Infrasound-Altered Pollination in a Common Western North American Plant: Evidence from Wind Turbines and Railways
Abstract
1. Introduction
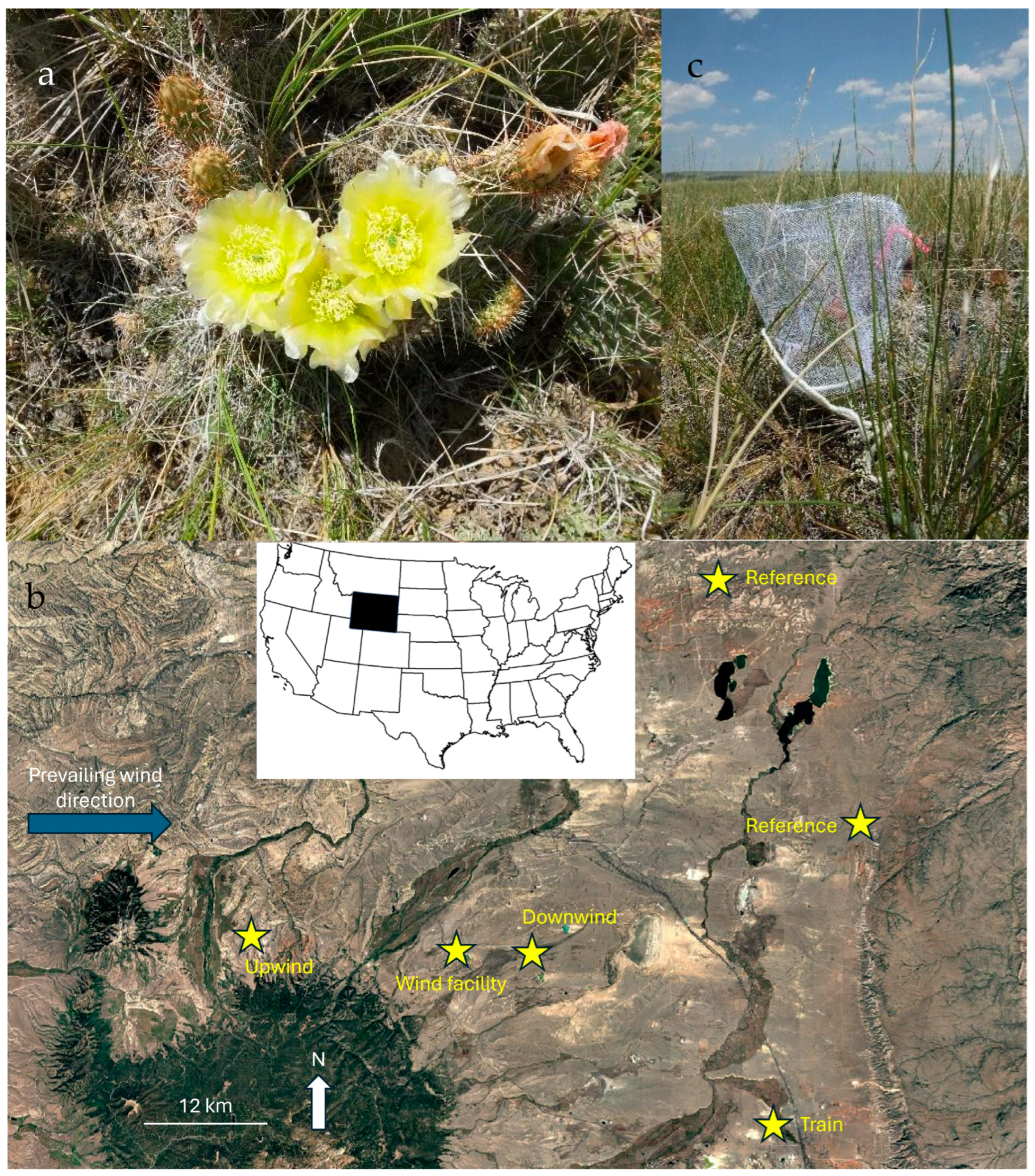
2. Materials and Methods
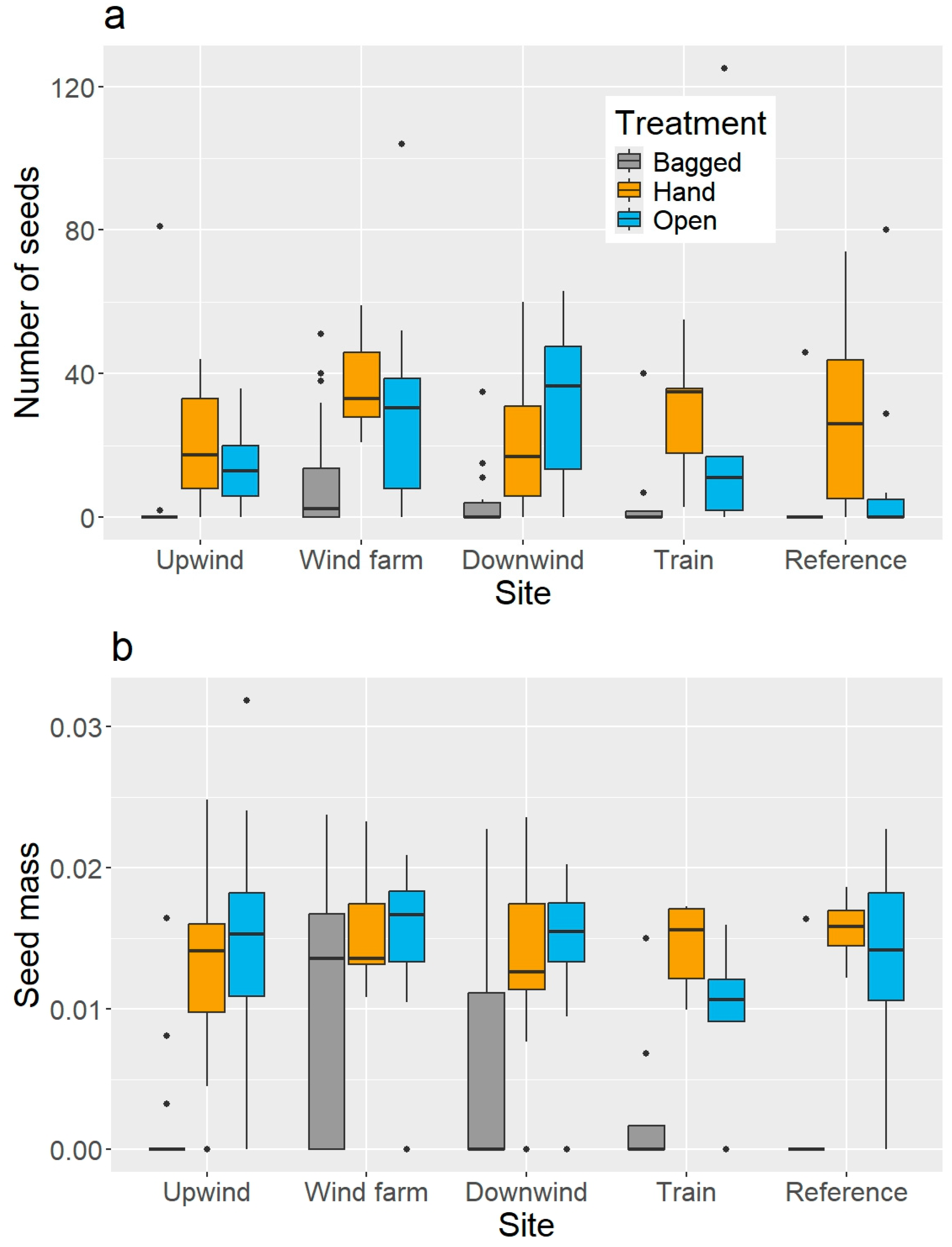
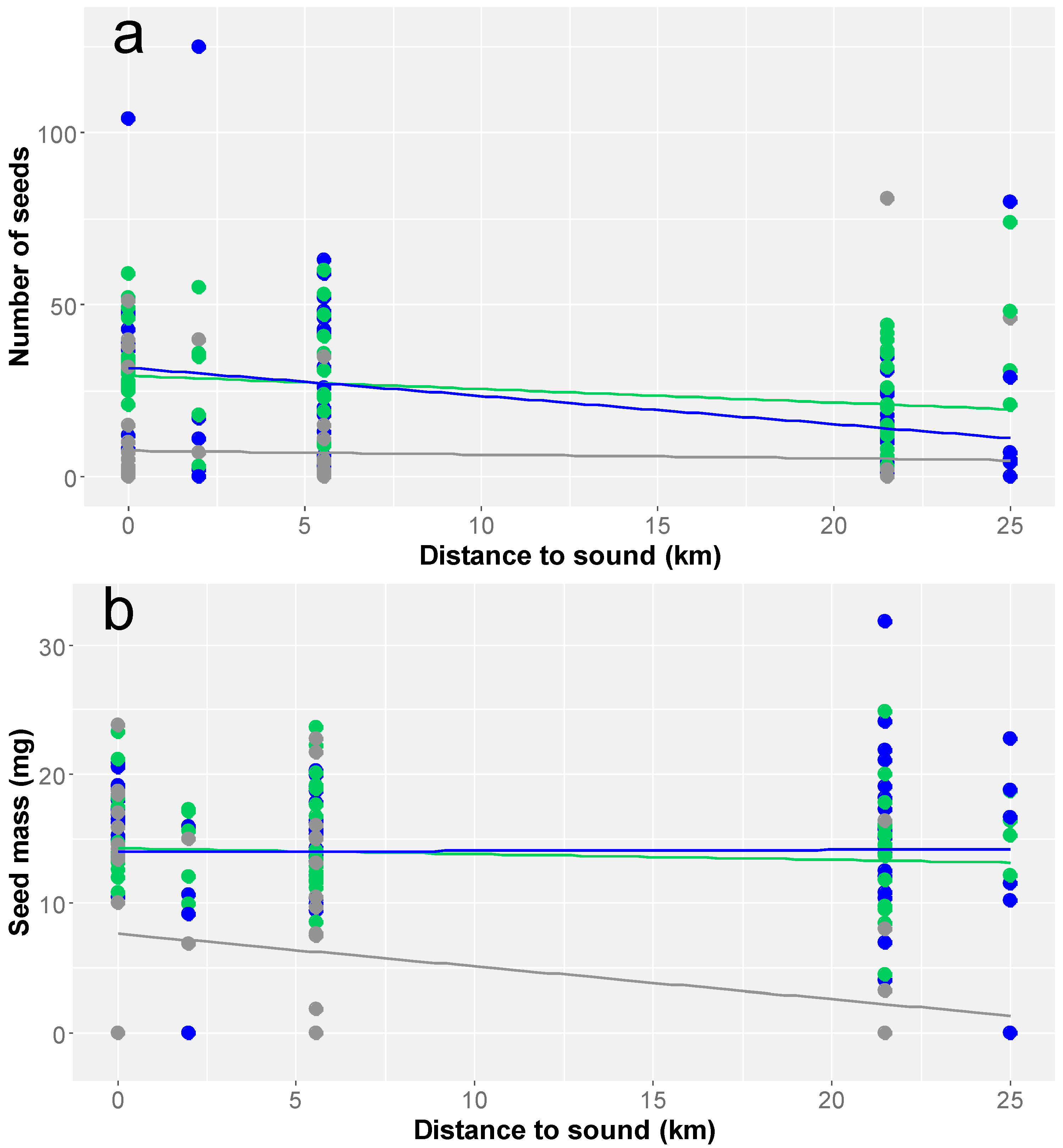
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFries, R.S.; Foley, J.A.; Asner, G.P. Land-use choices: Balancing human needs and ecosystem function. Front. Ecol. Environ. 2004, 2, 249–257. [Google Scholar] [CrossRef]
- Pijanowski, B.C.; Farina, A.; Gage, S.H.; Dumyahn, S.L.; Krause, B.L. What is soundscape ecology? An introduction and overview of an emerging new science. Landsc. Ecol. 2011, 26, 1213–1232. [Google Scholar] [CrossRef]
- Weschler, M.; Tronstad, L. Wind energy and insects: Reviewing the state of knowledge and identifying potential interactions. PeerJ 2024, 12, e18153. [Google Scholar] [CrossRef] [PubMed]
- Ryunosuke, K. Adverse impacts of wind power generation on collision behaviour of birds and anti-predator behaviour of squirrels. J. Nat. Conserv. 2008, 16, 44–55. [Google Scholar] [CrossRef]
- Meunier, M. Wind Farm—Long term noise and vibration measurements. Proc. Meet. Acoust. 2013, 19, 040075. [Google Scholar] [CrossRef]
- Zajamsek, B.; Hansen, K.L.; Doolan, C.J.; Hansen, C.H. Characterisation of wind farm infrasound and low-frequency noise. J. Sound Vib. 2016, 370, 176–190. [Google Scholar] [CrossRef]
- Payne, R.S. The role of infrasounds in maintaining whale herds. J. Acoust. Soc. Am. 2004, 115, 2554. [Google Scholar] [CrossRef]
- Garstang, M. Elephant infrasounds: Long-range communication. In Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach; Brudzynski, S.M., Ed.; Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 19, pp. 57–67. [Google Scholar]
- Mutschlecner, J.P.; Whitaker, R.W. Infrasound from earthquakes. J. Geophys. Res.-Atmos. 2005, 110, D01108. [Google Scholar] [CrossRef]
- Turnbull, C.; Turner, J.; Walsh, D. Measurement and level of infrasound from wind farms and other sources. Acoust. Aust. 2012, 40, 45–50. [Google Scholar]
- Donn, W.L.; Balachandran, N.K.; Kaschak, G. Atmospheric infrasound radiated by bridges. J. Acoust. Soc. Am. 1974, 56, 1367–1370. [Google Scholar] [CrossRef]
- Sandberg, U. Identification of infrasound generation mechanisms in a bus. J. Low Freq. Noise Vib. Act. Control 1988, 7, 110–117. [Google Scholar] [CrossRef]
- Iida, M.; Kikuchi, K.; Takami, H. Infrasound generated by a high-speed train running through a short tunnel. J. Low Freq. Noise Vib. Act. Control 2007, 26, 177–194. [Google Scholar] [CrossRef]
- Jakobsen, J. Infrasound emission from wind turbines. J. Low Freq. Noise Vib. Act. Control 2005, 24, 145–155. [Google Scholar] [CrossRef]
- Keith, S.E.; Daigle, G.A.; Stinson, M.R. Wind turbine low frequency and infrasound propagation and sound pressure level calculations at dwellings. J. Acoust. Soc. Am. 2018, 144, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Marcillo, O.; Arrowsmith, S.; Blom, P.; Jones, K. On infrasound generated by wind farms and its propagation in low-altitude tropospheric waveguides. J. Geophys. Res. Atmos. 2015, 120, 9855–9868. [Google Scholar] [CrossRef]
- Tronstad, L.M.; Weschler, M.; Storey, A.M.; Handley, J.; Tronstad, B.P. Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area. Wind 2025, 5, 15. [Google Scholar] [CrossRef]
- Malec, T.; Boczar, T.; Wotzka, D.; Kozioł, M. Measurement and Analysis of Infrasound Signals Generated by Operation of High-Power Wind Turbines. Energies 2021, 14, 6544. [Google Scholar] [CrossRef]
- Dorsey, B.; Olsson, M.; Rew, L.J. Ecological effects of railways on wildlife. In Handbook of Road Ecology; Wiley: Hoboken, NJ, USA, 2015; pp. 219–227. [Google Scholar]
- Lucas, P.S.; de Carvalho, R.G.; Grilo, C. Railway disturbances on wildlife: Types, effects, and mitigation measures. In Railway Ecology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–99. [Google Scholar]
- Popp, J.N.; Boyle, S.P. Railway ecology: Underrepresented in science? Basic Appl. Ecol. 2017, 19, 84–93. [Google Scholar] [CrossRef]
- Schuster, E.; Bulling, L.; Koppel, J. Consolidating the State of Knowledge: A Synoptical Review of Wind Energy’s Wildlife Effects. Environ. Manag. 2015, 56, 300–331. [Google Scholar] [CrossRef]
- Baidya Roy, S.; Traiteur, J.J. Impacts of wind farms on surface air temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 17899–17904. [Google Scholar] [CrossRef]
- Baidya Roy, S.; Pacala, S.W.; Walko, R. Can large wind farms affect local meteorology? J. Geophys. Res. Atmos. 2004, 109, D19101. [Google Scholar] [CrossRef]
- Roy, S.B. Simulating impacts of wind farms on local hydrometeorology. J. Wind Eng. Ind. Aerodyn. 2011, 99, 491–498. [Google Scholar]
- Fiedler, B.H.; Bukovsky, M.S. The effect of a giant wind farm on precipitation in a regional climate model. Environ. Res. Lett. 2011, 6, 045101. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, Y.; Baidya Roy, S.; Dai, Y.; Chen, H. Diurnal and seasonal variations of wind farm impacts on land surface temperature over western Texas. Clim. Dyn. 2013, 41, 307–326. [Google Scholar] [CrossRef]
- Urziceanu, M.; Anastasiu, P.; Rozylowicz, L.; Sesan, T.E. Local-scale impact of wind energy farms on rare, endemic, and threatened plant species. PeerJ 2021, 9, e11390. [Google Scholar] [CrossRef]
- Silva, M.; Pasos, I. Vegetation. In Wildlife and Wind Farms, Conflicts and Solutions: Volume 1—Onshore: Potential Effects; Perrow, M., Ed.; Pelagic Publishing: Exeter, UK, 2017; pp. 40–62. [Google Scholar]
- Brouček, J. Effect of noise on performance, stress, and behaviour of animals. Slovak J. Anim. Sci. 2014, 47, 111–123. [Google Scholar]
- Gagliano, M. Green symphonies: A call for studies on acoustic communication in plants. Behav. Ecol. 2013, 24, 789–796. [Google Scholar] [CrossRef]
- Trigg, J.; Naweed, A.; Kinnear, S. A scoping review of freight rail noise and vibration impacts on domestic animal health and welfare. Anim. Welf. 2022, 31, 69–77. [Google Scholar] [CrossRef]
- Gómez-Martínez, C.; González-Estévez, M.A.; Cursach, J.; Lázaro, A. Pollinator richness, pollination networks, and diet adjustment along local and landscape gradients of resource diversity. Ecol. Appl. 2022, 32, e2634. [Google Scholar] [CrossRef]
- Hotker, H.; Krone, O.; Nehls, G. Birds of Prey and Wind Farms: Analysis of Problems and Possible Solutions; Springer: Cham, Switzerland, 2017; p. 331. [Google Scholar]
- Kirol, C.P.; Smith, K.T.; Graf, N.E.; Dinkins, J.B.; Lebeau, C.W.; Maechtle, T.L.; Sutphin, A.L.; Beck, J.L. Greater sage-grouse response to the physical footprint of energy development. J. Wildl. Manag. 2020, 84, 989–1001. [Google Scholar] [CrossRef]
- Bolin, K.; Bluhm, G.; Eriksson, G.; Nilsson, M.E. Infrasound and low frequency noise from wind turbines: Exposure and health effects. Environ. Res. Lett. 2011, 6, 035103. [Google Scholar] [CrossRef]
- Hansen, C.H.; Doolan, C.J.; Hansen, K.L. Effects of Wind Farm Noise and Vibration on People. In Wind Farm Noise: Measurement, Assessment and Control; Wiley: Hoboken, NJ, USA, 2017; pp. 436–475. [Google Scholar]
- Velilla, E.; Collinson, E.; Bellato, L.; Berg, M.P.; Halfwerk, W. Vibrational noise from wind energy-turbines negatively impacts earthworm abundance. Oikos 2021, 130, 844–849. [Google Scholar] [CrossRef]
- Agnew, R.C.N.; Smith, V.J.; Fowkes, R.C. Wind turbines cause chronic stress in badgers (Meles meles) in Great Britain. J. Wildl. Dis. 2016, 52, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.-K.; Kim, J.Y.; Jeong, M.-J.; Ryu, C.-M. Beyond Chemical Triggers: Evidence for Sound-Evoked Physiological Reactions in Plants. Front. Plant Sci. 2018, 9, 25. [Google Scholar] [CrossRef]
- Veits, M.; Khait, I.; Obolski, U.; Zinger, E.; Boonman, A.; Goldshtein, A.; Saban, K.; Seltzer, R.; Ben-Dor, U.; Estlein, P.; et al. Flowers respond to pollinator sound within minutes by increasing nectar sugar concentration. Ecol. Lett. 2019, 22, 1483–1492. [Google Scholar] [CrossRef]
- Vallejo-Marín, M. Buzz pollination: Studying bee vibrations on flowers. New Phytol. 2019, 224, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Eickwort, G.C.; Ginsberg, H.S. Foraging and mating behavior in Apoidea. Annu. Rev. Entomol. 1980, 25, 421–446. [Google Scholar] [CrossRef]
- Hansen, C.; Doolan, C.; Hansen, K. Wind Farm Noise: Measurement, Assessment and Control; John Wiley and Sons Ltd.: West Sussex, UK, 2017. [Google Scholar]
- Dreller, C.; Kirchner, W.H. Hearing in honeybees—Localization of the auditory sense organ. J. Comp. Physiol. A-Sens. Neural Behav. Physiol. 1993, 173, 275–279. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Razanajatovo, M.; Maurel, N.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Van Kleunen, M. Plants capable of selfing are more likely to become naturalized. Nat. Commun. 2016, 7, 13313. [Google Scholar] [CrossRef]
- Crawford, M.S.; Handley, J.; Tronstad, L.M. An insect-pollinated species in a wind-pollinated genus: Case study of the endemic plant, Laramie chickensage Artemisia simplex. Nord. J. Bot. 2022, 2022, e03708. [Google Scholar] [CrossRef]
- Fernández, J.D.; Bosch, J.; Nieto-Ariza, B.; Gómez, J.M. Pollen limitation in a narrow endemic plant: Geographical variation and driving factors. Oecologia 2012, 170, 421–431. [Google Scholar] [CrossRef]
- Morales, C.L.; Traveset, A. Interspecific pollen transfer: Magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant Sci. 2008, 27, 221–238. [Google Scholar] [CrossRef]
- Tang, B.; Wu, D.; Zhao, X.; Zhou, T.; Zhao, W.; Wei, H. The Observed Impacts of Wind Farms on Local Vegetation Growth in Northern China. Remote Sens. 2017, 9, 332. [Google Scholar] [CrossRef]
- Xu, K.; He, L.; Hu, H.; Liu, S.; Du, Y.; Wang, Z.; Li, Y.; Li, L.; Khan, A.; Wang, G. Positive ecological effects of wind farms on vegetation in China’s Gobi desert. Sci. Rep. 2019, 9, 6341. [Google Scholar] [CrossRef]
- Pătru-Stupariu, I.; Calotă, A.-M.; Santonja, M.; Anastasiu, P.; Stoicescu, I.; Biriş, I.A.; Stupariu, M.-S.; Buttler, A. Do wind turbines impact plant community properties in mountain region? Biologia 2019, 74, 1613–1619. [Google Scholar] [CrossRef]
- Pohrib, M.D.; Niacsu, L. Natural Land Patterns Suitable for Wind Farms Placement in Eastern Romania. Case Study: The Padureni-Husi Area. Int. J. Eng. Technol. 2012, 4, 645. [Google Scholar] [CrossRef]
- Fargione, J.; Kiesecker, J.; Slaats, M.J.; Olimb, S. Wind and wildlife in the Northern Great Plains: Identifying low-impact areas for wind development. PLoS ONE 2012, 7, e41468. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kaldellis, J.K.; Garakis, K.; Kapsali, M. Noise impact assessment on the basis of onsite acoustic noise immission measurements for a representative wind farm. Renew. Energy 2012, 41, 306–314. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Schlindwein, C.; Wittmann, D. Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst. Evol. 1997, 204, 179–193. [Google Scholar] [CrossRef]
- Osborn, M.M.; Kevan, P.G.; Lane, M.A. Pollination biology of Opuntia polyacantha and Opuntia phaeacantha (Cactaceae) in southern Colorado. Plant Syst. Evol. 1988, 159, 85–94. [Google Scholar] [CrossRef]
- Love, L.D.; Christianson, A. Geologic Map of Wyoming; U.S. Geological Survey: Reston, VA, USA, 1985. [Google Scholar]
- Curtis, J.; Grimes, K. Wyoming Climate Atlas. Available online: https://www.wrds.uwyo.edu/sco/climateatlas/ (accessed on 24 February 2025).
- Moore, G.; Goldman, D.; Garland, M.; Taliga, C.; Hinshaw, J. PLANTS Database. Available online: https://plants.usda.gov/home (accessed on 11 January 2022).
- Starfinger, U.; Karrer, G. A standard protocol for testing viability with the Triphenyl Tetrazolium Chloride (TTC) Test. Jul.-Kühn-Arch. 2016, 65–66. [Google Scholar] [CrossRef]
- Delignette-Muller, M.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Rizopoulos, D. GLMMadaptive: Generalized Linear Mixed Models Using Adaptive Gaussian Quadrature. 2023. Available online: https://drizopoulos.github.io/GLMMadaptive/ (accessed on 25 July 2025).
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.E. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package. Version 1.7.2. 2022. Available online: https://rvlenth.github.io/emmeans/ (accessed on 25 July 2025).
- Wickham, H. The Split-Apply_Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Topić, J.; Stančić, Z. Extinction of fen and bog plants and their habitats in Croatia. Biodivers. Conserv. 2006, 15, 3371–3381. [Google Scholar] [CrossRef]
- Walker, K.J.; Preston, C.D. Ecological predictors of extinction risk in the flora of lowland England, UK. Biodivers. Conserv. 2006, 15, 1913–1942. [Google Scholar] [CrossRef]
- Takle, E.S. Climate. In Wildlife and Wind Farms, Conflicts and Solutions: Volume 1—Onshore: Potential Effects; Perrow, M., Ed.; Pelagic Publishing: Exeter, UK, 2017; pp. 24–39. [Google Scholar]
- Van Etten, M.L.; Brunet, J. The impact of global warming on floral traits that affect the selfing rate in a high-altitude plant. Int. J. Plant Sci. 2013, 174, 1099–1108. [Google Scholar] [CrossRef]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Cota-Sánchez, J.H.; Almeida, O.J.; Falconer, D.J.; Choi, H.J.; Bevan, L. Intriguing thigmonastic (sensitive) stamens in the Plains Prickly Pear Opuntia polyacantha (Cactaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 381–389. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the ‘buzz’about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, R.H.; Hou, T.-Z.; Li, Y.-F.; Li, B.-M. Advances in effects of sound waves on plants. J. Integr. Agric. 2014, 13, 335–348. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Bijlsma, R.; Bundgaard, J.; VanPutten, W. Environmental dependence of inbreeding depression and purging in Drosophila melanogaster. J. Evol. Biol. 1999, 12, 1125–1137. [Google Scholar] [CrossRef]
- Keller, L.; Waller, D. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]


| Response Variable | Coefficient | t or z-Values | p-Values |
|---|---|---|---|
| Number seeds | |||
| Intercept | 2.3 | 4.9 | <0.0001 |
| Train | −0.24 | −0.37 | 0.67 |
| Turbine | −0.14 | −0.37 | 0.71 |
| Distance | −0.028 | −2.1 | 0.03 |
| Hand-pollinated | 1.4 | 5.4 | <0.0001 |
| Open | 1.2 | 5.0 | <0.0001 |
| Seed mass | |||
| Intercept | 2.2 | 8.4 | <0.0001 |
| Train | −0.61 | −2.0 | 0.04 |
| Turbine | −0.16 | −0.71 | 0.48 |
| Distance | −0.018 | −2.9 | 0.004 |
| Hand-pollinated | 0.91 | 7.1 | <0.0001 |
| Open | 0.91 | 7.3 | <0.0001 |
| % Viable seeds | |||
| Intercept | −0.27 | −2.4 | 0.018 |
| Train | −0.033 | −0.26 | 0.84 |
| Turbine | −0.049 | −0.51 | 0.70 |
| Distance | −0.0037 | −1.4 | 0.39 |
| Hand-pollinated | 0.31 | 4.8 | <0.0001 |
| Open | 0.26 | 4.1 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tronstad, L.M.; Mazur, M.; Thelen-Wade, L.; Dority, D.; Lester, A.; Weschler, M.; Dillon, M.E. Infrasound-Altered Pollination in a Common Western North American Plant: Evidence from Wind Turbines and Railways. Environments 2025, 12, 266. https://doi.org/10.3390/environments12080266
Tronstad LM, Mazur M, Thelen-Wade L, Dority D, Lester A, Weschler M, Dillon ME. Infrasound-Altered Pollination in a Common Western North American Plant: Evidence from Wind Turbines and Railways. Environments. 2025; 12(8):266. https://doi.org/10.3390/environments12080266
Chicago/Turabian StyleTronstad, Lusha M., Madison Mazur, Lauren Thelen-Wade, Delina Dority, Alexis Lester, Michelle Weschler, and Michael E. Dillon. 2025. "Infrasound-Altered Pollination in a Common Western North American Plant: Evidence from Wind Turbines and Railways" Environments 12, no. 8: 266. https://doi.org/10.3390/environments12080266
APA StyleTronstad, L. M., Mazur, M., Thelen-Wade, L., Dority, D., Lester, A., Weschler, M., & Dillon, M. E. (2025). Infrasound-Altered Pollination in a Common Western North American Plant: Evidence from Wind Turbines and Railways. Environments, 12(8), 266. https://doi.org/10.3390/environments12080266






