Understanding PFAS Adsorption: How Molecular Structure Affects Sustainable Water Treatment
Abstract
1. Introduction
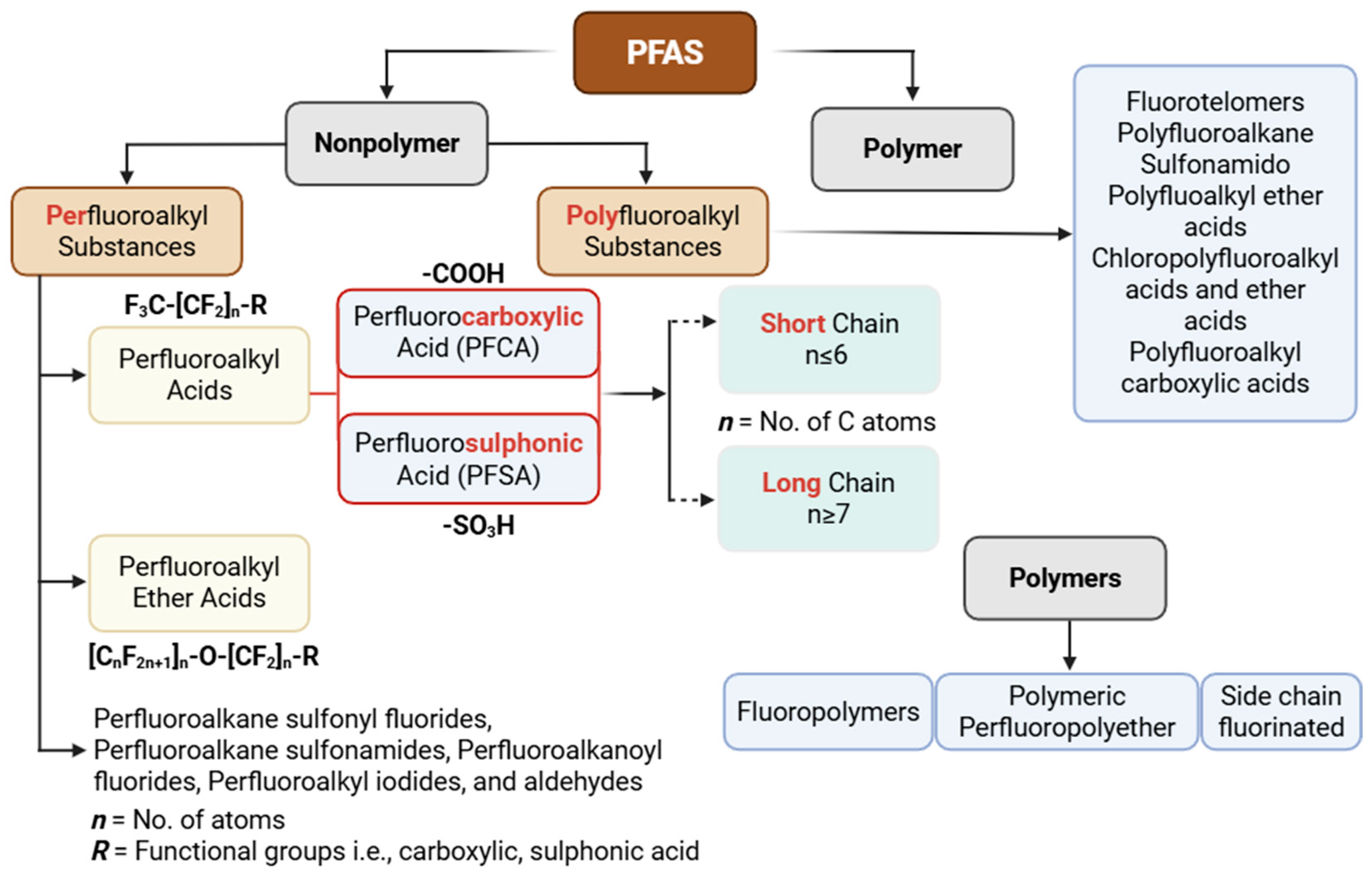
2. Classification of PFASs
2.1. Polymeric and Non-Polymeric
2.2. Per- and Polyfluoroalkyl Substances
2.2.1. Long-Chain
2.2.2. Short-Chain
2.2.3. Sulphonic Acids
2.2.4. Carboxylic Acids
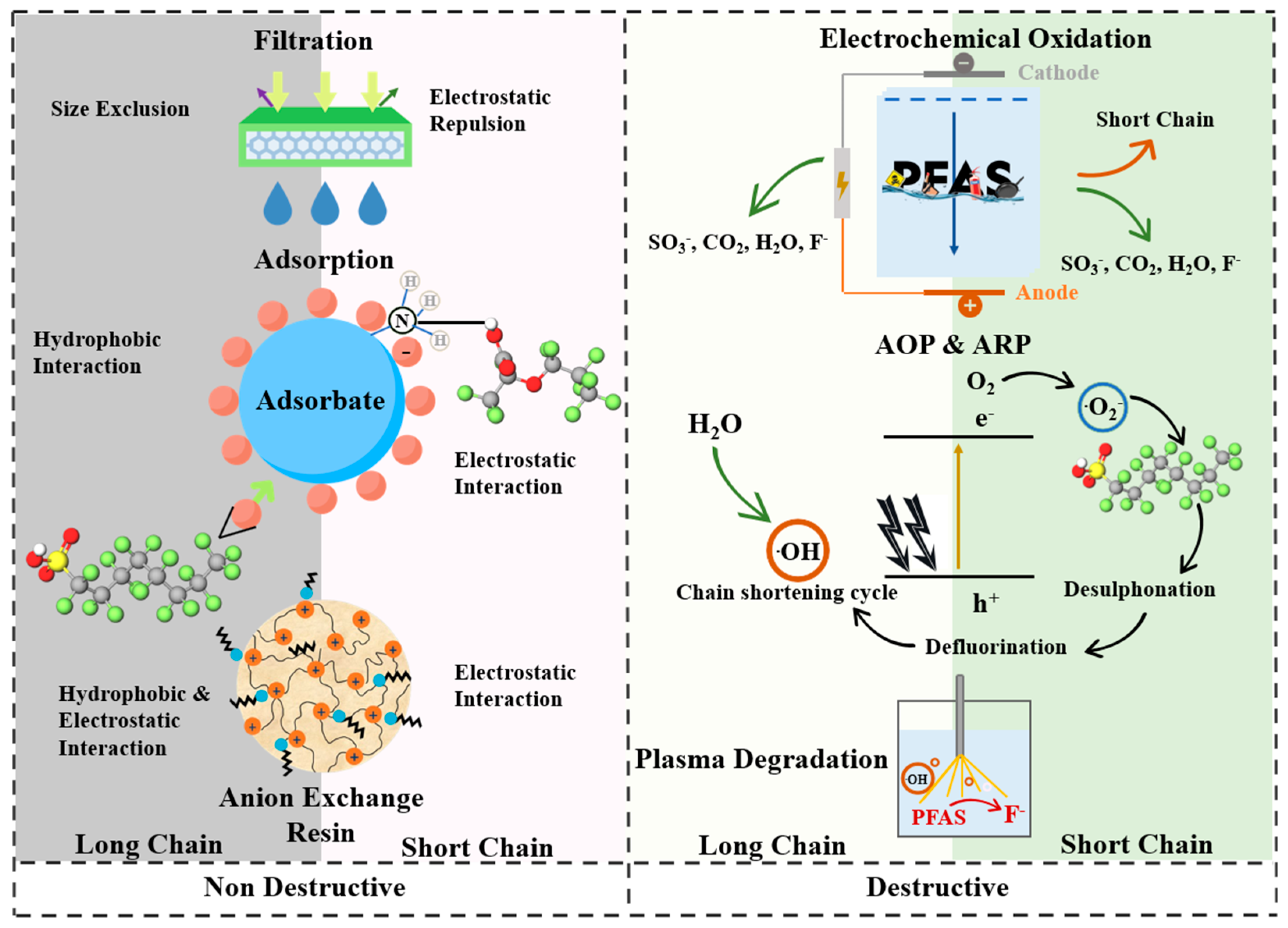
3. Methods for PFAS Removal
3.1. Non-Destructive Methods
3.2. Destructive Method
4. PFAS Removal by Adsorption
4.1. Sustainable Natural Polymers
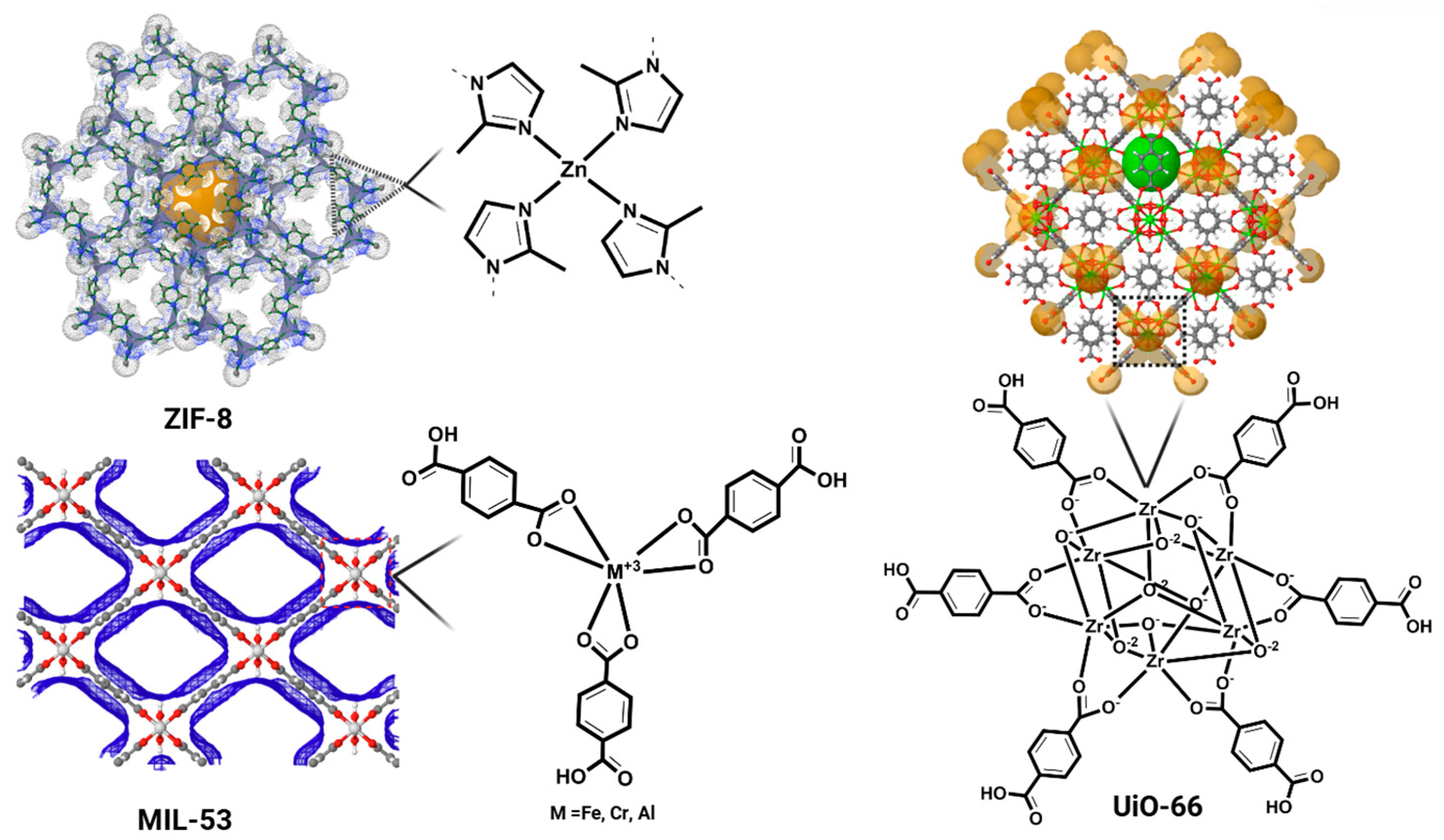
4.2. Synthetic Polymers
5. Key Parameters Affecting PFAS Adsorption
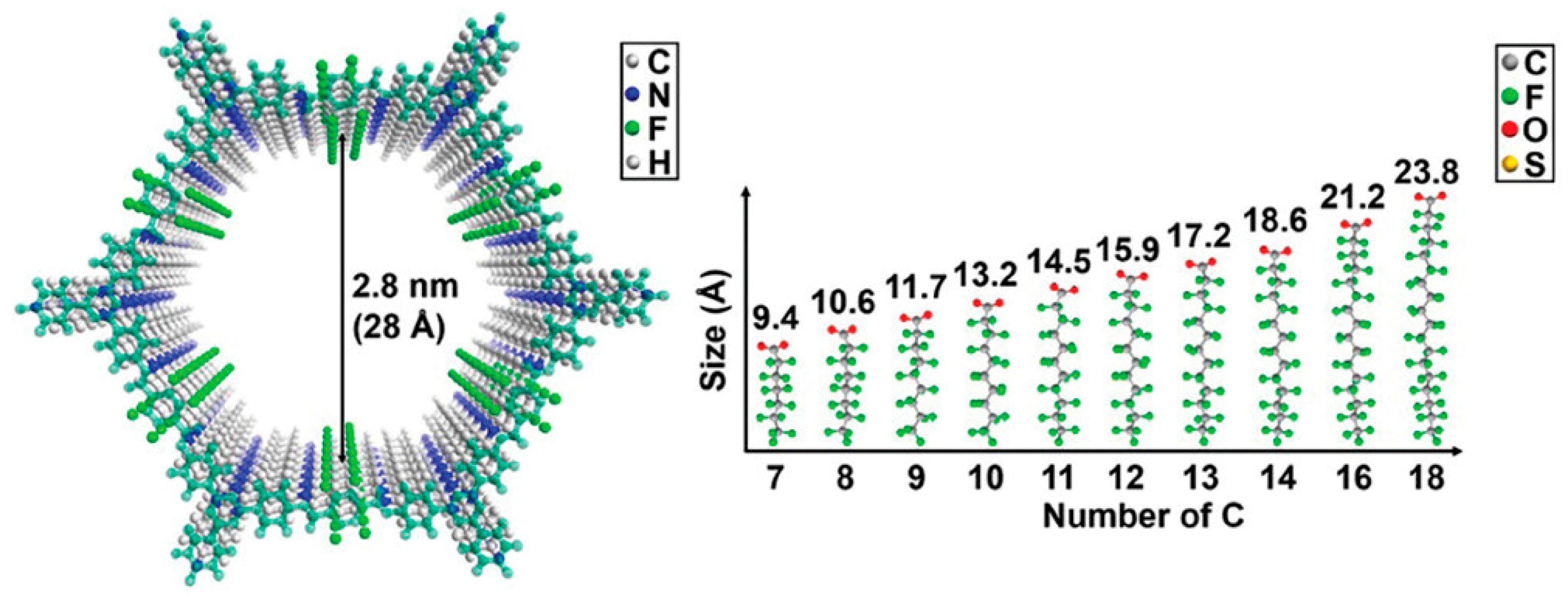
5.1. Effect of PFAS Chain Length
5.2. Effect of Functional Head Groups
5.3. Combined Effect of Chain Length and Functional Groups
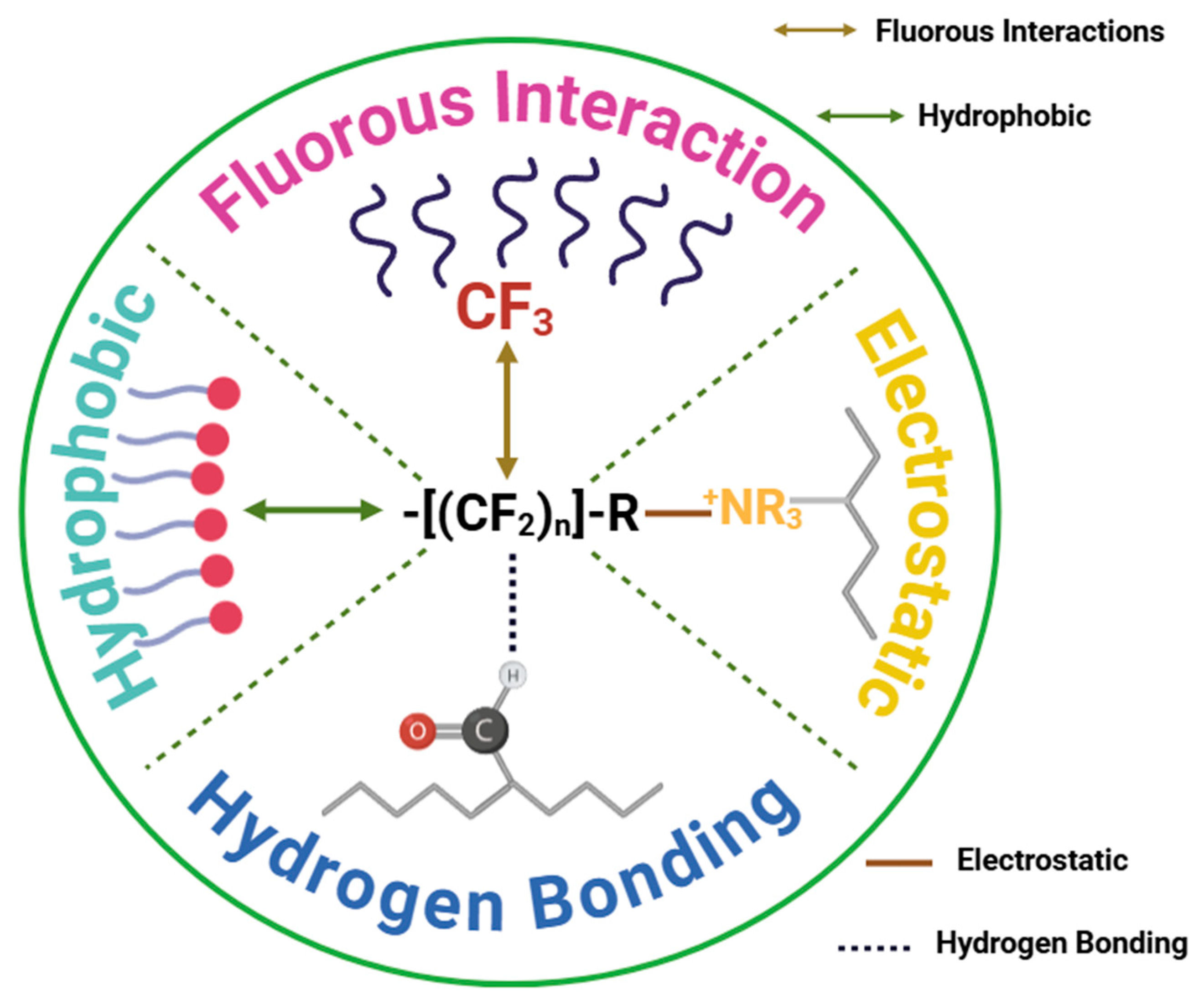
6. Adsorption Mechanism
7. Comparative Study of PFAS Removal Technologies
8. Conclusions and Future Perspective
8.1. Sustainable Approach
8.2. Principles for PFAS-Adsorbent Interactions
8.3. Current Research Gaps in PFAS Adsorption Studies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFASs | Per- and polyfluoroalkyl substances |
| CAS | Chemical Abstract Service |
| OECD | Organization of Co-operation and Development |
| AFFs | Aqueous film-forming foams |
| PFAAs | Perfluoroalkyl acids |
| PFOS | Perfluorooctanesulphonic acid |
| PFOA | Perfluorooctanoic acid |
| GenX | Hexafluoropropylene oxide dimer acid |
| PFHxS | Perfluorohexane sulphonic acid |
| PFNA | Perfluoronanoic acid |
| EPA | Environmental Protection Agency |
| NPDWR | National Primary Drinking Water Regulation |
| MCLs | Maximum contaminant levels |
| ppt | Parts per trillion |
| ppm | Parts per million |
| ppb | Parts per billion |
| PFBS | Perfluorobutane sulfonic acid |
| GAC | Granular activated carbon |
| SCWO | Super critical water oxidation |
| PTFE | Polytetrafluoroethylene |
| PFSAs | Perfluorosulfonic acids |
| PFCAs | Perfluoroalkyl carboxylic acids |
| LC/MS | Liquid chromatography/mass spectrometry |
| IX | Ion exchange resins |
| RO | Reverse osmosis |
| NF | Nanofiltration |
| MOFs | Metal–organic frameworks |
| AOPs | Advanced oxidation processes |
| ARPs | Advanced reduction processes |
| UV | Ultraviolet |
| C-F | Carbon–fluorine |
| F-F | Fluorine–fluorine |
| NTP | Non-thermal plasma |
| PFHxA | Perflourohexanoic acid |
| ADONA | 4,8-Dioxa-3H-perflourononanoic acid |
| PEI | Polyethyleneimine |
| TEA | Triethylamine |
| PFBA | Perfluorobutanoic acid |
| Kow | Octanol/water partition coefficient |
| ECH/EPI | Epichlorohydrin |
| P&P | Pulp and paper |
| Kd | Solid–water distribution coefficient |
| β-CDs | Beta cyclodextrins |
| HDI | Hexamethylene diisocyanate |
| Cl | Benzyl chloride |
| PEI-f-CMC | Polyethyleneimine functionalized cellulose microcrystals |
| N-Me-FOSAA | 2-(N-Methylperflourooctanesulfonamido) acetic acid |
| N-Et-FOSAA | 2-(N-Ethylperflourooctanesulfonamido) acetic acid |
| DAC | Alkylamine modified dialdehyde cellulose |
| QNC | Quaternized nanocellulose |
| QWP | Quaternized wood pulp |
| GCBs | Polyethyleneimine grafted chitosan beads |
| CBs | Crushed chitosan beads |
| CDPs | Cyclodextrin polymers |
| COFs | Covalent organic frameworks |
| MIPs | Molecularly imprinted polymers |
| DMAPAA-Q | N-[3-(dimethylamino)propyl]acrylamide methyl chloride quaternary |
| DFT | Density functional theory |
| QA-COFs | Quaternary amine functionalized covalent organic frameworks |
| ZIF | Zeolitic imidazolate framework |
| UiO | University of Oslo |
| MIL | Materials Institute Lavoisier |
| ΔG | Gibbs free energy |
| CMC | Critical micelle concentration |
| PEI-PVC | Polyethyleneimine-polyvinyl chloride |
| HSAB | Hard and soft acid–base |
| PFPeS | Perfluoropentane sulphonic acid |
| PFPeA | Perflouropentanoic acid |
| PFHpA | Perflouroheptanoic acid |
| PFHpS | Perflouroheptane sulphonic acid |
| PFDA | Perflourodecanoic acid |
| MPCA | Minnesota Pollution Control Agency |
| SC | Short-chain |
| LC | Long-chain |
| TRL | Technology Readiness Level |
References
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: A comprehensive review. Environ. Sci. Process. Impacts 2022, 24, 196–208. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Recent progress and challenges on the removal of per- and poly-fluoroalkyl substances (PFAS) from contaminated soil and water. Environ. Sci. Pollut. Res. 2022, 29, 58405–58428. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Stelben, P.; McDonough, C.A.; Dukes, D.A.; Aristizabal-Henao, J.J.; Nason, S.L.; Li, Y.; Sternberg, S.; Lin, E.; Beckmann, M. FluoroMatch 2.0—Making automated and comprehensive non-targeted PFAS annotation a reality. Anal. Bioanal. Chem. 2022, 414, 1201–1215. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per-and polyfluoroalkyl substances (PFAS) in PubChem: 7 million and growing. Environ. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L.G. Historical and current usage of per-and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Høisæter, Å.; Pfaff, A.; Breedveld, G.D. Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. J. Contam. Hydrol. 2019, 222, 112–122. [Google Scholar] [CrossRef]
- Jerič, P.; Likozar, B.; Novak, U. Biobased Hydrophobic Solutions for Natural Textiles—Moving Beyond PFAS. J. Compos. Sci. 2025, 9, 81. [Google Scholar] [CrossRef]
- Curtzwiler, G.W.; Silva, P.; Hall, A.; Ivey, A.; Vorst, K. Significance of perfluoroalkyl substances (PFAS) in food packaging. Integr. Environ. Assess. Manag. 2020, 17, 7–12. [Google Scholar] [CrossRef]
- Ramírez Carnero, A.; Lestido-Cardama, A.; Vazquez Loureiro, P.; Barbosa-Pereira, L.; Rodríguez Bernaldo de Quirós, A.; Sendón, R. Presence of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in food contact materials (FCM) and its migration to food. Foods 2021, 10, 1443. [Google Scholar] [CrossRef]
- Guerrero-Vacas, G.; Comino, F.; Rodríguez-Alabanda, O. Evaluation of the effectiveness and durability of commercial non-stick coatings. J. Food Eng. 2024, 370, 111959. [Google Scholar] [CrossRef]
- Dignes, C.; Nicolas, N.J.; Schroeder, T.B.; Aizenberg, J. Robust PFAS-Free Superhydrophobicity Exhibited in Hierarchically Nanostructured Coatings on Textiles. Adv. Eng. Mater. 2024, 27, 2401736. [Google Scholar] [CrossRef]
- Green, E.G. PFAS, Planes, and Problems: PFAS Regulation in the Aerospace and Aviation Industries. Okla. L. Rev. 2023, 76, 441. [Google Scholar]
- Tansel, B. PFAS use in electronic products and exposure risks during handling and processing of e-waste: A review. J. Environ. Manag. 2022, 316, 115291. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Wang, M.; He, M.; Ascencio, K.; Blotevogel, J.; Adamson, D.T.; Mahendra, S.; Mohanty, S.K. Perfluoroalkyl acids on suspended particles: Significant transport pathways in surface runoff, surface waters, and subsurface soils. J. Hazard. Mater. 2021, 417, 126159. [Google Scholar] [CrossRef] [PubMed]
- Sammut, G.; Sinagra, E.; Sapiano, M.; Helmus, R.; De Voogt, P. Perfluoroalkyl substances in the Maltese environment–(II) sediments, soils and groundwater. Sci. Total Environ. 2019, 682, 180–189. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical review of thermal decomposition of per-and polyfluoroalkyl substances: Mechanisms and implications for thermal treatment processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef]
- Drew, R.; Hagen, T.G.; Champness, D.; Sellier, A. Half-lives of several polyfluoroalkyl substances (PFAS) in cattle serum and tissues. Food Addit. Contam. Part A 2022, 39, 320–340. [Google Scholar] [CrossRef]
- Zhang, M.; Yazaydin, A.O. The effect of perfluoroalkyl chain length and the type of acid group on PFAS adsorption from water. Chem. Eng. J. 2024, 499, 155851. [Google Scholar] [CrossRef]
- Chowdhury, N.; Prabakar, S.; Choi, H. Dependency of the photocatalytic and photochemical decomposition of per-and polyfluoroalkyl substances (PFAS) on their chain lengths, functional groups, and structural properties. Water Sci. Technol. 2021, 84, 3738–3754. [Google Scholar] [CrossRef]
- de Paula Nunes, E.; Abou Dehn Pestana, B.; Pereira, B.B. Human biomonitoring and environmental health: A critical review of global exposure patterns, methodological challenges and research gaps. J. Toxicol. Environ. Health Part B 2025, 1–19. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, J. Exposure Pathways and Human Health Risks of PFAS. In Ecological and Human Health Impacts of Contaminated Food and Environments; CRC Press: Boca Raton, FL, USA, 2025; pp. 119–134. [Google Scholar]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Jha, G.; Kankarla, V.; McLennon, E.; Pal, S.; Sihi, D.; Dari, B.; Diaz, D.; Nocco, M. Per-and polyfluoroalkyl substances (PFAS) in integrated crop–livestock systems: Environmental exposure and human health risks. Int. J. Environ. Res. Public Health 2021, 18, 12550. [Google Scholar] [CrossRef] [PubMed]
- Pye, E.S.; Wallace, S.E.; Marangoni, D.G.; Foo, A.C. Albumin proteins as delivery vehicles for PFAS contaminants into respiratory membranes. ACS Omega 2023, 8, 44036–44043. [Google Scholar] [CrossRef]
- Perng, W.; Nakiwala, D.; Goodrich, J.M. What happens in utero does not stay in utero: A review of evidence for prenatal epigenetic programming by per-and polyfluoroalkyl substances (PFAS) in infants, children, and adolescents. Curr. Environ. Health Rep. 2023, 10, 35–44. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Sabaredzovic, A.; Namork, E.; Nygaard, U.C.; Granum, B.; Haug, L.S. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ. Int. 2016, 94, 687–694. [Google Scholar] [CrossRef]
- India-Aldana, S.; Yao, M.; Midya, V.; Colicino, E.; Chatzi, L.; Chu, J.; Gennings, C.; Jones, D.P.; Loos, R.J.; Setiawan, V.W. PFAS exposures and the human metabolome: A systematic review of epidemiological studies. Curr. Pollut. Rep. 2023, 9, 510–568. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.D.; Kleinman, M.T.; Bai, X.; Barlaz, M.; Abraczinskas, M.; Guidry, V.; Watson, J.; Chow, J. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manag. Assoc. 2021, 71, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a mechanistic understanding of poly-and perfluoroalkylated substances and cancer. Cancers 2022, 14, 2919. [Google Scholar] [CrossRef]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; DeWitt, J.C.; Knappe, D.R.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A. Scientific basis for managing PFAS as a chemical class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef]
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the safe operating space of a new planetary boundary for per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef]
- Figuière, R.; Miaz, L.T.; Savvidou, E.; Cousins, I.T. An overview of potential alternatives for the multiple uses of per-and polyfluoroalkyl substances. Environ. Sci. Technol. 2025, 59, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Uszkiewicz, K.L. Unveiling a Hidden Hazard: A Deep Dive into Forever Chemicals and the Urgent Need for Solutions. Vill. Env’t LJ 2025, 36, 161. [Google Scholar]
- Knutson, J.R. Informing Wisconsin’s Response to PFAS: Environmental Justice and Strategies for Identifying and Managing Sources of PFAS in Drinking Water and Wastewater; The University of Wisconsin-Madison: Madison, WI, USA, 2024. [Google Scholar]
- Gerrard, M.B.; McTiernan, E. Regulation of polyfluoroalkyl chemicals in New York. NYLJ 2022. Available online: https://scholarship.law.columbia.edu/faculty_scholarship/3216/ (accessed on 15 September 2025).
- Pollack, J.B.; Carey, I.Q.; Xu, V.Y. Regulation of Products with PFAS. Env’t L. Rep. 2024, 54, 10148. [Google Scholar]
- Longpré, D.; Lorusso, L.; Levicki, C.; Carrier, R.; Cureton, P. PFOS, PFOA, LC-PFCAS, and certain other PFAS: A focus on Canadian guidelines and guidance for contaminated sites management. Environ. Technol. Innov. 2020, 18, 100752. [Google Scholar] [CrossRef]
- Goldenman, G.; Fernandes, M.; Holland, M.; Tugran, T.; Nordin, A.; Schoumacher, C.; McNeill, A. The Cost of Inaction: A Socioeconomic Analysis of Environmental and Health Impacts Linked to Exposure to PFAS; Nordic Council of Ministers: Copenhagen, Denmark, 2019. [Google Scholar]
- Nzeribe, B.N.; Thagard, S.M.; Holsen, T.M.; Stratton, G.; Crimi, M. Oxidation and reduction approaches for treatment of perfluoroalkyl substances. In Perfluoroalkyl Substances in the Environment; CRC Press: Boca Raton, FL, USA, 2018; pp. 255–302. [Google Scholar]
- Buck, R.C.; Korzeniowski, S.H.; Laganis, E.; Adamsky, F. Identification and classification of commercially relevant per-and poly-fluoroalkyl substances (PFAS). Integr. Environ. Assess. Manag. 2021, 17, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Patlewicz, G.; Richard, A.M.; Williams, A.J.; Judson, R.S.; Thomas, R.S. Towards reproducible structure-based chemical categories for PFAS to inform and evaluate toxicity and toxicokinetic testing. Comput. Toxicol. 2022, 24, 100250. [Google Scholar] [CrossRef]
- Korzeniowski, S.H.; Buck, R.C.; Newkold, R.M.; kassmi, A.E.; Laganis, E.; Matsuoka, Y.; Dinelli, B.; Beauchet, S.; Adamsky, F.; Weilandt, K.; et al. A critical review of the application of polymer of low concern regulatory criteria to fluoropolymers II: Fluoroplastics and fluoroelastomers. Integr. Environ. Assess. Manag. 2023, 19, 326–354. [Google Scholar] [CrossRef]
- Malouchi, N.; Chatzimichailidou, S.; Tolkou, A.K.; Kyzas, G.Z.; Calgaro, L.; Marcomini, A.; Katsoyiannis, I.A. The Removal of Per-and Poly-Fluoroalkyl Substances from Water: A Review on Destructive and Non-Destructive Methods. Separations 2024, 11, 122. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Herzke, D.; Olsson, E.; Posner, S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway—A pilot study. Chemosphere 2012, 88, 980–987. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical properties and interactions of perfluoroalkyl substances (PFAS)-Challenges and opportunities in sensing and remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef]
- Chambers, W.S.; Hopkins, J.G.; Richards, S.M. A review of per-and polyfluorinated alkyl substance impairment of reproduction. Front. Toxicol. 2021, 3, 732436. [Google Scholar] [CrossRef]
- Bellia, G.R.; Bilott, R.A.; Sun, N.; Thompson, D.; Vasiliou, V. Use of clinical chemistry health outcomes and PFAS chain length to predict 28-day rodent oral toxicity. Toxicol. Mech. Methods 2023, 33, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; MacDonald Gibson, J. Predicting the occurrence of short-chain PFAS in groundwater using machine-learned Bayesian networks. Front. Environ. Sci. 2022, 10, 958784. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, L.; Tang, C.; Yang, Y.; Liang, S.; Wang, A.; Xu, J.; Huang, Q.; Lin, H. Broad-spectrum capture of hundreds of per-and polyfluoroalkyl substances from fluorochemical wastewater. Nat. Commun. 2025, 16, 1972. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.; Song, M.; Ikram, S.; Zahra, Z. Overview of per-and polyfluoroalkyl substances (PFAS), their applications, sources, and potential impacts on human health. Pollutants 2024, 4, 136–152. [Google Scholar] [CrossRef]
- Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E.B. A critical review on PFAS removal from water: Removal mechanism and future challenges. Sustainability 2023, 15, 16173. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Hussain, H.N.; Jilani, M.I.; Imtiaz, F.; Ahmed, T.; Arshad, M.B.; Mudassar, M.; Sharif, M.N. Advances in the removal of Polyfluoroalkyl Substances (PFAS) from water using destructive and non-destructive methods. Green Anal. Chem. 2025, 12, 100225. [Google Scholar] [CrossRef]
- Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Treatment Trends and Hybrid Methods for the Removal of Poly-and Perfluoroalkyl Substances from Water—A Review. Appl. Sci. 2024, 14, 2574. [Google Scholar] [CrossRef]
- Tushar, M.M.R.; Pushan, Z.A.; Aich, N.; Rowles, L.S. Balancing sustainability goals and treatment efficacy for PFAS removal from water. npj Clean Water 2024, 7, 130. [Google Scholar] [CrossRef]
- Jafarinejad, S. A mini-review of full-scale drinking water treatment plants for per-and polyfluoroalkyl substances (PFAS) removal: Possible solutions and future directions. Sustainability 2025, 17, 451. [Google Scholar] [CrossRef]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing conventional and advanced approaches for heavy metal removal in wastewater treatment: An in-depth review emphasizing filter-based strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef]
- Hopkins, Z.R.; Knappe, D.R. Predicting per-and polyfluoroalkyl substances removal in pilot-scale granular activated carbon adsorbers from rapid small-scale column tests. AWWA Water Sci. 2024, 6, e1369. [Google Scholar] [CrossRef]
- Maroli, A.S.; Zhang, Y.; Lubiantoro, J.; Venkatesan, A.K. Surfactant-enhanced coagulation and flocculation improves the removal of perfluoroalkyl substances from surface water. Environ. Sci. Adv. 2024, 3, 1714–1721. [Google Scholar] [CrossRef]
- Sanzana, S.; Fenti, A.; Iovino, P.; Panico, A. A review of PFAS remediation: Separation and degradation technologies for water and wastewater treatment. J. Water Process Eng. 2025, 74, 107793. [Google Scholar] [CrossRef]
- Johnson, J.K.; Hoffman, C.M., Jr.; Smith, D.A.; Xia, Z. Advanced filtration membranes for the removal of perfluoroalkyl species from water. Acs Omega 2019, 4, 8001–8006. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Faria, A.F.; Quiñones, K.Y.D.; Zhang, C.; He, Q.; Ma, J.; Shen, Y.; Zhi, Y. Evaluating the efficiency of nanofiltration and reverse osmosis membrane processes for the removal of per-and polyfluoroalkyl substances from water: A critical review. Sep. Purif. Technol. 2022, 302, 122161. [Google Scholar] [CrossRef]
- Das, S.; Ronen, A. A review on removal and destruction of per-and polyfluoroalkyl substances (PFAS) by novel membranes. Membranes 2022, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lei, Q.; Liu, F.; Song, Z.; Khusid, B.; Zhang, W. Evaluation of commercial nanofiltration and reverse osmosis membrane filtration to remove per-and polyfluoroalkyl substances (PFAS): Effects of transmembrane pressures and water matrices. Water Environ. Res. 2024, 96, e10983. [Google Scholar] [CrossRef]
- Fujioka, T.; Takeuchi, H.; Tahara, H.; Murakami, H.; Boivin, S. Effects of functional groups of polyfluoroalkyl substances on their removal by nanofiltration. Water Res. X 2024, 24, 100233. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced oxidation processes for water and wastewater treatment–Guidance for systematic future research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef]
- Kumar, R.; Dada, T.K.; Whelan, A.; Cannon, P.; Sheehan, M.; Reeves, L.; Antunes, E. Microbial and thermal treatment techniques for degradation of PFAS in biosolids: A focus on degradation mechanisms and pathways. J. Hazard. Mater. 2023, 452, 131212. [Google Scholar] [CrossRef]
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef]
- Zaman, D.; Tiwari, M.K.; Mishra, S. Low-cost adsorptive removal techniques for pharmaceuticals and personal care products. In Measurement, Analysis and Remediation of Environmental Pollutants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 397–421. [Google Scholar]
- Fouda-Mbanga, B.G.; Onotu, O.; Tywabi-Ngeva, Z. Advantages of the reuse of spent adsorbents and potential applications in environmental remediation: A review. Green Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Calore, F.; Badetti, E.; Bonetto, A.; Pozzobon, A.; Marcomini, A. Non-conventional sorption materials for the removal of legacy and emerging PFAS from water: A review. Emerg. Contam. 2024, 10, 100303. [Google Scholar] [CrossRef]
- Sheraz, N.; Shah, A.; Haleem, A.; Iftikhar, F.J. Comprehensive assessment of carbon-, biomaterial-and inorganic-based adsorbents for the removal of the most hazardous heavy metal ions from wastewater. RSC Adv. 2024, 14, 11284–11310. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Li, D.; Londhe, K.; Chi, K.; Lee, C.S.; Venkatesan, A.K.; Hsiao, B.S. Functionalized bio-adsorbents for removal of perfluoroalkyl substances: A perspective. AWWA Water Sci. 2021, 3, e1258. [Google Scholar] [CrossRef]
- Abeysinghe, H.; Ma, X.; Tsige, M. PFAS removal via adsorption: A synergistic review on advances of experimental and computational approaches. Chemosphere 2025, 377, 144323. [Google Scholar] [CrossRef]
- Cantoni, B.; Turolla, A.; Wellmitz, J.; Ruhl, A.S.; Antonelli, M. Perfluoroalkyl substances (PFAS) adsorption in drinking water by granular activated carbon: Influence of activated carbon and PFAS characteristics. Sci. Total Environ. 2021, 795, 148821. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, M.; Liu, Z.; Sauvé, S.; Barbeau, B. Comparative assessment of powdered versus granular activated carbon for PFAS removal in drinking water treatment plants. ACS EST Water 2025, 5, 851–861. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ekpe, O.D.; Lee, H.-J.; Oh, J.-E. Perfluoroalkyl substances and pharmaceuticals removal in full-scale drinking water treatment plants. J. Hazard. Mater. 2020, 400, 123235. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Kosaka, K.; Yoshida, N.; Asami, M.; Matsui, Y. Long-term removal of perfluoroalkyl substances via activated carbon process for general advanced treatment purposes. Water Res. 2023, 245, 120559. [Google Scholar] [CrossRef]
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.; Ahrens, L. Removal of per-and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef]
- Gonzalez, D.; Thompson, K.; Quiñones, O.; Dickenson, E.; Bott, C. Granular activated carbon-based treatment and mobility of per-and polyfluoroalkyl substances in potable reuse for aquifer recharge. AWWA Water Sci. 2021, 3, e1247. [Google Scholar] [CrossRef]
- Kempisty, D.M.; Arevalo, E.; Spinelli, A.M.; Edeback, V.; Dickenson, E.R.; Husted, C.; Higgins, C.P.; Summers, R.S.; Knappe, D.R. Granular activated carbon adsorption of perfluoroalkyl acids from ground and surface water. AWWA Water Sci. 2022, 4, e1269. [Google Scholar] [CrossRef]
- Najm, I.; Gallagher, B.; Vishwanath, N.; Blute, N.; Gorzalski, A.; Feffer, A.; Richardson, S. Per-and polyfluoroalkyl substances removal with granular activated carbon and a specialty adsorbent: A case study. AWWA Water Sci. 2021, 3, e1245. [Google Scholar] [CrossRef]
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per-and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Børresen, M.H.; Schlabach, M.; Cornelissen, G. Sorption of perfluorinated compounds from contaminated water to activated carbon. J. Soils Sediments 2010, 10, 179–185. [Google Scholar] [CrossRef]
- Jais, F.M.; Ibrahim, M.S.I.; El-Shafie, A.; Choong, C.E.; Kim, M.; Yoon, Y.; Jang, M. Updated review on current approaches and challenges for poly-and perfluoroalkyl substances removal using activated carbon-based adsorbents. J. Water Process Eng. 2024, 64, 105625. [Google Scholar] [CrossRef]
- Mian, M.M.; Zhu, J.; Jiang, X.; Deng, S. Recent advances in activated carbon driven PFAS removal: Structure-adsorption relationship and new adsorption mechanisms. Front. Environ. Sci. Eng. 2025, 19, 78. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Ma, S.; Zhang, Q. Adsorption of per-and polyfluoroalkyl substances (PFAS) from water with porous organic polymers. Chemosphere 2024, 346, 140600. [Google Scholar] [CrossRef]
- Boyer, T.H.; Fang, Y.; Ellis, A.; Dietz, R.; Choi, Y.J.; Schaefer, C.E.; Higgins, C.P.; Strathmann, T.J. Anion exchange resin removal of per-and polyfluoroalkyl substances (PFAS) from impacted water: A critical review. Water Res. 2021, 200, 117244. [Google Scholar] [CrossRef]
- Appleman, T.D.; Higgins, C.P.; Quiñones, O.; Vanderford, B.J.; Kolstad, C.; Zeigler-Holady, J.C.; Dickenson, E.R. Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems. Water Res. 2014, 51, 246–255. [Google Scholar] [CrossRef]
- Rahman, M.F.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Ion-exchange treatment of perfluorinated carboxylic acids in water: Comparison of polystyrenic and polyacrylic resin structures and impact of sulfate on their performance. ACS EsT Water 2022, 2, 1195–1205. [Google Scholar] [CrossRef]
- Tshangana, C.; Nhlengethwa, S.; Glass, S.; Denison, S.; Kuvarega, A.; Nkambule, T.; Mamba, B.; Alvarez, P.J.; Muleja, A. Technology status to treat PFAS-contaminated water and limiting factors for their effective full-scale application. npj Clean Water 2025, 8, 41. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Bezerra de Souza, B.; Casarini, M.M.; Kewalramani, J.A. A review of PFAS destruction technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Pinto da Silva, L.; Esteves da Silva, J.C. Nanomaterial-based advanced oxidation/reduction processes for the degradation of PFAS. Nanomaterials 2023, 13, 1668. [Google Scholar] [CrossRef]
- Alalm, M.G.; Boffito, D.C. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- Umar, M. Reductive and oxidative UV degradation of PFAS—Status, needs and future perspectives. Water 2021, 13, 3185. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, T.; Yang, X.; Ding, S.; Ma, M. Insights into photo/electrocatalysts for the degradation of per-and polyfluoroalkyl substances (PFAS) by advanced oxidation processes. Catalysts 2023, 13, 1308. [Google Scholar] [CrossRef]
- Gyaljen Tamang, S.; Umlauf, G.; Barz, J.; Ghomi, M.R. Degradation of PFOA solutions and PFAS-contaminated groundwater using atmospheric non-thermal plasma treatment. Water Pract. Technol. 2024, 19, 2645–2654. [Google Scholar] [CrossRef]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma water treatment for PFAS: Study of degradation of perfluorinated substances and their byproducts by using cold atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Takemine, S.; Yamamoto, K.; Haga, Y.; Takata, M. Residual organic fluorinated compounds from thermal treatment of PFOA, PFHxA and PFOS adsorbed onto granular activated carbon (GAC). J. Mater. Cycles Waste Manag. 2016, 18, 625–630. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Prospects of Novel Technologies for PFAS Destruction in Water and Wastewater. Appl. Sci. 2025, 15, 9311. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Bergmann, D.; Bulatovic, T.; Surapaneni, A.; Gray, S. Review of influence of critical operation conditions on by-product/intermediate formation during thermal destruction of PFAS in solid/biosolids. Sci. Total Environ. 2023, 854, 158796. [Google Scholar] [CrossRef] [PubMed]
- Mirabediny, M.; Sun, J.; Yu, T.T.; Åkermark, B.; Das, B.; Kumar, N. Effective PFAS degradation by electrochemical oxidation methods-recent progress and requirement. Chemosphere 2023, 321, 138109. [Google Scholar] [CrossRef] [PubMed]
- Bekchanov, D.; Mukhamediev, M.; Yarmanov, S.; Lieberzeit, P.; Mujahid, A. Functionalizing natural polymers to develop green adsorbents for wastewater treatment applications. Carbohydr. Polym. 2024, 323, 121397. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L.F. Cellulose–chitosan functional biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef]
- Li, D.; Das, R.; Zhang, Y.; Zheng, S.; Oltulu, M.; Venkatesan, A.K.; Hsiao, B.S. Alkylamine-Modified Dialdehyde Cellulose Nanofibers for PFAS Adsorption. ACS EST Water 2025, 5, 1582–1594. [Google Scholar] [CrossRef]
- Harris, J.T.; de la Garza, G.D.; Devlin, A.M.; McNeil, A.J. Rapid removal of poly-and perfluoroalkyl substances with quaternized wood pulp. ACS EST Water 2022, 2, 349–356. [Google Scholar] [CrossRef]
- Huang, J.; Fu, K.; Fang, Z.; Luo, J. Enhanced selective removal of PFAS at trace level using quaternized cellulose-functionalized polymer resin: Performance and mechanism. Water Res. 2025, 272, 122937. [Google Scholar] [CrossRef]
- Shahrokhi, R.; Park, J. Enhanced removal of short-and long-chain per-and poly-fluoroalkyl substances from aqueous phase using crushed grafted chitosan beads: Performance and mechanisms. Environ. Pollut. 2024, 340, 122836. [Google Scholar] [CrossRef]
- García-Castrillo, M.; Barandika, G.; Lizundia, E. Low Environmental Impact Magnetic Chitosan and Chitin Cryogels for PFAS Remediation. Adv. Funct. Mater. 2024, 34, 2405298. [Google Scholar] [CrossRef]
- Wittwer, P.; Roesch, P.; Vogel, C.; Simon, F.; Gehrenkemper, L.; Feldmann, I.; Simon, F.-G. Less Is More: Influence of Cross-Linking Agent Concentration on PFOS Adsorption in Chitosan. Appl. Sci. 2024, 14, 11145. [Google Scholar] [CrossRef]
- Khan, P.; Ali, S.; Jan, R.; Kim, K.-M. Lignin Nanoparticles: Transforming Environmental Remediation. Nanomaterials 2024, 14, 1541. [Google Scholar] [CrossRef]
- Mel, M.; Lau, B.; Hockaday, W.C. Sorption of per-and polyfluoroalkyl substances by lignin in pulp and paper wastewater. J. Hazard. Mater. 2024, 480, 136016. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, Z.-W.; Klemes, M.J.; Ateia, M.; Trang, B.; Wang, J.; Ching, C.; Helbling, D.E.; Dichtel, W.R. A tunable porous β-cyclodextrin polymer platform to understand and improve anionic PFAS removal. ACS Cent. Sci. 2022, 8, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Abaie, E.; Kumar, M.; Kumar, N.; Sun, Y.; Guelfo, J.; Shen, Y.; Reible, D. Application of β-cyclodextrin adsorbents in the removal of mixed per-and polyfluoroalkyl substances. Toxics 2024, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Attia, M.F.; Maroli, A.; Tharayil, N.; Alexis, F.; Whitehead, D.C.; Karanfil, T. Rapid removal of poly-and perfluorinated alkyl substances by poly (ethylenimine)-functionalized cellulose microcrystals at environmentally relevant conditions. Environ. Sci. Technol. Lett. 2018, 5, 764–769. [Google Scholar] [CrossRef]
- Li, D.; Lee, C.-S.; Zhang, Y.; Das, R.; Akter, F.; Venkatesan, A.K.; Hsiao, B.S. Efficient removal of short-chain and long-chain PFAS by cationic nanocellulose. J. Mater. Chem. A 2023, 11, 9868–9883. [Google Scholar] [CrossRef]
- Deng, S.; Zheng, Y.; Xu, F.; Wang, B.; Huang, J.; Yu, G. Highly efficient sorption of perfluorooctane sulfonate and perfluorooctanoate on a quaternized cotton prepared by atom transfer radical polymerization. Chem. Eng. J. 2012, 193, 154–160. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef]
- Ching, C.; Lin, Z.-W.; Dichtel, W.R.; Helbling, D.E. Evaluating the performance of novel cyclodextrin polymer granules to remove perfluoroalkyl acids (PFAAs) from water. ACS EST Eng. 2023, 3, 661–670. [Google Scholar] [CrossRef]
- Gomri, C.; Benkhaled, B.T.; Cretin, M.; Semsarilar, M. Adsorbent Material Used for the Treatment of Per-and Poly-Fluoroalkyl Substances (PFAS): A Short Review. Macromol. Chem. Phys. 2024, 225, 2400012. [Google Scholar] [CrossRef]
- Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environ. Sci. Eur. 2023, 35, 12. [Google Scholar] [CrossRef]
- Ateia, M.; Arifuzzaman, M.; Pellizzeri, S.; Attia, M.F.; Tharayil, N.; Anker, J.N.; Karanfil, T. Cationic polymer for selective removal of GenX and short-chain PFAS from surface waters and wastewaters at ng/L levels. Water Res. 2019, 163, 114874. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jia, Y.; Zhou, S.; Deng, S. Removal of typical PFAS from water by covalent organic frameworks with different pore sizes. J. Hazard. Mater. 2023, 460, 132522. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Z.; Shao, H.; Zhou, S.; Yu, G.; Deng, S. Cationic covalent organic framework for efficient removal of PFOA substitutes from aqueous solution. Chem. Eng. J. 2021, 412, 127509. [Google Scholar] [CrossRef]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef]
- Ilango, A.K.; Mekkat, R.; Jeyalakshmi, V.; Pervez, M.N.; Jiang, T.; Chand, P.; Kumaran, Y.; Efstathiadis, H.; Sukalingum, D.; Soos, M. Enhanced removal of PFAS in water using activated ZIF-8 carbons: High adsorption efficiency, repeatable regenerability and reusability. Chem. Eng. J. 2025, 507, 160192. [Google Scholar] [CrossRef]
- Hua, L.; Solomon, M.B.; Deanna, M.A.; Donald, W.A. Dual-Functional Metal Organic Frameworks for Adsorptive Removal and Ultra-Trace Quantitation of 50 Per-and Polyfluoroalkyl Substances in Water. J. Hazard. Mater. 2025, 494, 138679. [Google Scholar] [CrossRef]
- Hedbom, D.; Gaiser, P.; Günther, T.; Cheung, O.; Strømme, M.; Åhlén, M.; Sjödin, M. A fluorinated zirconium-based metal–organic framework as a platform for the capture and removal of perfluorinated pollutants from air and water. J. Mater. Chem. A 2025, 13, 1731–1737. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, Y.; Xiao, F.; Ma, J.; Zou, Y.; Tang, W. Perfluorooctane sulfonate removal by metal-organic frameworks (MOFs): Insights into the effect and mechanism of metal nodes and organic ligands. Chem. Eng. J. 2021, 406, 126852. [Google Scholar] [CrossRef]
- Karbassiyazdi, E.; Kasula, M.; Modak, S.; Pala, J.; Kalantari, M.; Altaee, A.; Esfahani, M.R.; Razmjou, A. A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents. Chemosphere 2023, 311, 136933. [Google Scholar] [CrossRef] [PubMed]
- Tasfaout, A.; Ibrahim, F.; Morrin, A.; Brisset, H.; Sorrentino, I.; Nanteuil, C.; Laffite, G.; Nicholls, I.A.; Regan, F.; Branger, C. Molecularly imprinted polymers for per-and polyfluoroalkyl substances enrichment and detection. Talanta 2023, 258, 124434. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, L.; Ren, X.; Sun, H. Synthesis of a perfluorooctanoic acid molecularly imprinted polymer for the selective removal of perfluorooctanoic acid in an aqueous environment. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. Tunable macromolecular-based materials for the adsorption of perfluorooctanoic and octanoic acid anions. J. Colloid Interface Sci. 2013, 402, 196–203. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Tian, Y.; Wu, F.; Deng, C.; Guo, Q.; Sun, H.; Lu, S. Synthesis and evaluation of molecularly imprinted polymers with binary functional monomers for the selective removal of perfluorooctanesulfonic acid and perfluorooctanoic acid. J. Chromatogr. A 2017, 1516, 42–53. [Google Scholar] [CrossRef]
- Du, L.; Wu, Y.; Zhang, X.; Zhang, F.; Chen, X.; Cheng, Z.; Wu, F.; Tan, K. Preparation of magnetic molecularly imprinted polymers for the rapid and selective separation and enrichment of perfluorooctane sulfonate. J. Sep. Sci. 2017, 40, 2819–2826. [Google Scholar] [CrossRef]
- Pulster, E.L.; Bowman, S.R.; Keele, L.; Steevens, J. Guide to Per-and Polyfluoroalkyl Substances (PFAS) Sampling Within Natural Resource Damage Assessment and Restoration; US Geological Survey: Reston, VA, USA, 2024.
- Sleep, J.A.; Miklavcic, S.J.; Juhasz, A.L. Modelling of PFAS-surface interactions: Effect of surface charge and solution ions. Chemosphere 2023, 319, 137910. [Google Scholar] [CrossRef]
- Behnami, A.; Pourakbar, M.; Ayyar, A.S.-R.; Lee, J.-W.; Gagnon, G.; Benis, K.Z. Treatment of aqueous per-and poly-fluoroalkyl substances: A review of biochar adsorbent preparation methods. Chemosphere 2024, 357, 142088. [Google Scholar] [CrossRef]
- Fatima, M.; Kelso, C.; Hai, F. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water 2025, 17, 1401. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Higgins, C.P. Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ. Sci. Technol. 2013, 47, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Sukeesan, S.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Evaluation of the adsorption behavior of mixed perfluoroalkyl and polyfluoroalkyl substances onto granular activated carbon and styrene-divinylbenzene resins. Remediat. J. 2023, 33, 297–308. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, F.; Shen, C.; Chen, J.; Park, C.M.; Sun, Y.; Flury, M.; Wang, D. Per-and polyfluoroalkyl substances (PFAS) in subsurface environments: Occurrence, fate, transport, and research prospect. Rev. Geophys. 2022, 60, e2021RG000765. [Google Scholar] [CrossRef]
- Alves, A.V.; Tsianou, M.; Alexandridis, P. Fluorinated surfactant adsorption on mineral surfaces: Implications for PFAS fate and transport in the environment. Surfaces 2020, 3, 516–566. [Google Scholar] [CrossRef]
- Manayil Parambil, A.; Priyadarshini, E.; Paul, S.; Bakandritsos, A.; Sharma, V.K.; Zboril, R. Emerging nanomaterials for the detection of per-and poly-fluorinated substances. J. Mater. Chem. A 2025, 13, 8246–8281. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Li, H.; Dong, Q.; Zhang, M.; Gong, T.; Zan, R.; Wang, W. Transport behavior difference and transport model of long-and short-chain per-and polyfluoroalkyl substances in underground environmental media: A review. Environ. Pollut. 2023, 327, 121579. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Liang, Y. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution-A review. Sci. Total Environ. 2019, 694, 133606. [Google Scholar] [CrossRef]
- Kang, S.B.; Wang, Z.; Zhang, W.; Kim, K.-Y.; Won, S.W. Removal of short-and long-chain PFAS from aquatic systems using electrostatic attraction of polyethylenimine-polyvinyl chloride electrospun nanofiber adsorbent. Sep. Purif. Technol. 2023, 326, 124853. [Google Scholar] [CrossRef]
- Han, D.; Ma, Y.; Huang, C.; Zhang, X.; Xu, H.; Zhou, Y.; Liang, S.; Chen, X.; Huang, X.; Liao, H. Occurrence and source apportionment of perfluoroalkyl acids (PFAAs) in the atmosphere in China. Atmos. Chem. Phys. 2019, 19, 14107–14117. [Google Scholar] [CrossRef]
- Ho, T.-L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Li, R.; Adarsh, N.N.; Lu, H.; Wriedt, M. Metal-organic frameworks as platforms for the removal of per-and polyfluoroalkyl substances from contaminated waters. Matter 2022, 5, 3161–3193. [Google Scholar] [CrossRef]
- Xiang, Q.; Shan, G.; Wu, W.; Jin, H.; Zhu, L. Measuring log Kow coefficients of neutral species of perfluoroalkyl carboxylic acids using reversed-phase high-performance liquid chromatography. Environ. Pollut. 2018, 242, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Eichler, C.M.; Little, J.C. A framework to model exposure to per-and polyfluoroalkyl substances in indoor environments. Environ. Sci. Process. Impacts 2020, 22, 500–511. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. A comparative assessment of octanol-water partitioning and distribution constant estimation methods for perfluoroalkyl carboxylates and sulfonates. Nat. Preced. 2009, 4, 1–27. [Google Scholar] [CrossRef]
- He, Y.; Cheng, X.; Gunjal, S.J.; Zhang, C. Advancing PFAS sorbent design: Mechanisms, challenges, and perspectives. ACS Mater. Au 2023, 4, 108–114. [Google Scholar] [CrossRef]
- Wackett, L.P. Evolutionary obstacles and not C–F bond strength make PFAS persistent. Microb. Biotechnol. 2024, 17, e14463. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Hermann, R.B. Theory of hydrophobic bonding. II. Correlation of hydrocarbon solubility in water with solvent cavity surface area. J. Phys. Chem. 1972, 76, 2754–2759. [Google Scholar] [CrossRef]
- Blokzijl, W.; Engberts, J.B. Hydrophobic effects. Opinions and facts. Angew. Chem. Int. Ed. Engl. 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Kancharla, S.; Alexandridis, P.; Tsianou, M. Sequestration of per-and polyfluoroalkyl substances (PFAS) by adsorption: Surfactant and surface aspects. Curr. Opin. Colloid Interface Sci. 2022, 58, 101571. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, A.; Apul, O.; Venkatesan, A.K. Coexisting ions and long-chain per-and polyfluoroalkyl substances (PFAS) inhibit the adsorption of short-chain PFAS by granular activated carbon. J. Hazard. Mater. 2023, 460, 132378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhang, X.; Penn, L.; Gulliver, J.S.; Simcik, M.F. Effects of monovalent cations on the competitive adsorption of perfluoroalkyl acids by kaolinite: Experimental studies and modeling. Environ. Sci. Technol. 2011, 45, 10028–10035. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.A.; Janisse, S.E.; Heffern, M.C.; Kinyua, M.; Velázquez, J.M. Adsorption of perfluorooctanoic acid from water by pH-modulated Brönsted acid and base sites in mesoporous hafnium oxide ceramics. Iscience 2022, 25, 104138. [Google Scholar] [CrossRef]
- Lobitz, A.; Steuber, A.; Jia, S.; Guo, L. Harnessing Fluorine Chemistry: Strategies for Per-and Polyfluoroalkyl Substances Removal and Enrichment. ChemPlusChem 2025, 90, 2400784. [Google Scholar] [CrossRef]
- Pollice, R.; Chen, P. Origin of the Immiscibility of Alkanes and Perfluoroalkanes. J. Am. Chem. Soc. 2019, 141, 3489–3506. [Google Scholar] [CrossRef]
- Tan, X.; Zhong, J.; Fu, C.; Dang, H.; Han, Y.; Král, P.; Guo, J.; Yuan, Z.; Peng, H.; Zhang, C. Amphiphilic perfluoropolyether copolymers for the effective removal of polyfluoroalkyl substances from aqueous environments. Macromolecules 2021, 54, 3447–3457. [Google Scholar] [CrossRef]
- Kumarasamy, E.; Manning, I.M.; Collins, L.B.; Coronell, O.; Leibfarth, F.A. Ionic fluorogels for remediation of per-and polyfluorinated alkyl substances from water. ACS Cent. Sci. 2020, 6, 487–492. [Google Scholar] [CrossRef]
- Smaili, H.; Ng, C. Adsorption as a remediation technology for short-chain per-and polyfluoroalkyl substances (PFAS) from water—A critical review. Environ. Sci. Water Res. Technol. 2023, 9, 344–362. [Google Scholar] [CrossRef]
- Li, F.; Fang, X.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Adsorption of perfluorinated acids onto soils: Kinetics, isotherms, and influences of soil properties. Sci. Total Environ. 2019, 649, 504–514. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Zarei, A.; Khazdooz, L.; Zadehnazari, A.; Amirjalayer, S.; Addicoat, M.A.; Khosropour, A.; Abbaspourrad, A. Mechanistic study of PFAS adsorption using a QPPTA linked viologen-modified covalent organic framework. J. Mater. Chem. A 2025, 13, 8180–8192. [Google Scholar] [CrossRef]
- Liang, S.; Mora, R.; Huang, Q.; Casson, R.; Wang, Y.; Woodard, S.; Anderson, H. Field demonstration of coupling ion-exchange resin with electrochemical oxidation for enhanced treatment of per-and polyfluoroalkyl substances (PFAS) in groundwater. Chem. Eng. J. Adv. 2022, 9, 100216. [Google Scholar] [CrossRef]
- Murray, C.C.; Marshall, R.E.; Liu, C.J.; Vatankhah, H.; Bellona, C.L. PFAS treatment with granular activated carbon and ion exchange resin: Comparing chain length, empty bed contact time, and cost. J. Water Process Eng. 2021, 44, 102342. [Google Scholar] [CrossRef]
- Ellis, A.C.; Boyer, T.H.; Fang, Y.; Liu, C.J.; Strathmann, T.J. Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per-and polyfluoroalkyl substances (PFASs). Water Res. 2023, 243, 120324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, Z.; Wang, D.; Liu, G.; Wang, W.; Mu, S.; Yu, G.; Deng, S. Pilot-scale removal of PFAS from chromium-plating wastewater by anion exchange resin and activated carbon: Adsorption difference between PFOS and 6: 2 fluorotelomer sulfonate. Chem. Eng. J. 2024, 481, 148569. [Google Scholar] [CrossRef]
- Barr Engineering Co.; Hazen; Sawyer. Evaluation of Current Alternatives and Estimated Cost Curves for PFAS Removal and Destruction from Municipal Wastewater, Bio-Solids, Landfill Leachate, and Compost Contact Water; Minnesota Pollution Control Agency (MPCA): St Paul, MN, USA, 2023.
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Woodard, S.; Nickelsen, M.; Mededovic Thagard, S.; Holsen, T.M. Removal of poly-and per-fluorinated compounds from ion exchange regenerant still bottom samples in a plasma reactor. Environ. Sci. Technol. 2020, 54, 13973–13980. [Google Scholar] [CrossRef]
- Soriano, A.; Gorri, D.; Urtiaga, A. Membrane preconcentration as an efficient tool to reduce the energy consumption of perfluorohexanoic acid electrochemical treatment. Sep. Purif. Technol. 2019, 208, 160–168. [Google Scholar] [CrossRef]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Veciana, M.; Bräunig, J.; Farhat, A.; Pype, M.-L.; Freguia, S.; Carvalho, G.; Keller, J.; Ledezma, P. Electrochemical oxidation processes for PFAS removal from contaminated water and wastewater: Fundamentals, gaps and opportunities towards practical implementation. J. Hazard. Mater. 2022, 434, 128886. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sathiyan, K.; Zidki, T.; Nadagouda, M.N.; Sharma, V.K. Electrochemical degradation of per-and poly-fluoroalkyl substances in the presence of natural organic matter. Sep. Purif. Technol. 2023, 325, 124639. [Google Scholar] [CrossRef]



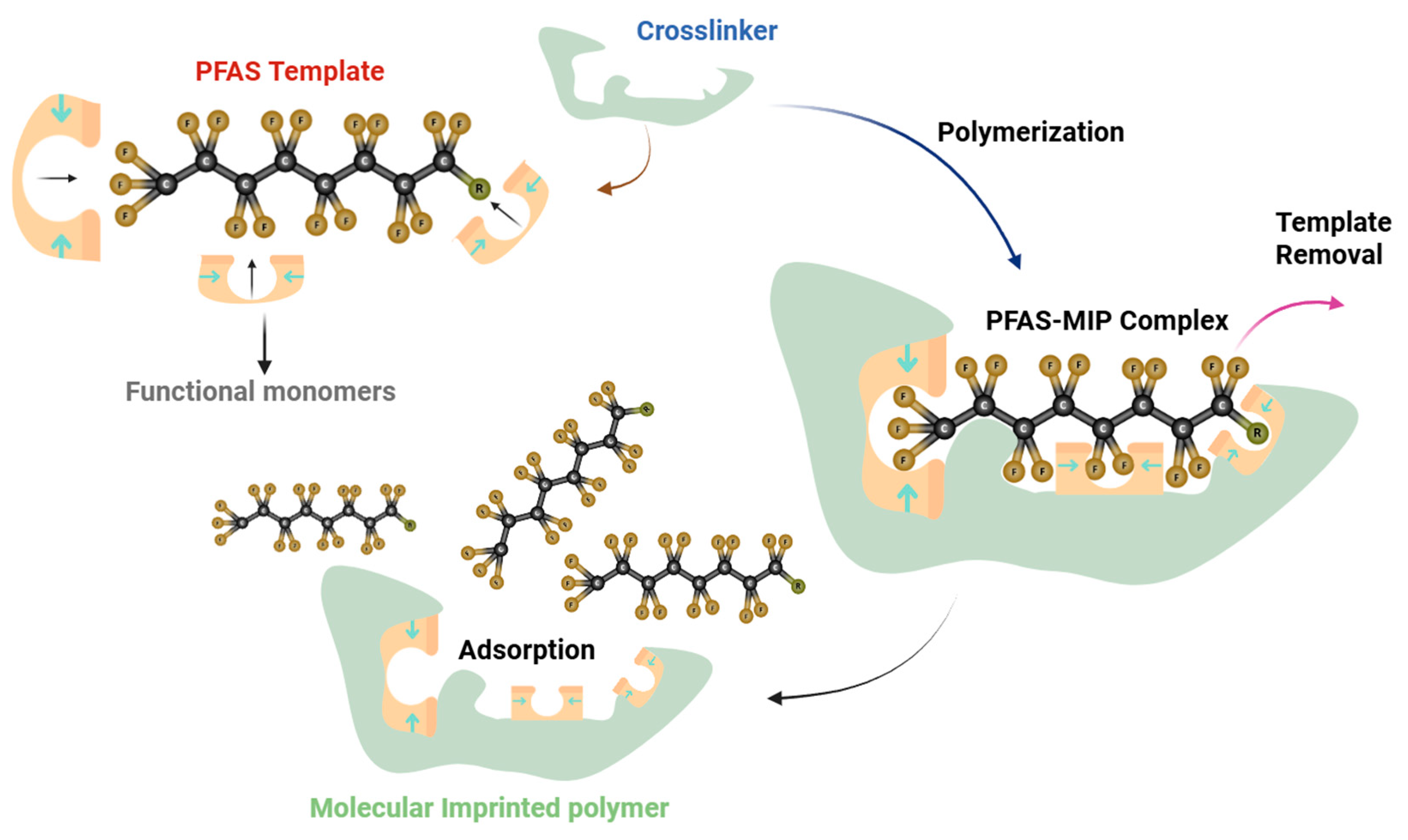

| Biopolymer Adsorbents | PFAS | Initial PFAS Concentration (mg/L) | qmax (mg/g) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|
| Polyethyleneimine functionalized cellulose microcrystals (PEI-f-CMC) | PFCA (C4-C13), PFSA (C4-C10), ADONA, N-MeFOSAA, N-EtFOSAA | 0.002–0.050 | 2.32 (PFOA) | N/A * | [123] |
| Alkylamine modified dialdehyde cellulose (DAC) | PFOS PFOA | 5–50 | 576–697 235–346 132 | >Electrostatic <Hydrophobic Interaction | [113] |
| PFBA | 25–150 | ||||
| Quaternized nanocellulose (QNC) | PFOS PFOA PFBS PFBA | 60 | 559 405 319 121 | >Hydrophobic <Electrostatic Interaction | [124] |
| Quaternized wood pulp (QWP) | PFOS PFOA | 0.0025 | 763 605 | Hydrophobic and Electrostatic Interaction | [114] |
| Quaternized cotton | PFOS PFOA | 95.02–460.12 78.67–380.95 | 1650.43 1283.62 | Hydrophobic Interaction | [125] |
| Alkylamine-modified dialdehyde cellulose (DAC) | PFOS PFOA PFBA | 5–50 25–150 | 576–697 235–346 132 | Hydrophobic and Electrostatic Interaction | [113] |
| Polyethyleneimine grafted chitosan beads (GCBs) | PFOS PFOA PFBA PFBS | 0.001 | 500 555.5 1428.6 769.2 | Hydrophobic and Electrostatic Interaction | [116] |
| Crushed chitosan beads (CBs) | PFOS PFOA PFBA PFBS | 384.6 312.5 476.2 303.1 | |||
| MIP-Chitosan beads | PFOS | 20–550 | 3202.1 | Electrostatic Interaction | [126] |
| Kraft/alkali Lignin | PFBA PFBS PFOA PFOS | 0.01 | N/A | Hydrophobic and Ion-Dipole Interactions | [120] |
| β-cyclodextrins-HDI | PFDA PFPS PFPA PFOSA PFOS PFOA PFNS PFNA PFHxA PFHxS PFHpA PFHpS PFDS | 0.0001–0.001 | N/A | Weak Hydrophobic + Electrostatic Interaction | [122] |
| β-cyclodextrins-EPI | Weak Hydrophobic + Electrostatic Interaction | ||||
| β-cyclodextrins-Cl | Strong Hydrophobic Interaction | ||||
| Cyclodextrin polymers (CDPs) | PFAAs | 0.01–5 | 92.54 (PFOA) 93 (PFBA) | Hydrophobic Interaction | [127] |
| Synthetic Adsorbents | PFAS | Initial PFAS Concentration (mg/L) | qmax (mg/g) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|
| Poly DMAPAA-Q hydrogel a | PFOS PFOA PFBS PFBA GenX ADONA | 0.001 | N/A * | Hydrophobic and Electrostatic Interaction | [130] |
| COFs b | PFBS PFBA PFHxS PFHxA PFOS PFOA | 21.76 15.52 29.01 22.77 36.26 30.04 | N/A * | Hydrogen Bonding, Hydrophobic and Electrostatic Interaction | [131] |
| QA-COFs c | GenX HFPO-TA | 16.50–198.02 20.70–248.42 | 679.27 894.29 | Electrostatic Interaction | [132] |
| Amine-f-COFs d | GenX | 0.2–100 | 130–200 | Electrostatic Interaction | [133] |
| Nitrogen doped ZIF-8 e | PFSA PFCA | 0.01 | 788.43 1115.11 | Hydrophobic and Electrostatic Interaction | [134] |
| UiO f-67 | PFSA PFCA PFASA FTs | N/A * | N/A * | Hydrophobic and Charge-Pairing Interaction | [135] |
| UiO f-67-F2 | PFOA | 1000 | 928 | Hydrophobic and Fluorophilic Interaction | [136] |
| MIL g-53 | PFOS | 20–80 | ~220 | Electrostatic and Coordination Interaction | [137] |
| MIPs | Monomers | PFAS Template | Crosslinker | Initial PFAS Conc. (mg/L) | qmax (mg/g) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan-based MIP | Chitosan | PFOS | Epichlorohydrin (ECH) | 20–550 | 3202.1 | Electrostatic Interaction | [126] |
| PFOA-MIP | Acrylic acid | PFOA | Ethylene glycol dimethacrylate (EGDMA) | 0.02–0.1 | 5.45 | N/A * | [140] |
| HDI-1 | polyurethane and β-cyclodextrin | PFOA | Hexamethylene diisocyanate (HDI) | 2.07–2070.35 | 1089.9 | Ion-Dipole and Hydrophobic Interaction | [141] |
| Bi-functional MIP | 2-(trifluoromethyl) acrylic acid, 4-vinyl pyridine | PFOA PFOS | Ethylene glycol dimethacrylate (EGDMA) | 0.05–10 | 6.42 6.27 | Hydrophobic Interaction | [142] |
| Magnetic-MIP-PFOS | Acrylamide + Fe3O4@SiO2NPs | PFOS | Ethylene glycol dimethacrylate (EGDMA) | 0.1–0.7 | 2.401 | Hydrophobic, Electrostatic Interaction, and Hydrogen Bonding | [143] |
| Category | Name | Molecular Structures | Molecular Weights a | -CF2 Units | pKa | Log Kow (Neutral) | ΔGhydrophobic (kJ mol−1) d | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-chain | PFBS | 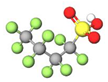 | 300.09 | (-CF2-)4 | −3.3 a | −3.3 e | 1.98 c | 2.6 e | - |
| PFBA | 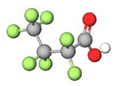 | 214.04 | (-CF2-)3 | 0.4 a | 1.1 e | 1.05 b | 2.3 e | −10.1 | |
| PFPeS |  | 350.10 | (-CF2-)5 | 0.14 a | −3.3 e | - | 3.3 e | - | |
| PFPeA | 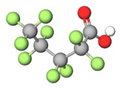 | 264.05 | (-CF2-)4 | 0.17 a | 0.34 e | 3.19 b | 3.0 e | −13.4 | |
| PFHxS | 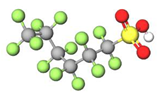 | 400.11 | (-CF2-)6 | −3.34 a | −3.3 e | 3.44 c | 4.0 e | - | |
| PFHxA | 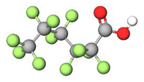 | 314.05 | (-CF2-)5 | −0.16 a | −0.78 e | 3.99 b | 3.7 e | −16.8 | |
| PFHpA | 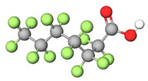 | 364.06 | (-CF2-)6 | −0.19 a | −2.3 e | 4.40 b | 4.4 e | −20.1 | |
| Long-chain | PFHpS | 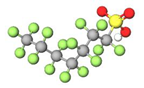 | 450.12 | (-CF2-)7 | −2.29 a | −3.3 e | 4.06 c | 4.7 e | - |
| PFOS |  | 500.13 | (-CF2-)8 | −3.27 a | −3.3 e | 4.05 c | 5.4 e | - | |
| PFOA | 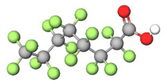 | 414.07 | (-CF2-)7 | −0.2 a | −0.5 e | 4.67 b | 5.1 e | −23.5 | |
| PFNA | 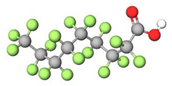 | 464 | (-CF2-)8 | 0.52 a | −6.5 e | 5.02 b | 5.8 e | −26.8 | |
| PFDA | 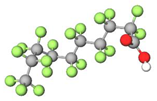 | 514.80 | (-CF2-)9 | 0.4 a | −5.2 e | 5.44 b | 6.5 e | −30.2 | |
| Technology | Removal Efficiency | Cost | Scalability | Limitations | References | |
|---|---|---|---|---|---|---|
| Non-Destructive | Sustainable polymer adsorbent (aerogel and hydrogel) | >99% | In research | Lab → Pilot scale | Upscaling, disposal of spent adsorbent | - |
| Activated carbon (GAC) a | >90% | USD 0.44/m3 WW b | Full-scale TRL c-9 | Less removal of SC d, interference of NOM e | [182] | |
| Ion exchange resins (regenerable) | >90% | USD 0.40/ m3 TW f | Full scale TRL c-9 | Regeneration cost | [182] | |
| Membranes (NF g, MF h and RO i) | >99% RO 90–99% NF g | NF USD 0.016–0.16/m3 TW f | Full scale TRL c-9 | Membrane fouling, high energy consumption | [184] | |
| Coagulation/ flocculation | 1–50% | N/A n | DT j | Less removal efficiency | [184] | |
| Destructive | Electrochemical oxidation | 60–99% | High cost | Bench → Pilot scale TRL c-7 | Less effective for SC d, high cost, electrode stability, and hazardous byproducts | [99] |
| Advanced oxidation and reduction process | 75% AOP k >90% ARP l | High cost | Full scale TRL c-9 AOP TRL c-5 ARP | Do not fully defluorinate the tail, less efficiency for SC | [184] | |
| Plasma degradation | >99%, LC m >99%, SC d | - | Lab → Pilot scale | pH sensitive, NOM e interference, long treatment time | [99,185] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.; Ayinla, R.T.; Elsayed, I.; Hassan, E.B. Understanding PFAS Adsorption: How Molecular Structure Affects Sustainable Water Treatment. Environments 2025, 12, 330. https://doi.org/10.3390/environments12090330
Hamza M, Ayinla RT, Elsayed I, Hassan EB. Understanding PFAS Adsorption: How Molecular Structure Affects Sustainable Water Treatment. Environments. 2025; 12(9):330. https://doi.org/10.3390/environments12090330
Chicago/Turabian StyleHamza, Muhammad, Ridwan T. Ayinla, Islam Elsayed, and El Barbary Hassan. 2025. "Understanding PFAS Adsorption: How Molecular Structure Affects Sustainable Water Treatment" Environments 12, no. 9: 330. https://doi.org/10.3390/environments12090330
APA StyleHamza, M., Ayinla, R. T., Elsayed, I., & Hassan, E. B. (2025). Understanding PFAS Adsorption: How Molecular Structure Affects Sustainable Water Treatment. Environments, 12(9), 330. https://doi.org/10.3390/environments12090330







