All passive samplers were analyzed by spiking internal standard and surrogate solution onto the sorbent and adding 2.0 mL of CS
2 directly to each storage vial or the sampler housing. The vial or sampler was then placed on a shaker for approximately 30 min, and the extract was transferred to an autosampler vial for analysis by GC/MS by US EPA Method 8260 [
22], in synchronous SIM/Scan mode with a 6% cyanopropyl phenyl, 94% polydimethylsiloxane, 30 m × 0.25 mm × 1.4 um column. Field blanks, trip blanks, and laboratory blanks were used to evaluate false positives and/or high bias due to transport, storage, sample handling, and sorbent contamination.
2.4.1. Radiello® 130
The uptake rates used to generate sample concentrations were published by the Radiello
® manufacturer, Fondazione Salvatore Maugeri (Padova, Italy), based on measurements made in a standard atmosphere chamber [
18]. The rates were corrected for the average temperature recorded over the sampling duration using the equation:
where K is the measured temperature in degrees Kelvin, Q
K is the uptake rate at temperature K, and Q
298 is the published reference rate at 298 K.
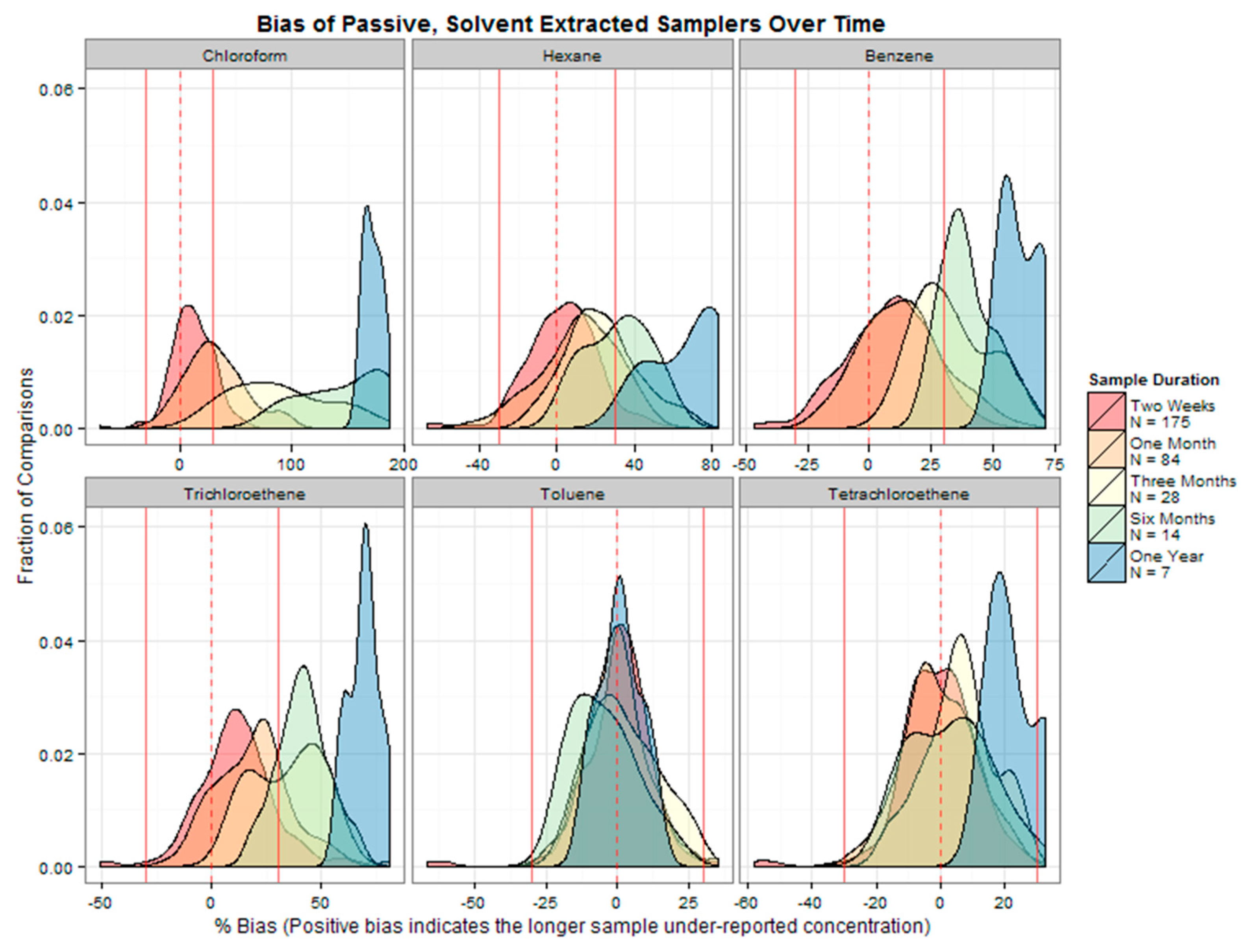
Evaluation of the passive sampler performance over the exposure period was determined by comparing the numerical average of the shorter time segments (e.g., 1 week and 2 weeks) to the concurrent integrated measurement (e.g., biweekly and monthly). For each interval evaluated, the relative percent difference (%Bias) was calculated using the following equation:
where C
A = average concentration of the shorter exposure period sample and C
I = measured concentration of the integrated sample over the longer exposure period.
A positive %Bias indicated the average concentration of the shorter duration measurements was higher than the longer integrated sample concentration. A negative %Bias indicated that the shorter duration measurement underestimated the actual vapor concentration. The acceptance criterion to demonstrate equivalency between concentrations and durations was ±30%. If the reported concentration was a nondetect, the %Bias calculation was performed using half of the reporting limit for the corresponding concentration.
Field blanks were collected using a blank Radiello® 130 cartridge from the media sample batch sent to the field from the laboratory. The cartridge was removed from the sealed storage vial and transferred to the diffusive housing in a similar manner to sample deployment. The cartridge was then immediately removed from the housing, returned to the storage vial, and sealed for shipment back to the laboratory with the field samples. In general, a field blank was collected with each shipment to the laboratory. A total of 47 field blanks were submitted over the duration of the project.
Blank Radiello® 130 cartridges from the media batches were also assigned as trip blanks, measures of exposure during transportation to the laboratory. The cartridge was not opened or removed from the storage vial but was sent back to the laboratory along with the field samples. There were 22 trip blanks submitted for analysis.
In the case of the laboratory blank, a Radiello
® 130 cartridge was extracted with each analytical batch to measure background from the sorbent and the extraction process. A total of 73 unique laboratory blanks were analyzed and reported over the duration of the project. To assist in data interpretation, all blank and field sample results were evaluated down to the method detection limit (MDL). The results of the field, trip, and laboratory blanks for the Radiello
® 130 sampler are summarized in
Table A1,
Table A2 and
Table A3. The number of blanks with detections above the reporting limit (RL) and MDL are tabulated. Summary statistics were then calculated on this subset of positive detections.
Hexane and toluene were commonly detected in the field, trip, and laboratory blanks above the MDL. In the case of the field blanks, several had concentrations above the RL for hexane and toluene. All detections in the trip and laboratory blanks were below the RL but above the MDL. Positive biases for benzene, hexane, and toluene were anticipated for the daily Radiello® 130 samples due to the blank levels. Sample concentrations were not adjusted for concentrations detected in any blank. For the daily passive samples, the average mass collected on the blank sorbent was 0.11, 0.10, and 0.04 µg for benzene, hexane, and toluene, respectively. A positive bias was expected for hexane for the weekly samples as well because the mass collected in the samples was generally less than 10 times the associated blank levels. Blank levels of toluene were not significant when evaluating the weekly samples because the mass collected in the samples was generally greater than 10 times the associated blank levels. Longer duration samples would normally collect more mass and thus would not be significantly affected.
No detections of chloroform or cis-1,2-dichloroethene (cis-1,2-DCE) were measured in any of the blanks. For a small percentage of the blanks, low-concentration detections above the MDL but below the RL were measured for PCE and TCE.
To monitor extraction efficiency, 5.0 µg of toluene-d8 was spiked into each field sample and quality control (QC) sample Radiello 130 cartridge immediately prior to extraction. The recoveries were evaluated against laboratory limits of 70% to 130%. All surrogate recoveries met the laboratory criterion, and summary statistics are presented in
Table A4.
Accuracy of the extraction and analysis step for the target compounds was evaluated by analyzing a laboratory control sample (LCS). An unused Radiello cartridge was spiked with a standard containing 5.0 µg of each compound of interest. The laboratory acceptance criterion for LCS recovery was 70 to 130%. All LCS recoveries met the control limits of 70 to 130%, and summary statistics are presented in
Table A5.
Sample precision was evaluated by collecting field duplicates and by analyzing laboratory control sample duplicates (LCSDs). Field duplicates were collected for approximately every 10 field samples, and an LCSD was prepared and analyzed with each sample analytical batch. Because the LCSD was a second cartridge prepared and extracted in the same manner as the LCS, the relative percentage difference (%RPD) represents the precision of the analytical method from extraction through analysis. The method precision data are summarized in
Table A6 and
Table A7. The laboratory acceptance criterion of %RPD < 25% for the field duplicates was met for all compounds except for TCE in one duplicate sample. The LCS/LCSD met the %RPD < 25% except for benzene in two analytical batches and hexane in five analytical batches.
2.4.2. SKC 575 Badge Sampler
A field/trip blank was collected using a blank SKC 575 badge sampler from the media sample batch sent to the field from the laboratory. The blank sampler was opened during preparation of field samples for deployment and then sealed and stored at ambient temperature until sample shipment. A total of 1 field blank was submitted over the duration of the project. In the case of the laboratory blank, a SKC 575 badge sampler was extracted with each analytical batch to measure background from the sorbent and the extraction process. A total of 2 laboratory blanks were analyzed and reported over the duration of the project. To assist in data interpretation, all blank samples and all field sample results were evaluated down to the MDL. The results of the QC samples for the SKC 575 badge samplers are summarized in
Table A8 and
Table A9. Summary statistics were then calculated on the subset of samples with positive detections. Benzene was detected in the field/trip and laboratory blank above the MDL. A positive bias for benzene was anticipated for the 6-month and 1-year SKC575 badge samples due to the blank levels. The average mass of benzene collected on the sorbent for the 6-month and 1-year SKC 575 badge samples was 0.94 and 1.2 ug/m
3, respectively. No detections of any of the other target analytes were measured in any of the blanks.
Accuracy of the extraction and analysis step for the target compounds was evaluated by analyzing an LCS. An unused SKC 575 badge sampler was spiked with a standard containing 5.0 µg of each compound of interest. The laboratory acceptance criterion for LCS recovery was 70 to 130%. All LCS recoveries met the control limits of 70 to 130%, and summary statistics are presented in
Table A10.
Sample precision was evaluated by collecting field duplicates and by analyzing LCSDs. Field duplicates were collected for approximately every 10 field samples, and an LCSD was prepared and analyzed with each sample preparation batch. Because the LCSD was a second badge that was prepared and extracted in the same manner as the LCS, the %RPD represents the precision of the analytical method from extraction through analysis. The method precision is summarized in
Table A11 and
Table A12. The laboratory acceptance criterion of %RPD < 25% was met for all compounds except for benzene, chloroform, and hexane in one field duplicate sample and hexane in two analytical batches.
To monitor extraction efficiency, 5.0 µg of toluene-d8 was spiked into each field sample and QC sample immediately prior to extraction. The recoveries were evaluated against laboratory limits of 70% to 130%. All surrogate recoveries met the laboratory criterion, and summary statistics are presented in
Table A13.
2.4.3. WMSTM
A field/trip blank was collected using a blank WMSTM sampler from the media sample batch sent to the field from the laboratory. The blank sampler was opened during preparation of field samples for deployment and then sealed and stored at ambient temperature until sample shipment. A total of 1 field/trip blank was submitted over the duration of the project. Benzene was detected above the MDL but below the reporting limit. No other compounds were detected in the field/trip blank.
For a laboratory blank, a WMS
TM sampler was extracted with each analytical batch to measure background from the sorbent and the extraction process. A total of 2 laboratory blanks were analyzed and reported over the duration of the project. To assist in data interpretation, all blank samples and all field sample results were evaluated down to the MDL. The results of the field and laboratory blanks for the WMS
TM samplers are summarized in
Table A14 and
Table A15. Summary statistics were then calculated on the subset of sample with positive detections.
Benzene was the only target analyte detected in the one field/trip and the laboratory blanks above the MDL. The blank level of benzene is significant when evaluating the 6-month and 1-year samples with which it is associated, because the concentrations detected in the samples were less than 10 times the amount detected in the blank. No detections of chloroform, cis-1,2-DCE, hexane, toluene, PCE, or TCE were measured in any of the blanks. To monitor extraction efficiency, 5.0 µg of toluene-d8 was spiked into each field sample and QC sample WMS vial immediately prior to extraction. The recoveries were evaluated against laboratory limits of 70% to 130%. All surrogate recoveries met the laboratory criterion, and summary statistics are presented in
Table A16.
Accuracy of the extraction and analysis steps for the target compounds was evaluated by analyzing an LCS. An unused WMS
TM sampler was spiked with a standard containing 5.0 µg of each compound of interest. The laboratory acceptance criterion for LCS recovery was 70 to 130%. All LCS recoveries met the control limits of 70 to 130%, and summary statistics are presented in
Table A17.
Sample precision was evaluated by collecting field duplicates and by analyzing LCSDs. Field duplicates were collected for approximately every 10 field samples, and an LCSD was prepared and analyzed with each sample preparation batch. Because the LCSD was a second cartridge prepared and extracted in the same manner as the LCS, the %RPD represents the precision of the analytical method from extraction through analysis. The method precision is summarized in
Table A18 and
Table A19. The laboratory acceptance criterion of %RPD < 25% was met for all compounds except for hexane in one field duplicate and one LCSD.
The WMS
TM sample concentrations were adjusted for uptake rate variations due to temperature as recorded onsite by an Onset HOBO
® data logging system placed on each wooden sample rack. All measurements were made in accordance with an approved quality assurance project plan. Although some deviations were identified, the deviances did not affect the interpretation of our results [
17].