Backward and Historical PFOA Exposure Estimation in an Adult Population Highly Exposed in the Veneto Region
Abstract
1. Introduction
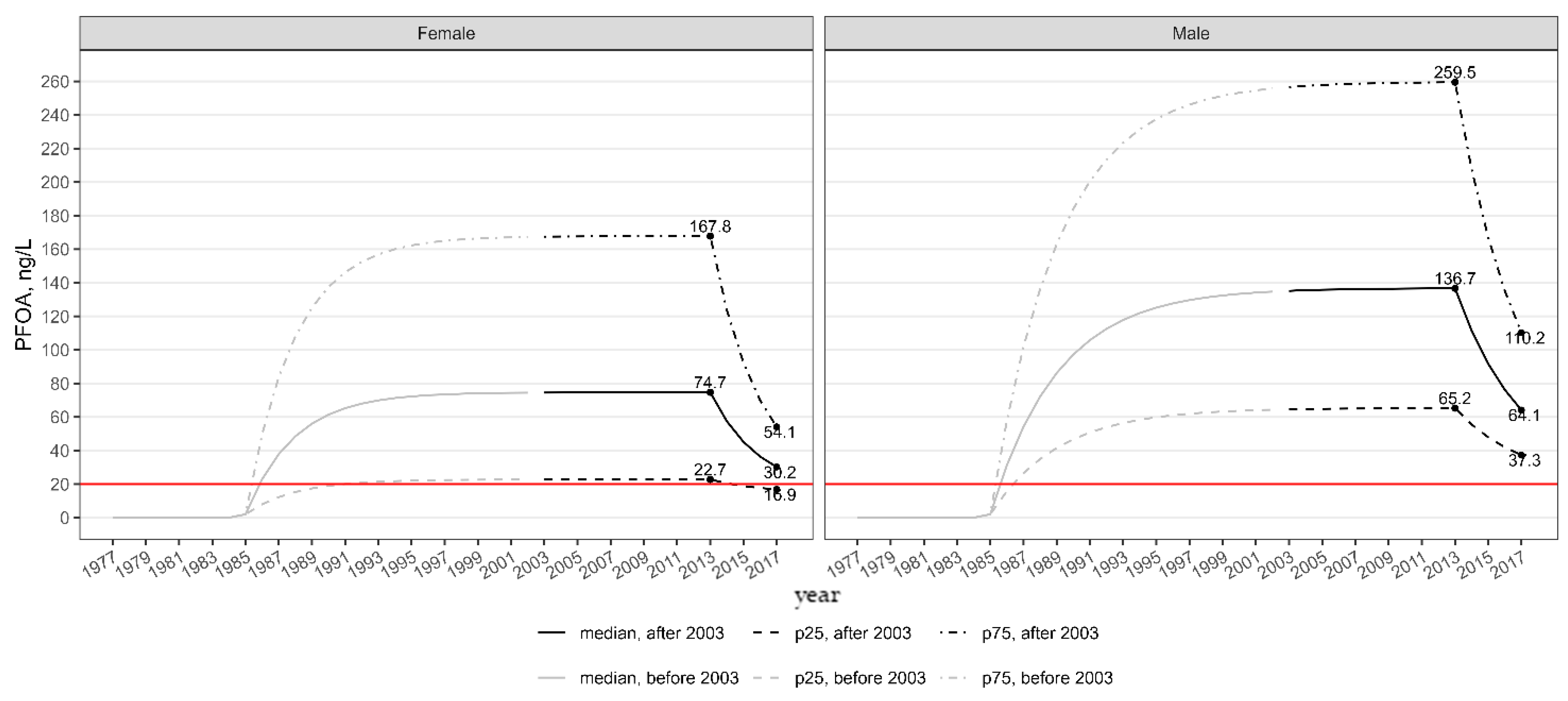
- An estimate of the time of arrival of PFOA contamination at the central drinking water distribution facility (Madonna di Lonigo), which serves the most affected area. This was estimated at 17 years based on the average groundwater flow velocity and a retardation factor of 1.9. The estimated year of arrival at the waterworks is 1986 [9].
- The date when single activated carbon filtration was installed at the same water distribution plant (September 2013) [10].
- An estimate of the PFOA daily intake rate (ng/kg-day) from public drinking water and non-local food after the installation of carbon filtration (September 2013 to December 2017). This was carried out by the Istituto Superiore di Sanità, based on contamination measurements from 1591 drinking water samples collected in the affected area and food intake estimates from northeastern Italy (thus excluding locally sourced foods) [10,11].
- PFOA serum level measurements collected during the Regional Health Surveillance Plan among 18,345 exposed males and females 14 to 39 years of age; enrollment was launched on 1 January 2017, and about four-fifths of the samples were collected before August 2018, with an estimated participation rate of 63.5%. The screening was then completed in May 2023 [12].
- An estimate of PFOA half-life in the 2871 males (2.83 years, 95% CI: 2.78–2.89) and 2989 females (2.04 years, 95% CI: 2.00–2.08) residing in the impacted area and participating in the Regional Health Surveillance Plan [13].
2. Methodology
- Css is the serum concentration of PFOA at steady state (ng/mL), resulting from PFOA daily intake rate via drinking water and food;
- C0 is the initial or baseline serum concentration of PFOA (ng/mL);
- k is the elimination rate constant (year−1), calculated as k = ln(2)/t1/2, where t1/2 is the elimination half-life (in years)
- IR is the daily intake rate (ng/kg-day) of PFOA via drinking water and food consumption;
- Cl is the clearance rate of PFOA (L/kg-day), calculated as Cl = k × Vd, where Vd is the volume of distribution per body weight (0.17 L/kg for PFOA) and k is the elimination half-life;
- CB is the background serum concentration of PFOA resulting from other sources of exposure (e.g., dust ingestion, inhalation and direct contact). CB was estimated by multiplying the serum concentration observed in residents of the Veneto Region never exposed to contaminated water or local food by the percent contribution (Pc) from these alternative sources in the general population [8,15].
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up; The National Academies Press: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- IARC. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). In IARC Monographs on the Identification of Carcinogenic Hazards to Humans; ARC: Lyon, France, 2025; Volume 135, 754p. [Google Scholar]
- Li, H.; Hammarstrand, S.; Midberg, B.; Xu, Y.; Li, Y.; Olsson, D.S.; Fletcher, T.; Jakobsson, K.; Andersson, E.M. Cancer incidence in a Swedish cohort with high exposure to perfluoroalkyl substances in drinking water. Environ. Res. 2022, 204 Pt C, 112217. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Vieira, V.M.; Ryan, P.B.; Detwiler, R.; Sanders, B.; Steenland, K.; Bartell, S.M. Environmental fate and transport modeling for perfluorooctanoic acid emitted from the Washington Works Facility in West Virginia. Environ. Sci. Technol. 2011, 45, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Vieira, V.M.; Ryan, P.B.; Steenland, K.; Bartell, S.M. Retrospective exposure estimation and predicted versus observed serum perfluorooctanoic acid concentrations for participants in the C8 Health Project. Environ. Health Perspect. 2011, 119, 1760–1765, Erratum in Environ. Health Perspect. 2013, 121, A113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, S.; Bartell, S.M. Serum PFAS Calculator for Adults, Version 1.2. 2020. Available online: www.ics.uci.edu/~sbartell/pfascalc.html (accessed on 1 July 2025).
- ATSDR PFAS Blood Level Estimation Tool. 2024. Available online: https://www.atsdr.cdc.gov/pfas/blood-testing/estimation-tool.html (accessed on 1 July 2025).
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Zare Jeddi, M.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 27007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ARPAV–Dipartimento Regionale Per La Sicurezza Del Territorio. Stima Dei Tempi Di Propagazione Dell’inquinamento Da Sostanze Perfluoroalchiliche (PFAS) Nelle Acque Sotterranee In Provincia Di Vicenza, Padova, Verona. 2025. Available online: https://www.arpa.veneto.it/arpav/chi-e-arpav/file-e-allegati/pfas/nt_0516_stima_tempi_propagazione_pfas_nella_acque_sotterranee_v04.pdf/@@display-file/file (accessed on 23 June 2025).
- Istituto Superiore Di Sanità, Dipartimento Di Sicurezza Alimentare, Nutrizione E Sanità Pubblica Veterinaria. Contaminazione Da Sostanze Perfluoroalchiliche In Veneto. Valutazione Dell’esposizione Alimentare E Caratterizzazione Del Rischio. Relazione Finale. 2019. Available online: https://www.sivempveneto.it/wp-content/uploads/2019/07/Relazione-PFAS_ISS-2019_finale.pdf (accessed on 23 June 2025).
- ARPAV Contaminazione Da PFAS: Azioni ARPAV Regione Veneto. Periodi Di Riferimento: Dal 14 Giugno 2013 Al 31 Dicembre 2017. 2018. Available online: https://www.arpa.veneto.it/arpav/pagine-generiche/allegati-pagine-generiche/pfas-relazioni-attivita-arpav/aggiornamento-relazione-pfas--gennaio-2018.pdf/@@display-file/file (accessed on 23 June 2025).
- Regione del Veneto. Piano Di Sorveglianza Sanitaria Sulla Popolazione Esposta A PFAS. Rapporto N. 17–Maggio 2023. Available online: https://www.regione.veneto.it/documents/10793/12935055/Bollettino+PFAS+n.+17+-+maggio+2023.pdf/95cd8c4c-8790-4725-b5b0-f3cd089b51cc (accessed on 1 July 2025).
- Batzella, E.; Rosato, I.; Pitter, G.; Da Re, F.; Russo, F.; Canova, C.; Fletcher, T. Determinants of PFOA Serum Half-Life After End of Exposure: A Longitudinal Study on Highly Exposed Subjects in the Veneto Region. Environ. Health Perspect. 2024, 132, 27002, Erratum in Environ. Health Perspect. 2025, 133, 49001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Marra, V.; Russo, F.; Vazzoler, M.; Testai, E.; et al. Serum concentrations of perfluorinated alkyl substances in farmers living in areas affected by water contamination in the Veneto Region (Northern Italy). Environ. Int. 2020, 136, 105435. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Shin, H.M.; Saugo, M. Impact of local food consumption on exposure to perfluorooctanoic acid and perfluorooctane sulfonate in a contaminated community in North-Eastern Italy. Epidemiol. Prev. 2024, 48, 326–332. (In English) [Google Scholar] [CrossRef] [PubMed]
- Vaccari, L.; Ranzi, A.; Canova, C.; Ghermandi, G.; Giannini, S.; Pitter, G.; Russo, F.; Stefanelli, J.; Teggi, S.; Vantini, A.; et al. Reliability of toxicokinetic modelling for PFAS exposure assessment in contaminated water in northern Italy. Heliyon 2024, 31, e35288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ingelido, A.M.; Marra, V.; Abballe, A.; Valentini, S.; Iacovella, N.; Barbieri, P.; Porpora, M.G.; di Domenico, A.; De Felip, E. Perfluorooctanesulfonate and perfluorooctanoic acid exposures of the Italian general population. Chemosphere 2010, 80, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Girardi, P.; Merler, E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ. Res. 2019, 179 Pt A, 108743. [Google Scholar] [CrossRef] [PubMed]
- TAR-Veneto-Sez.-IV-6-Maggio-2024-N.-896. Rivista Giuridica Online. Available online: https://rgaonline.it/wp-content/uploads/2024/08/TAR-Veneto-Sez.-IV-6-maggio-2024-n.-896.pdf (accessed on 1 July 2025).
- Frisbee, S.J.; Brooks, A.P., Jr.; Maher, A.; Flensborg, P.; Arnold, S.; Fletcher, T.; Steenland, K.; Shankar, A.; Knox, S.S.; Pollard, C.; et al. The C8 health project: Design, methods, and participants. Environ. Health Perspect. 2009, 117, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flesch-Janys, D.; Becher, H.; Gurn, P.; Jung, D.; Konietzko, J.; Manz, A.; Päpke, O. Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. J. Toxicol. Environ. Health 1996, 47, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Jin, C.; MacNeil, J.; Lally, C.; Ducatman, A.; Vieira, V.; Fletcher, T. Predictors of PFOA levels in a community surrounding a chemical plant. Environ. Health Perspect. 2009, 117, 1083–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, R.B.; Ducatman, A. Serum concentrations of selected perfluoroalkyl substances for US females compared to males as they age. Sci. Total Environ. 2022, 10, 156891. [Google Scholar] [CrossRef] [PubMed]
- WHO Europe. Keeping Our Water Clean: The Case of Water Contamination in the Veneto Region, Italy; WHO: Geneva, Switzerland, 2017; ISBN 9789289052467. [Google Scholar]
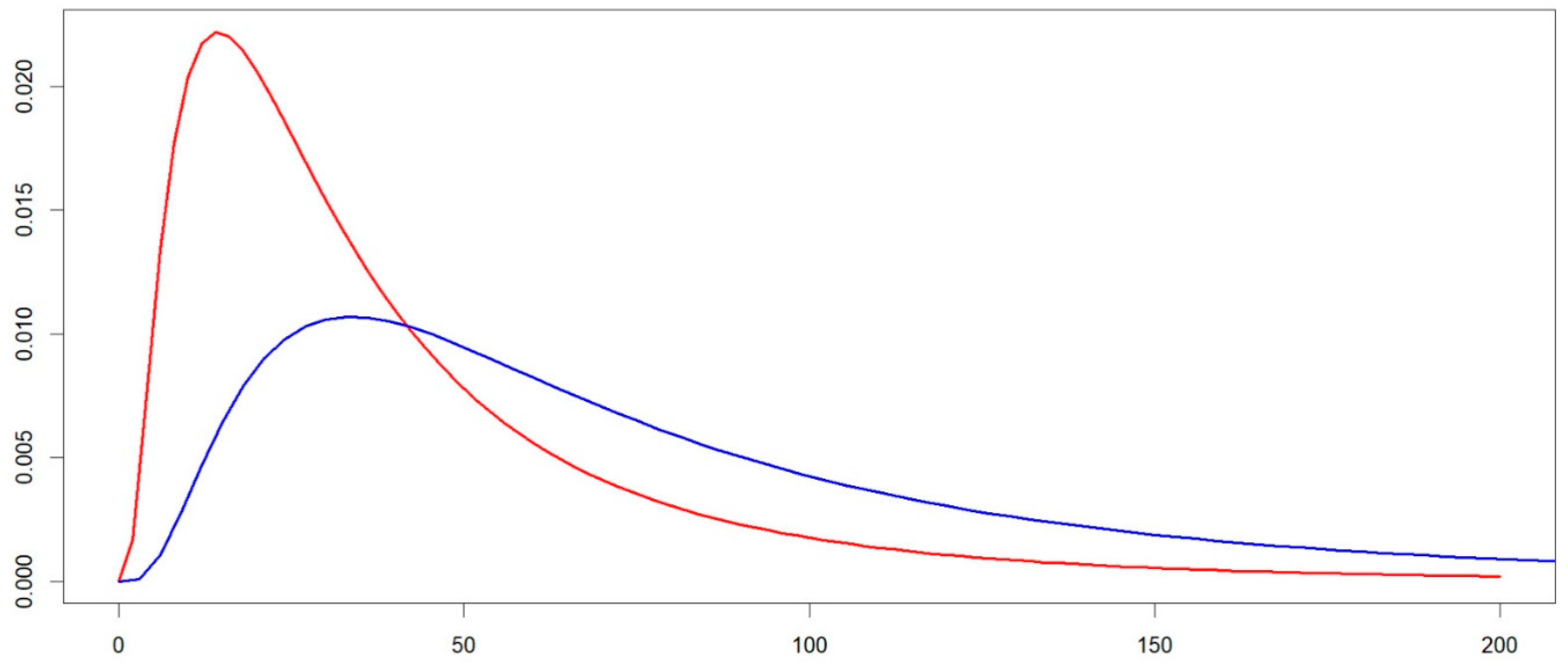
| Measures from the Regional Health Surveillance Plan | Estimates from ln-Normal Approximation | |||
|---|---|---|---|---|
| M | F | M | F | |
| p5 | 9.0 | 3.8 | 17.1 | 7.3 |
| p25 | - | - | 37.3 | 16.9 |
| p50 | 64.1 | 30.2 | 64.1 | 30.2 |
| p75 | - | - | 110.2 | 54.1 |
| p95 | 240.3 | 125.2 | 240.4 | 125.3 |
| Criterion | Expected Impact | Notes |
|---|---|---|
| PFOA half-life duration | ± | An overall modest impact is expected based on the point estimates, as the local half-life estimate is very precise. However, the half-life derived in a population includes inter-individual variability |
| Choice of pharmacokinetic model | ± | Steady-state serum concentrations are higher in multi-compartment models [14]. |
| PFOA daily intake rate | ± | Scenarios involving the use of private wells or local food consumption were excluded. The average daily intake rate was used, which is higher than the median in right-skewed distributions, thus leading to a higher Css estimate. Separate estimates for males and females are not available, and estimates for red subareas are very close. |
| Measures of PFOA serum levels in the Regional Health Surveillance Plan | ± | Possible selection bias in participation. Exclusion of the first and fourth quartiles reduced the dispersion observed in test results. |
| Timing of PFOA contamination arrival in drinking water wells | ± | A delay factor of 1.9 (proposed by ARPAV) was applied. Had the arrival time been shorter, the resulting duration of human exposure would be increased. |
| Absolute PFOA amounts reaching the drinking water wells | + | Insufficient data available for environmental modelling. PFOA production increased until the early 2000s, then ceased in 2017. If less pollutant reached the wells within a given time interval, the intake rate would be proportionally lower. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolotta, P.; Ducatman, A.; Ioverno, E.; Olivieri, A.; Saugo, M. Backward and Historical PFOA Exposure Estimation in an Adult Population Highly Exposed in the Veneto Region. Environments 2025, 12, 291. https://doi.org/10.3390/environments12090291
Bartolotta P, Ducatman A, Ioverno E, Olivieri A, Saugo M. Backward and Historical PFOA Exposure Estimation in an Adult Population Highly Exposed in the Veneto Region. Environments. 2025; 12(9):291. https://doi.org/10.3390/environments12090291
Chicago/Turabian StyleBartolotta, Patrizia, Alan Ducatman, Enrico Ioverno, Armando Olivieri, and Mario Saugo. 2025. "Backward and Historical PFOA Exposure Estimation in an Adult Population Highly Exposed in the Veneto Region" Environments 12, no. 9: 291. https://doi.org/10.3390/environments12090291
APA StyleBartolotta, P., Ducatman, A., Ioverno, E., Olivieri, A., & Saugo, M. (2025). Backward and Historical PFOA Exposure Estimation in an Adult Population Highly Exposed in the Veneto Region. Environments, 12(9), 291. https://doi.org/10.3390/environments12090291







