Adsorption of Phosphates from Wastewater Using MgAlFe-Layered Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of the MgAlFe-LDH Material
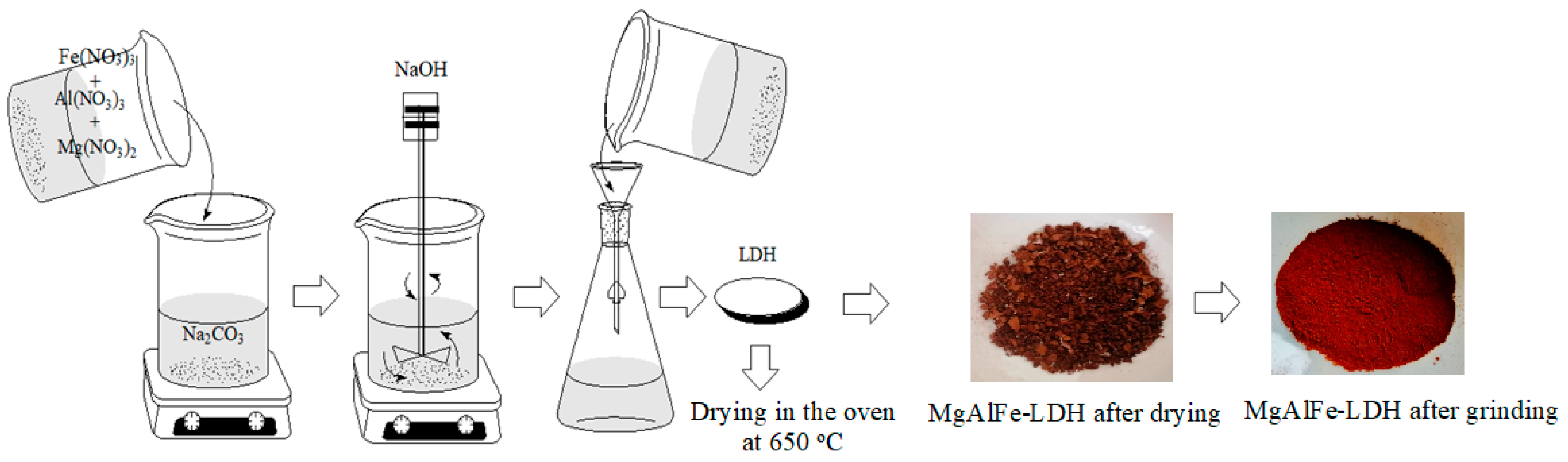
2.2.1. Synthesis of MgAlFe-LDH Material
2.2.2. Characterization of the MgAlFe-LDH Material
2.3. Preparation of the Simulated Wastewater Solution
2.4. Equilibrium Adsorption Isotherms
2.4.1. Langmuir Model
2.4.2. Freundlich Model
2.5. Adsorption Kinetics
2.6. Adsorbent Reusability
3. Results and Discussion
3.1. Characterization of MgAlFe-LDH Materials
3.1.1. Digital Microscope Analysis
3.1.2. X-Ray Diffraction (XRD) Analysis
3.1.3. Specific Surface Area
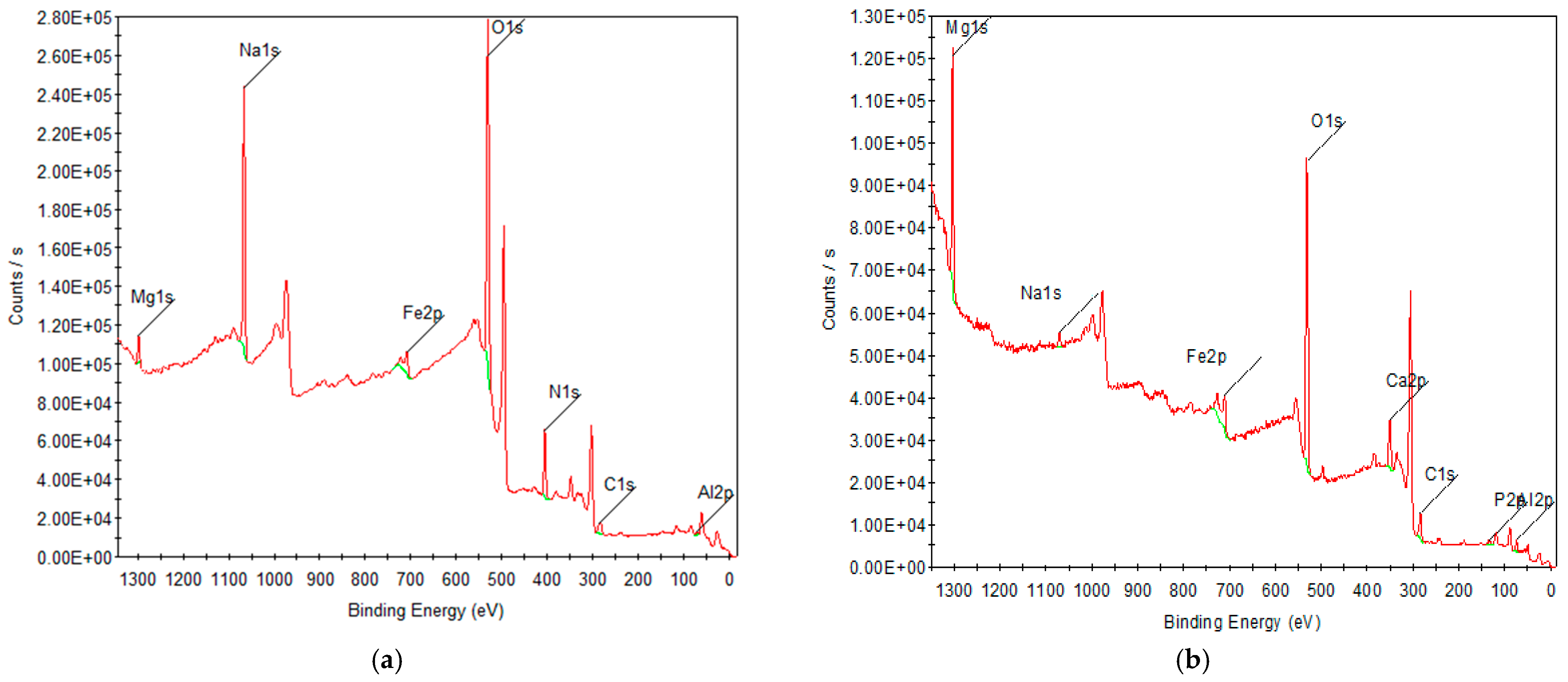
3.1.4. XPS Analysis
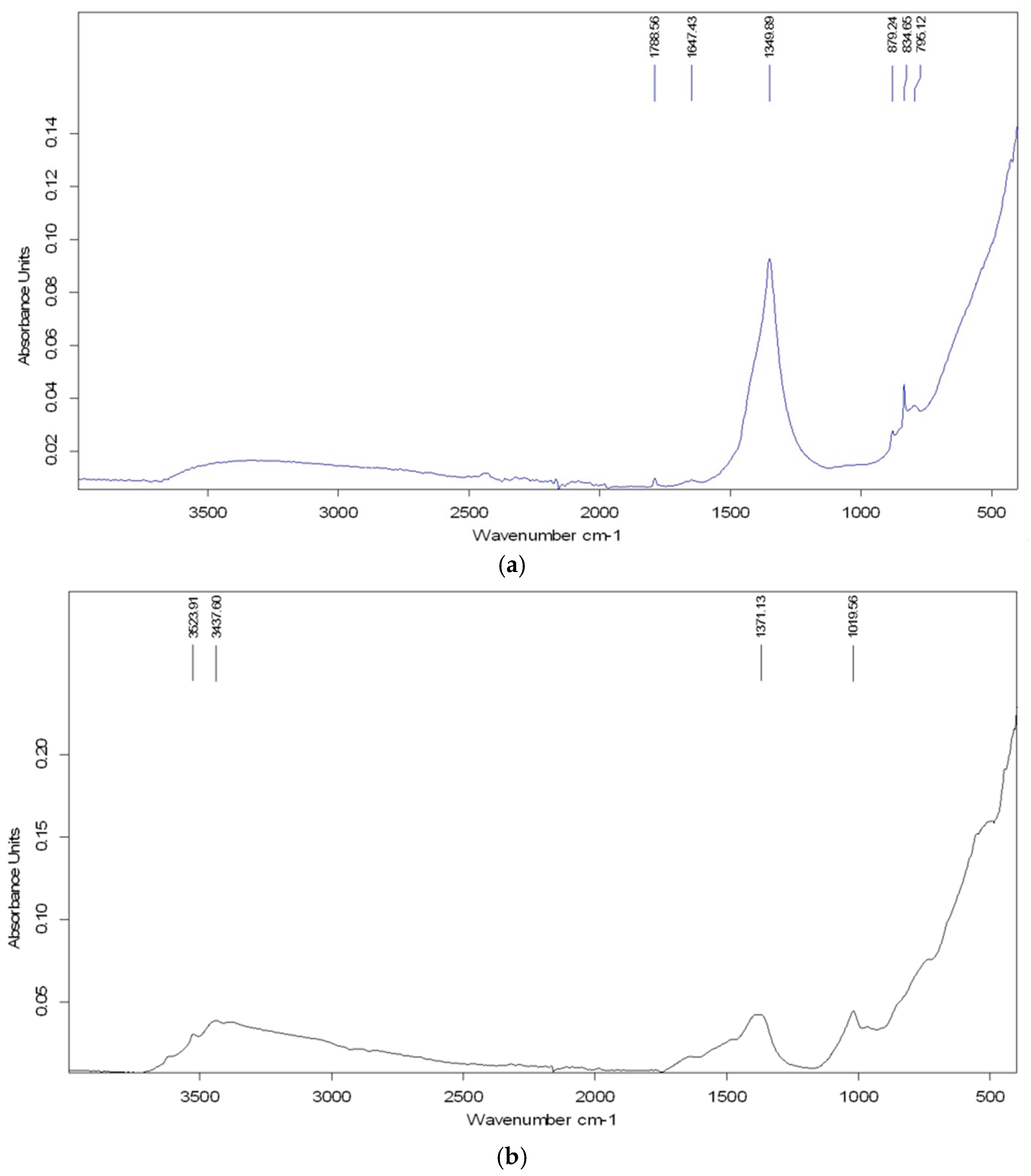
3.1.5. FTIR Analysis
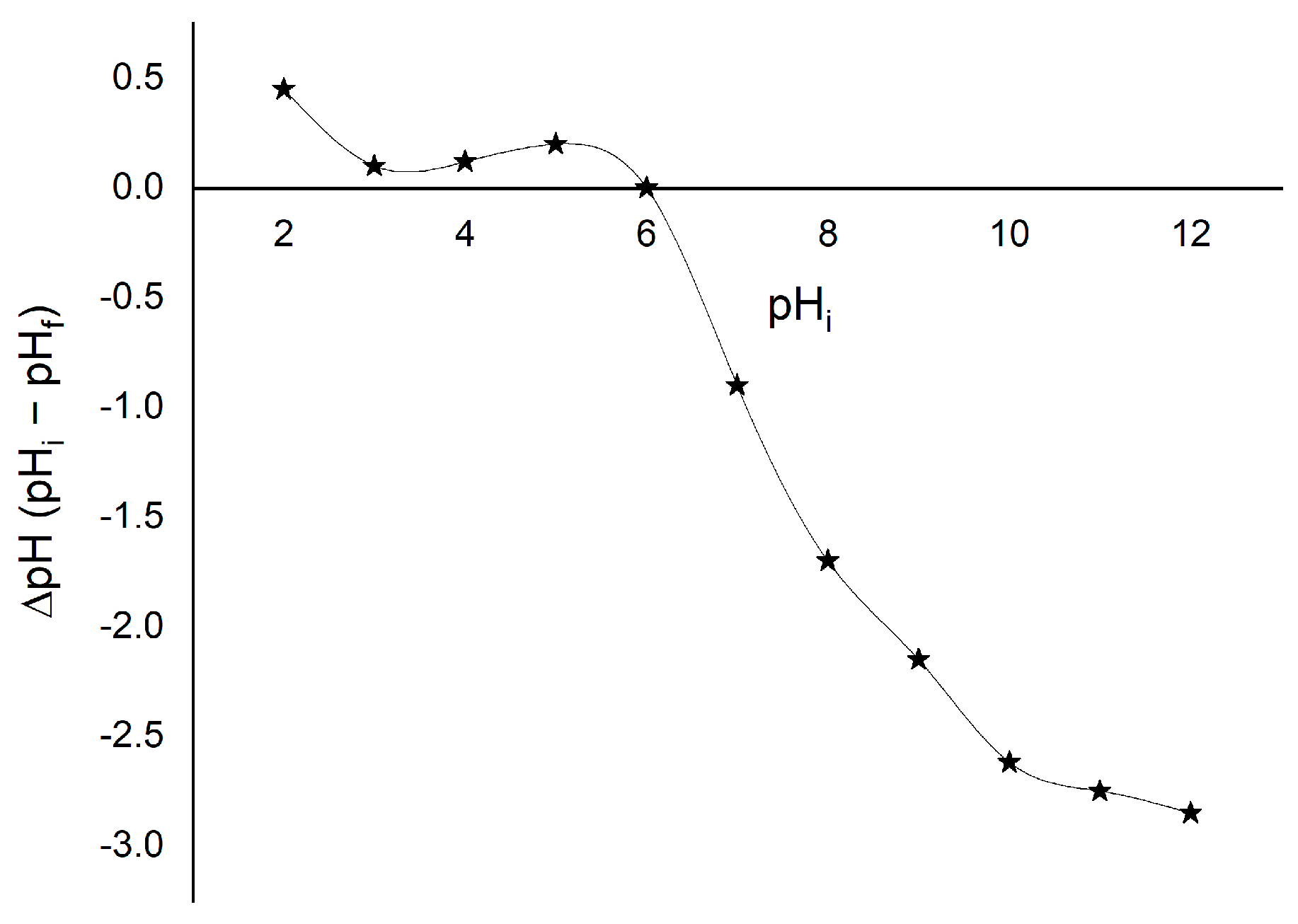
3.1.6. The Point of Zero Charge (pHpzc) of the MgAlFe-LDH
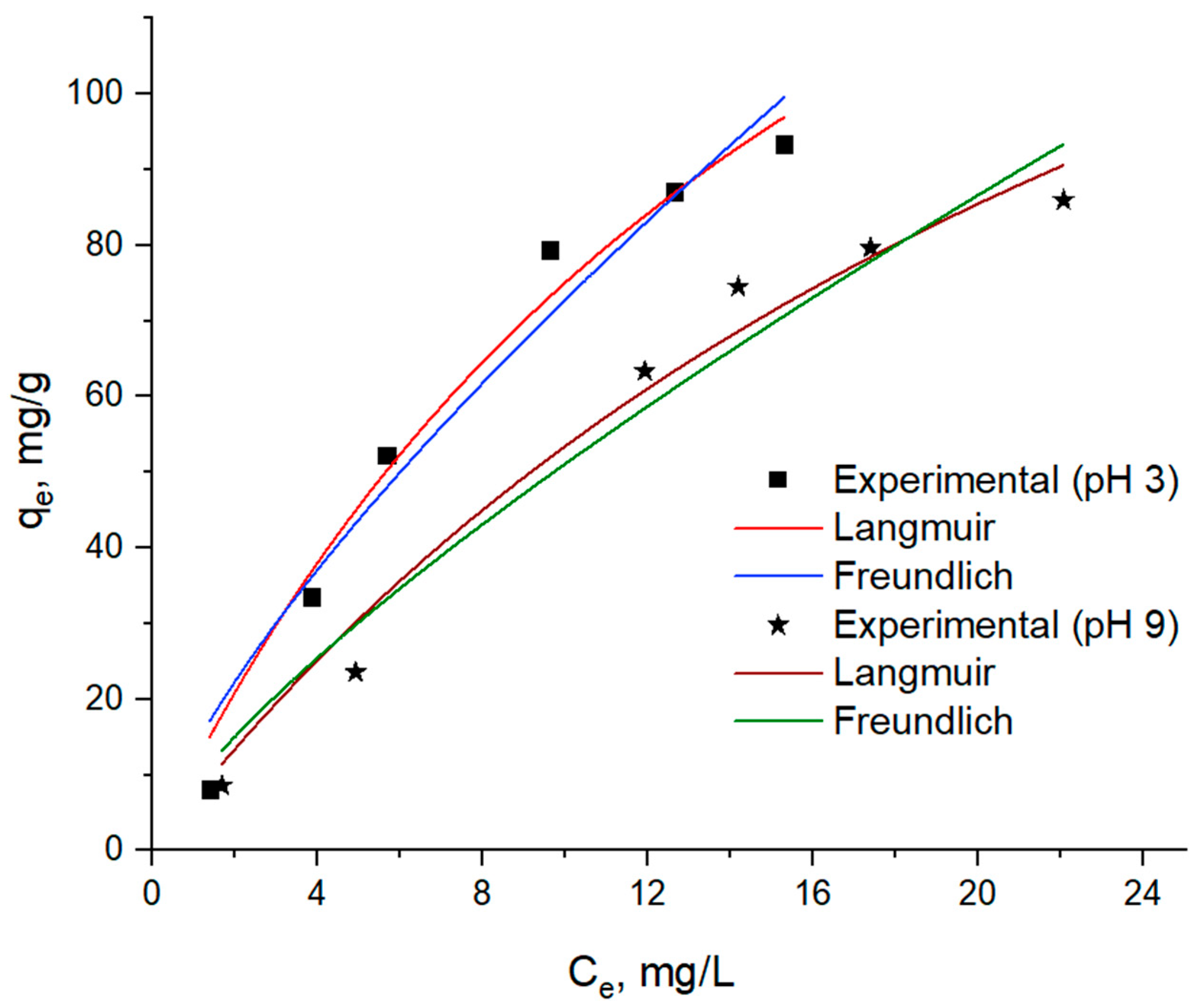
3.2. Adsorption Isotherms
3.3. Kinetics
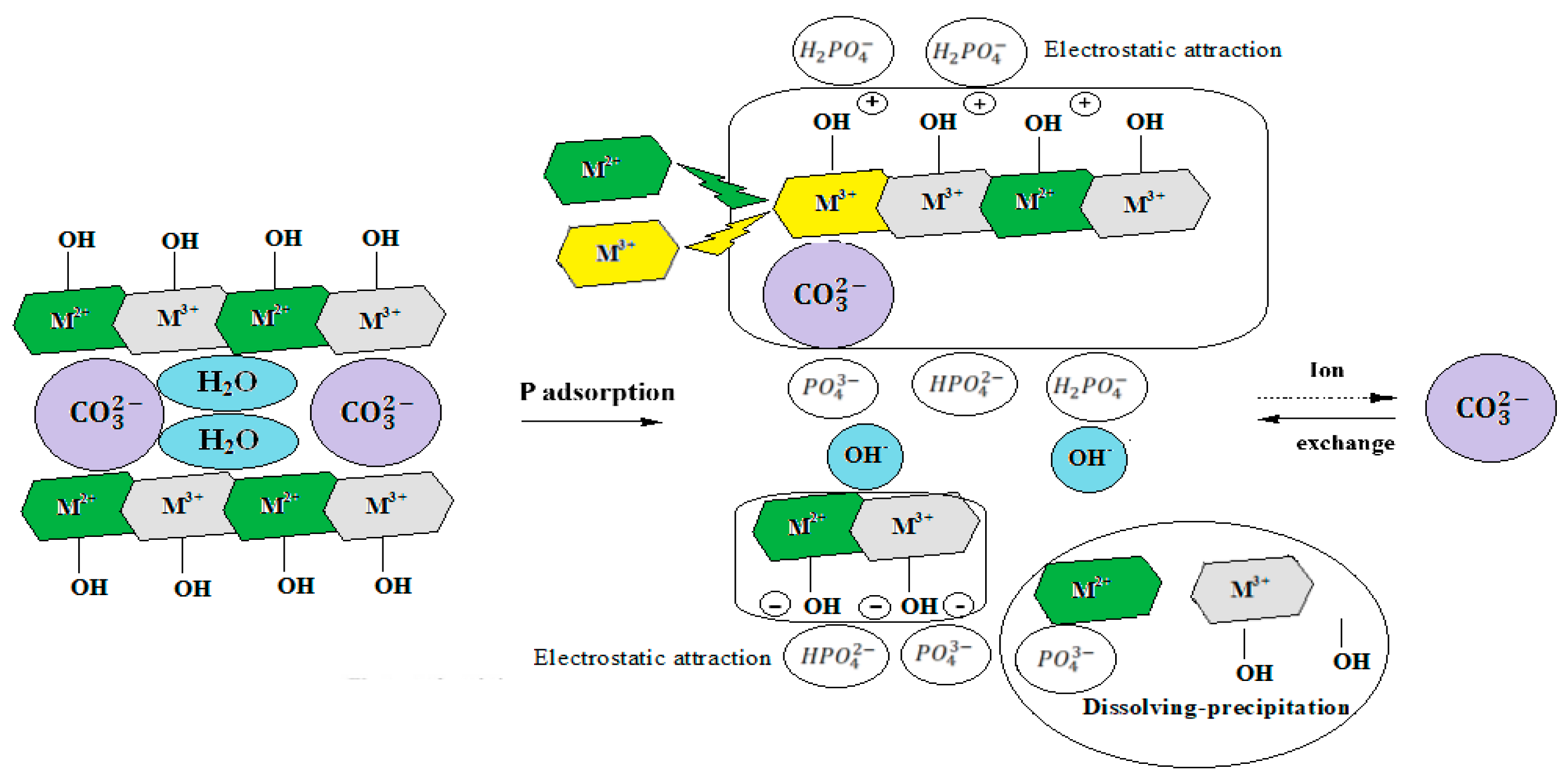
3.4. Adsorption Mechanism
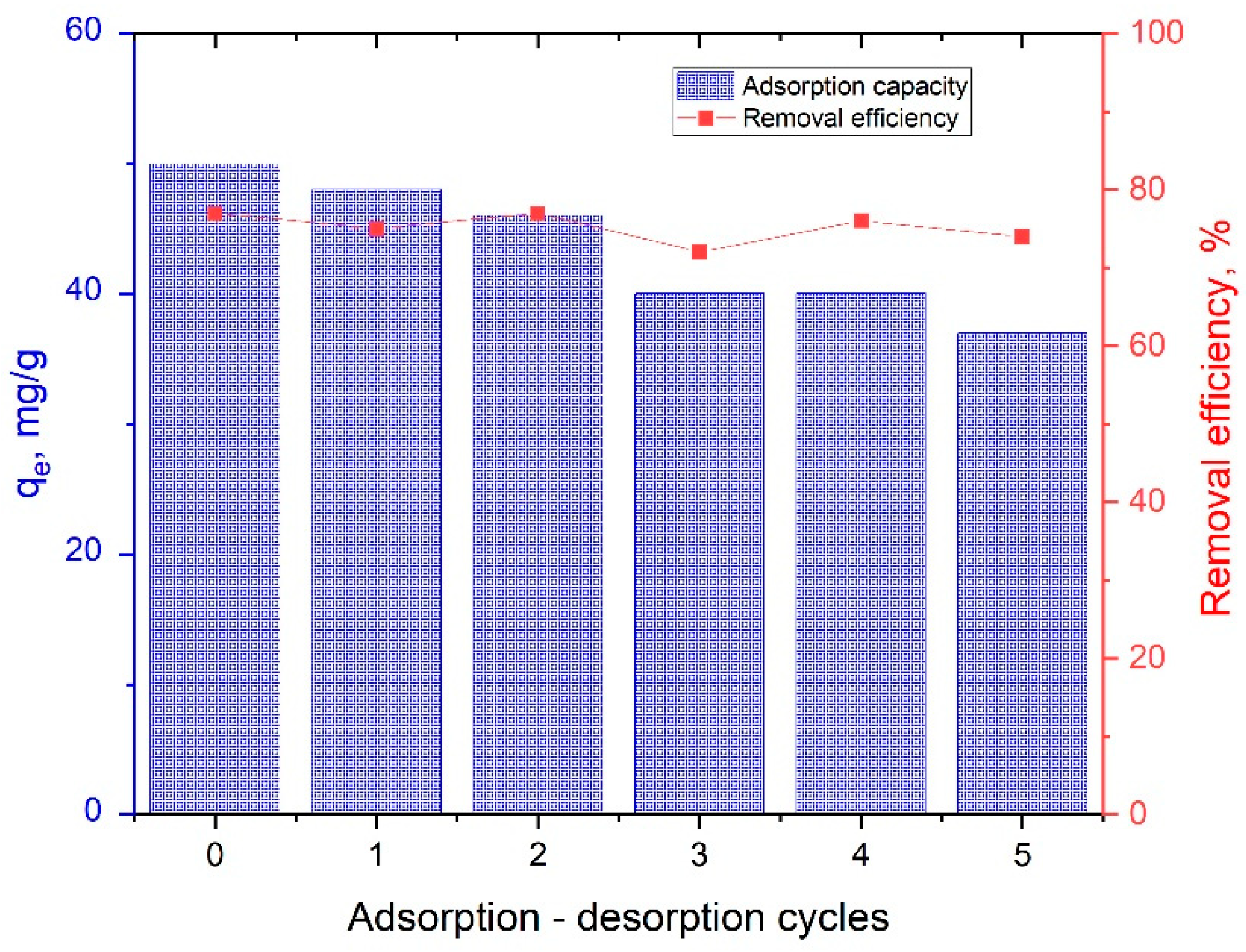
3.5. Reusability of MgAlFe-LDH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barao, A.L.P. Carbon Nitrogen and Phosphorus Soil Cycle Modelling—Dissertação para Obtenção do Grau de Mestre; Universidade Técnica de Lisboa: Lisboa, Portugal, 2007. [Google Scholar]
- Madjar, R.M.; Scăeteanu, G.V.; Sandu, M.A. Nutrient Water Pollution from Unsustainable Patterns of Agricultural Systems, Effects and Measures of Integrated Farming. Water 2024, 16, 3146. [Google Scholar] [CrossRef]
- Paerl, H.W.; Plaas, H.E.; Nelson, L.M.; Korbobo, A.S.; Cheshire, J.H.; Yue, L.; Preece, E.P. Dual Nitrogen and Phosphorus Reductions Are Needed for Long-Term Mitigation of Eutrophication and Harmful Cyanobacterial Blooms in the Hydrologically-Variable San Francisco Bay Delta, CA. Sci. Total Environ. 2024, 957, 177499. [Google Scholar] [CrossRef] [PubMed]
- Oduor, B.O.; Campo-Bescos, M.A.; Lana-Renault, N.; Kyllmar, K.; Mårtensson, K.; Casalí, J. Quantification of agricultural best management practices impacts on sediment and phosphorous export in a small catchment in southeastern Sweden. Agric. Water Manag. 2023, 290, 108595. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, D.; Adam, N.A.; Mirzalevens, S.; Zhang, G. Exploring the nexus between coastal tourism growth and eutrophication: Challenges for environmental management. Mar. Pollut. Bull. 2025, 216, 117922. [Google Scholar] [CrossRef]
- Selerio, E.F. Configuration of urban water eutrophication abatement systems using a novel regression-based optimization scheme. J. Environ. Manag. 2022, 319, 115667. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zhang, J.; Yu, C.; Xu, Z. Impacts of watershed nutrient loads on eutrophication risks under multiple socio-economic development scenarios in the Pearl River Estuary, China. J. Clean. Prod. 2025, 496, 145133. [Google Scholar] [CrossRef]
- Zill, J.; Perujo, N.; Fink, P.; Mallast, U.; Siebert, C.; Weitere, M. Contribution of groundwater-borne nutrients to eutrophication potential and the share of benthic algae in a large lowland river. Sci. Total Environ. 2024, 951, 175617. [Google Scholar] [CrossRef]
- Wang, C.; He, R.; Wu, Y.; Lürling, M.; Cai, H.; Jiang, H.L.; Liu, X. Bioavailable phosphorus (P) reduction is less than mobile P immobilization in lake sediment for eutrophication control by inactivating agents. Water Res. 2017, 109, 196–206. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, Y.; Zhang, W.; Wei, S.; Zhang, S. Developing zirconium xerogel coagulants for deep removal of phosphorous. Environ. Surf. Interfaces 2024, 2, 66–74. [Google Scholar] [CrossRef]
- Sisay, G.B.; Atisme, T.B.; Negie, Z.W.; Workie, Y.A.; Mekonnen, M.L. Mg/Zr modified nanobiochar from spent coffee grounds for phosphate recovery and its application as a phosphorous release fertilizer. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100766. [Google Scholar] [CrossRef]
- Lei, Y.; Remmers, J.C.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Is There a Precipitation Sequence in Municipal Wastewater Induced by Electrolysis? Environ. Sci. Technol. 2018, 52, 8399–8407. [Google Scholar] [CrossRef]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P.; et al. Side stream enhanced biological phosphorus removal (S2EBPR) process improves system performance-a full-scale comparative study. Water Res. 2019, 167, 115109. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Altaf, M.M.; Khan, N.A.; Khan, A.H.; Khan, A.A.; Ahmed, S.; Kumar, P.S.; Naushad, M.; Rajapaksha, A.U.; Iqbal, J.; et al. Recent technologies for nutrient removal and recovery from wastewaters: A review. Chemosphere 2021, 277, 130328. [Google Scholar] [CrossRef] [PubMed]
- Hashim, K.S.; Ewadh, H.M.; Muhsin, A.A.; Zubaidi, S.L.; Kot, P.; Muradov, M.; Aljefery, M.; Al-Khaddar, R. Phosphate removal from water using bottom ash: Adsorption performance, coexisting anions and modelling studies. Water Sci. Technol. 2021, 83, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Rui, X.; Xu, Y.; Gao, Y.; Lee, C.T.; Li, C. Enhanced precipitation performance for treating high-phosphorus wastewater using novel magnetic seeds from coal fly ash. J. Environ. Manag. 2022, 315, 115168. [Google Scholar] [CrossRef]
- Sichler, T.C.; Becker, R.; Sauer, A.; Barjenbruch, M.; Ostermann, M.; Adam, C. Determination of the phosphorus content in sewage sludge: Comparison of different aqua regia digestion methods and ICP-OES, ICP-MS, and photometric determination. Environ. Sci. Eur. 2022, 34, 99. [Google Scholar] [CrossRef]
- Ahranjani, P.J.; Dehghan, K.; Farhoudi, S.; Bidhendi, M.E.; Korrani, Z.S.; Rezania, S. Effective removal of nitrate and phosphate ions from water using nickel-doped calcium alginate beads. Process Saf. Environ. Prot. 2025, 194, 486–496. [Google Scholar] [CrossRef]
- Fizir, M.; Touil, S.; Richa, A.; Wei, L.; Douadia, S.; Taibi, R.; Cherifi, S.; Mansuroglu, D.S.; Dramou, P. Kaolin-iron cross-linked alginate beads for efficient phosphate removal from water: An initiation towards sustainable treatment of domestic and hydroponic wastewaters. Appl. Clay Sci. 2024, 256, 107430. [Google Scholar] [CrossRef]
- Cheng, K.; You, Q.; Zou, L.; Zhang, Y.; Wang, P.; Zhang, W. High-temperature calcination modified red clay as an efficient adsorbent for phosphate removal from water. Environ. Res. 2025, 268, 120704. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, M.; Song, W.; Yang, G.; Feng, P.; Chulalaksananukul, W.; Zhu, S.; Huang, K.; Wang, Z. Innovative iron-manganese modified microalgae biochar for efficient phosphate iron removal from water: Preparation and adsorption mechanisms. J. Water Process Eng. 2024, 66, 106051. [Google Scholar] [CrossRef]
- Qu, J.; Peng, W.; Wang, M.; Cui, K.; Zhang, J.; Bi, F.; Zhang, G.; Hu, Q.; Wang, Y.; Zhang, Y. Metal-doped biochar for selective recovery and reuse of phosphate from water: Modification design, removal mechanism, and reutilization strategy. Bioresour. Technol. 2024, 407, 131075. [Google Scholar] [CrossRef]
- Isidoro Ribeiro, N.; Barreto Pessanha, O.; Gomes Soares Pessanha, M.L.; Guimaraes, D. Efficient phosphate adsorption by a composite composed of Mg6Al2(CO3) (OH)16⋅4H2O LDH and Chitosan: Kinetic, thermodynamic, desorption, and characterization studies. Sep. Purif. Technol. 2023, 307, 122717. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, X.; Xie, S.; Wang, C.; Bai, J.; Li, X.; Zhang, R.; Xiao, X.; Hu, J.; Jiang, X. Mechanistic insights into efficient phosphorus adsorption and recovery from water using functional ZnO/ZnFe-LDHs alginate hydrogels. J. Environ. Chem. Eng. 2025, 13, 115091. [Google Scholar] [CrossRef]
- Jimenez, A.; Guerra, M.; Pascual, D.; Trujillano, R.; Rives, V.; Vicente, M.A.; Gil, A. Photodegradation of paracetamol on CaAlGa and ZnAlTi mixed metal oxides (MMO) synthesized via LDH from Al–saline slags. J. Environ. Chem. Eng. 2025, 13, 116143. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Liu, S.; Zhang, J.; Chen, H.; Qiu, Z. Effective adsorption of Congo red by an innovative biochar/LDH-derived MIL-100(Al): Investigation of coexisting pollutants and mechanism revelation. Sep. Purif. Technol. 2025, 359, 130670. [Google Scholar] [CrossRef]
- He, C.; Moa, W.; Yang, Y.; Guo, W.; Huang, Y.; Yu, C.; Su, X.; Yang, J.; Feng, J.; He, A.; et al. Microwave-assisted modification of magnetic Mg/Al/Fe-LDH hydrotalcite for efficient arsenic removal from water. J. Environ. Manag. 2025, 381, 125228. [Google Scholar] [CrossRef]
- Sadeghi, M.; Moradi, M. Mg–Al LDH@MoS2 nanocomposite as promising recyclable nanosorbent for high-efficient removal of endocrine disrupting chemicals from aqueous environment. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136247. [Google Scholar] [CrossRef]
- Kumar, S.; Bar, A.; Sarkar, S.; Singh, J.; Upadhyay, C. Efficient removal of Congo Red dye using MoS-modified Mg-Al LDH nanocomposites for efficient wastewater remediation. Surf. Interfaces 2025, 64, 106351. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Improved performance of 3D hierarchical NiAl-LDHs micro-flowers via a surface anchored ZIF-8 for rapid multiple-pollutants simultaneous removal and residues monitoring. J. Hazard. Mater. 2020, 395, 122635. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Cui, H.; Zhu, H.; Shutes, B.; Yan, B.; Hou, S. Layered double hydroxides, an effective nanomaterial to remove phosphorus from wastewater: Performance, mechanism, factors and reusability. Sci. Total Environ. 2023, 884, 163757. [Google Scholar] [CrossRef]
- Qi, R.; Qian, C.; Li, Y.; Wang, Y. Biofilm formation on MgFe-LDH@quartz sand as novel wetland substrate under varied C/N ratios for BDE-47 removal. Environ. Pollut. 2024, 361, 124779. [Google Scholar] [CrossRef]
- Yuan, M.; Qiu, S.; Li, M.; Di, Z.; Feng, M.; Guo, C.; Fu, W.; Zhang, K.; Hu, W.; Wang, F. Enhancing phosphate removal performance in water using La–Ca/Fe–LDH: La loading alleviates ineffective stacking of laminates and increases the number of active adsorption sites. J. Clean. Prod. 2023, 388, 135857. [Google Scholar] [CrossRef]
- Hao, M.; Gao, P.; Yang, D.; Chen, X.; Xiao, F.; Yang, S. Highly efficient adsorption behavior and mechanism of Urea-Fe3O4@LDH for triphenyl phosphate. Environ. Pollut. 2020, 267, 114142. [Google Scholar] [CrossRef] [PubMed]
- SR 11411-2:1998; Îngrășăminte Chimice Complexe. Determinarea Fosforului. Metoda Spectrofotometrică (Complex Chemical Fertilizers. Determination of Phosphorus. Spectrophotometric Method). Romanian Standards Association (ASRO): Bucharest, Romania, 1998.
- Guo, X.; Wang, J. Comparison of Linearization Methods for Modeling the Langmuir Adsorption Isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Diaconu, E.; Orbuleţ, O.D.; Miron, A.R.; Modrogan, C. Forecasting the sorption of phosphates in soil with artificial neural networks. U.P.B. Sci. Bull. Ser. B 2010, 72, 175–182. [Google Scholar]
- Aziam, R.; Stefan, D.S.; Nouaa, S.; Chiban, M.; Boșomoiu, M. Adsorption of Metal Ions from Single and Binary Aqueous Systems on Bio-Nanocomposite, Alginate-Clay. Nanomaterials 2024, 14, 362. [Google Scholar] [CrossRef]
- Ndankou, C.S.D.; Ștefan, D.S.; Nsami, N.J.; Daouda, K.; Bosomoiu, M. Evaluation of Phenobarbital Adsorption Efficiency on Biosorbents or Activated Carbon Obtained from Adansonia Digitata Shells. Materials 2024, 17, 1591. [Google Scholar] [CrossRef]
- Luengo, C.V.; Volpe, M.A.; Avena, M.J. High sorption of phosphate on Mg-Al layered double hydroxides: Kinetics and equilibrium. J. Environ. Chem. Eng. 2017, 5, 4656–4662. [Google Scholar] [CrossRef]
- Rousselot, I.; Taviot-Guého, C.; Besse, J.P. Synthesis and characterization of mixed Ga/Al-containing layered double hydroxides: Study of their basic properties through the Knoevenagel condensation of benzaldehyde and ethyl cyanoacetate, and comparison to other LDHs. Int. J. Inorg. Mater 1999, 1, 165–174. [Google Scholar] [CrossRef]
- Monti, M.; Benito, P.; Basile, F.; Fornasari, G.; Gazzano, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. Electrosynthesis of Ni/Al and Mg/Al Layered Double Hydroxides on Pt and FeCrAlloy supports: Study and control of the pH near the electrode surface. Electrochim. Acta 2013, 108, 596–604. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers, Inc.: New York, NY, USA, 2001; ISBN 978-1-59033-060-6. [Google Scholar]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-G.; Yang, Y.-M.; Yu, S.-J.; Shan, R.-R.; Yu, H.-Q.; Zhu, B.-C.; Du, B. Adsorptive Removal of Phosphate by Mg–Al and Zn–Al Layered Double Hydroxides: Kinetics, Isotherms and Mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, S.; Wang, L.; Liu, Q.; Zhang, W.; Chen, Y. Phosphate removal from aqueous solution using MgFe-layered double hydroxide modified biochar. J. Environ. Chem. Eng. 2020, 8, 103593. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, Y.; Luan, Y.N.; Wang, Q.; Zhao, Z.; Guo, Z.; Liu, C. Efficient phosphate removal by Mg-La binary layered double hydroxides: Synthesis optimization, adsorption performance, an inner mechanism. Environ. Sci. Pollut. Res. 2024, 31, 29132–29147. [Google Scholar] [CrossRef]
- Mariska, S.; Zhang, J.-W.; Hoang, H.C.; Duong, M.N.; Nguyen, D.H.; Chao, H.-P. Adsorptive Removal of Phosphate and Nitrate by Layered Double Hydroxides through the Memory Effect and In Situ Synthesis. Appl. Water Sci. 2025, 15, 42. [Google Scholar] [CrossRef]
- Qing, Z.; Qin, Q.; Wang, L.; Jiang, C.; Yang, Z.; Liu, Y.; Zhang, S.; Chen, J. Rapid and Efficient Removal of Phosphate by La-Doped Layered Double Hydroxide/Biochar from Aqueous Solution. New J. Chem. 2024, 48, 3208–3220. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]

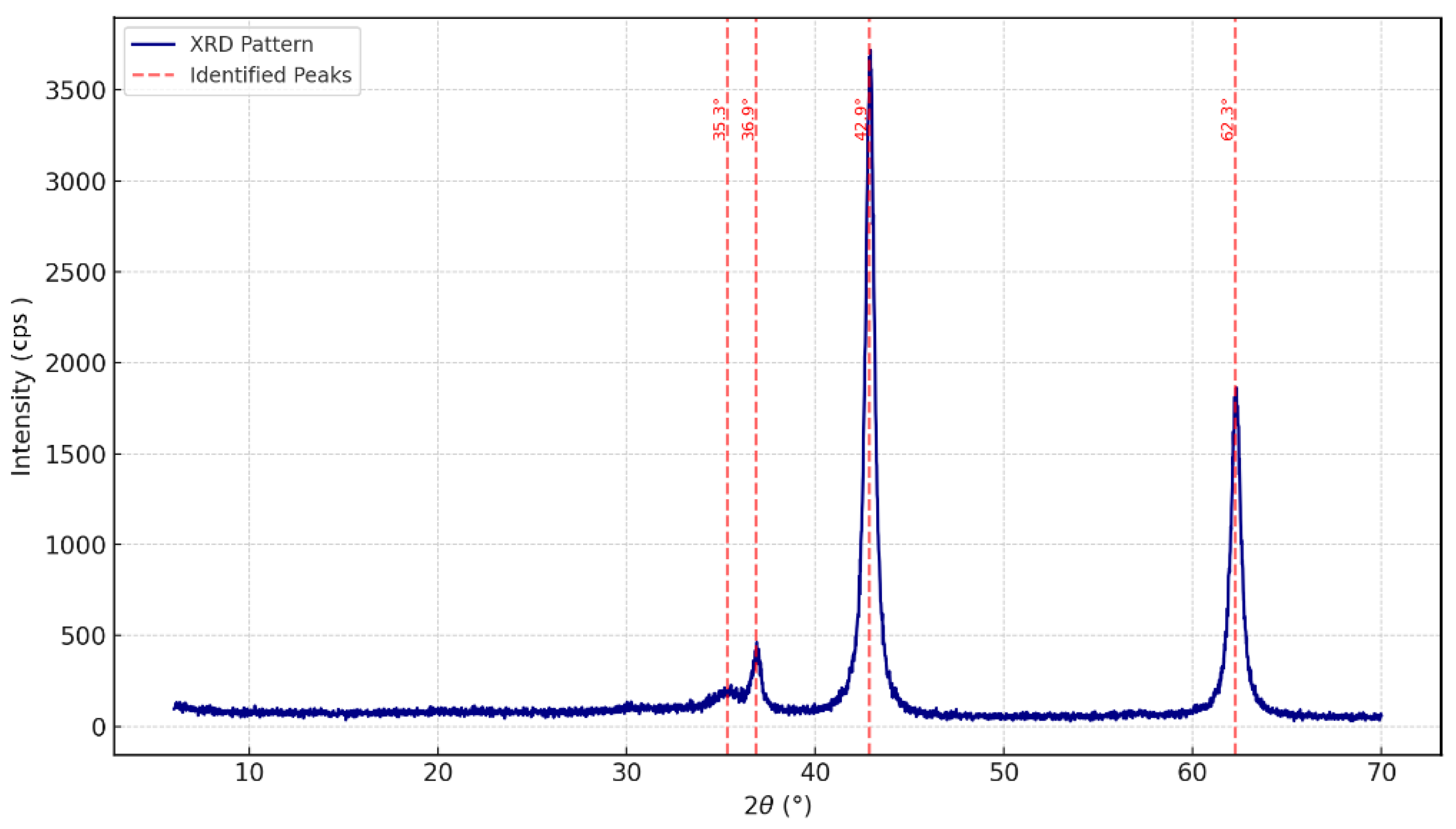

| 2θ (deg) | d (Å) | Height (cps) | FWHM (°) | Size(Å) |
|---|---|---|---|---|
| 35.3369 | 2.53793 | 50.29 | 1.8911 | 46.05 |
| 36.8636 | 2.43624 | 177.19 | 0.5715 | 153.02 |
| 42.8816 | 2.10724 | 2482.47 | 0.5535 | 161.05 |
| 62.2742 | 1.48965 | 1221.87 | 0.6042 | 160.43 |
| Material | SBET (m2/g) | Vt (cm3/g) | Reference |
|---|---|---|---|
| LDHs/Alginate | 2.76 | 0.001293 | [43] |
| FeMg-LDH | 0.62 | - | [32] |
| CaAl0.25Ga0.75-LDH | 4 | - | [42] |
| MgAlFe-LDH | 4.9 | 0.0018 | Present work |
| Element | Peak BE | Area (P) CPS·eV | Area (N) KE^0.6 | Atomic % | Relative Concentration (%) |
|---|---|---|---|---|---|
| Mg1s | 1302.96 | 229,336.8 | 898.53 | 22.04 | 28.35 |
| O1s | 531.1 | 362,606.8 | 2015.62 | 49.45 | 66.83 |
| Ca2p | 351.4 | 74,768.17 | 216.59 | 5.31 | 9.24 |
| Fe2p | 711.06 | 78,045.53 | 87.74 | 2.15 | 9.65 |
| C1s | 284.52 | 31,397.89 | 445.58 | 10.93 | 3.88 |
| Al2p | 74.17 | 12,601.85 | 302.32 | 7.42 | 1.55 |
| Na1s | 74.17 | 14,109.69 | 44.45 | 1.09 | 1.75 |
| P2p | 132.94 | 5881.45 | 65.21 | 1.6 | 0.75 |
| pH | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/g) | R2 | % RMSE | KF, (mg/g)⋅(L/mg)1/n | n | R2 | % RMSE | |
| 3 | 0.0535 | 215.179 | 0.9750 | 2.849 | 13.3574 | 1.3585 | 0.9518 | 9.653 |
| 9 | 0.0333 | 213.829 | 0.9719 | 3.563 | 8.8425 | 1.3128 | 0.9505 | 12.523 |
| Material | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| La–MgAl–LDH/BC | 249.3 | [51] |
| La/Fe-chitosan | 87.23 | [49] |
| Alginate/kaolin | 22.51 | [20] |
| AC/MgAl-3 LDH | 337.2 | [52] |
| CO3–LDHs | 184.0 | [50] |
| MgAlFe–LDH | 215.18 | Present work |
| Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | k1 (min−1) | qe (mg/g) | R2 | % RMSE | k2 (g/(min · mg)) | qe (mg/g) | R2 | % RMSE |
| 3 | 0.010290 | 101.944 | 0.9882 | 2.315 | 1.2283 × 10−4 | 110.351 | 0.9563 | 7.654 |
| 9 | 0.006765 | 102.410 | 0.9914 | 2.695 | 1.2685 × 10−4 | 96.302 | 0.9417 | 8.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orbuleţ, O.D.; Bobirică, L.; Enache, M.; Pațac, R.C.; Bosomoiu, M.; Modrogan, C. Adsorption of Phosphates from Wastewater Using MgAlFe-Layered Double Hydroxides. Environments 2025, 12, 316. https://doi.org/10.3390/environments12090316
Orbuleţ OD, Bobirică L, Enache M, Pațac RC, Bosomoiu M, Modrogan C. Adsorption of Phosphates from Wastewater Using MgAlFe-Layered Double Hydroxides. Environments. 2025; 12(9):316. https://doi.org/10.3390/environments12090316
Chicago/Turabian StyleOrbuleţ, Oanamari Daniela, Liliana Bobirică, Mirela Enache (Cişmaşu), Ramona Cornelia Pațac, Magdalena Bosomoiu, and Cristina Modrogan. 2025. "Adsorption of Phosphates from Wastewater Using MgAlFe-Layered Double Hydroxides" Environments 12, no. 9: 316. https://doi.org/10.3390/environments12090316
APA StyleOrbuleţ, O. D., Bobirică, L., Enache, M., Pațac, R. C., Bosomoiu, M., & Modrogan, C. (2025). Adsorption of Phosphates from Wastewater Using MgAlFe-Layered Double Hydroxides. Environments, 12(9), 316. https://doi.org/10.3390/environments12090316








