Selective Adsorption Performance of a High-Capacity Mesoporous Silica Aerogel for Fluoroquinolones
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
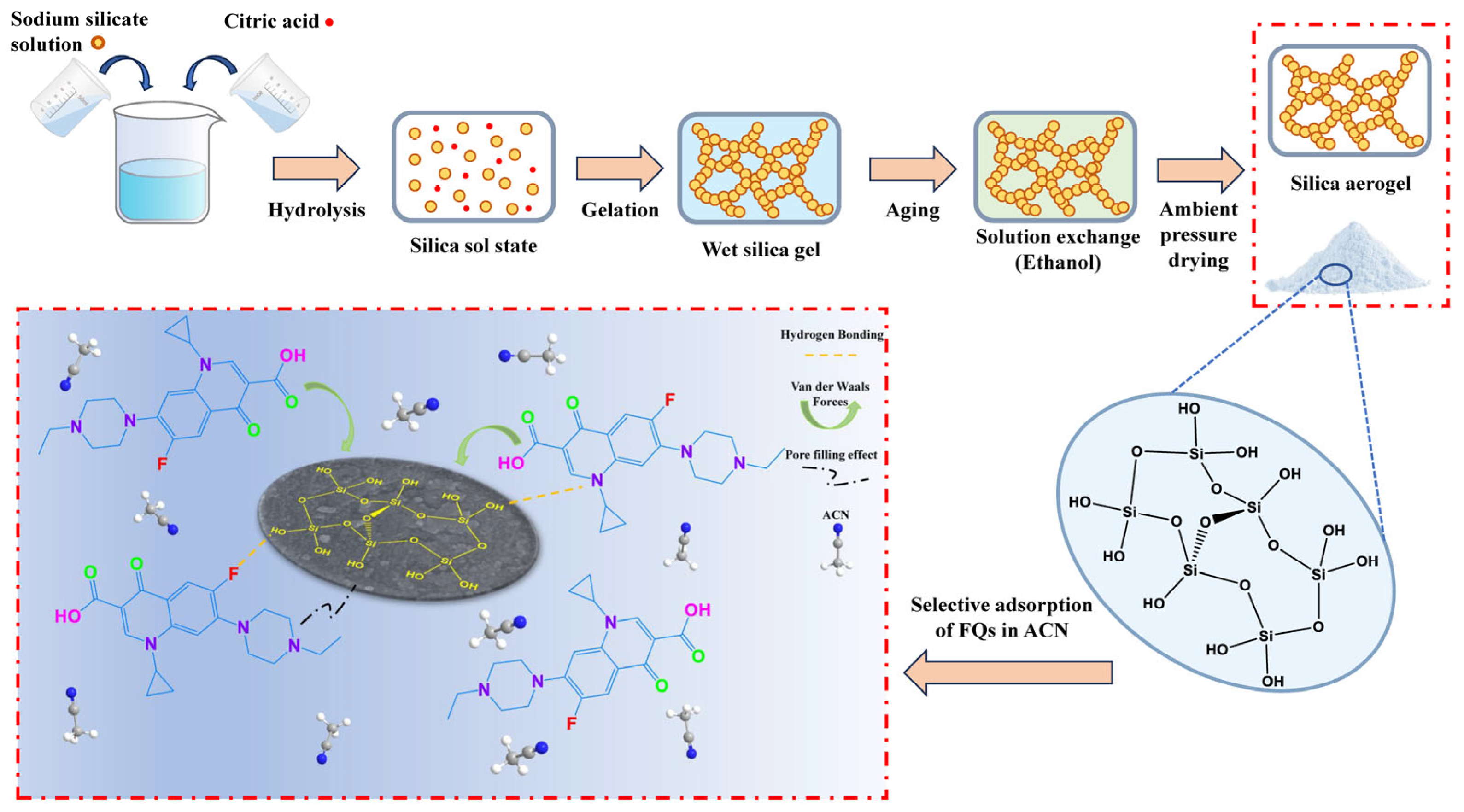
2.2. Preparation of SAs and SA Composites
2.3. Characterization
2.4. Adsorption Experiments
2.5. Application in OWLs Treatment
3. Results and Discussion
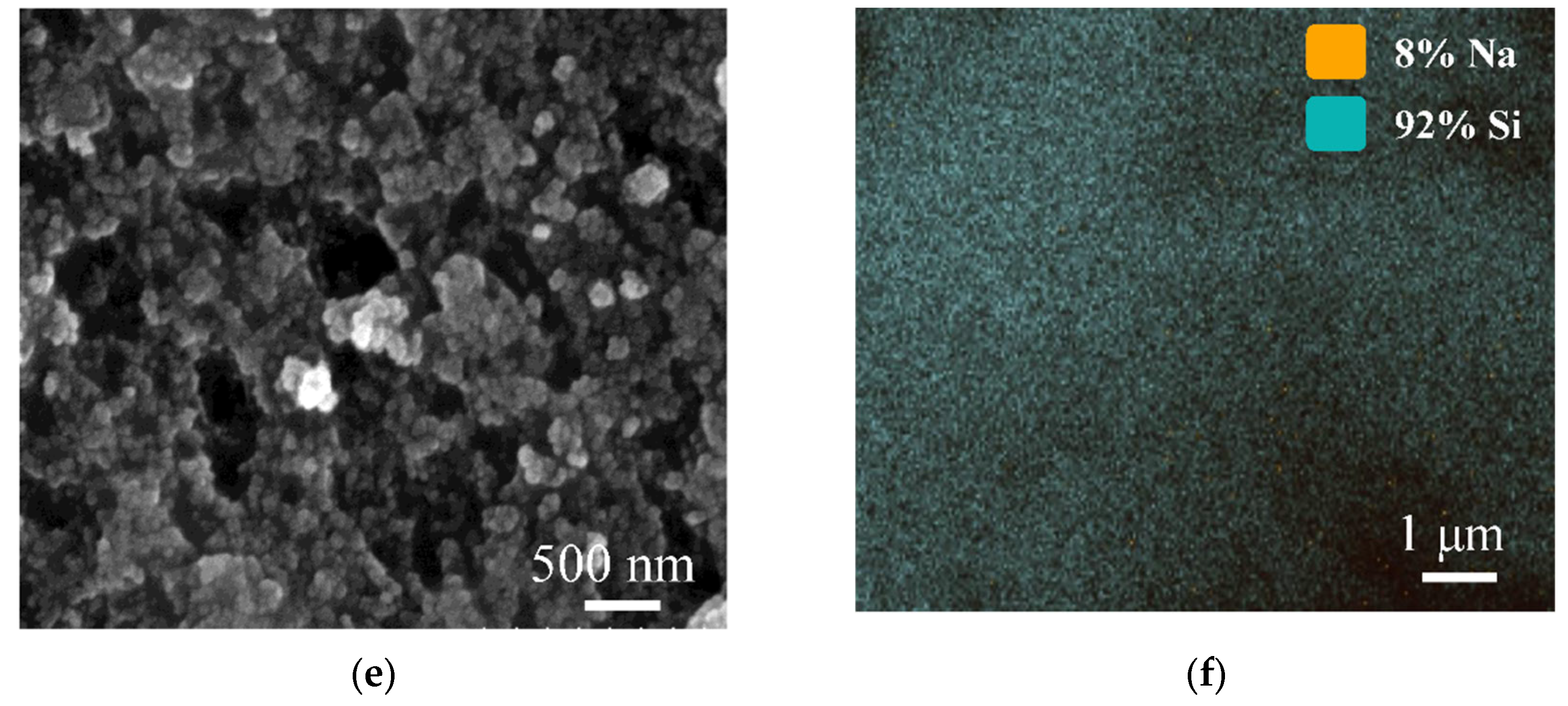
3.1. Characterization of SA-60
3.2. Adsorption Performance Under Different Conditions
3.2.1. Effect of Drying Temperature and Metal Doping
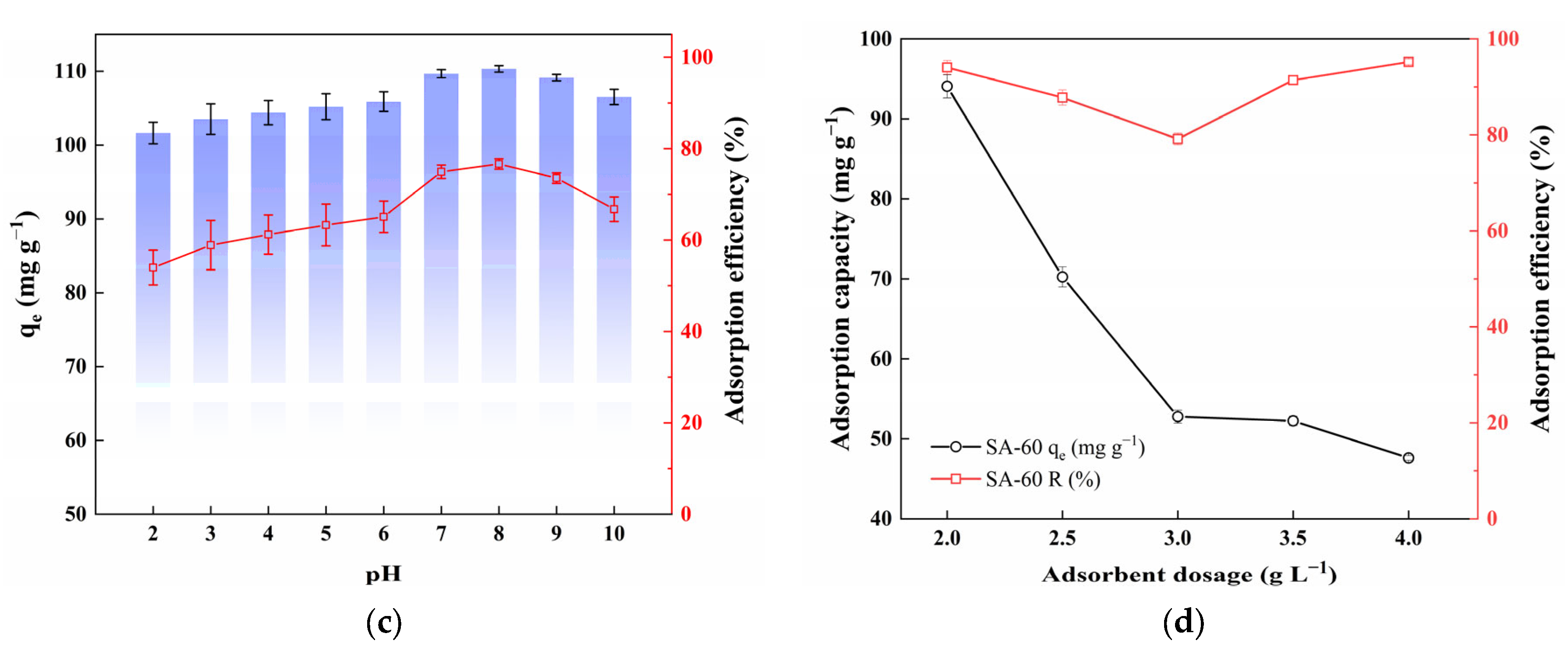
3.2.2. Effect of pH
3.2.3. Effect of Adsorbent Dosage
3.2.4. Selective Adsorption in Mixed Systems
3.2.5. Reusability
3.3. Adsorption Isotherms and Thermodynamics
3.3.1. Adsorption Isotherms
3.3.2. Adsorption Kinetics
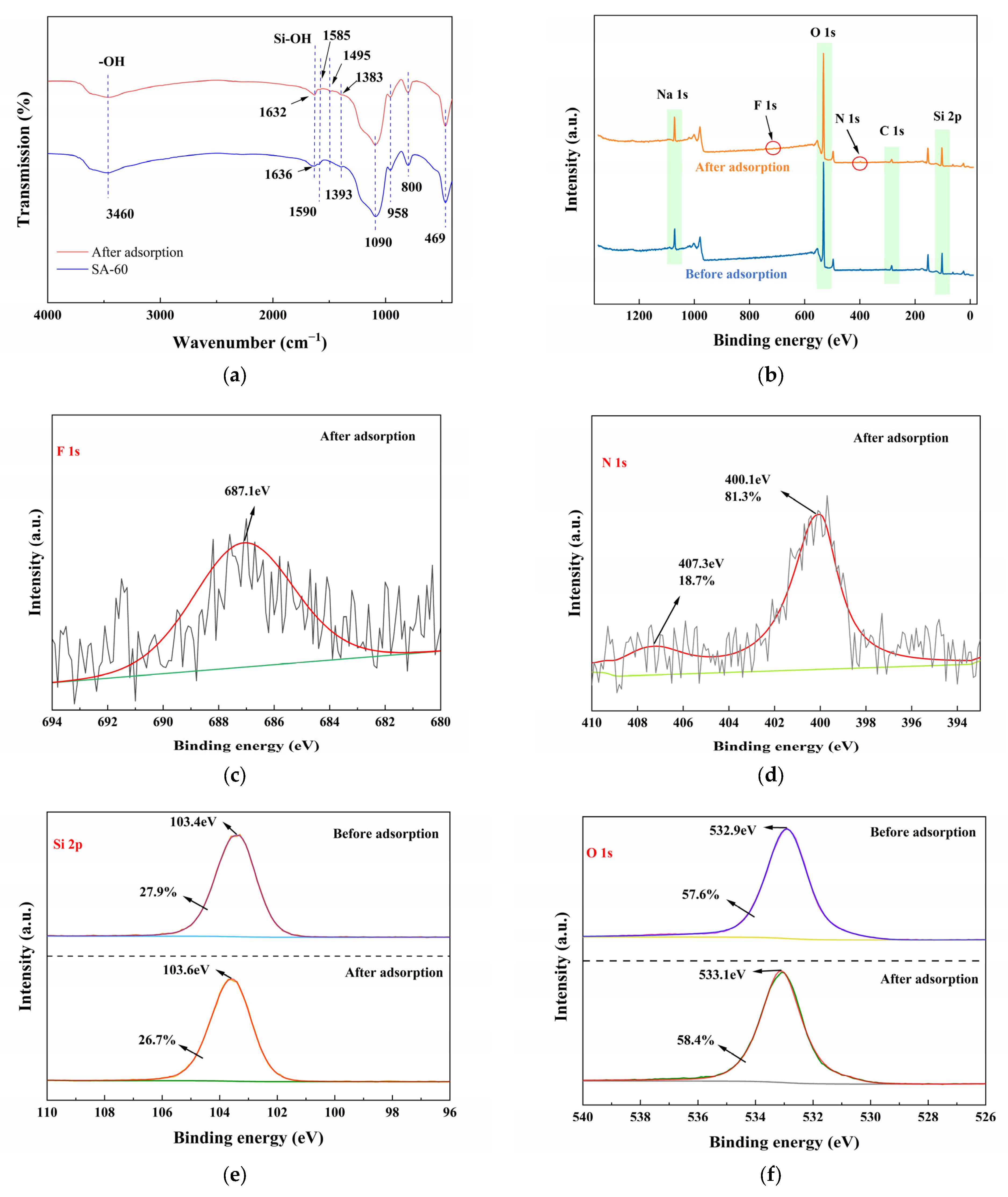
3.3.3. Mechanistic Insights from Spectroscopy
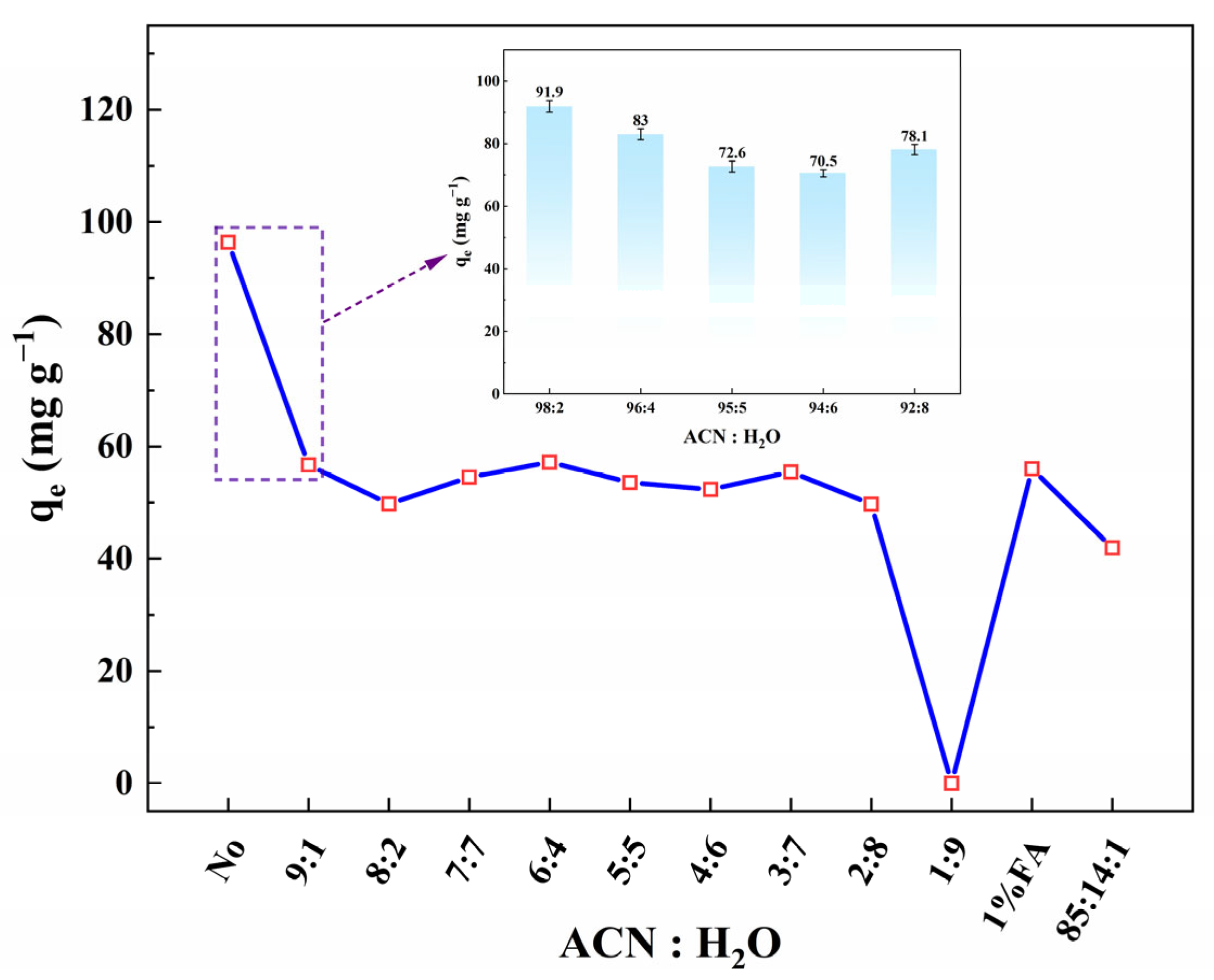
3.3.4. Application in Treating ACN-Water Waste
3.4. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FQs | Fluoroquinolone antibiotics |
| OWLs | Organic waste liquids |
| SAs | Silica aerogels |
| APD | Ambient pressure drying |
| FeCl2 | Ferrous chloride |
| MgSO4 | Magnesium sulfate |
| ZnCl2 | Zinc chloride |
| CuCl2 | Copper (II) chloride |
| NH3·H2O | Ammonia solution |
| HCl | Hydrochloric acid |
| FA | Formic acid |
| HAc | Acetic acid |
| ACN | Acetonitrile |
| MeOH | Methanol |
| EtOH | Absolute ethanol |
| IPA | Isopropanol |
| EA | Ethyl acetate |
| DMSO | Dimethyl sulfoxide |
| ENR | Enrofloxacin |
| CBF | Carbofuran |
| NASONEX | Mometasone furoate |
| SD | Sulfadiazine |
| LCM | Lincomycin |
| NZP | Nitrazepam |
| SEM | Scanning electron microscopy |
| BET | Brunauer–Emmett–Teller |
| XRD | X-ray diffraction |
| FT-IR | Fourier transform infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| TGA | Thermogravimetric analysis |
| HPLC-HRMS | High-performance liquid chromatography coupled with high-resolution mass spectrometry |
| EDS | Energy-dispersive X-ray spectroscopy |
Appendix A
Appendix A.1. Characterization
Appendix A.2. Adsorption Experiments
Appendix A.3. Adsorption Isotherms
Appendix A.4. Adsorption Thermodynamics
Appendix A.5. Adsorption Kinetics
Appendix A.6. Selectivity and Repeatability Experiment
References
- Suyamud, B.; Chen, Y.; Quyen, D.T.T.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial resistance in aquaculture: Occurrence and strategies in Southeast Asia. Sci. Total Environ. 2024, 907, 167942. [Google Scholar] [CrossRef]
- Cuprys, A.; Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Fluoroquinolones metal complexation and its environmental impacts. Coord. Chem. Rev. 2018, 376, 46–61. [Google Scholar] [CrossRef]
- Feng, M.; Wang, Z.; Dionysiou, D.D.; Sharma, V.K. Metal-mediated oxidation of fluoroquinolone antibiotics in water: A review on kinetics, transformation products, and toxicity assessment. J. Hazard. Mater. 2018, 344, 1136–1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, P.; Li, J.; Huang, Y.; Zhao, Y.; Jiang, L.; Fang, X.; Yang, T.; Huang, Z.; Huang, C. Separation-and-Recovery Technology for Organic Waste Liquid with a High Concentration of Inorganic Particles. Engineering 2018, 4, 406–415. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Oladipo, A.O.; Idris, A.O.; Usisipho, F.; Azizi, S.; Maaza, M.; Lebelo, S.L.; Mamba, B.B. Advancements in electrochemical technologies for the removal of fluoroquinolone antibiotics in wastewater: A review. Sci. Total Environ. 2023, 881, 163522. [Google Scholar] [CrossRef]
- Chao, S.-J.; Chung, K.-H.; Lai, Y.-F.; Lai, Y.-K.; Chang, S.-H. Keratin particles generated from rapid hydrolysis of waste feathers with green DES/KOH: Efficient adsorption of fluoroquinolone antibiotic and its reuse. Int. J. Biol. Macromol. 2021, 173, 211–218. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Zou, Y.; Tian, M.; Wang, L.; Li, L.; Wang, M.; Tao, Y.; Wang, J.; Wen, Z.; et al. Covalent assembly synthesis of covalent organic framework and MXene based composite for the adsorption of fluoroquinolones. J. Environ. Chem. Eng. 2023, 11, 110975. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Y.; Chen, Y.; Shen, J.; Wei, Y.; Wang, C. Fluorinated/sulfonated dual-functional three-component covalent organic polymer for adsorption of fluoroquinolones: Preparation, adsorption performance and mechanism. Sep. Purif. Technol. 2025, 354, 128763. [Google Scholar] [CrossRef]
- Soni, H.; Kanjariya, P.; Ballal, S.; Panigrahi, R.; Ariffin, I.A.; Tantawi, D.; Abosaoda, M.K.; Nathiya, D.; Jayabalan, K.; Chauhan, A.S. Recent advances in the synthesis of magnetic nanocomposites for the adsorption of heavy metal ions from wastewater. J. Mol. Struct. 2025, 1345, 143019. [Google Scholar] [CrossRef]
- Thai, V.-A.; Dang, V.D.; Thuy, N.T.; Pandit, B.; Vo, T.-K.-Q.; Khedulkar, A.P. Fluoroquinolones: Fate, effects on the environment and selected removal methods. J. Clean. Prod. 2023, 418, 137762. [Google Scholar] [CrossRef]
- Shah, S.N.; Mo, K.H.; Yap, S.P.; Radwan, M.K.H. Towards an energy efficient cement composite incorporating silica aerogel: A state of the art review. J. Build. Eng. 2021, 44, 103227. [Google Scholar] [CrossRef]
- Çok, S.S.; Koç, F.; Gïzlï, N. Lightweight and highly hydrophobic silica aerogels dried in ambient pressure for an efficient oil/organic solvent adsorption. J. Hazard. Mater. 2021, 408, 124858. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-D.; Cheng, X.; Zheng, Y.-M. Facile co-precursor sol-gel synthesis of a novel amine-modified silica aerogel for high efficiency carbon dioxide capture. J. Colloid Interface Sci. 2018, 530, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Matias, P.M.C.; Paixão, J.A.; Murtinho, D.; Valente, A.J.M.; Durães, L. Chitosan–Silica Composite Aerogel for the Adsorption of Cupric Ions. Gels 2024, 10, 192. [Google Scholar] [CrossRef]
- Choi, H.; Han, H.H.; Parale, V.G.; Kim, T.; Park, W.; Kim, Y.; Kim, J.; Choi, Y.; Bae, Y.-S.; Park, H.-H. Rigid amine-incorporated silica aerogel for highly efficient CO2 capture and heavy metal removal. Chem. Eng. J. 2024, 483, 149357. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Parale, V.G.; Choi, H.; Kim, T.; Lee, K.-Y.; Phadtare, V.D.; Park, H.-H. Epoxy-thiol crosslinking for enhanced mechanical strength in silica aerogels and highly efficient dye adsorption. Appl. Surf. Sci. 2024, 642, 158619. [Google Scholar] [CrossRef]
- Sert Çok, S.; Koç, F.; Len, A.; Gizli, N.; Dudás, Z. The role of surface and structural properties on the adsorptive behavior of vinyl-methyl decorated silica aerogel-like hybrids for oil/organic solvent clean-up practices. Sep. Purif. Technol. 2024, 334, 125958. [Google Scholar] [CrossRef]
- Li, L.; Xu, T.; Zhang, F.; Du, C.; He, S. Preparation of Super-Flexible Silica Aerogel and Its Application in Oil–Water Separation. Gels 2023, 9, 739. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, M.; Chen, Z.; Lu, L.; Ma, Z.; Ding, Y.; Yin, L.; Liu, T.; Li, M.; Yang, L.; et al. A layered aerogel composite with silica fibers, SiC nanowires, and silica aerogels ternary networks for thermal insulation at high-temperature. J. Mater. Sci. Technol. 2025, 204, 71–80. [Google Scholar] [CrossRef]
- Chen, Y.X.; Hendrix, Y.; Schollbach, K.; Brouwers, H.J.H. A silica aerogel synthesized from olivine and its application as a photocatalytic support. Constr. Build. Mater. 2020, 248, 118709. [Google Scholar] [CrossRef]
- Jabbari-Gargari, A.; Moghaddas, J.; Hamishehkar, H.; Jafarizadeh-Malmiri, H. Carboxylic acid decorated silica aerogel nanostructure as drug delivery carrier. Microporous Mesoporous Mater. 2021, 323, 111220. [Google Scholar] [CrossRef]
- Gupta, S.; Prajapati, A.; Kumar, A.; Acharya, S. Synthesis of silica aerogel and its application for removal of crystal violet dye by adsorption. Watershed Ecol. Environ. 2023, 5, 241–254. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Kataoka, T.; Orita, Y.; Shimoyama, Y. Photo-thermal CO2 desorption from amine-modified silica/carbon aerogel for direct air capture. Chem. Eng. J. 2024, 482, 148710. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, J.; Liu, W.; Yan, W.; Zhu, K.; Kong, Y.; Jiang, X.; Shen, X. Facile Synthesis of Polymer-Reinforced Silica Aerogel Microspheres as Robust, Hydrophobic and Recyclable Sorbents for Oil Removal from Water. Polymers 2023, 15, 3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, F.; Bao, Y.; Zhan, S. A Mini Review on the Synthesis of Mesoporous Silica and its Application in Antibiotic Removal. Adv. Sustain. Syst. 2025, 9, 2400634. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Ahankari, S.S. Nanocellulose-based aerogels for water purification: A review. Carbohydr. Polym. 2023, 309, 120677. [Google Scholar] [CrossRef]
- Fan, Y.; Zeng, G.; Ma, X. Multi-templates surface molecularly imprinted polymer for rapid separation and analysis of quinolones in water. Environ. Sci. Pollut. Res. 2019, 27, 7177–7187. [Google Scholar] [CrossRef]
- Xu, L.; Chen, P.; Zhang, X.; Lan, D.L.; Liu, Y.; Lai, W.; Shehzad, H.; Zhou, L.; Ouyang, J. Synthesis of Zr-based metal-organic framework/MWCNTs composite for adsorption of enrofloxacin from aqueous solution. Sep. Purif. Technol. 2024, 334, 126004. [Google Scholar] [CrossRef]
- Çok, S.S.; Koç, F.; Len, A.; Kriechbaum, M.; Dudás, Z. Antibiotic adsorption from aqueous media by using silica aerogels derived from amine-bridged silsesquioxane networks. J. Water Process Eng. 2024, 68, 106538. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, K.; Ma, D.; Lin, H.; Liu, Z.; Qin, S.; Luo, Y. Synthesis of high specific surface area silica aerogel from rice husk ash via ambient pressure drying. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 399–406. [Google Scholar] [CrossRef]
- Gong, D.; Qu, M.; Wang, X.; Ai, X.; Tang, P.; Zhao, W.; Wang, X.; Bin, Y. Preparation of Epoxy-Enhanced Silica Aerogels with Thermal Insulation and Hydrophobicity by Ambient Pressure Drying. ACS Appl. Polym. Mater. 2025, 7, 2997–3007. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.; Li, L.; Jiang, X.; Wang, P.; Fang, K.; Li, J.; Hao, D.; Zhu, H.; Wang, Q.; et al. Synergistic mechanisms for efficient and safe antibiotic removal: Effective adsorption and photocatalytic degradation using aerogels. Sep. Purif. Technol. 2025, 354, 129455. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. Synthesis of sustainable silica xerogels/aerogels using inexpensive steel slag and bean pod ash: A comparison study. Adv. Powder Technol. 2020, 31, 926–936. [Google Scholar] [CrossRef]
- Xie, J.; Sun, W.; Yan, Z.; Ji, X.; Hu, H.; Wei, W. Selective adsorption of organic dyes by porous hydrophilic silica aerogels from aqueous system. Water Sci. Technol. 2018, 78, 402–414. [Google Scholar] [CrossRef]
- Stanec, A.M.; Anderson, A.M.; Avanessian, C.; Carroll, M.K. Analysis and characterization of etched silica aerogels. J. Sol-Gel Sci. Technol. 2020, 94, 406–415. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Wang, X.; Xu, K.; Li, Y.; Guo, S. Dissolution and leaching mechanisms of calcium ions in cement based materials. Constr. Build. Mater. 2018, 180, 103–108. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, M.; Zhang, Y.; Liu, F.; Xu, Z. Rapid adsorption of directional cellulose nanofibers/3-glycidoxypropyltrimethoxysilane/polyethyleneimine aerogels on microplastics in water. Int. J. Biol. Macromol. 2023, 235, 123884. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Esmorís, C.; Rodríguez-López, L.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Influence of pH on the adsorption-desorption of doxycycline, enrofloxacin, and sulfamethoxypyridazine in soils with variable surface charge. Environ. Res. 2022, 214, 114071. [Google Scholar] [CrossRef]
- Li, D.; Zuo, S.; Liu, Z.; Xiong, K.; Ren, L.; Zhang, X. Adsorption of tetracycline on copper alginate-graphene oxide aerogels prepared by secondary lyophilization under CuSO4 crosslinking. J. Mol. Struct. 2025, 1338, 142289. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; He, J.; Wu, Y.; Sun, J.; Wen, X. Waste cotton-based activated carbon with excellent adsorption performance towards dyes and antibiotics. Chemosphere 2025, 376, 144292. [Google Scholar] [CrossRef]
- Cui, C.; Yang, M.; Zhai, J.; Bai, W.; Dai, L.; Liu, L.; Jiang, S.; Wang, W.; Ren, E.; Cheng, C.; et al. Bamboo cellulose–derived activated carbon aerogel with controllable mesoporous structure as an effective adsorbent for tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2023, 30, 12558–12570. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Phuong, V.N.; Van, T.N.; Thi, P.N.; Dinh Thi Lan, P.; Pham, H.T.; Cao, H.T. Low-cost hydrogel derived from agro-waste for veterinary antibiotic removal: Optimization, kinetics, and toxicity evaluation. Environ. Technol. Innov. 2020, 20, 101098. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Lv, S.; Wei, D.; Liu, L. Three-dimensional graded porous structure and compressible KGM-GO/CS/SA composite aerogel spheres for efficient adsorption of antibiotics. Sep. Purif. Technol. 2024, 346, 127547. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-T.; Nguyen, T.-B.; Chen, W.-H.; Chen, C.-W.; Kumar Patel, A.; Bui, X.-T.; Chen, L.; Singhania, R.R.; Dong, C.-D. Phosphoric acid-activated biochar derived from sunflower seed husk: Selective antibiotic adsorption behavior and mechanism. Bioresour. Technol. 2023, 371, 128593. [Google Scholar] [CrossRef]
- Ai, S.; Xu, Y.; Zhou, H.; Cui, Z.; Wu, T.; Tian, D. Superelastic and ultralight covalent organic framework composite aerogels modified with different functional groups for ultrafast adsorbing organic pollutants in water. Chin. Chem. Lett. 2024, 36, 110761. [Google Scholar] [CrossRef]
- Gordi, Z.; Ghorbani, M.; Ahmadian Khakhiyani, M. Adsorptive removal of enrofloxacin with magnetic functionalized graphene oxide@ metal–organic frameworks employing D-optimal mixture design. Water Environ. Res. 2020, 92, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J.; Fang, Y.; Cao, C.; Liu, Z.; Li, L.; Huang, Y.; Lin, J.; Tang, C. Selective adsorption behavior/mechanism of antibiotic contaminants on novel boron nitride bundles. J. Hazard. Mater. 2019, 364, 654–662. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Ramkumar, K.; Meenakshi, S. Effective removal of organic pollutants by adsorption onto chitosan supported graphene oxide-hydroxyapatite composite: A novel reusable adsorbent. J. Mol. Liq. 2020, 318, 114200. [Google Scholar] [CrossRef]
- Babas, H.; Khachani, M.; Warad, I.; Ajebli, S.; Guessous, A.; Guenbour, A.; Safi, Z.; Berisha, A.; Bellaouchou, A.; Abdelkader, Z.; et al. Sofosbuvir adsorption onto activated carbon derived from argan shell residue: Optimization, kinetic, thermodynamic and theoretical approaches. J. Mol. Liq. 2022, 356, 119019. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Zhu, L.; Tang, J.; Liu, Z.; Dai, Y. Adsorption interactions between typical microplastics and enrofloxacin: Relevant contributions to the mechanism. Chemosphere 2024, 351, 141181. [Google Scholar] [CrossRef] [PubMed]
- Ransing, A.A.; Wang, Q.; Dhavale, R.P.; Choi, H.; Bangi, U.K.H.; Parale, V.G.; Lee, W.; Kanamori, K.; Park, H.-H. Ionotropically-engineered synthesis of mechanically robust hybrid sodium alginate–silica aerogels with enhanced specific surface area for high-capacity and selective dye adsorption from wastewater. Chem. Eng. J. 2025, 507, 160590. [Google Scholar] [CrossRef]
- Li, N.; Tao, K.; Xia, W.; Yu, C.; Yang, H. A novel cellulose/lignin/montmorillonite ternary hybrid aerogel for efficiently adsorptive removal of antibiotics from water. Chem. Eng. J. 2023, 466, 143265. [Google Scholar] [CrossRef]
- Ma, M.; Ye, Z.; Zhang, J.; Wang, Y.; Ning, S.; Yin, X.; Fujita, T.; Chen, Y.; Wu, H.; Wang, X. Synthesis and fabrication of segregative and durable MnO2@chitosan composite aerogel beads for uranium(VI) removal from wastewater. Water Res. 2023, 247, 120819. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, T.; Yang, L.; Wu, L.; Li, P.; Tang, J.; Chen, Y.; Gao, F.; Cui, S.; Qi, X.; et al. Efficient adsorptive removal of fluoroquinolone antibiotics from water by alkali and bimetallic salts co-hydrothermally modified sludge biochar. Environ. Pollut. 2022, 298, 118833. [Google Scholar] [CrossRef]
- Huang, T.; Shao, Y.-W.; Zhang, Q.; Deng, Y.-F.; Liang, Z.-X.; Guo, F.-Z.; Li, P.-C.; Wang, Y. Chitosan-Cross-Linked Graphene Oxide/Carboxymethyl Cellulose Aerogel Globules with High Structure Stability in Liquid and Extremely High Adsorption Ability. ACS Sustain. Chem. Eng. 2019, 7, 8775–8788. [Google Scholar] [CrossRef]
- Pu, J.-C.; Doka Dari, M.; Tang, X.-Q.; Yuan, P.-Q. Diffusion of benzene through water film confined in silica mesopores: Effect of competitive adsorption of solvent. Chem. Eng. Sci. 2020, 224, 115793. [Google Scholar] [CrossRef]
- Shchur, Y.; Pavlyuk, O.; Andrushchak, A.S.; Vitusevich, S.; Kityk, A.V. Porous Si Partially Filled with Water Molecules—Crystal Structure, Energy Bands and Optical Properties from First Principles. Nanomaterials 2020, 10, 396. [Google Scholar] [CrossRef]
- Mehraban Khaledi, S.; Taherimehr, M.; Hassaninejad-Darzi, S.K. Porous Fe-Porphyrin as an Efficient Adsorbent for the Removal of Ciprofloxacin from Water. ACS Omega 2024, 9, 15950–15958. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, W.; Chu, M.; Zou, M.; Zhao, J. Molecular imprinting functionalization of magnetic biochar to adsorb sulfamethoxazole: Mechanism, regeneration and targeted adsorption. Process Saf. Environ. Prot. 2023, 171, 238–249. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Li, D.; Zhang, W.; Zhao, K.; Tang, Y. Elastic BN aerogel for efficient adsorption of tetracycline. Diamond Relat. Mater. 2024, 149, 111620. [Google Scholar] [CrossRef]
- Wei, F.; Gong, J.; Ren, Q.; Yu, X.; Wang, Y.; Chen, H.; Liang, Z. Preparation of Zn/Zr-MOFs by microwave-assisted ball milling and adsorption of lomefloxacin hydrochloride and levofloxacin hydrochloride in wastewater. Environ. Res. 2024, 252, 118941. [Google Scholar] [CrossRef]
- Ma, H.; Yu, B.; Wang, Q.; Owens, G.; Chen, Z. Enhanced removal of pefloxacin from aqueous solution by adsorption and Fenton-like oxidation using NH2-MIL-88B. J. Colloid Interface Sci. 2021, 583, 279–287. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, W.; Yang, Z.; Cao, J.; Jia, M.; Xiang, Y.; Hu, Q.; Xu, Z. Facile fabrication of three-dimensional hierarchical porous ZIF-L/gelatin aerogel: Highly efficient adsorbent with excellent recyclability towards antibiotics. Chem. Eng. J. 2021, 426, 130798. [Google Scholar] [CrossRef]
- Ma, P.; Yao, S.; Wang, Z.; Qi, F.; Liu, X. Preparation of nitrogen-doped hierarchical porous carbon aerogels from agricultural wastes for efficient pollution adsorption. Sep. Purif. Technol. 2023, 311, 123250. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Peng, C.; Deng, Q.; Yu, Y.; He, X.; Hu, T.; Jiang, L.; Shan, S.; Zheng, Y.; et al. Microwave-assisted synthesis of l-aspartic acid-based metal organic aerogel (MOA) for efficient removal of oxytetracycline from aqueous solution. Appl. Surf. Sci. 2023, 610, 155608. [Google Scholar] [CrossRef]
- Samanta, A.; Ghosh, S.; Das, P. Extraction of silica and biochar from biomass waste for the synthesis of aerogel and its application for the removal of dye. Biomass Convers. Biorefin. 2025, 1–19. [Google Scholar] [CrossRef]
- Zeng, Y.; Tang, X.; Qin, Y.; Maimaiti, A.; Zhou, X.; Guo, Y.; Liu, X.; Zhang, W.; Gao, J.; Zhang, L. Enhanced removal of methylene blue from wastewater by alginate/carboxymethyl cellulose-melamine sponge composite. Int. J. Biol. Macromol. 2023, 244, 125280. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, Z.; Zhang, X.; Yang, P.; Ma, J. Lanthanum modification κ-carrageenan/sodium alginate dual-network aerogels for efficient adsorption of ciprofloxacin hydrochloride. Environ. Technol. Innov. 2021, 24, 102052. [Google Scholar] [CrossRef]
- Geng, R.; Wang, J.; Zhang, Z.; Dong, Q.; Wu, F.; Chen, S.; Su, T.; Qi, X. Adsorption of antibiotics by polydopamine-modified salecan hydrogel: Performance, kinetics and mechanism studies. Chem. Eng. J. 2023, 454, 140446. [Google Scholar] [CrossRef]
- Brião, G.d.V.; da Silva, M.G.C.; Vieira, M.G.A. Efficient and Selective Adsorption of Neodymium on Expanded Vermiculite. Ind. Eng. Chem. Res. 2021, 60, 4962–4974. [Google Scholar] [CrossRef]



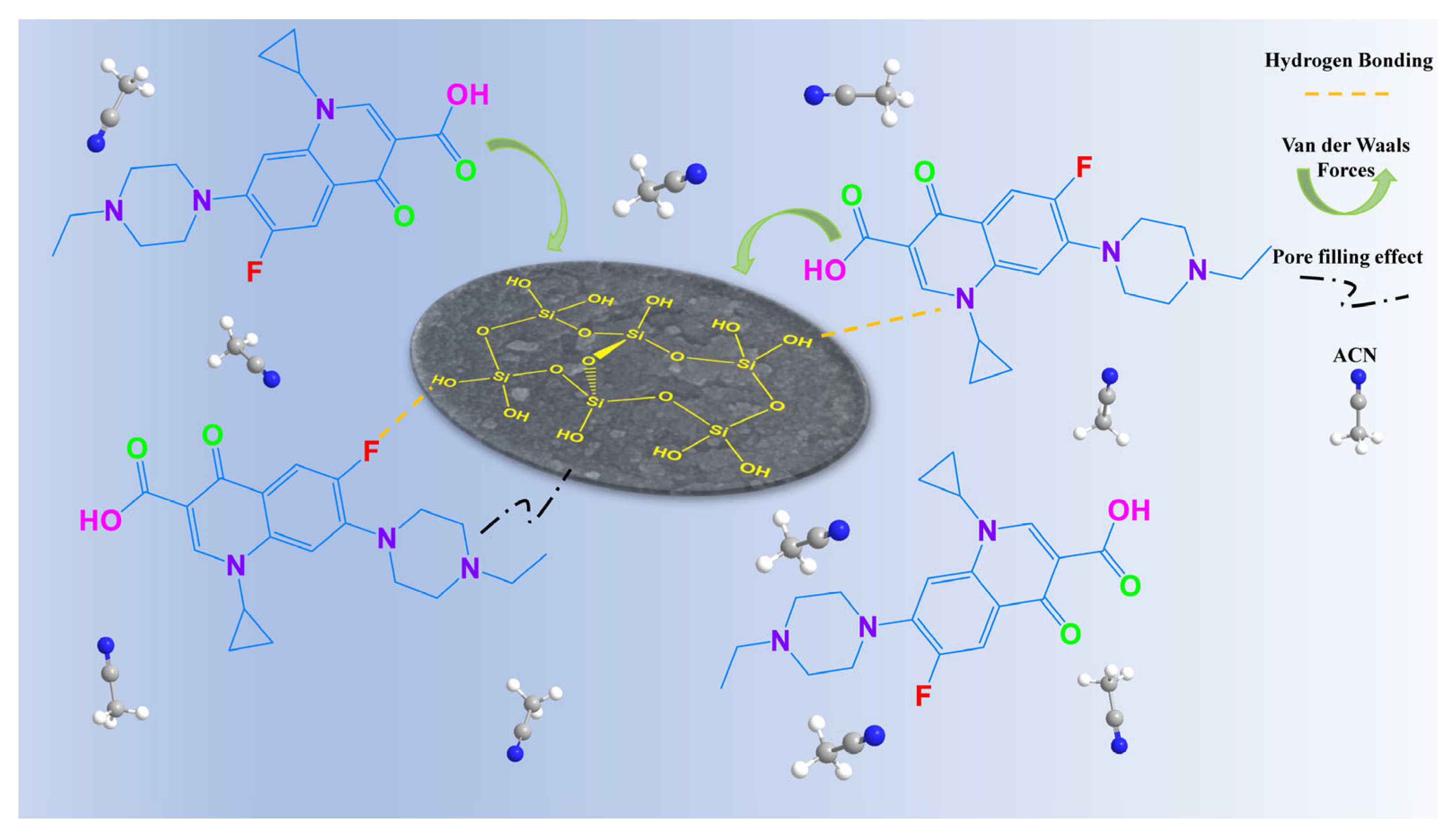
| Adsorbent Specification | Type of Antibiotic | Maximum Capacity (mg g−1) | Reference |
|---|---|---|---|
| Copper alginate-graphene oxide aerogels | Tetracycline | 238.15 | [43] |
| Novel amine-substituted silica aerogels | Ciprofloxacin | 101.00 | [31] |
| Waste cotton-based activated carbon | Ciprofloxacin hydrochloride | 824.00 | [44] |
| Bamboo-derived activated carbon aerogel | Tetracycline hydrochloride | 863.80 | [45] |
| Biochar and chitosan hydrogel | Enrofloxacin | 100.43 | [46] |
| KGM-GO/CS/SA composite aerogel spheres | Ofloxacin | 427.63 | [47] |
| Phosphoric acid-activated biochar derived from sunflower seed husk | Sulfamethoxazole | 251.30 | [48] |
| Covalent organic framework composite aerogels | Ciprofloxacin | 405.70 | [49] |
| Magnetic functionalized graphene oxide MOFs | Enrofloxacin | 344.83 | [50] |
| SA-60 | Enrofloxacin | 630.18 | This work |
| Target Compound Category | (µg · g−1) | MW | ||
|---|---|---|---|---|
| Fluoroquinolone class * | 30.60 | 429.78 | - | 319.33–399.39 |
| Lincomycin | 3.88 | 218.62 | 7.88 | 406.54 |
| Nitrazepam | 0.82 | 70.73 | 37.14 | 281.27 |
| Carbofuran | 0.59 | 52.57 | 52.09 | 221.25 |
| Sulfadiazine | 0.36 | 33.82 | 84.34 | 250.28 |
| Mometasone furoate | 0.29 | 27.51 | 105.11 | 521.43 |
| Adsorbents | Solvents | Type | The Dielectric Constant | Adsorption Rate |
|---|---|---|---|---|
| Drying SA-60 | H2O | protic solvent | 78.38 | 0.00% |
| ACN | dipolar aprotic solvent | 37.50 | 93.66% | |
| MeOH | protic solvent | 32.66 | 69.58% | |
| EtOH | protic solvent | 24.30 | 69.03% | |
| IPA | protic solvent | 18.30 | 70.40% | |
| EA | aprotic solvent | 6.02 | 88.91% | |
| 90% ACN-water | mixed solvent | N.A. | 56.72% | |
| After wetting, drying SA-60 | ACN | dipolar aprotic solvent | 37.50 | 86.21% |
| Wetting SA-60 | ACN | dipolar aprotic solvent | 37.50 | 68.57% |
| 90% ACN-water | mixed solvent | N.A. | 26.84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Gu, L.; Liu, Z.; Zhang, J.; Xia, W.; Wang, P.; Zhai, W.; Yang, G.; Shen, X.; Fan, C.; et al. Selective Adsorption Performance of a High-Capacity Mesoporous Silica Aerogel for Fluoroquinolones. Environments 2025, 12, 300. https://doi.org/10.3390/environments12090300
Zhao Y, Gu L, Liu Z, Zhang J, Xia W, Wang P, Zhai W, Yang G, Shen X, Fan C, et al. Selective Adsorption Performance of a High-Capacity Mesoporous Silica Aerogel for Fluoroquinolones. Environments. 2025; 12(9):300. https://doi.org/10.3390/environments12090300
Chicago/Turabian StyleZhao, Yifan, Lin Gu, Zhihan Liu, Junyu Zhang, Wei Xia, Peng Wang, Wenlei Zhai, Guangxin Yang, Xiaosheng Shen, Chengqi Fan, and et al. 2025. "Selective Adsorption Performance of a High-Capacity Mesoporous Silica Aerogel for Fluoroquinolones" Environments 12, no. 9: 300. https://doi.org/10.3390/environments12090300
APA StyleZhao, Y., Gu, L., Liu, Z., Zhang, J., Xia, W., Wang, P., Zhai, W., Yang, G., Shen, X., Fan, C., & Kong, C. (2025). Selective Adsorption Performance of a High-Capacity Mesoporous Silica Aerogel for Fluoroquinolones. Environments, 12(9), 300. https://doi.org/10.3390/environments12090300








