Floral Characteristics Alter the Abundance and Richness of Bees Captured in Passive Traps
Abstract
1. Introduction
2. Materials and Methods
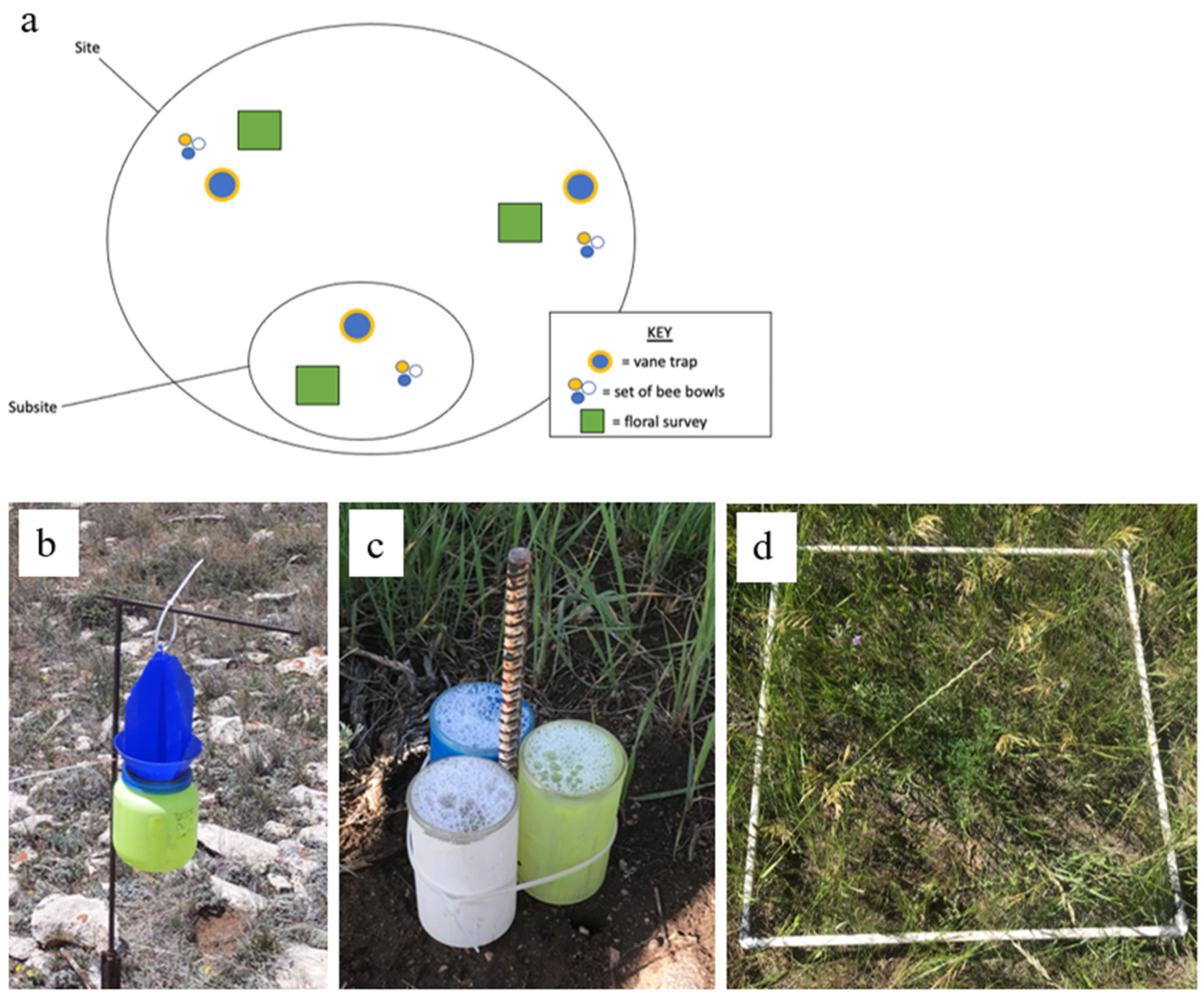
2.1. Study Sites
2.2. Field Sampling
2.3. Statistical Analyses
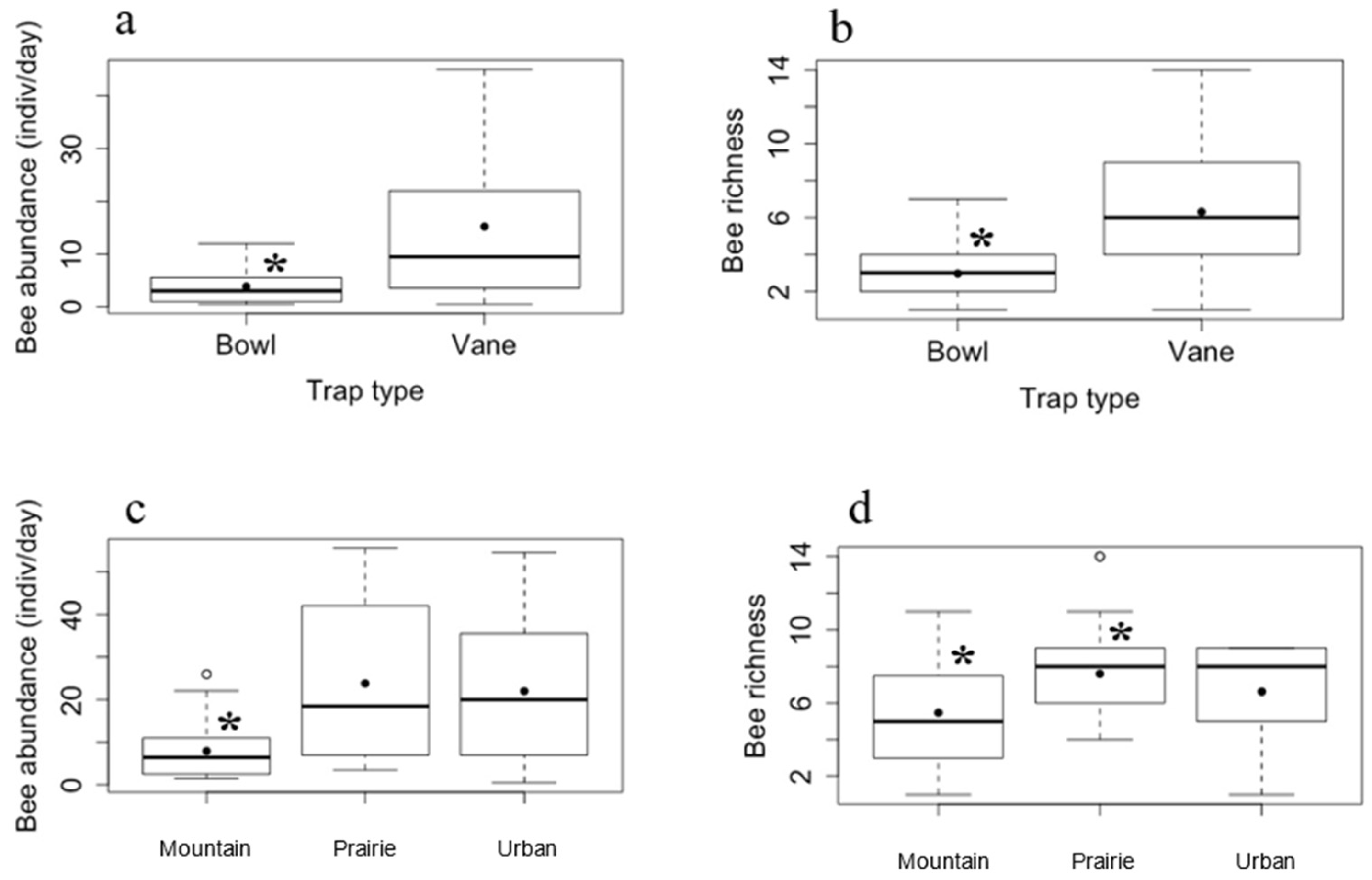
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa | Individual Flower Surface Area (mm2/m2) | Number of Flowers | Number of Specimens |
|---|---|---|---|
| Alliaceae | 307 | 10 | |
| Allium | 14.9 | 307 | 10 |
| Anthericaceae | 9 | 4 | |
| Leucocrinum | 572.6 | 9 | 4 |
| Apiaceae | 124 | 5 | |
| Cymopterus | 5.8 | 124 | 5 |
| Asteraceae | 1760 | 75 | |
| Achillea | 9.5 | 339 | 19 |
| Anaphalis | 12.6 | 280 | 2 |
| Antennaria | 20.7 | 521 | 11 |
| Cirsium | 641.4 | 4 | 3 |
| Erigeron | 189.4 | 6 | 4 |
| Grindelia | 323.4 | 28 | 7 |
| Gutierrezia | 18.7 | 484 | 8 |
| Heterotheca | 147.2 | 82 | 10 |
| Senecio | 328 | 11 | 3 |
| Solidago | 115.5 | 1 | 3 |
| Taraxacum | 423.6 | 3 | 3 |
| Townsendia | 207.8 | 1 | 2 |
| Boraginaceae | 483 | 13 | |
| Cryptantha | 15.5 | 392 | 5 |
| Cynoglossum | 19.6 | 28 | 1 |
| Mertensia | 201.5 | 59 | 6 |
| Myosotis | 19.6 | 4 | 1 |
| Brassicaceae | 72 | 5 | |
| Physaria | 45.1 | 72 | 5 |
| Cactaceae | 4 | 6 | |
| Opuntia | 1943.9 | 4 | 6 |
| Calochortaceae | 1 | 2 | |
| Calochortus | 353.5 | 1 | 2 |
| Campanulaceae | 11 | 3 | |
| Campanula | 645.3 | 11 | 3 |
| Caryophyllaceae | 560 | 18 | |
| Cerastium | 122.2 | 36 | 8 |
| Eremogone | 56.7 | 524 | 10 |
| Crassulaceae | 26 | 3 | |
| Sedum | 100 | 26 | 3 |
| Euphorbiaceae | 704 | 3 | |
| Chamaesyce | 12.6 | 505 | 1 |
| Euphorbia | 78.5 | 199 | 2 |
| Fabaceae | 3353 | 31 | |
| Astragalus | 20 | 405 | 3 |
| Lupinus | 35.5 | 519 | 17 |
| Melilotus | 7.3 | 577 | 5 |
| Oxytropis | 7 | 52 | 1 |
| Trifolium | 24 | 1800 | 5 |
| Linaceae | 6 | 2 | |
| Linum | 304.7 | 6 | 2 |
| Melanthiaceae | 19 | 3 | |
| Zigadenus | 22.2 | 19 | 3 |
| Onagraceae | 14 | 5 | |
| Calylophus | 865.9 | 7 | 2 |
| Chamerion | 176.7 | 6 | 1 |
| Oenothera | 628.3 | 1 | 2 |
| Plantaginaceae | 1876 | 3 | |
| Plantago | 7.1 | 1876 | 3 |
| Polemoniaceae | 151 | 4 | |
| Phlox | 136.7 | 147 | 3 |
| Polemonium | 153.9 | 4 | 1 |
| Polygonaceae | 1036 | 8 | |
| Erigonum | 11.3 | 430 | 3 |
| Polygonum | 3.5 | 606 | 5 |
| Portulanceae | 3 | 2 | |
| Lewisii | 39.3 | 3 | 2 |
| Ranunculaceae | 48 | 9 | |
| Anemone | 314.2 | 1 | 1 |
| Delphinium | 227.3 | 47 | 8 |
| Rosaceae | 85 | 15 | |
| Pentaphylloides | 128.8 | 7 | 4 |
| Potentilla | 175.7 | 78 | 11 |
| Rubiaceae | 204 | 1 | |
| Galium | 12.6 | 204 | 1 |
| Santalaceae | 126 | 2 | |
| Comandra | 24 | 126 | 2 |
| Scrophulariaceae | 424 | 21 | |
| Castilleja | 125.5 | 49 | 4 |
| Collinsia | 8 | 7 | 1 |
| Linaria | 132 | 1 | 1 |
| Orthocarpus | 88 | 1 | 1 |
| Pedicularis | 129 | 62 | 1 |
| Penstemon | 84.2 | 304 | 13 |
| Violaceae | 2 | 1 | |
| Viola | 227 | 2 | 1 |
| Total | NA | 11,406 | 254 |
| Taxa | No. Specimens Vane | No. Specimens Bowl |
|---|---|---|
| Andrenidae | 33 | 23 |
| Andrena | 23 | 10 |
| Perdita | 10 | 10 |
| Halictidae | 904 | 521 |
| Agapostemon | 221 | 59 |
| Halictus | 107 | 36 |
| Lasioglossum | 547 | 420 |
| Augochlorella | 10 | 4 |
| Dufourea | 8 | 1 |
| Sphecodes | 11 | 1 |
| Megachilidae | 355 | 40 |
| Anthidium | 19 | 3 |
| Ashmeadiella | 0 | 1 |
| Dianthidium | 20 | 5 |
| Hoplitis | 61 | 9 |
| Megachile | 47 | 2 |
| Osmia | 202 | 20 |
| Coelioxys | 1 | 0 |
| Heriades | 1 | 0 |
| Stelis | 4 | 0 |
| Apidae | 1944 | 114 |
| Anthophora | 250 | 29 |
| Apis | 10 | 1 |
| Bombus | 554 | 12 |
| Eucera | 747 | 51 |
| Melissodes | 199 | 10 |
| Ceratina | 39 | 4 |
| Diadasia | 108 | 6 |
| Habropoda | 5 | 0 |
| Melecta | 6 | 0 |
| Nomada | 5 | 1 |
| Svastra | 17 | 0 |
| Tetraloniella | 4 | 0 |
| Colletidae | 16 | 7 |
| Colletes | 6 | 1 |
| Hylaeus | 10 | 6 |
References
- Matias, D.M.; Leventon, J.; Rau, A.L.; Borgemeister, C.; von Wehrden, H. A review of ecosystem service benefits from wild bees across social contexts. Ambio 2017, 46, 456–467. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Biol. Soc. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.R.; Isaacs, R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl. Ecol. 2014, 15, 701–711. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Lebuhn, G.; Droege, S.; Connor, E.F.; Gemmill-Herren, B.; Potts, S.G.; Minckley, R.L.; Griswold, T.; Jean, R.; Kula, E.; Roubik, D.W.; et al. Detecting insect pollinator declines on regional and global scales. Conserv. Biol. 2013, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L.; Robinson, G.E. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Wood, T.J.; Gibbs, J.; Graham, K.K.; Isaacs, R. Narrow pollen diets are associated with declining Midwestern bumble bee species. Ecology 2019, 100, e02697. [Google Scholar] [CrossRef]
- Meeus, I.; Brown, M.J.; De Graaf, D.C.; Smagghe, G. Effects of invasive parasites on bumble bee declines. Conserv. Biol. 2011, 25, 662–671. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Woodard, S.H.; Federman, S.; James, R.R.; Danforth, B.N.; Griswold, T.L.; Inouye, D.; McFrederick, Q.S.; Morandin, L.; Paul, D.L.; Sellers, E.; et al. Towards a U.S. national program for monitoring native bees. Biol. Conserv. 2020, 252, 108821. [Google Scholar] [CrossRef]
- Graves, T.A.; Janousek, W.M.; Gaulke, S.M.; Nicholas, A.C.; Keinath, D.A.; Bell, C.M.; Cannings, S.; Hatfield, R.G.; Heron, J.M.; Koch, J.B.; et al. Western bumble bee: Declines in the continental United States and range-wide information gaps. Ecosphere 2020, 11, e03141. [Google Scholar] [CrossRef]
- Tronstad, L.; Bell, C.; Crawford, M. Choosing collection methods and sample sizes for monitoring bees. Agric. For. Entomol. 2022, 24, 531–539. [Google Scholar] [CrossRef]
- Kuhlman, M.P.; Burrows, S.; Mummey, D.L.; Ramsey, P.W.; Hahn, P.G. Relative bee abundance varies by collection method and flowering richness: Implications for understanding patterns in bee community data. Ecol. Solut. Evid. 2021, 2, e12071. [Google Scholar] [CrossRef]
- Bell, C.; Tronstad, L.; Hotaling, S. Tailoring your bee sampling protocol: Comparing three methods reveals the best approaches to capturing bees. Agric. For. Entomol. 2023, 25, 477–488. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Oliver, T.H.; Breeze, T.D.; O’Connor, R.S.; Potts, S.G.; Roberts, S.P.M.; Garratt, M.P.D. Inventorying and monitoring crop pollinating bees: Evaluating the effectiveness of common sampling methods. Insect Conserv. Divers. 2022, 15, 299–311. [Google Scholar] [CrossRef]
- Krahner, A.; Schmidt, J.; Maixner, M.; Porten, M.; Schmitt, T. Evaluation of four different methods for assessing bee diversity as ecological indicators of agroecosystems. Ecol. Indic. 2021, 125, 107573. [Google Scholar] [CrossRef]
- Pei, C.K.; Hovick, T.J.; Duquette, C.A.; Limb, R.F.; Harmon, J.P.; Geaumont, B.A. Two common bee-sampling methods reflect different assemblages of the bee (Hymenoptera: Apoidea) community in mixed-grass prairie systems and are dependent on surrounding floral resource availability. J. Insect Conserv. 2021, 26, 69–83. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Menz, M.H.M.; Dixon, K.W.; Bateman, P.W. The relative performance of sampling methods for native bees: An empirical test and review of the literature. Ecosphere 2020, 11, e03076. [Google Scholar] [CrossRef]
- Stephen, W.P.; Rao, S. Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). J. Kans. Entomol. Soc. 2005, 78, 373–380. [Google Scholar] [CrossRef]
- Hall, M. Blue and yellow vane traps differ in their sampling effectiveness for wild bees in both open and wooded habitats. Agric. For. Entomol. 2018, 20, 487–495. [Google Scholar] [CrossRef]
- Rhoades, P.; Griswold, T.; Waits, L.; Bosque-Pérez, N.A.; Kennedy, C.M.; Eigenbrode, S.D. Sampling technique affects detection of habitat factors influencing wild bee communities. J. Insect Conserv. 2017, 21, 703–714. [Google Scholar] [CrossRef]
- McCravy, K.W.; Ruholl, J.D. Bee (Hymenoptera: Apoidea) Diversity and Sampling Methodology in a Midwestern USA Deciduous Forest. Insects 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Scheper, J.; Bommarco, R.; Holzschuh, A.; Potts, S.G.; Riedinger, V.; Roberts, S.P.M.; Rundlöf, M.; Smith, H.G.; Steffan-Dewenter, I.; Wickens, J.B.; et al. Local and landscape-level floral resources explain effects of wildflower strips on wild bees across four European countries. J. Appl. Ecol. 2015, 52, 1165–1175. [Google Scholar] [CrossRef]
- Bruckman, D.; Campbell, D.R. Floral neighborhood influences pollinator assemblages and effective pollination in a native plant. Oecologia 2014, 176, 465–476. [Google Scholar] [CrossRef]
- Filipiak, M. A Better Understanding of Bee Nutritional Ecology Is Needed to Optimize Conservation Strategies for Wild Bees-The Application of Ecological Stoichiometry. Insects 2018, 9, 85. [Google Scholar] [CrossRef]
- Elliott, S.E.; Irwin, R.E. Effects of flowering plant density on pollinator visitation, pollen receipt, and seed production in Delphinium barbeyi (Ranunculaceae). Am. J. Bot. 2009, 96, 912–919. [Google Scholar] [CrossRef]
- Potts, S.G.; Willmer, P. Abiotic and biotic factors influencing nest-site selection by Halictus rubicundus, a ground-nesting halictine bee. Ecol. Entomol. 1997, 22, 319–328. [Google Scholar] [CrossRef]
- Hachuy-Filho, L.; Ballarin, C.S.; Amorim, F.W. Changes in plant community structure and decrease in floral resource availability lead to a high temporal β-diversity of plant–bee interactions. Arthropod-Plant Interact. 2020, 14, 571–583. [Google Scholar] [CrossRef]
- Joshi, N.K.; Leslie, T.; Rajotte, E.G.; Kammerer, M.A.; Otieno, M.; Biddinger, D.J. Comparative Trapping Efficiency to Characterize Bee Abundance, Diversity, and Community Composition in Apple Orchards. Ann. Entomol. Soc. Am. 2015, 108, 785–799. [Google Scholar] [CrossRef]
- Tuell, J.K.; Ascher, J.S.; Isaacs, R. Wild Bees (Hymenoptera: Apoidea: Anthophila) of the Michigan Highbush Blueberry Agroecosystem. Ann. Entomol. Soc. Am. 2009, 102, 275–287. [Google Scholar] [CrossRef]
- Droege, S.A.M.; Tepedino, V.J.; Lebuhn, G.; Link, W.; Minckley, R.L.; Chen, Q.; Conrad, C. Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv. Divers. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Michener, C.D.; McGinley, R.J.; Danforth, B.N. The Bee Genera of North and Central America (Hymenoptera: Apoidea); Smithsonian Institution Press: Washington, WA, USA, 1994. [Google Scholar]
- Dorn, R.D. Vascular Plants of Wyoming; Mountain West Pub.: Ann Arbor, MI, USA, 1988. [Google Scholar]
- R Core Development Team. R: A Language for Environment and Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community ecology package. R Package Version 2020, 2, 5–6. [Google Scholar]
- Bates, D.; Maechler, M. Matrix: Sparse and dense matrix classes and methods. R Package Version 2019, 1, 1–4. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting lienar mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Osborne, J.L.; Martin, A.P.; Carreck, N.L.; Swain, J.L.; Knight, M.E.; Goulson, D.; Hale, R.J.; Sanderson, R.A. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 2008, 77, 406–415. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee Foraging Ranges and Their Relationship to Body Size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gibbs, J.; Gratton, C. Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc. Ecol. 2016, 31, 1523–1535. [Google Scholar] [CrossRef]
- Frasnelli, E.; Robert, T.; Chow, P.K.Y.; Scales, B.; Gibson, S.; Manning, N.; Philippides, A.O.; Collett, T.S.; Hempel de Ibarra, N. Small and Large Bumblebees Invest Differently when Learning about Flowers. Curr. Biol. 2021, 31, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.; Sipes, S. Characterizing floral specialization by bees: Analytical methods and a revised lexicon for oligolecty. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2007. [Google Scholar]
- Milet-Pinheiro, P.; Ayasse, M.; Schlindwein, C.; Dobson, H.E.M.; Dotterl, S. Host location by visual and olfactory floral cues in an oligolectic bee: Innate and learned behavior. Behav. Ecol. 2012, 23, 531–538. [Google Scholar] [CrossRef]
- Tsujimoto, S.G.; Ishii, H.S. Effect of flower perceptibility on spatial-reward associative learning by bumble bees. Behav. Ecol. Sociobiol. 2017, 71, 105, Erratum in Behav. Ecol. Sociobiol. 2017, 71, 130. [Google Scholar] [CrossRef]
- Fowler, J. Pollen Specialist Bees of the Western United States. Available online: https://jarrodfowler.com/pollen_specialist.html (accessed on 1 March 2022).
- Gonzalez, V.H.; Park, K.E.; Çakmak, I.; Hranitz, J.M.; Barthell, J.F. Bee bowls and bee body size in unmanaged urban habitats. J. Hymenopt. Res. 2016, 51, 241–247. [Google Scholar] [CrossRef]
- McCabe, L.M.; Colella, E.; Chesshire, P.; Smith, D.; Cobb, N.S. The transition from bee-to-fly dominated communities with increasing elevation and greater forest canopy cover. PLoS ONE 2019, 14, e0217198. [Google Scholar] [CrossRef]
- Packer, L.; Darla-West, G. Bees: How and Why to Sample Them; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Shapiro, L.H.; Tepedino, V.J.; Minckley, R.L. Bowling for bees: Optimal sample number for “bee bowl” sampling transects. J. Insect Conserv. 2014, 18, 1105–1113. [Google Scholar] [CrossRef]



| Model | AIC | ΔAIC | K | Weight |
|---|---|---|---|---|
| Site flower surface area + Trap type | 492.48 | 6 | 0.87 | |
| Subsite flower surface area + Trap type | 496.33 | 3.85 | 6 | 0.13 |
| Ecosystem | 560.94 | 68.46 | 6 | 0.00 |
| Ecosystem + Site flower density | 564.46 | 71.98 | 7 | 0.00 |
| Ecosystem + Site flower surface area | 564.62 | 72.14 | 7 | 0.00 |
| Model | AIC | ΔAIC | K | Weight |
|---|---|---|---|---|
| Subsite flower surface area + Trap type | 459.66 | 6 | 0.68 | |
| Site flower surface area + Trap type | 461.15 | 1.50 | 6 | 0.32 |
| Site flower surface area | 586.68 | 127.03 | 5 | 0.00 |
| Subsite flower surface area | 587.15 | 127.50 | 5 | 0.00 |
| Ecosystem | 587.22 | 127.56 | 6 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, M.; Bell, C.; Dillon, M.E.; Tronstad, L.M. Floral Characteristics Alter the Abundance and Richness of Bees Captured in Passive Traps. Environments 2025, 12, 301. https://doi.org/10.3390/environments12090301
Mazur M, Bell C, Dillon ME, Tronstad LM. Floral Characteristics Alter the Abundance and Richness of Bees Captured in Passive Traps. Environments. 2025; 12(9):301. https://doi.org/10.3390/environments12090301
Chicago/Turabian StyleMazur, Madison, Christine Bell, Michael E. Dillon, and Lusha M. Tronstad. 2025. "Floral Characteristics Alter the Abundance and Richness of Bees Captured in Passive Traps" Environments 12, no. 9: 301. https://doi.org/10.3390/environments12090301
APA StyleMazur, M., Bell, C., Dillon, M. E., & Tronstad, L. M. (2025). Floral Characteristics Alter the Abundance and Richness of Bees Captured in Passive Traps. Environments, 12(9), 301. https://doi.org/10.3390/environments12090301






