Enhanced Degradation of 4-Nitrophenol via a Two-Stage Co-Catalytic Fenton Packed-Bed Reactor with External Circulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and DPW
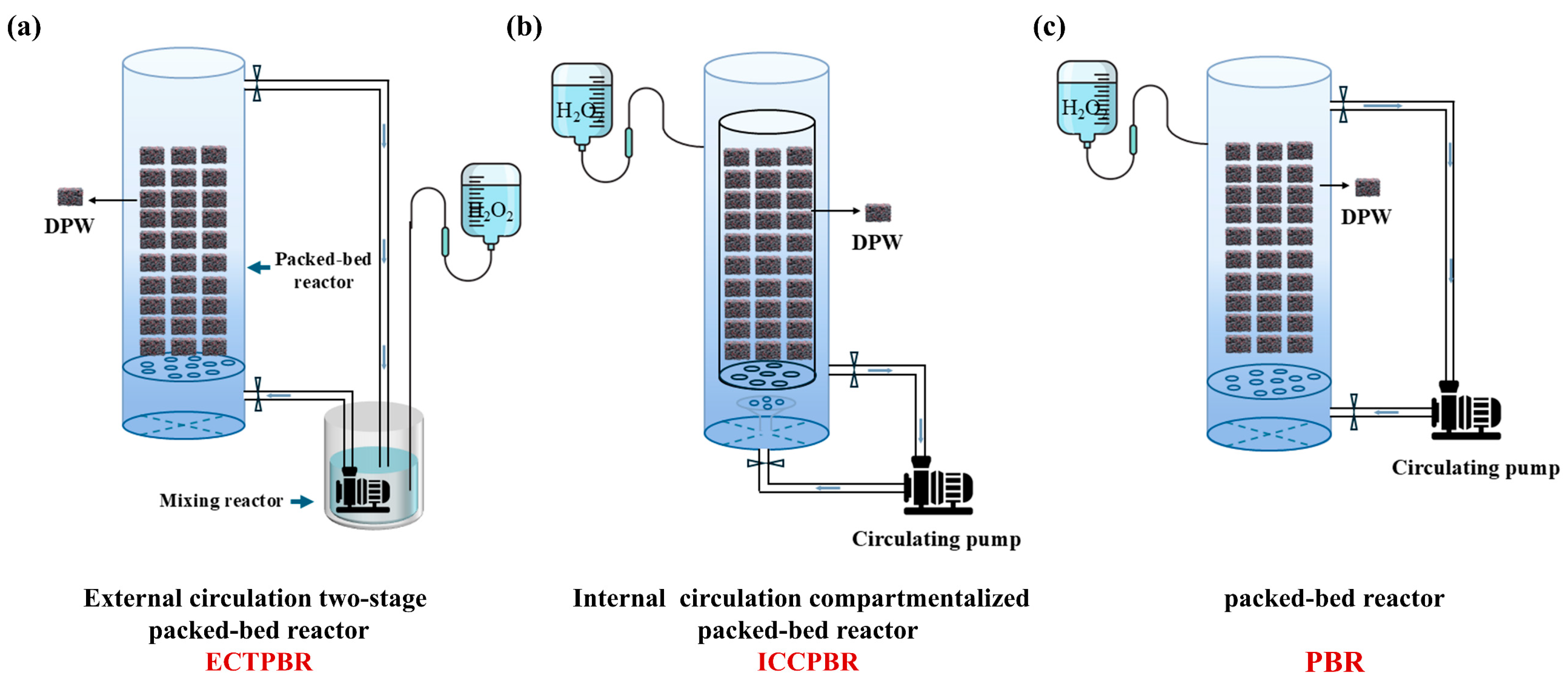
2.2. Design and Operation of Reactors
2.2.1. External Circulation Two-Stage Packed-Bed Reactor (ECTPBR)
2.2.2. Internal Circulation Compartmentalized Packed-Bed Reactor (ICCPBR)
2.2.3. Packed-Bed Reactor (PBR)
2.2.4. Operation Process of Reactors
2.3. Analytical Methods
3. Results and Discussion
3.1. 4-NP Degradation Performance of ECTPBR
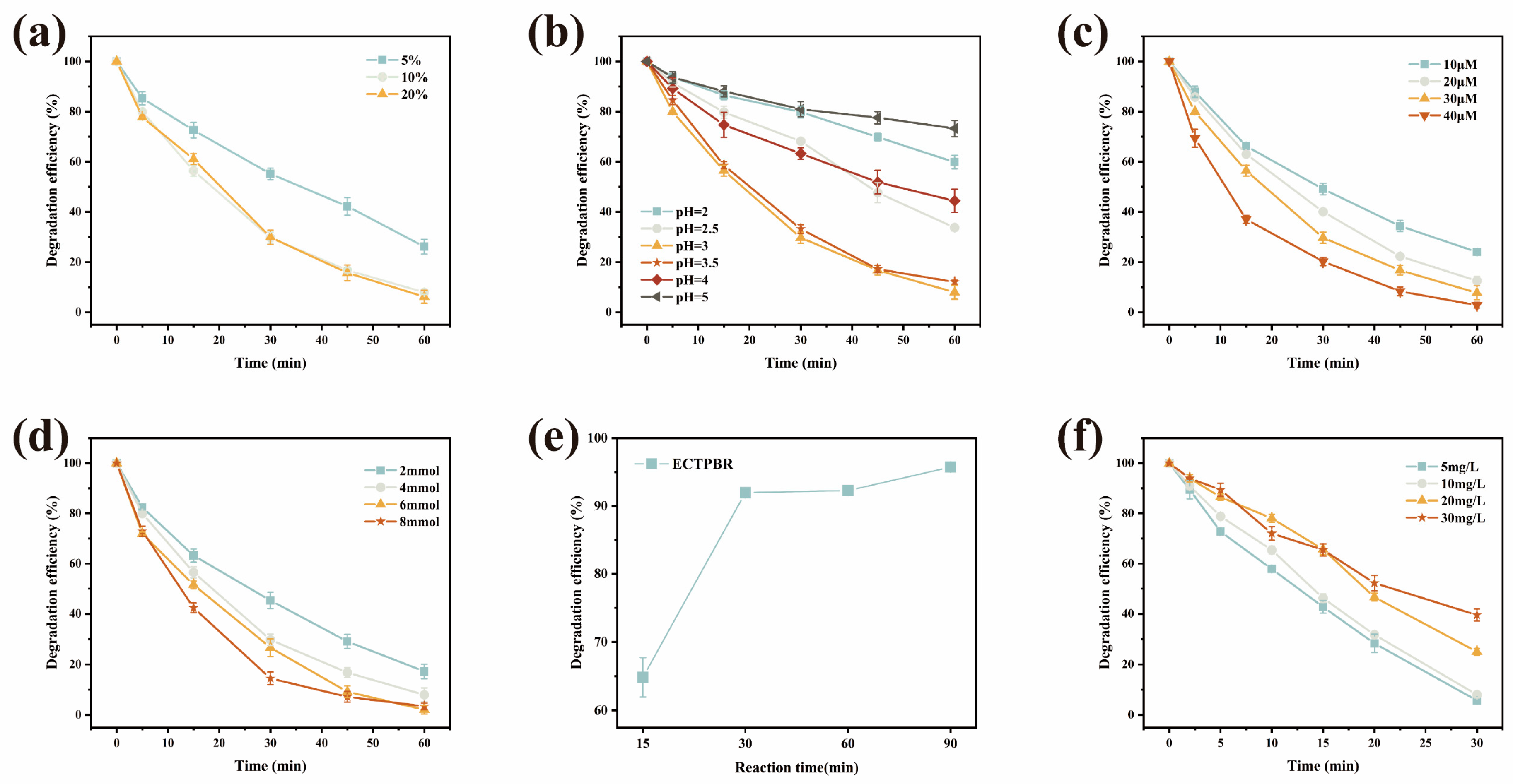
3.1.1. Effect of H2O2 Addition Method on 4-NP Degradation Performance of ECTPBR
3.1.2. Effect of Main Factors on 4-NP Degradation by Reactors
3.1.3. Circulating and Reaction Equilibrium of ECTPBR
3.2. Process Mechanism of ECTPBR
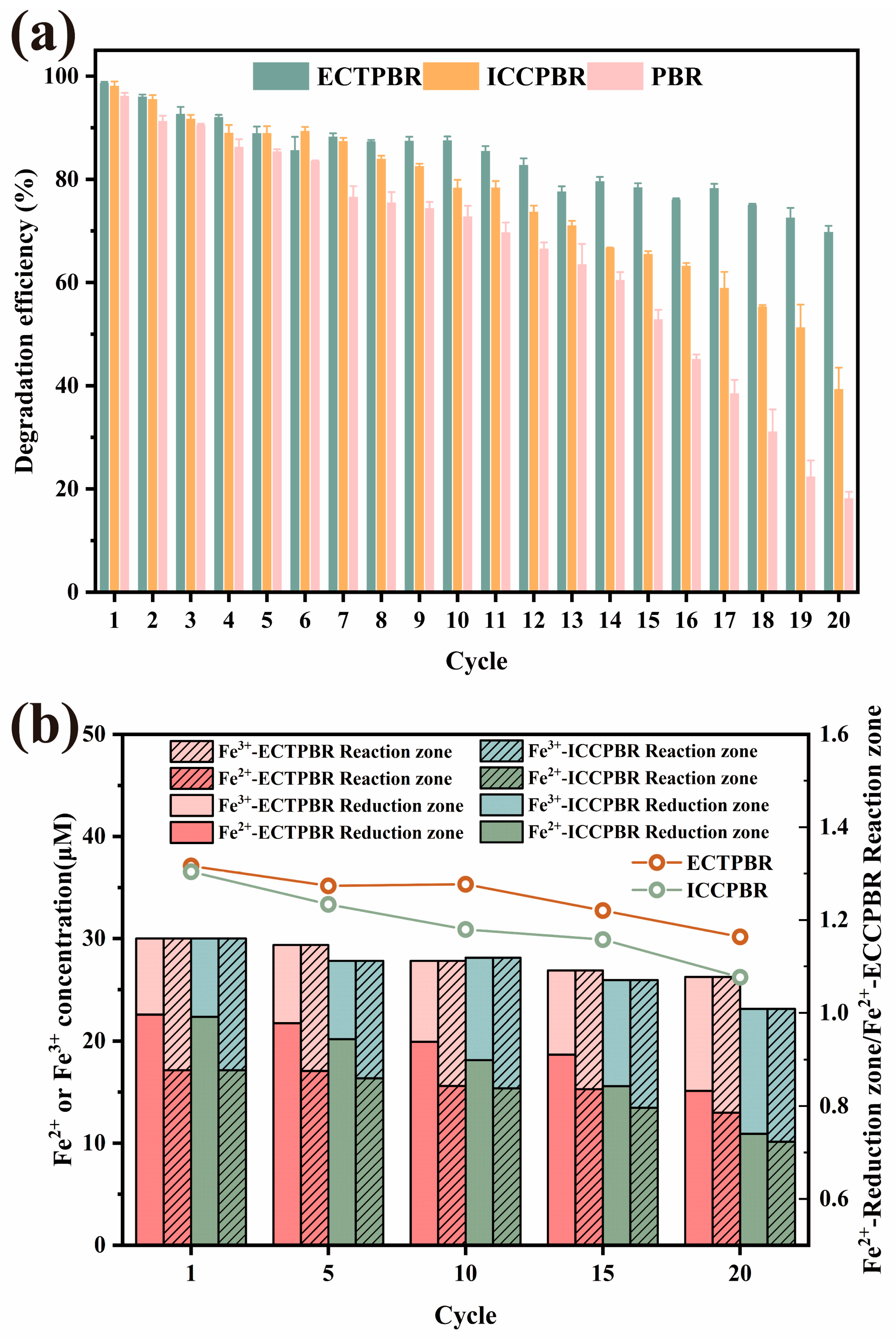
3.2.1. Verification of the Reliability of Partition Reaction Strategy
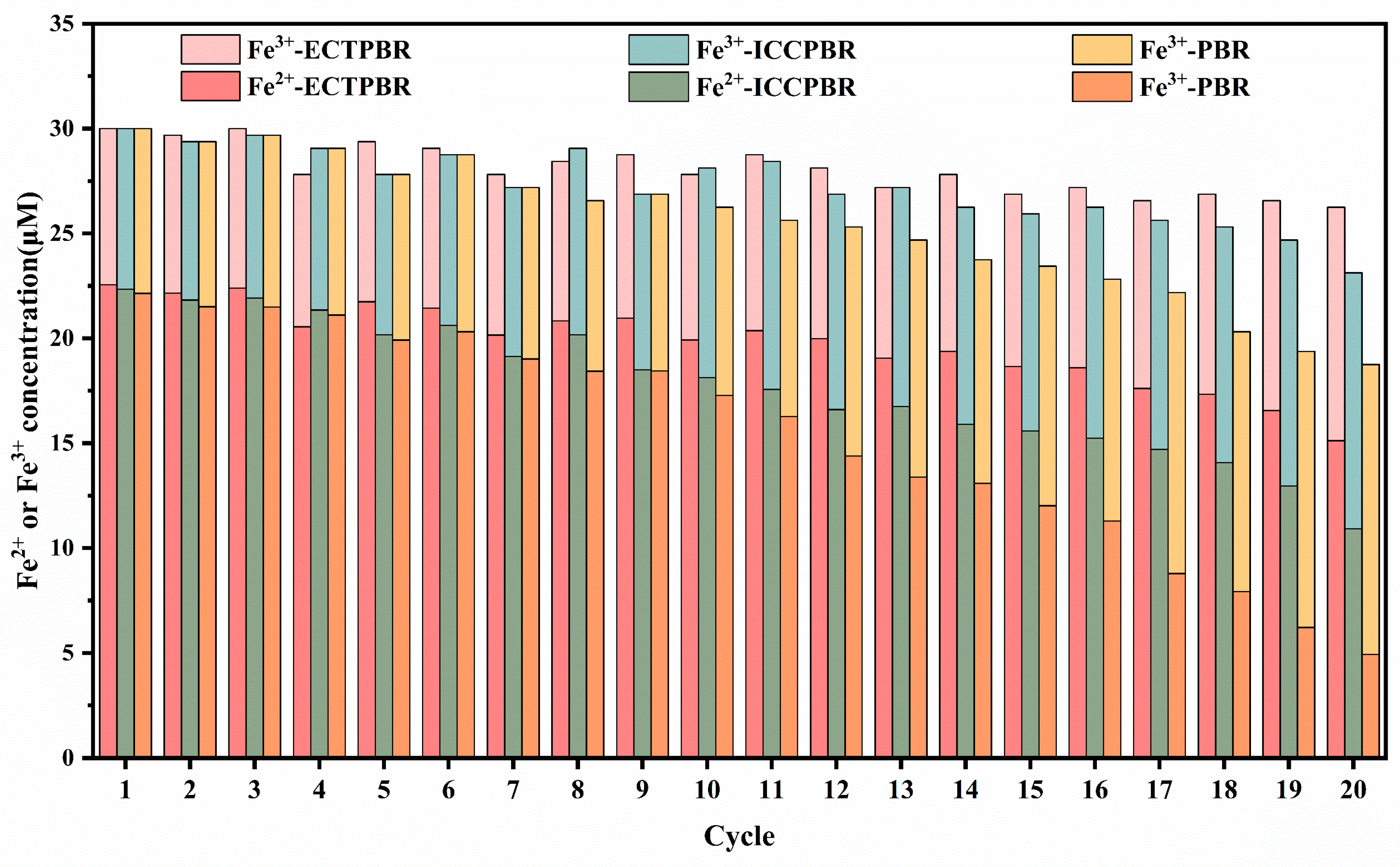
3.2.2. Fe2+/Fe3+ Circulation and Iron Sludge
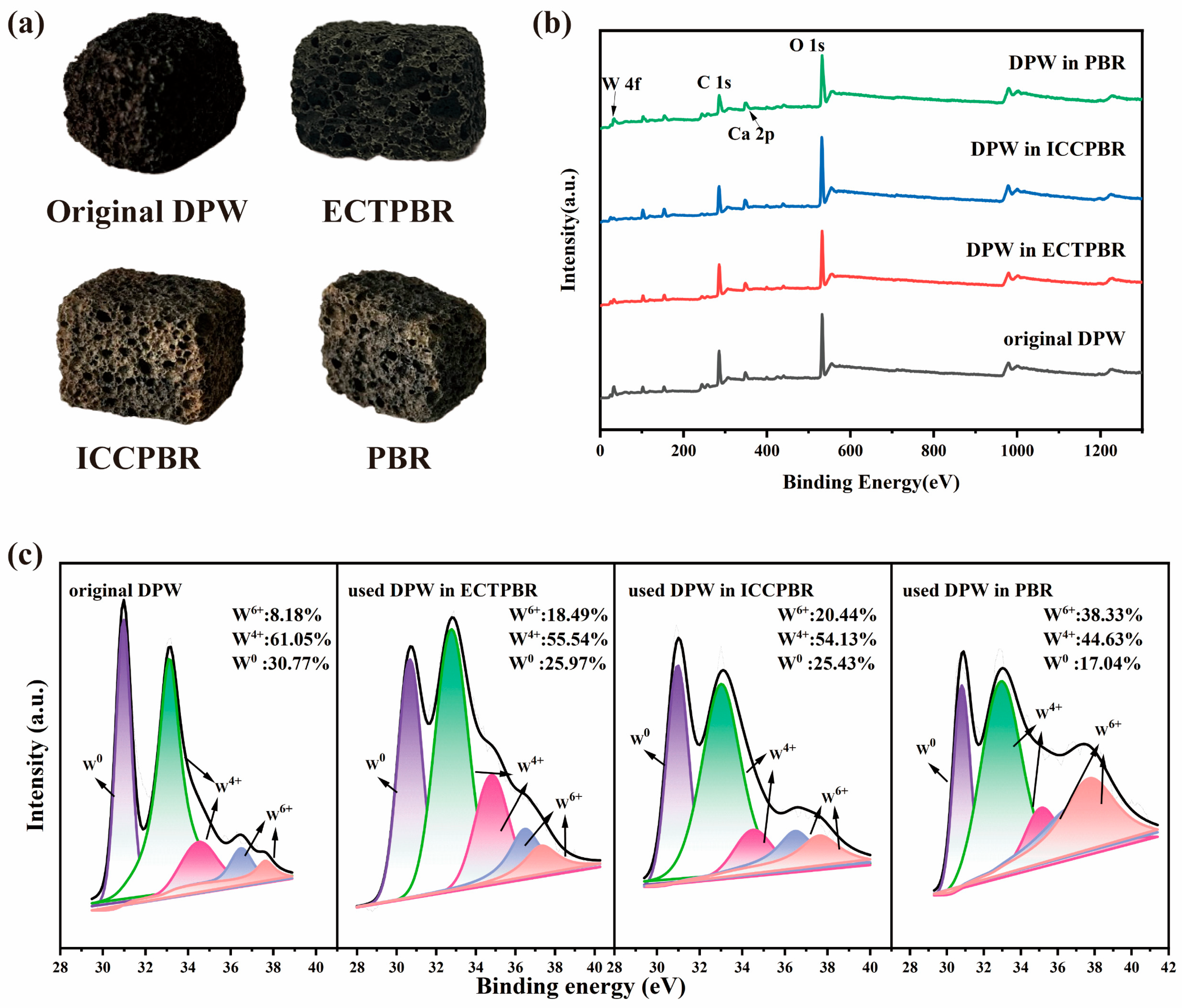
3.2.3. Stability of DPW and ECTPBR
3.2.4. Mechanism of Degradation of 4-NP
Identification of ROS
Degradation Products and Toxicity Evaluation
3.2.5. Mechanism of Degradation of 4-NP by ECTPBR
3.2.6. Regulation Strategies of ECTPBR
3.3. Tungsten Release and Potential Post-Treatment Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WC | Tungsten carbide |
| ECTPBR | External circulation two-stage packed-bed reactor |
| ICCPBR | Internal circulation compartmentalized packed-bed reactor |
| PBR | Packed-bed reactor |
| DPW | diatomite plate@polydopamine@WC |
| 4-NP | 4-nitrophenol |
References
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal Sulfides as Excellent Co-Catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Dong, C.; Ji, J.; Shen, B.; Xing, M.; Zhang, J. Enhancement of H2O2 Decomposition by the Co-Catalytic Effect of WS2 on the Fenton Reaction for the Synchronous Reduction of Cr(VI) and Remediation of Phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, Y.; Li, X.; Xiao, C.; Liu, J.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; et al. 3D N-Doped Graphene Aerogel Sponge-Loaded CoS2 Co-Catalytic Fenton System for Ciprofloxacin Degradation. J. Clean. Prod. 2022, 380, 135008. [Google Scholar] [CrossRef]
- Atri, S.; Zazimal, F.; Gowrisankaran, S.; Dyrcikova, Z.; Caplovicova, M.; Roch, T.; Dvoranova, D.; Homola, T.; Plesch, G.; Brigante, M.; et al. Mxene-Decorated Spinel Oxides as Innovative Activators of Peroxymonosulfate for Degradation of Caffeine in WWTP Effluents: Insights into Mechanisms. Chem. Eng. J. 2024, 502, 157814. [Google Scholar] [CrossRef]
- Xiao, Y.; Ji, J.; Zhu, L.; Bao, Y.; Liu, X.; Zhang, J.; Xing, M. Regeneration of Zero-Valent Iron Powder by the Cocatalytic Effect of WS2 in the Environmental Applications. Chem. Eng. J. 2020, 383, 123–158. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Xing, M. Cocatalytic Fenton Reaction for Pollutant Control. Cell Rep. Phys. Sci. 2020, 1, 100149. [Google Scholar] [CrossRef]
- Tian, Q.; Chang, J.; Yu, B.; Jiang, Y.; Gao, B.; Yang, J.; Li, Q.; Gao, Y.; Xu, X. Co-Catalysis Strategy for Low-Oxidant-Consumption Fenton-like Chemistry: From Theoretical Understandings to Practical Applications and Future Guiding Strategies. Water Res. 2024, 267, 122488. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Ma, S.; Xiao, C.; Liu, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. MoS2 Sponge Co-Catalytic Fenton Reaction for Efficient Degradation of Antibiotics: Performance, Mechanism and Reactor Operation. J. Water Process Eng. 2024, 67, 106161. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 Sponge as Excellent Cocatalysts in Advanced Oxidation Processes for Pollutant Control. Angew. Chem. Int. Ed. 2020, 59, 13968–13976. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, Y.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. Efficient Degradation of Ciprofloxacin by 3D Oxygen-Doped MoS2/Polyacrylamide/Graphene Oxide Composite Hydrogel Co-Catalytic Fenton Reaction. J. Environ. Chem. Eng. 2024, 12, 111759. [Google Scholar] [CrossRef]
- Zhang, D.; An, Z.; Zhang, Y.; Hu, Y.; Zhan, J.; Zhou, H.; Wu, M. MoS2@SiO2 Enhanced Persulfate Oxidation for the Degradation of Triazine Herbicides in Fixed-Bed Reactor. J. Water Process Eng. 2023, 52, 103523. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liu, Y.; Tang, C.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. Effective Degradation of Norfloxacin by 3D Diatomite plate@polydopamine@WC Co-Catalytic Fenton at near-Neutral pH. Chem. Eng. J. 2024, 486, 150038. [Google Scholar] [CrossRef]
- An, Z.; Hu, Y.; Zhang, D.; Zhou, H.; Zhan, J.; Wu, M. Preparation of MoS2/SiO2 Composites as Fixed-Bed Reactors for Fenton-like Advanced Oxidation of Sulfonamides in Water. J. Environ. Chem. Eng. 2022, 10, 107867. [Google Scholar] [CrossRef]
- Ding, Z.; An, Z.; Zhang, Y.; Zhou, H.; Liu, L.; Wu, M. Advanced Oxidation Treatment of Aqueous Atrazine by Fixed-Bed Reactor Packed with Carbon-Supported Molybdenum Disulfide. J. Water Process Eng. 2024, 58, 104857. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Pan, Y.; Liu, B.; Chen, B.; Zhang, M.; Liu, X.; Wang, Z. Simultaneous Decomplexation of Pb-EDTA and Elimination of Free Pb Ions by MoS2/H2O2: Mechanisms and Applications. J. Hazard. Mater. 2024, 471, 134292. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, Y.; Xia, B.-Y.; Wang, J.-Y.; Wang, X. Novel Tungsten Carbide Nanorods: An Intrinsic Peroxidase Mimetic with High Activity and Stability in Aqueous and Organic Solvents. Biosens. Bioelectron. 2014, 54, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Duesterberg, C.K.; Waite, T.D. Process Optimization of Fenton Oxidation Using Kinetic Modeling. Environ. Sci. Technol. 2006, 40, 4189–4195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Li, X.; Xiao, C.; Shi, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. High-Efficient Degradation of Chloroquine Phosphate by Oxygen Doping MoS2 Co-Catalytic Fenton Reaction. J. Hazard. Mater. 2023, 458, 131894. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; et al. Degradation of Sulfamethoxazole by Super-Hydrophilic MoS2 Sponge Co-Catalytic Fenton: Enhancing Fe2+/Fe3+ Cycle and Mass Transfer. J. Hazard. Mater. 2023, 458, 131878. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.; Huang, C. Treatment of Landfill Leachate by Fenton’s Reagent in a Continuous Stirred Tank Reactor. J. Hazard. Mater. 2006, 136, 618–623. [Google Scholar] [CrossRef]
- Bai, L.; Mei, J. Low Amount of Au Nanoparticles Deposited ZnO Nanorods Heterojunction Photocatalysts for Efficient Degradation of P-Nitrophenol. J. Sol-Gel Sci. Technol. 2020, 94, 468–476. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, L.; Shi, H.; Chen, W.; Zhong, Y.; Zhang, Y.; Zhou, Y.; Huang, K. Simultaneous Degradation of p-Nitrophenol and Reduction of Cr(VI) in One Step Using Microwave Atmospheric Pressure Plasma. Water Res. 2022, 212, 118124. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Gaware, G.J.; Chinthala, M. Heterojunction Photocatalysts for the Removal of Nitrophenol: A Systematic Review. Chemosphere 2023, 310, 136853. [Google Scholar] [CrossRef]
- Kuang, S.; Le, Q.; Hu, J.; Wang, Y.; Yu, N.; Cao, X.; Zhang, M.; Sun, Y.; Gu, W.; Yang, Y.; et al. Effects of P-Nitrophenol on Enzyme Activity, Histology, and Gene Expression in Larimichthys crocea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 228, 108638. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Afridi, M.N.; Kumar, V.; Lee, J.; Ali, I.; Kim, K.-H.; Kim, J.-O. Photocatalytic Degradation Performance of Various Types of Modified TiO2 against Nitrophenols in Aqueous Systems. J. Clean. Prod. 2019, 231, 899–912. [Google Scholar] [CrossRef]
- Takatsuka, M.; Goto, S.; Moritake, K.; Shimada, Y.; Tsuchida, T. Antioxidant Capacity of Edaravone, Quercetin, and Myricetin Involving Probabilistic Fluctuations Using Eosin-Y and Eosin-B as Fluorescent Probes in the ORAC Assay. J. Photochem. Photobiol. A 2025, 460, 116142. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, C.; Duan, W.; Lang, D.; Pan, B. Coupling Adsorption and Degradation in p-Nitrophenol Removal by Biochars. J. Clean. Prod. 2020, 271, 122550. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial Degradation of Nitrophenols and Their Derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Yi, Z.; Lin, D.; Zhu, L.; Yang, K. Enhanced Sorption of Naphthalene and p-Nitrophenol by Nano-SiO2 Modified with a Cationic Surfactant. Water Res. 2013, 47, 4006–4012. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Lin, M.; Zhou, L.; Wen, H.; Zhong, T.; Zhao, H.; Tian, S.; He, C. Boron-Doped Porous Carbon Boosts Electron Transport Efficiency for Enhancing Fenton-like Oxidation Capacity: High-Speed Driving of Fe(III) Reduction. Appl. Catal. B 2024, 343, 123535. [Google Scholar] [CrossRef]
- He, D.; Wang, D.; Luo, H.; Zeng, Y.; Zeng, G.; Li, J.; Pan, X. Tungsten Disulfide (WS2) Is a Highly Active Co-Catalyst in Fe(III)/H2O2 Fenton-like Reactions for Efficient Acetaminophen Degradation. Sci. Total Environ. 2023, 871, 162151. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Oxidative Degradation of the Antibiotic Oxytetracycline by Cu@Fe3O4 Core-Shell Nanoparticles. Sci. Total Environ. 2018, 631–632, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Pliego, G.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Application of Intensified Fenton Oxidation to the Treatment of Sawmill Wastewater. Chemosphere 2014, 109, 34–41. [Google Scholar] [CrossRef]
- Le, S.-T.; Israpanich, A.; Phenrat, T. Using Sequential H2O2 Addition to Sustain 1,2-Dichloroethane Detoxification by a Nanoscale Zerovalent Iron-Induced Fenton’s System at a Natural pH. Chemosphere 2022, 305, 135376. [Google Scholar] [CrossRef]
- Primo, O.; Rivero, M.J.; Ortiz, I. Photo-Fenton Process as an Efficient Alternative to the Treatment of Landfill Leachates. J. Hazard. Mater. 2008, 153, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Hameed, B.H. Fenton-like Oxidation of Acid Red 1 Solutions Using Heterogeneous Catalyst Based on Ball Clay. Int. J. Environ. Sci. Dev. 2011, 2, 218–222. [Google Scholar] [CrossRef]
- Xiao, C.; Li, X.; Li, Q.; Hu, Y.; Cheng, J.; Chen, Y. Ni-Doped FeC2O4 for Efficient Photo-Fenton Simultaneous Degradation of Organic Pollutants and Reduction of Cr(VI): Accelerated Fe(III)/Fe(II) Cycle, Enhanced Stability and Mechanism Insight. J. Clean. Prod. 2022, 340, 130775. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Xiao, C.; Hu, Y.; Liu, H.; Chen, Y.; Cheng, J. Manipulating the Morphology of Self-Assembly Broccoli-like Cobalt Nickel Spinel for Enhancing the Peroxydisulfate Activation towards Highly-Effective Ciprofloxacin Degradation: Radical and Non-Radical Pathways, Mechanism and Toxicity Evaluation. Appl. Surf. Sci. 2023, 617, 156593. [Google Scholar] [CrossRef]
- Haji, S.; Sabea, M.R.; Aljawder, T.; Al-Aradi, M.H.; Al-Khateeb, W.M.; Elkanzi, E.; Ahmed, S. Modeling Catalyst Deactivation in Heterogeneous Fenton-like Oxidation Reactions. Chem. Eng. J. 2021, 416, 128279. [Google Scholar] [CrossRef]
- Bolan, S.; Wijesekara, H.; Ireshika, A.; Zhang, T.; Pu, M.; Petruzzelli, G.; Pedron, F.; Hou, D.; Wang, L.; Zhou, S.; et al. Tungsten Contamination, Behavior and Remediation in Complex Environmental Settings. Environ. Int. 2023, 181, 108276. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient Removal of Bisphenol A by Superoxide Radical and Singlet Oxygen Generated from Peroxymonosulfate Activated with Fe0-Montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Z.; Qiu, L.; Chen, Z.; Xiao, X.; Mo, X.; Cui, L. Recycling of Fenton Sludge Containing Ni as an Efficient Catalyst for Tetracycline Degradation through Peroxymonosulfate Activation. J. Clean. Prod. 2020, 268, 122174. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, B.; Gao, Y.; Zhu, H.; Chen, Y.; Li, X.; Zhang, Q. Mott–Schottky Effect in Core–Shell W@WC Heterostructure: Boosting Both Electronic/Ionic Kinetics for Lithium Storage. Small 2023, 19, 2300955. [Google Scholar] [CrossRef]
- Fu, B.-G.; Zhou, X.; Lu, Y.; Quan, W.-Z.; Li, C.; Cheng, L.; Xiao, X.; Yu, Y.-Y. Interfacial OOH* Mediated Fe(II) Regeneration on the Single Atom Co-N-C Catalyst for Efficient Fenton-like Processes. J. Hazard. Mater. 2024, 470, 134214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J. Carbon-Doped Defect MoS2 Co-Catalytic Fe3+/Peroxymonosulfate Process for Efficient Sulfadiazine Degradation: Accelerating Fe3+/Fe2+ Cycle and 1O2 Dominated Oxidation. Sci. Total Environ. 2023, 858, 159587. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, Y.; Sun, B.; Song, W.; Sun, J.; Gao, N.; Qiao, J.; Guan, X. Enhancement of the Advanced Fenton Process by Weak Magnetic Field for the Degradation of 4-Nitrophenol. RSC Adv. 2015, 5, 13357–13365. [Google Scholar] [CrossRef]
- Liu, T.; Chen, N.; Deng, Y.; Chen, F.; Feng, C. Degradation of P-Nitrophenol by Nano-Pyrite Catalyzed Fenton Reaction with Enhanced Peroxide Utilization. RSC Adv. 2020, 10, 15901–15912. [Google Scholar] [CrossRef]
- Gao, Q.; Ullah, H.; Ming, Z.; Zhao, S.; Xu, A.; Li, X.; Khan, A. Synergistic Degradation of P-Nitrophenol Using Peroxymonosulfate Activated with a Bimetallic Mn3O4–Cu2O Catalyst: Investigation of Strong Interactions between Metal Oxides. J. Environ. Chem. Eng. 2024, 12, 113958. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Ji, F.; Lai, B. Heterogeneous Catalytic Oxidation for the Degradation of P-Nitrophenol in Aqueous Solution by Persulfate Activated with CuFe2O4 Magnetic Nano-Particles. Chem. Eng. J. 2017, 324, 63–73. [Google Scholar] [CrossRef]
- Jiteshwaran, T.; Janani, B.; Syed, A.; Elgorban, A.M.; Abid, I.; Wong, L.S.; Khan, S.S. Interfacial Engineering of BiVO4/PANI p-n Heterojunction for Enhanced Photocatalytic Degradation of p-Nitrophenol: Pathway, Toxicity Evaluation and Mechanistic Insights. Surf. Interfaces 2024, 49, 104361. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Chen, L.; Fan, G.; Long, Y. Spatial Confinement and Erbium-Mediated Electronic Modulation for Enhanced 4-Nitrophenol Destruction through Peroxymonosulfate Activation. Sep. Purif. Technol. 2025, 353, 128437. [Google Scholar] [CrossRef]
- Li, Z.; Kang, J.; Tang, Y.; Jin, C.; Luo, H.; Li, S.; Liu, J.; Wang, M.; Lv, C. The Enhanced P-Nitrophenol Degradation with Fe/Co3O4 Mesoporous Nanosheets via Peroxymonosulfate Activation and Its Mechanism Insight. J. Alloys Compd. 2021, 858, 157739. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Mo, L.; Li, J.; Xu, J. Removal of Tungsten from Electroplating Wastewater by Acid- and Heat-Treated Sepiolite. Desalination Water Treat. 2015, 56, 232–238. [Google Scholar] [CrossRef]
- Zaguzin, V.P.; Ksenzova, V.I.; Pogrebnyak, Y.F. Chemical-Spectral Determination of Tungsten, Molybdenum and Tin in Natural Waters. Zhurnal Anal. Khimii 1980, 35, 1143–1147. [Google Scholar]
- Plattes, M.; Bertrand, A.; Schmitt, B.; Sinner, J.; Verstraeten, F.; Welfring, J. Removal of Tungsten Oxyanions from Industrial Wastewater by Precipitation, Coagulation and Flocculation Processes. J. Hazard. Mater. 2007, 148, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Ogi, T.; Makino, T.; Okuyama, K.; Stark, W.J.; Iskandar, F. Selective Biosorption and Recovery of Tungsten from an Urban Mine and Feasibility Evaluation. Ind. Eng. Chem. Res. 2016, 55, 2903–2910. [Google Scholar] [CrossRef]
- Sastry, C.S.P.; Lingeswara Rao, J.S.V.M. Determination of Doxorubicin Hydrochloride by Visible Spectrophotometry. Talanta 1996, 43, 1827–1835. [Google Scholar] [CrossRef]





| Element | Original DPW | ECTPBR | ICCPBR |
|---|---|---|---|
| C (Atomic %) | 57.82 | 54.24 | 33.72 |
| O (Atomic %) | 40.35 | 43.26 | 64.78 |
| W (Atomic %) | 1.83 | 1.76 | 0.64 |
| Fe (Atomic %) | N.A. | 0.73 | 0.86 |
| Cycle | ECTPBR (mg/L) | ICCPBR (mg/L) | PBR (mg/L) |
|---|---|---|---|
| 1 | 0.1451 | 0.4140 | 0.4751 |
| 5 | 0.9827 | 1.9375 | 1.9573 |
| 10 | 1.6580 | 2.7081 | 2.8532 |
| 15 | 2.5453 | 4.4810 | 4.7892 |
| 20 | 3.6363 | 6.1206 | 6.2678 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, J.; Hu, Y.; Shi, Y.; Tang, C.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. Enhanced Degradation of 4-Nitrophenol via a Two-Stage Co-Catalytic Fenton Packed-Bed Reactor with External Circulation. Environments 2025, 12, 280. https://doi.org/10.3390/environments12080280
Liu Y, Liu J, Hu Y, Shi Y, Tang C, Cheng J, Zhu X, Wang G, Xie J. Enhanced Degradation of 4-Nitrophenol via a Two-Stage Co-Catalytic Fenton Packed-Bed Reactor with External Circulation. Environments. 2025; 12(8):280. https://doi.org/10.3390/environments12080280
Chicago/Turabian StyleLiu, Yan, Jingyu Liu, Yongyou Hu, Yueyue Shi, Chaoyang Tang, Jianhua Cheng, Xiaoqiang Zhu, Guobin Wang, and Jieyun Xie. 2025. "Enhanced Degradation of 4-Nitrophenol via a Two-Stage Co-Catalytic Fenton Packed-Bed Reactor with External Circulation" Environments 12, no. 8: 280. https://doi.org/10.3390/environments12080280
APA StyleLiu, Y., Liu, J., Hu, Y., Shi, Y., Tang, C., Cheng, J., Zhu, X., Wang, G., & Xie, J. (2025). Enhanced Degradation of 4-Nitrophenol via a Two-Stage Co-Catalytic Fenton Packed-Bed Reactor with External Circulation. Environments, 12(8), 280. https://doi.org/10.3390/environments12080280





