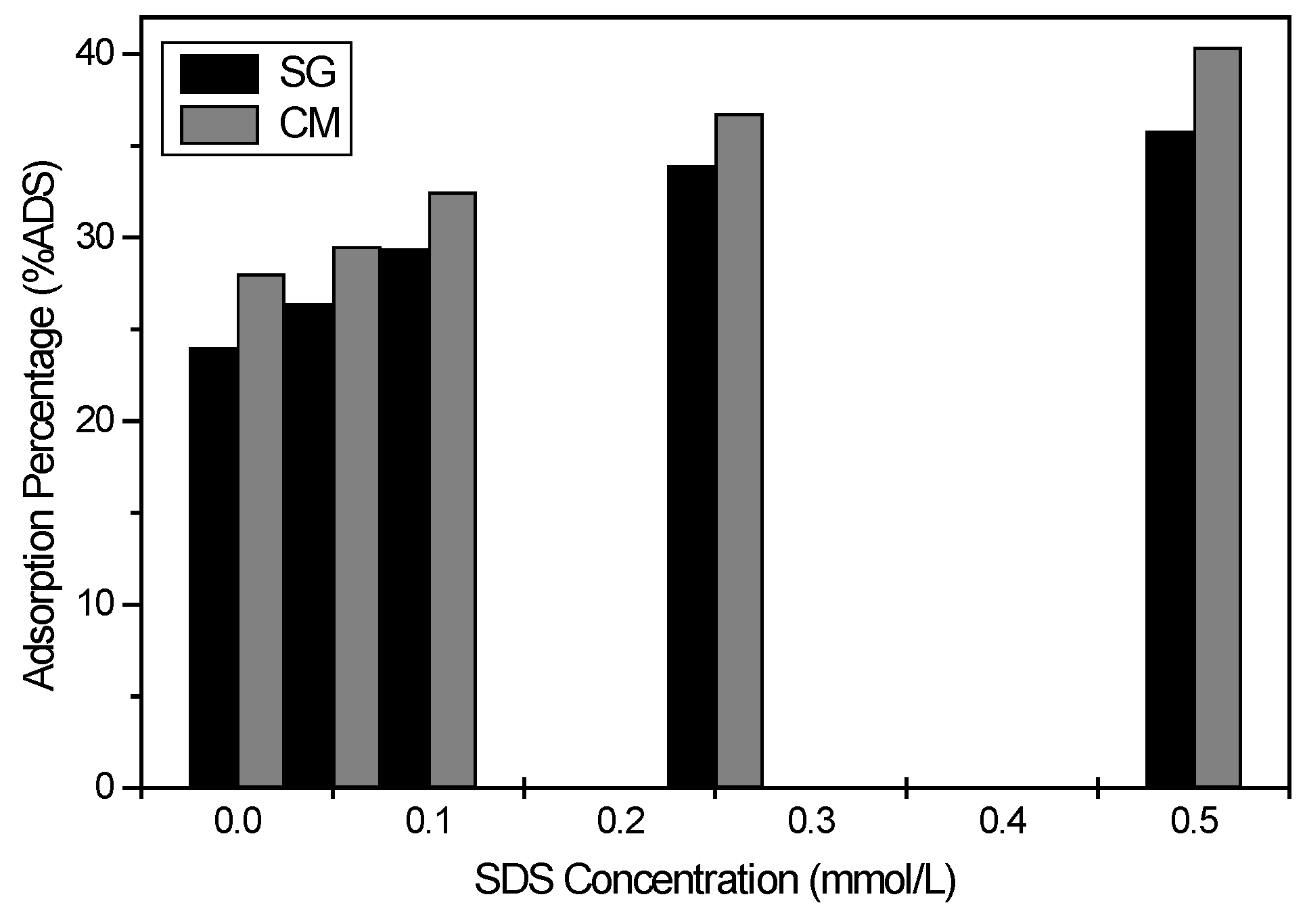
3.2.5. Effect of the Presence of Detergent
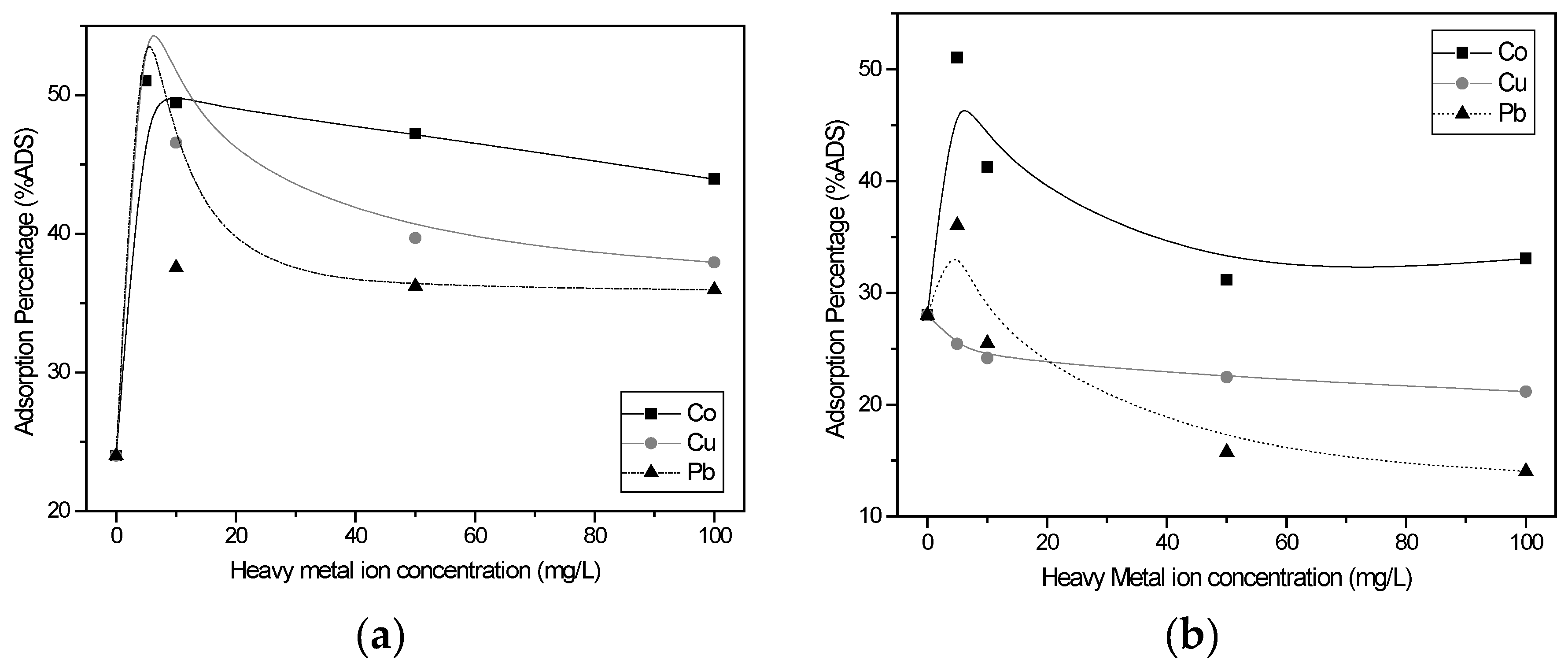
3.2.6. Effect of the Presence of Heavy Metal Ions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- US Environmental Protection Agency. Toxic Release Inventory and Community Right to Know; US EPA: Washington, DC, USA, 2002.
- US Environmental Protection Agency. Hazard Information on Toxic Chemicals Added to EPCRA Section 313 under Chemical Expansion; US EPA: Washington, DC, USA, 2002.
- Halden, R. Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; American Chemical Society: Washington, DC, USA, 2010. [Google Scholar]
- Navarro, A.; Chang, E.; Chang, P.; Yoon, S.; Manrique, A. Separation of dyes from aqueous solutions by magnetic alginate beads. Trends Chromatogr. 2013, 8, 31–41. [Google Scholar]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 9, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Srivastava, V.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Fundamentals and Applications of Biosorption Isotherms, Kinetics and Thermodynamics; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Lazo, J.; Navarro, A.E.; Sun-Kou, R.; Llanos, B. Synthesis and Characterization of organophilic clays and their application as phenol adsorbents. Rev. Soc. Quím. Perú 2008, 74, 3–19. [Google Scholar]
- Navarro, A.E.; Cuizano, N.; Lazo, J.; Sun-Kou, R.; Llanos, B. Comparative study of the removal of phenolic compounds on biological and non-biological adsorbents. J. Hazard. Mater. 2008, 164, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.E.; Cuizano, N.; Lazo, J.; Sun-Kou, R.; Llanos, B. Insights into Removal of phenol from aqueous solutions by low cost adsorbents: Clays versus algae. Sep. Sci. Technol. 2009, 44, 2491–2509. [Google Scholar] [CrossRef]
- Deng, H.; Liu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Cuizano, N.; Reyes, U.; Dominguez, S.; Llanos, B.; Navarro, A.E. Relevance of the pH on the adsorption of metallic ions by brown seaweeds. Rev. Soc. Quím. Perú 2010, 76, 123–130. [Google Scholar]
- Reyes, U.; Navarro, A.E.; Llanos, B. Use of seaweeds for Biosorption of Cupric ions in aqueous solutions. Rev. Soc. Quím. Perú 2009, 75, 353–361. [Google Scholar]
- Cuizano, N.; Llanos, B.; Navarro, A.E. Application of marine seaweeds as lead (II) biosorbents: Analysis of the equilibrium state. Rev. Soc. Quím. Perú 2009, 75, 33–43. [Google Scholar]
- Navarro, A.E.; Cuizano, N.; Portales, R.; Llanos, B. Adsorptive Removal of 2-nitrophenol and 2-chlorophenol by cross-linked algae from aqueous solutions. Sep. Sci. Technol. 2008, 43, 3183–3199. [Google Scholar] [CrossRef]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Sastre de Vicente, M. Biosorption of phenolic compounds by the brown alga Sargassum. muticum. J. Chem. Technol. Biotechnol. 2006, 81, 1093–1099. [Google Scholar] [CrossRef]
- Kotrba, P.; Mackova, M.; Macek, T. Microbial Biosorption of Metals; Springer: New York, NY, USA, 2011. [Google Scholar]
- Volesky, B. Sorption and Biosorption; BV SORBEX Inc.: Montreal, QC, Canada, 2004. [Google Scholar]
- Serrano, K.; Musaev, H.; Irkakhujaev, S.; Navarro, A.E. Use of lignocellulosic wastes as adsorbents of divalent cobalt (II) ions from contaminated solutions. Trends Chromatog. 2014, 9, 65–72. [Google Scholar]
- Zarzar, A.; Hong, M.; Llanos, B.; Navarro, A.E. Insights into the eco-friendly adsorption of caffeine from contaminated solutions by using hydrogel beads. J. Environ. Anal. Chem. 2015, 2, 4. [Google Scholar]
- Bellatin, L.; Herrera, O.; Navarro, A.E.; Sun, R.; Llanos, B. Study on the biosorption of basic yellow 57 dye, basic blue 99 and acid red 18 from hair dyes onto green tea leaves. Rev. Soc. Quim. Perú 2014, 80, 9–23. [Google Scholar]
- Rawajfih, Z.; Nsour, N. Characteristics of phenol and chlorinated phenols sorption onto surfactant-modified bentonite. J. Colloid Interface Sci. 2006, 298, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The Egg-Box model revisited. Biomacromolecules 2001, 2, 1089. [Google Scholar] [CrossRef] [PubMed]
- Gales, M.; Booth, R. Automated 4AAP phenolic method. J. AWWA 1976, 68, 540. [Google Scholar]
- Cuizano, N.; Llanos, B.; Navarro, A.E. Elimination of 2-chlorophenol from aqueous solutions by marine algae: Evidences of adsorption mechanism. Rev. Soc. Quím. Perú 2009, 75, 213–220. [Google Scholar]
- Japhe, T.; Zhdanova, K.; Rodenburg, L.; Roberson, L.; Navarro, A.E. Factors affecting the Biosorption of 2-Chlorophenol using spent tea leaf wastes as adsorbents. J. J. Environ. Sci. 2015, 1, 010. [Google Scholar]
- Choi, Y.; Isaac, P.; Irkakhujaev, S.; Masud, M.E.; Navarro, A.E. Use of spent tea wastes-chitosan capsules for the removal of divalent copper ions. J. J. Environ. Sci. 2015, 1, 003. [Google Scholar]
- Sponza, A.; Fernandez, N.; Yang, D.; Ortiz, K.; Navarro, A.E. Comparative Sorption of methylene blue onto hydrophobic clays. Environments 2015, 2, 388–398. [Google Scholar] [CrossRef]
- Navarro, A.E.; Portales, R.; Sun-Kou, R.; Llanos, B. Effect of pH on phenol biosorption by seaweeds. J. Hazard. Mater. 2008, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, J.; Sastre de Vicente, M. Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive microalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef]



| Isotherm Model | SG | CM |
|---|---|---|
| Langmuir | ||
| qmax (mg·g−1) | 82.10 | 17.69 |
| b (L/mg) | 0.0038 | 0.0064 |
| R2 | 0.98 | 0.98 |
| SD2 | 5.1 × 10−5 | 9.64 × 10−4 |
| p | <0.0001 | <0.0001 |
| Freundlich | ||
| kF | 0.579 | 0.199 |
| 1/n | 0.787 | 0.767 |
| R2 | 0.99 | 0.98 |
| SD2 | 5.1 × 10−3 | 9.22 × 10−3 |
| p | <0.0001 | <0.0001 |
| Tempkin | ||
| aT | 0.0711 | 0.083 |
| bT | 209.42 | 696.201 |
| R2 | 0.962 | 0.95 |
| SD2 | 3.36 | 0.575 |
| p | <0.0001 | <0.0001 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, A.E.; Hernandez-Vega, A.; Masud, M.E.; Roberson, L.M.; Diaz-Vázquez, L.M. Bioremoval of Phenol from Aqueous Solutions Using Native Caribbean Seaweed. Environments 2017, 4, 1. https://doi.org/10.3390/environments4010001
Navarro AE, Hernandez-Vega A, Masud ME, Roberson LM, Diaz-Vázquez LM. Bioremoval of Phenol from Aqueous Solutions Using Native Caribbean Seaweed. Environments. 2017; 4(1):1. https://doi.org/10.3390/environments4010001
Chicago/Turabian StyleNavarro, Abel E., Anibal Hernandez-Vega, Md Emran Masud, Loretta M. Roberson, and Liz M. Diaz-Vázquez. 2017. "Bioremoval of Phenol from Aqueous Solutions Using Native Caribbean Seaweed" Environments 4, no. 1: 1. https://doi.org/10.3390/environments4010001
APA StyleNavarro, A. E., Hernandez-Vega, A., Masud, M. E., Roberson, L. M., & Diaz-Vázquez, L. M. (2017). Bioremoval of Phenol from Aqueous Solutions Using Native Caribbean Seaweed. Environments, 4(1), 1. https://doi.org/10.3390/environments4010001






