Analysis of Selected Potentially Harmful Metal Elements in Soils and Vegetables in Gold Mining Region: Case Study Evaluated in Kenya, Africa
Abstract
1. Introduction
2. Materials and Methods
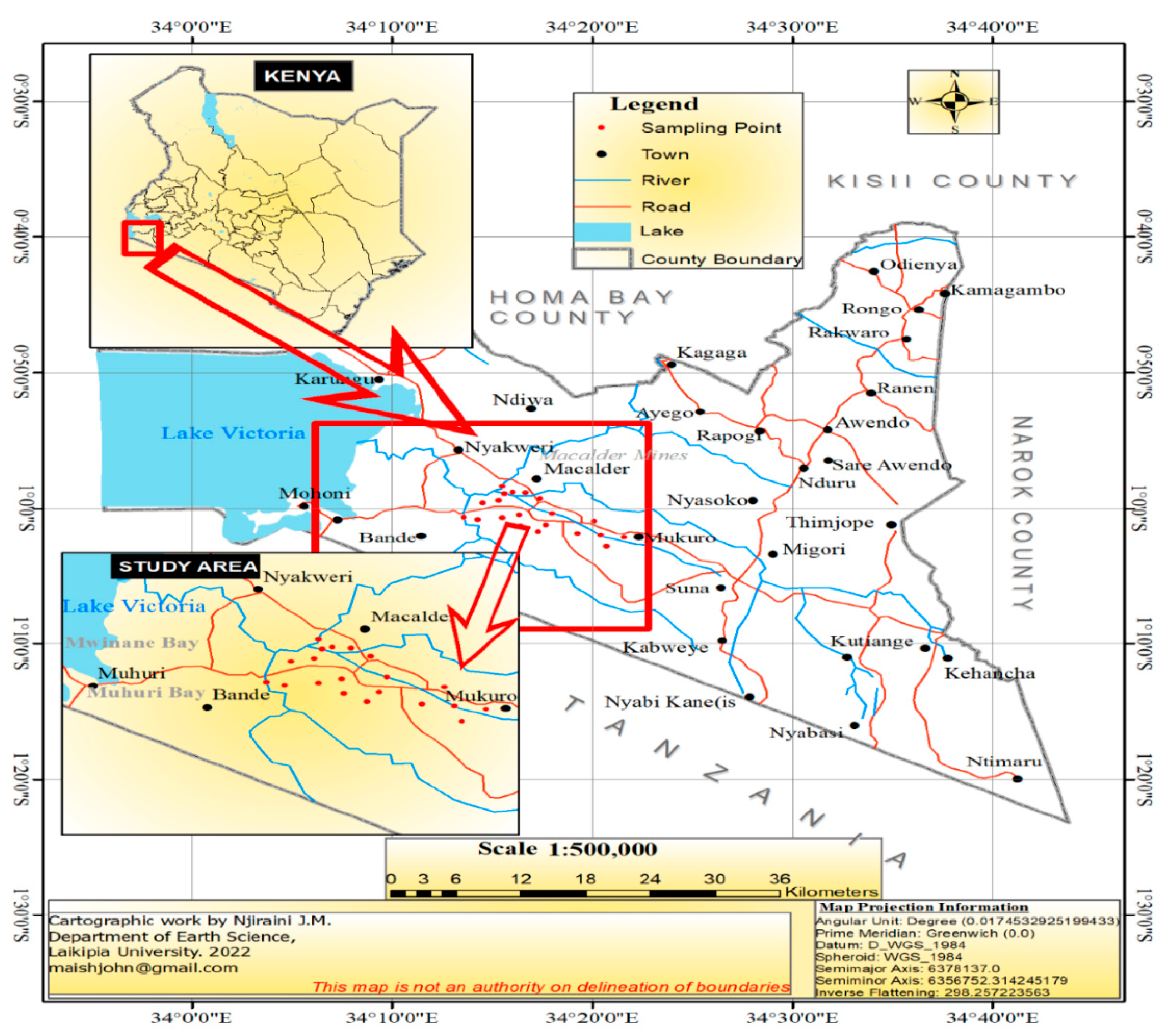
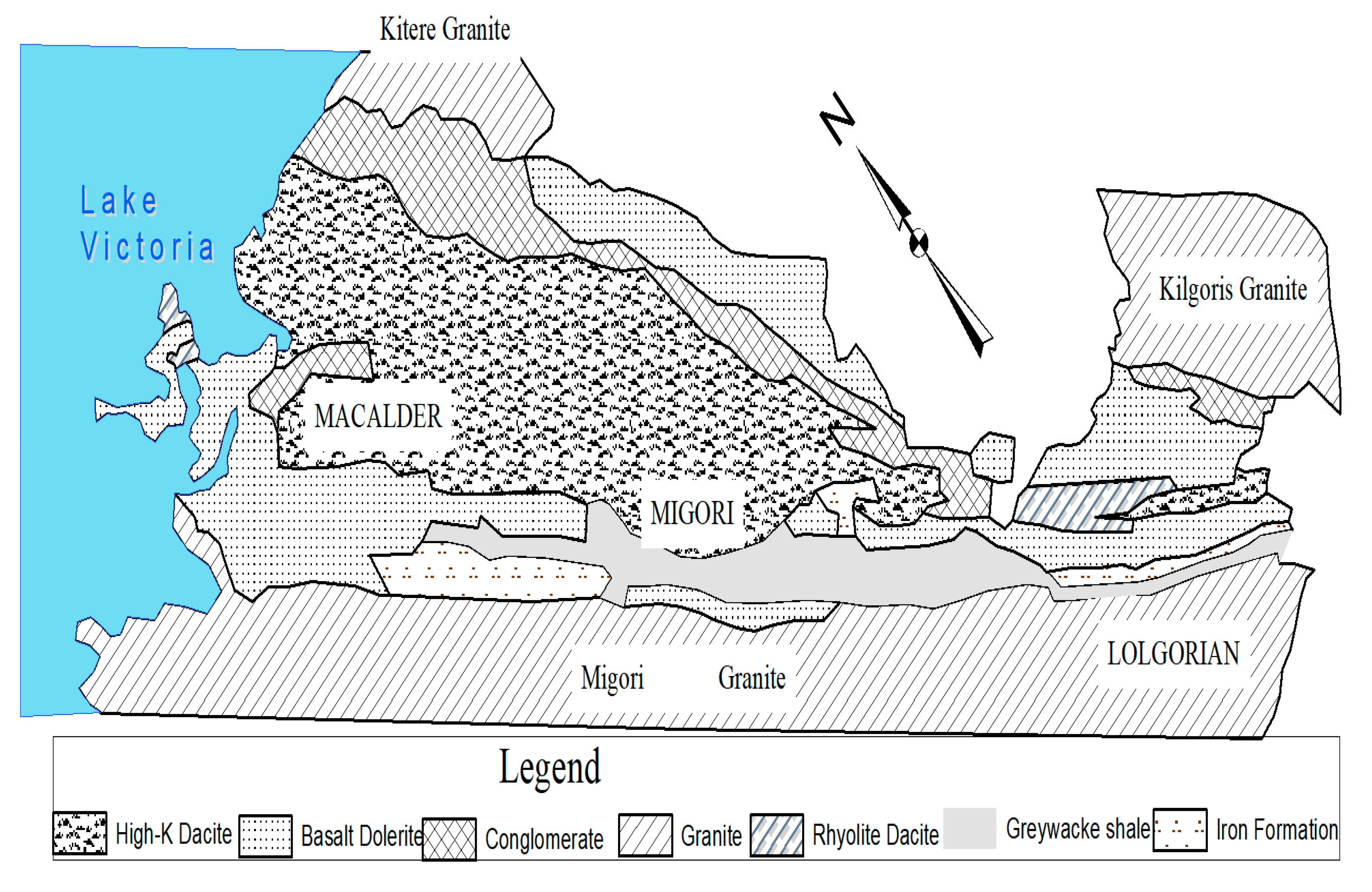
2.1. Study Area Setting
2.2. Sampling Design and Procedures
2.3. Preparation and Analysis of Samples
2.4. Calibration Process
2.5. Certified Reference Materials (CRMs) and Quality Control Measures
2.6. Instrument Efficiency and Drift Monitoring
2.7. Coefficient of Soil–Plant Transfer in Percent
2.8. Estimated Daily Intake (EDI) of Heavy Metals Studied
2.9. Hazard Quotient (HQ)
2.10. Hazard Index (HI)
3. Data Analysis
4. Results and Discussion
4.1. Heavy Metal Concentration in Soil
4.2. Heavy Metal and Trace Element Levels in Edible Leafy Vegetables
4.3. Coefficients of Soil–Plant Transfer
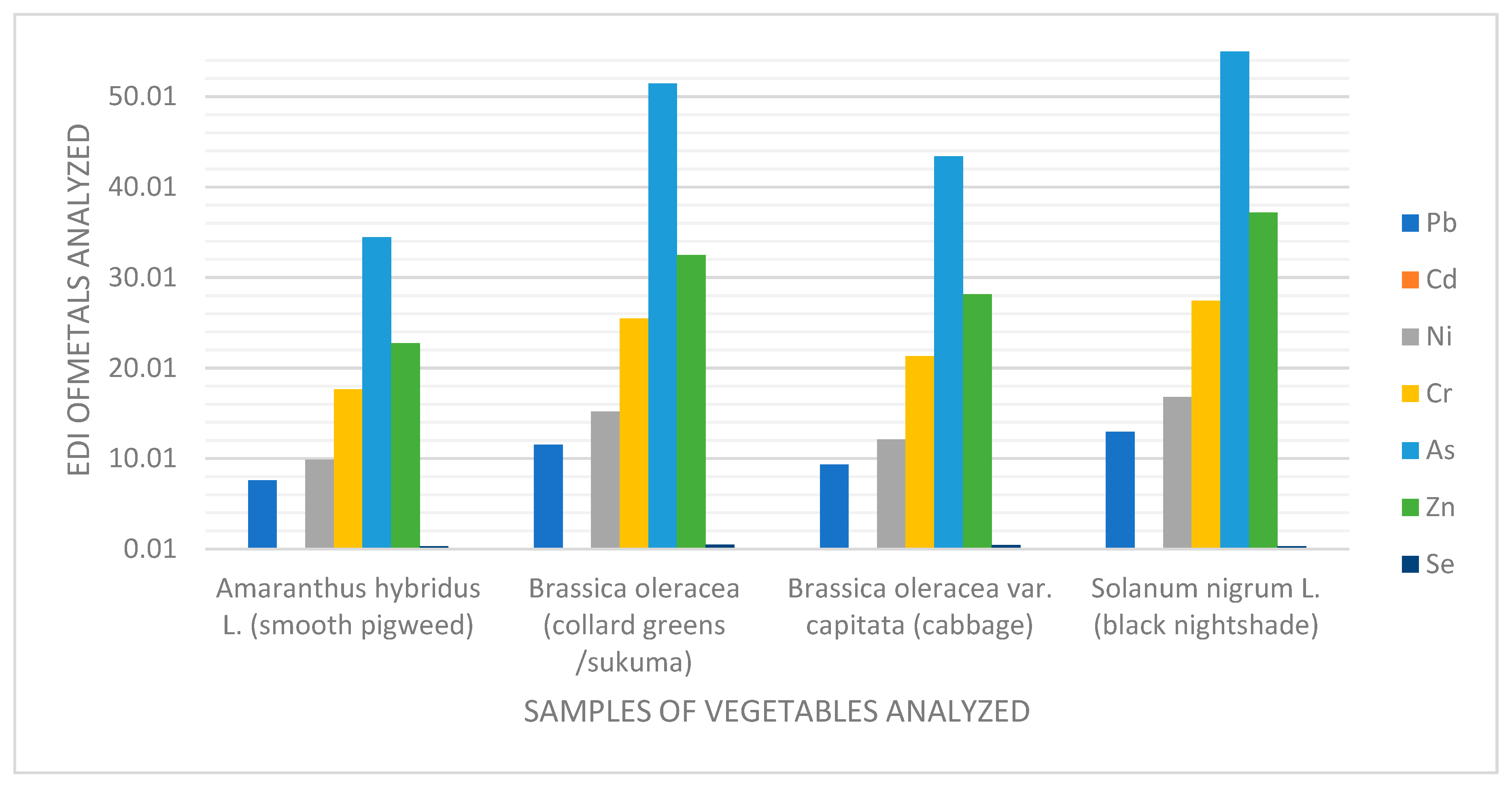
4.4. Estimated Daily Intakes (EDIs) of Heavy Metals and Trace Elements
4.5. Hazard Quotient
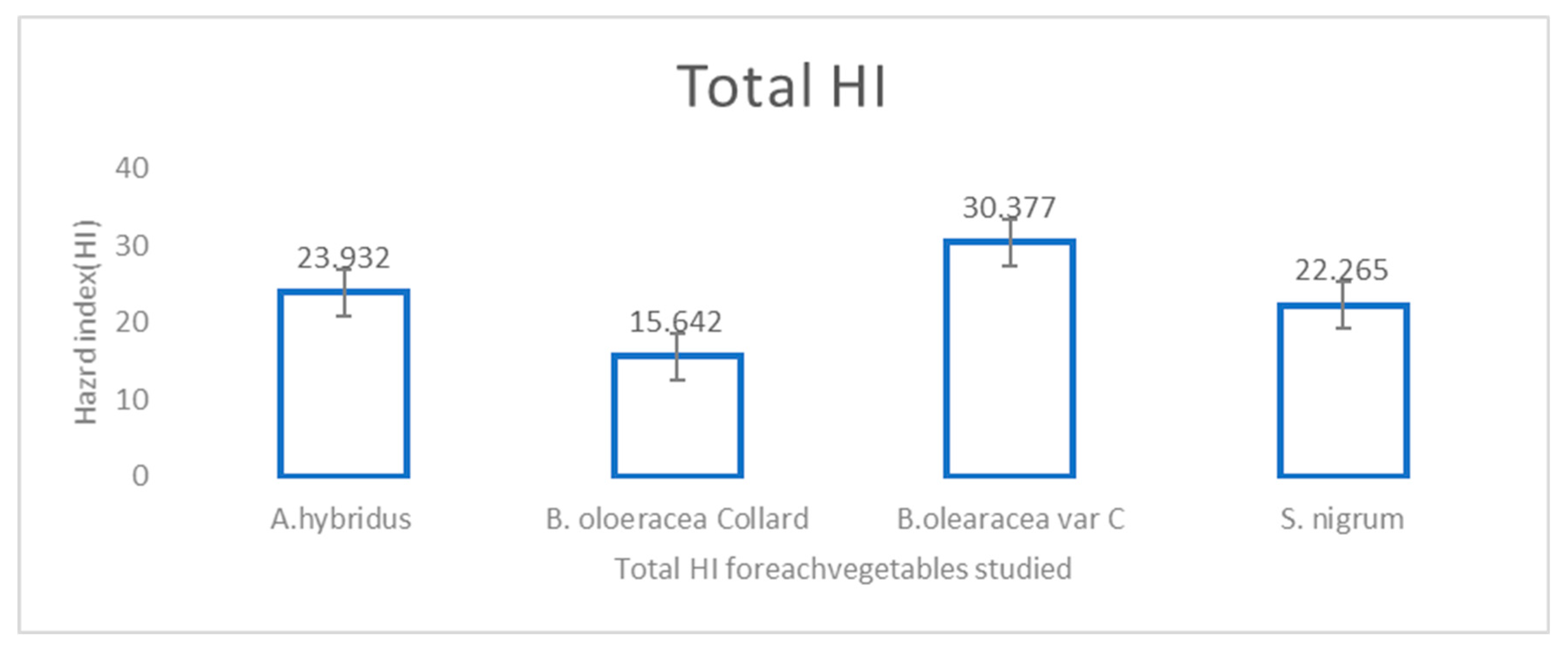
4.6. Hazard Index (HI) Analysis
4.7. Correlation Analysis of Heavy Metals and Trace Elements Studied in Vegetables
4.8. Conclusions
5. Study Limitations: Clarification on Soil Classification Limitation
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramya, V.; Patel, P. Health benefits of vegetables. Int. J. Chem. Stud. 2019, 7, 82–87. [Google Scholar]
- Javed, I.M.; Waseem, A.M.; Ammad, R. Vegetables as a source of important nutrients and bioactive compounds: Their human health benefits. MOJ Food Process. Technol. 2019, 7, 136–146. [Google Scholar] [CrossRef]
- Singh, V.; Garg, A.N. Availability of essential trace elements in Indian cereals, vegetables and spices using INAA and the contribution of spices to daily dietary intake. Food Chem. 2006, 94, 81–89. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Zhang, J.; Zhang, H.; Qiao, L.; Men, Y. Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. J. Environ. Sci. 2010, 22, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Ametepey, S.T.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis, Ghana. Int. J. Food Contam. 2018, 5, 5. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.W.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J.N. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Mwegoha, W.J.S.; Kihampa, C. Heavy metal contamination in agricultural soils and water in Dar es Salaam city, Tanzania. Afr. J. Environ. Sci. Technol. 2010, 4, 763–769. Available online: https://academicjournals.org/journal/AJEST/article-abstract/2B845D113610 (accessed on 17 May 2023).
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Ren, Z.; Xiao, R.; Zhang, Z.; Lv, X.; Fei, X. Risk assessment and source identification of heavy metals in agricultural soil: A case study in the coastal city of Zhejiang Province, China. Stoch. Environ. Res. Risk Assess. 2019, 33, 2109–2118. [Google Scholar] [CrossRef]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar]
- Gjorgieva Ackova, D. Heavy metals and their general toxicity on plants. Plant Sci. Today 2018, 5, 15–19. [Google Scholar] [CrossRef]
- Ngure, V.; Kinuthia, G. Health risk implications of lead, cadmium, zinc, and nickel for consumers of food items in Migori Gold mines, Kenya. J. Geochem. Explor. 2020, 209, 106430. [Google Scholar] [CrossRef]
- Saleh, H.N.; Panahande, M.; Yousefi, M.; Asghari, F.B.; Conti, G.O.; Talaee, E.; Mohammadi, A.A. Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biol. Trace Elem. Res. 2019, 190, 251–261. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1291107. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Framework for Cumulative Risk Assessment (Risk Assessment Forum, EPA/630/P-02/001F). Washington, DC; May 2003. Available online: https://www.epa.gov/sites/default/files/2014-11/documents/frmwrk_cum_risk_assmnt.pdf (accessed on 17 May 2023).
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Bhargava, P.; Gupta, N.; Vats, S.; Goel, R. Health issues and heavy metals. Austin J. Environ. Toxicol. 2017, 3, 3018. [Google Scholar]
- Ichang’l, D.W.; MacLean, W.H. The Archean voilcanic facies in the Migori segment, Nyanza greenstone belt. Kenya: Stratigraphy, geochemistry and mineralization. J. Afr. Earth Sci. 1994, 13, 277–290. [Google Scholar] [CrossRef]
- Darnley, A.G.; Bjorklund, A.; Bolviken, B.; Gustavsson, N.; Koval, P.V.; Plant, J.A.; Steenfelt, A.; Tauchid, M.; Xie, X. A global geochemical database for environmental and resource management. In Recommendations for International Geochemical Mapping; Final Report of IGCP Project 259; Earth Sciences 19; UNESCO: Paris, France, 1995; p. 16. [Google Scholar]
- Jolly, Y.N.; Islam, A.; Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus 2013, 2, 385. [Google Scholar] [CrossRef] [PubMed]
- Ara, M.H.; Mondal, U.K.; Dhar, P.K.; Uddin, M. Presence of heavy metals in vegetables collected from Jashore, Bangladesh: Human health risk assessment. J. Chem. Health Risks 2018, 8, 277–287. [Google Scholar]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants: Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 1989; p. 64. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Evaluation of Certain Food Additives and Contaminants: Forty-First Report of the Joint FAO; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Arsenic. CASnumber:7440-38-2. 2019. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx/chemID=1863 (accessed on 17 May 2023).
- WHO. Cadmium in Drinking-Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003; WHO/SDE/WSH/03.04/80. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Third [73rd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives: Eighty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series No. 1020; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241210294 (accessed on 17 May 2023).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998; World Health Organization: Geneva, Switzerland, 2004; Available online: http://whqlibdoc.who.int/publications/2004/9241546123.pdf (accessed on 17 May 2023).
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Zinc. 2019. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx/chemID=4197 (accessed on 17 May 2023).
- U.S. EPA (United States Environmental Protection Agency). Human Health Evaluation Manual, Supplemental Guidance: Standard Default Exposure Factors; USEPA: Washington, DC, USA, 1999.
- US EPA (United States Environmental Protection Agency). Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures; EPA/630/R-00/002; Risk Assessment Forum, U.S. Environmental Protection Agency: Washington, DC, USA, August 2000.
- DEA (Department of Environmental Affairs). The Framework for the Management of Contaminated Land, South Africa. 2010. Available online: http://sawic.environment.gov.za/documents/562.pdf (accessed on 5 February 2021).
- Yang, J.; Ma, S.; Zhou, J.; Song, Y.; Li, F. Heavy metal contamination in soils and vegetables and health risk assessment of inhabitants in Daye, China. J. Int. Med. Res. 2018, 46, 3374–3387. [Google Scholar] [CrossRef]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1987; pp. 158–160. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall International Inc.: Englewood Cliffs, NJ, USA, 1996; p. 662. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Bini, C.; Sartori, G.; Wahsha, M.; Fontana, S. Background levels of trace elements and soil geochemistry at regional level in NE Italy. J. Geochem. Explor. 2011, 109, 125–133. [Google Scholar] [CrossRef]
- Alfaro, M.R.; Montero, A.; Ugarte, O.M.; do Nascimento, C.W.A.; Accioly, A.M.d.A.; Biondi, C.M.; da Silva, Y.J.A.B. Background concentrations and reference values for heavy metals in soils of Cuba. Environ. Monit. Assess. 2015, 187, 4198. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; de Caritat, P. Establishing geochemical background variation and threshold values for 59 elements in Australian surface soil. Sci. Total Environ. 2017, 578, 633–648. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Murrill, M.; Das, R.; Siddayya Patil, S.G.; Sarkar, A.; Dadapeer, H.J.; Yendigeri, S.; Ahmed, R.; Das, K.K. Environmental arsenic contamination and its health effects in a historic gold mining area of the Mangalur greenstone belt of Northeastern Karnataka, India. J. Hazard. Mater. 2013, 262, 1048–1055. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, J.S.; Chon, H.T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- De Matos, A.T.; Fontes, M.P.F.; Da Costa, L.M.; Martinez, M.A. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ. Pollut. 2001, 111, 429–435. [Google Scholar] [CrossRef]
- Vejvodová, K.; Ash, C.; Dajčl, J.; Tejnecký, V.; Johanis, H.; Spasić, M.; Polák, F.; Praus, L.; Borůvka, L.; Drábek, O. Assessment of potential exposure to As, Cd, Pb and Zn in vegetable garden soils and vegetables in a mining region. Sci. Rep. 2022, 12, 13495. [Google Scholar] [CrossRef]
- Zafarzadeh, A.; Rahimzadeh, H.; Mahvi, A.H. Health risk assessment of heavy metals in vegetables in an endemic esophageal cancer region in Iran. Health Scope 2018, 7, e12340. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, F.; Wu, B. Mechanism of effects of nickel or nickel compounds on intestinal mucosal barrier. Chemosphere 2022, 305, 135429. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- El-deeb, A.K.; El-Bialy, B.E.; El-Borai, N.B.; Elsabbagh, H.S. Nickel: A Review on Environmental Distribution, Toxicokinetics, and Potential Health Impacts. J. Curr. Vet. Res. 2025, 7, 113–129. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Achmad, R.T.; Auerkari, E.I. Effects of chromium on human body. Annu. Res. Rev. Biol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Benthem, J.; Staal, Y.C.; Ezendam, J.; Piersma, A.H.; Hessel, E.V. Occupational exposure to hexavalent chromium. Part II. Hazard assessment of carcinogenic effects. Regul. Toxicol. Pharmacol. 2021, 126, 105045. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, S.; Gupta, D.K. Environmental arsenic exposure and human health risk. In Arsenic Water Resources Contamination: Challenges and Solutions; Fares, A., Singh, S.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 103–129. [Google Scholar] [CrossRef]
- Wei, W.; Ma, R.; Sun, Z.; Zhou, A.; Bu, J.; Long, X.; Liu, Y. Effects of mining activities on the release of heavy metals (HMs) in a typical mountain headwater region, the Qinghai-Tibet Plateau in China. Int. J. Environ. Res. Public Health 2018, 15, 1987. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H. Aberrance of zinc metalloenzymes-induced human diseases and its potential mechanisms. Nutrients 2021, 13, 4456. [Google Scholar] [CrossRef]
- Silva, C.S.; Moutinho, C.; Ferreira da Vinha, A.; Matos, C. Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. Int. J. Sci. Res. Methodol. 2019, 13, 57–80. [Google Scholar]
- Huang, J.Q.; Li, D.L.; Zhao, H.; Sun, L.H.; Xia, X.J.; Wang, K.N.; Luo, X.; Lei, X.G. The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J. Nutr. 2011, 141, 1605–1610. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Roba, C.; Roşu, C.; Piştea, I.; Ozunu, A.; Baciu, C. Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ. Sci. Pollut. Res. 2016, 23, 6062–6073. [Google Scholar] [CrossRef]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 2020, 15, e0227883. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Yu, H.Y.; Chen, J.J.; Li, F.B.; Zhang, H.H.; Liu, C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Makokha, V.A.; Qi, Y.; Shen, Y.; Wang, J. Concentrations, distribution, and ecological risk assessment of heavy metals in the East Dongting and Honghu Lake, China. Expo. Health 2016, 8, 31–41. [Google Scholar] [CrossRef]
- Ugbede, F.O.; Aduo, B.C.; Ogbonna, O.N.; Ekoh, O.C. Natural radionuclides, heavy metals and health risk assessment in surface water of Nkalagu river dam with statistical analysis. Sci. Afr. 2020, 8, e00439. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, P.; Zhan, J.; Gao, Y.; Dou, C.; Sun, Q. Assessment of trace metal bioavailability in garden soils and health risks via consumption of vegetables in the vicinity of Tongling mining area, China. Ecotoxicol. Environ. Saf. 2013, 90, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Z.; Yu, T.; Jiang, Z.; Huang, Q.; Yang, Y.; Liu, X.; Ma, X.; Li, B.; Lin, K.; et al. Cadmium accumulation in paddy soils affected by geological weathering and mining: Spatial distribution patterns, bioaccumulation prediction, and safe land usage. J Hazard Mater. 2023, 460, 132483. [Google Scholar] [CrossRef] [PubMed]
- Agan, L.; Khazenzi, J.; Kandioura, N.; Osano, O. Lead and cadmium pollution: Implications for health in artisanal and small-scale gold mining in Senegal and Kenya. Afr. J. Educ. Sci. Technol. 2024, 8, 221–239. [Google Scholar] [CrossRef]
- Ondayo, M.A.; Watts, M.J.; Hamilton, E.M.; Mitchell, C.; Mankelow, J.; Osano, O. Artisanal gold mining in Kakamega and Vihiga counties, Kenya: Potential human exposure and health risk. Environ. Geochem. Health 2023, 45, 6543–6565. [Google Scholar] [CrossRef]
- Ondayo, M.A.; Watts, M.J.; Humphrey, O.S.; Osano, O. Public health assessment of Kenyan ASGM communities using multi-element biomonitoring, dietary and environmental evaluation. Ecotoxicol. Environ. Saf. 2024, 277, 116323. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Mngadi, S.; Nomngongo, P.N.; Moja, S. Elemental composition and potential health risk of vegetable cultivated in residential area situated close to abandoned gold mine dump: Characteristics of soil quality on the vegetables. J. Environ. Sci. Health Part B 2024, 59, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Afriyie, R.Z.; Arthur, E.K.; Gikunoo, E.; Baah, D.S.; Dziafa, E. Potential health risk of heavy metals in some selected vegetable crops at an artisanal gold mining site: A case study at Moseaso in the Wassa Amenfi West District of Ghana. J. Trace Elem. Miner. 2023, 4, 100075. [Google Scholar] [CrossRef]
| Pb | Cd | Ni | Cr | As | Zn | Se | Hg | |
|---|---|---|---|---|---|---|---|---|
| MDL | 0.01 | 0.01 | 0.10 | 0.50 | 0.1 | 0.10 | 0.10 | 0.01 |
| Observed mean in soil samples | 171.08 | 0.86 | 15.50 | 39.10 | 5.90 | 313.50 | 0.20 | 1370 |
| Permissible limits for metals in soil (mean values) | 29 | 0.6 | 34 | 84 | 11 | 60 | 0.4 | 0.1 |
| Permissible limits for heavy metals in plants (mg/kg) | 2 | 0.02 | 10 | 1.30 | 0.60 | 0.000786–0.005714 (RDI) | 0.5 | |
| MDL RDI | Minimum Detection Limit Recommended Daily Intake | |||||||
| Vegetable Name | Moisture Content (%) | Pb | Cd | Ni | Cr | As | Zn | Se | Hg |
|---|---|---|---|---|---|---|---|---|---|
| MDL | 0.01 | 0.01 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.01 | |
| Smooth pigweed | 85.89 | 16.59 | 0.074 | 21.6 | 38.53 | 75.2 | 49.6 | 0.69 | 18.8 |
| Collard greens | 78.23 | 16.29 | 0.065 | 21.5 | 35.4 | 72.7 | 46.1 | 0.74 | 22.9 |
| Cabbage | 82.13 | 16.10 | 0.069 | 20.9 | 36.73 | 74.8 | 48.5 | 0.76 | 26.6 |
| Black nightshade | 76.34 | 16.87 | 0.071 | 21.9 | 35.73 | 71.5 | 47.1 | 0.67 | 16.3 |
| Mean level in the vegetables | 16.625 | 0.0685 | 21.55 | 34.83 | 71.3 | 46.55 | 0.705 | 21.15 | |
| WHOMPL | 2.0 | 0.02 | 10 | 1.3 | 0.1 | 0.6 | - | - | |
| Metal trend in vegetables in descending order | As > Zn > Cr >Ni >> Hg > Pb > Se > Cd | ||||||||
| Vegetable Name | Pb | Cd | Ni | Cr | As | Zn | Se | Hg | Efficacy |
|---|---|---|---|---|---|---|---|---|---|
| Smooth pigweed | 9.70 | 8.6 | 139.4 | 98.54 | 1274.6 | 15.8 | 34.5 | 8.71 | 186.05 |
| Collard greens | 9.52 | 7.56 | 138.7 | 90.54 | 1232.2 | 14.7 | 37.0 | 8.31 | 218.6 |
| Cabbage | 9.41 | 8.03 | 134.8 | 93.94 | 1367.8 | 15.5 | 38.0 | 8.85 | 239.6 |
| Black nightshade | 9.86 | 8.26 | 141.3 | 91.38 | 1211.9 | 15.0 | 33.5 | 9.41 | 215.9 |
| Average | 9.6 | 8.11 | 138.6 | 93.6 | 1271.6 | 15.25 | 35.8 | 8.51 |
| Vegetable Name | Calculated % DW of Vegetables | Pb | Cd | Ni | Cr | As | Zn | Se | Hg |
|---|---|---|---|---|---|---|---|---|---|
| Smooth pigweed | 0.1411 | 7.6 | 0.034 | 9.91 | 17.67 | 34.48 | 22.75 | 0.32 | 5.1 |
| Collard greens | 0.2177 | 11.53 | 0.046 | 15.21 | 25.5 | 51.43 | 32.51 | 0.52 | 6.22 |
| Cabbage | 0.1787 | 9.35 | 0.040 | 12.14 | 21.33 | 43.44 | 28.17 | 0.44 | 7.12 |
| Black nightshade | 0.2366 | 12.97 | 0.055 | 16.84 | 27.47 | 54.98 | 37.22 | 0.34 | 4.1 |
| Average (mg/kg/day) | 10.36 | 0.044 | 13.56 | 22.99 | 46.08 | 30.16 | 0.41 | 5.63 | |
| Tolerable daily intake for adults (µg/kg bw/day) except Zn (mg/kg/bw/day) | 0.1–3 [47] | 1 [47] | 0.03–0.13 [47] | 0.02–3 [47] | 2.8 [47] | 0.3–1.0 [47] | 0.9 [47] | 1 μg/kg bw/wk [28] | |
| Bw = body weight = 60 kgs. This study used 60 kg to calculate the EDI. | |||||||||
| Vegetables: 325 g/day of vegetables. | |||||||||
| Vegetables Sampled | Pb | Pb HQ | Cd | Cd HQ | Ni | Ni HQ | Cr | Cr HQ | As | AS HQ | Zn | Zn HQ | Se | Se HQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RfD mg/kg/day | 0.004 | 0.001 | 0.91 | 1.5 | 0.014 | 0.3 | 0.005 | |||||||
| Smooth pigweed | 7.6 | 1900 | 0.034 | 34 | 9.91 | 10.9 | 17.67 | 11.8 | 34.48 | 2462 | 22.75 | 75 | 0.32 | 64 |
| Collard greens | 11.53 | 1882 | 0.046 | 46 | 15.21 | 16.7 | 25.5 | 17 | 51.43 | 3673 | 32.51 | 108 | 0.52 | 104 |
| Cabbage | 9.35 | 2337.5 | 0.040 | 40 | 12.14 | 13.3 | 21.33 | 14.2 | 43.44 | 3102 | 28.17 | 93.9 | 0.44 | 88 |
| Black nightshade | 12.97 | 3242.5 | 0.055 | 55 | 16.84 | 18.5 | 27.47 | 18.3 | 54.98 | 3927 | 37.22 | 124 | 0.34 | 68 |
| Average HQ for metals | 2340.5 | 43.6 | 14.9 | 15.3 | 3291 | 100.2 | 81 |
| Vegetables Sampled | Pb HQ | Cd HQ | Ni HQ | Cr HQ | AS HQ | Zn HQ | Se HQ | Total HQ for Each Vegetable (HI) |
|---|---|---|---|---|---|---|---|---|
| Smooth pigweed | 1.900 | 3.4 | 1.09 | 1.18 | 2.46 | 7.5 | 6.4 | 23.932 |
| Collard greens | 1.882 | 4.6 | 1.67 | 1.7 | 3.67 | 1.08 | 1.04 | 15.642 |
| Cabbage | 2.337 | 4.0 | 1.33 | 1.42 | 3.10 | 9.39 | 8.8 | 30.377 |
| Black nightshade | 3.242 | 5.5 | 1.85 | 1.83 | 3.93 | 1.24 | 6.8 | 22.265 |
| Metals | Cr | Se | Ni | Zn | As | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|
| Cr | 1 | 0.204 | 0.632 b | 0.173 | 0.092 | 0.209 | 0.099 | 0.342 a |
| Se | 1 | 0.706 b | 0.343 a | 0.199 | 0.405 b | 0.384 b | 0.214 | |
| Ni | 1 | 0.757 b | 0.566 b | 0.549 b | 0.109 | 0.073 | ||
| Zn | 1 | 0.489 b | 0.559 b | −0.052 | 0.678 b | |||
| As | 1 | 0.179 | −0.397 b | 0.891 b | ||||
| Cd | 1 | 0.092 | 0.431 a | |||||
| Pb | 1 | 0.979 | ||||||
| Hg | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macharia, J.M.; Veronica, N.; Wangare, L.; Bence, R.L. Analysis of Selected Potentially Harmful Metal Elements in Soils and Vegetables in Gold Mining Region: Case Study Evaluated in Kenya, Africa. Environments 2025, 12, 317. https://doi.org/10.3390/environments12090317
Macharia JM, Veronica N, Wangare L, Bence RL. Analysis of Selected Potentially Harmful Metal Elements in Soils and Vegetables in Gold Mining Region: Case Study Evaluated in Kenya, Africa. Environments. 2025; 12(9):317. https://doi.org/10.3390/environments12090317
Chicago/Turabian StyleMacharia, John M., Ngure Veronica, Lareen Wangare, and Raposa L. Bence. 2025. "Analysis of Selected Potentially Harmful Metal Elements in Soils and Vegetables in Gold Mining Region: Case Study Evaluated in Kenya, Africa" Environments 12, no. 9: 317. https://doi.org/10.3390/environments12090317
APA StyleMacharia, J. M., Veronica, N., Wangare, L., & Bence, R. L. (2025). Analysis of Selected Potentially Harmful Metal Elements in Soils and Vegetables in Gold Mining Region: Case Study Evaluated in Kenya, Africa. Environments, 12(9), 317. https://doi.org/10.3390/environments12090317








