Chemometric Evaluation of 16 Priority PAHs in Soil and Roots of Syringa vulgaris and Ficus carica from the Bor Region (Serbia): An Insight into the Natural Plant Potential for Soil Phytomonitoring and Phytoremediation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sampling Area
2.2. Sample Collection and Pretreatment
2.3. Extraction of PAHs from Soil and Plant Samples and Chemical Analyses
2.3.1. Used Reagents and Measured Soil Parameters
2.3.2. Extraction and Instrumental Analysis of PAHs with QA/QC
2.4. Data Processing
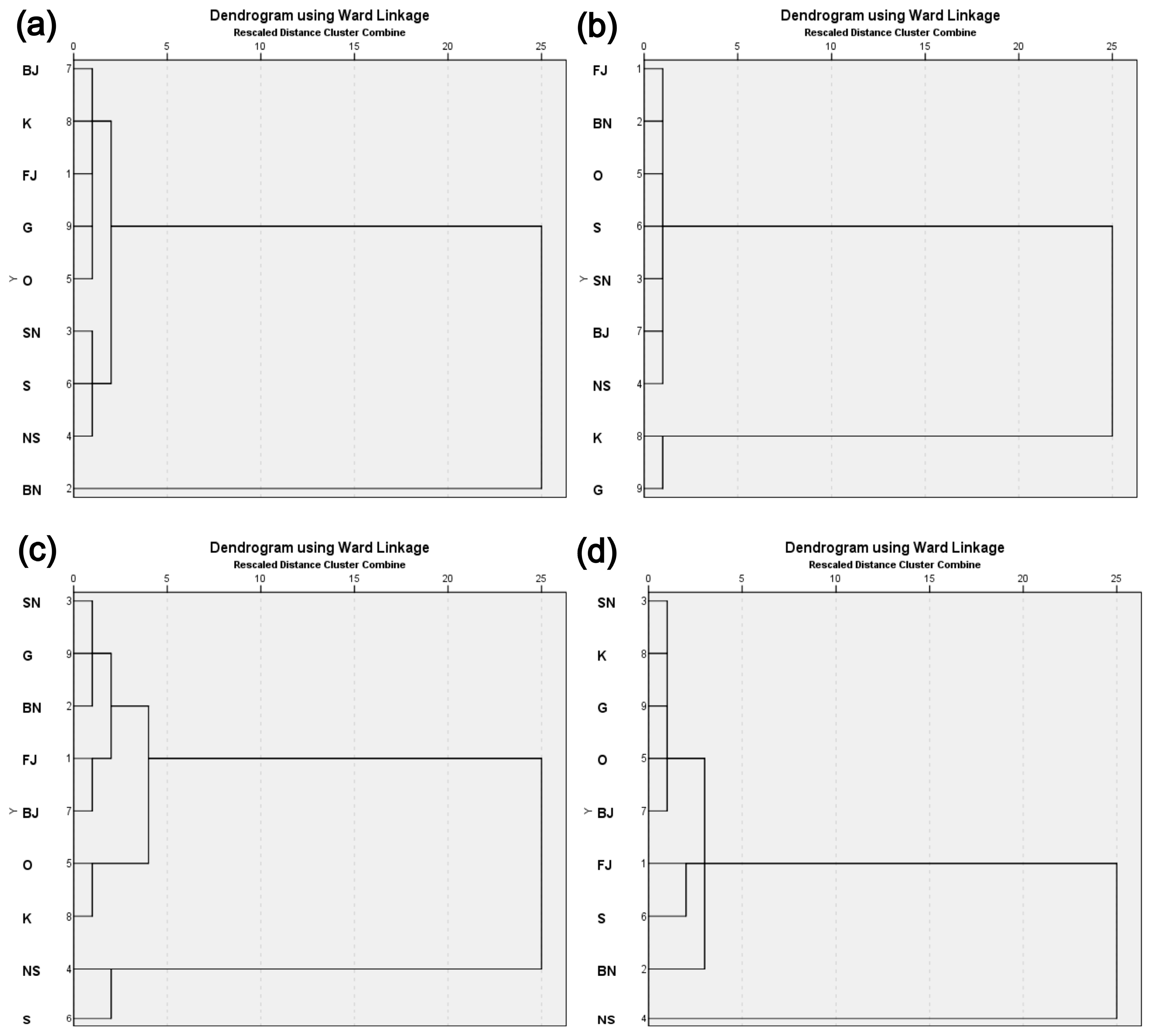
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishnoi, K.; Rani, P.; Bishnoi, N.R. Polycyclic aromatic hydrocarbons in sewage-irrigated vegetables from industrial cities in Haryana, India. Environ. Monit. Assess. 2024, 196, 337. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Jin, X.; Zeng, Y.; Deng, L.; Wang, X.; Chen, Z. Uptake, translocation, and risk assessment of PAHs in contaminated soil-air-vegetable systems based on a field simulation experiment. Environ. Pollut. 2021, 271, 116361. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, L.; Yu, Y.; Tan, L.; Wang, Z.; Suo, S.; Liu, C.; Qin, Y.; Peng, X.; Lu, H.; et al. Distribution, source, risk and phytoremediation of polycyclic aromatic hydrocarbons (PAHs) in typical urban landscape waters recharged by reclaimed water. J. Environ. Manag. 2023, 330, 117214. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, K.; Rameshpathy, M. Bioremediation of polycyclic aromatic hydrocarbons contaminated soils: Recent progress, perspectives and challenges. Environ. Monit. Assess. 2023, 195, 1441. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Alagić, S.Č.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Res. 2015, 17, 597–614. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, A.; Bindra, S.; Sharma, A. Phytoremediation: An emerging green technology for dissipation of PAHs from soil. J. Geochem. Explor. 2024, 259, 107426. [Google Scholar] [CrossRef]
- Kicińska, A.; Dmytrowski, P. Anthropogenic impact on soils of protected areas—Example of PAHs. Sci. Rep. 2023, 13, 1524. [Google Scholar] [CrossRef]
- Meištininkas, R.; Vaškevičiene, I.; Dikšaityte, A.; Pedišius, N.; Žaltauskaite, J. Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species. Environments 2024, 11, 64. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desa, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic aromatic hydrocarbons: Soil pollution and remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- USEPA. United States Environmental Protection Agency (2024): Risk-Based Screening Table—Generic Tables; Regional Screening Level, RSL Summary Table (TR=1x10-6 and THQ=1.0) May 2024. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 23 January 2025).
- Petruzzelli, G.; Pezzarossa, B.; Pedron, F. The Fate of Chemical Contaminants in Soil with a View to Potential Risk to Human Health: A Review. Environments 2025, 12, 183. [Google Scholar] [CrossRef]
- Cvetkovic, J.S.; Mitic, V.D.; Stankov Jovanovic, V.P.; Dimitrijevic, M.V.; Petrovic, G.M.; Nikolic Mandic, S.D.; Stojanovic, G.S. Optimization of the QuEChERS extraction procedure for the determination of polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 1711–1720. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B. The Impact of Organic Matter on Polycyclic Aromatic Hydrocarbon (PAH) Availability and Persistence in Soils. Molecules 2020, 25, 2470. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Liu, R.; Jin, C.; Dai, Y. Efficiency of five ornamental plant species in the phytoremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecol. Eng. 2015, 75, 384–391. [Google Scholar] [CrossRef]
- Tarigholizadeh, S.; Sushkova, S.; Rajput, V.D.; Ranjan, A.; Arora, J.; Dudnikova, T.; Barbashev, A.; Mandzhieva, S.; Minkina, T.; Wong, M.H. Transfer and Degradation of PAHs in the Soil–Plant System: A Review. J. Agric. Food Chem. 2024, 72, 46–64. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Liu, J.; Kong, H. Metabolism and subcellular distribution of anthracene in tall fescue (Festuca arundinacea Schreb.). Plant Soil 2013, 365, 171–182. [Google Scholar] [CrossRef]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Włóka, D.; Placek, A.; Smol, M.; Rorat, A.; Hutchison, D.; Kacprzak, M. The efficiency and economic aspects of phytoremediation technology using Phalaris arundinacea L. and Brassica napus L. combined with compost and nano SiO2 fertilization for the removal of PAHs from soil. J. Environ. Manag. 2019, 234, 311–319. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Alagić, S.Č.; Stankov Jovanović, V.P.; Mitić, V.D.; Cvetković, J.S.; Petrović, G.M.; Stojanović, G.S. Bioaccumulation of HMW PAHs in the roots of wild blackberry from the Bor region (Serbia): Phytoremediation and biomonitoring aspects. Sci. Total Environ. 2016, 562, 561–570. [Google Scholar] [CrossRef]
- Alagić, S.Č.; Stankov Jovanović, V.P.; Mitić, V.D.; Nikolić, J.S.; Petrović, G.M.; Tošić, S.B.; Stojanović, G.S. The effect of multiple contamination of soil on LMW and MMW PAHs accumulation in the roots of Rubus fruticosus L. naturally growing near The Copper Mining and Smelting Complex Bor (East Serbia). Environ. Sci. Pollut. Res. 2017, 24, 15609–15621. [Google Scholar] [CrossRef]
- Muratova, A.; Lyubun Sungurtseva, Y.I.; Turkovskaya, O.; Nurzhanova, A. Physiological and biochemical characteristic of Miscanthus × giganteus grown in heavy metal-oil sludge co-contaminated soil. J. Environ. Sci. 2022, 115, 114–125. [Google Scholar] [CrossRef]
- Qiu, Y.-W.; Qiu, H.-L.; Li, J.; Zhang, G. Bioaccumulation and Cycling of Polycyclic Aromatic Hydrocarbons (PAHs) in Typical Mangrove Wetlands of Hainan Island, South China. Arch. Environ. Contam. Toxicol. 2018, 75, 464–475. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Tarigholizadeh, S.; Antonenko, E.; Konstantinova, E.; Gülser, C.; Dudnikova, T.; Barbashev, A.; Kızılkaya, R. PAHs accumulation in soil-plant system of Phragmites australis Cav. in soil under long-term chemical contamination. Eurasian J. Soil Sci. 2020, 9, 242–253. [Google Scholar] [CrossRef]
- Zhen, M.; Chen, H.; Liu, Q.; Song, B.; Wang, Y.; Tang, J. Combination of rhamnolipid and biochar in assisting phytoremediation of petroleum hydrocarbon contaminated soil using Spartina anglica. J. Environ. Sci. 2019, 85, 107–118. [Google Scholar] [CrossRef]
- Ratola, N.; Amigo, J.M.; Alves, A. Comprehensive assessment of pine needles as bioindicators of PAHs using multivariate analysis. The importance of temporal trends. Chemosphere 2010, 81, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.F.; Esen, F.; Tasdemir, Y. Biomonitoring and Source Identification of Polycyclic Aromatic Hydrocarbons (PAHs) Using Pine Tree Components from Three Different Sites in Bursa, Turkey. Arch. Environ. Contam. Toxicol. 2020, 78, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, C.; Arrouays, D.; Bernoux, M. Comparison between analytical methods for organic carbon and organic matter determination in sandy Spodosols of France. Commun. Soil Sci. Plant Anal. 1998, 29, 2227–2233. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieślik, E. Comparison of different modifications on QuEChERS sample preparation method for PAHs determination in black, green, red and white tea. Environ. Sci. Pollut. Res. 2013, 21, 1326–1338. [Google Scholar] [CrossRef]
- Environment Agency Head Office (EAHO). The Determination of Polycyclic Aromatichydrocarbons in Soil by Dichloromethane Extraction Using Gaschromatography with Mass Spectrometric Detection; Standing Committee of Analysts Environment Agency (National Laboratory Service): Nottingham, UK, 2003. Available online: https://www.gov.uk/government/organisations/environment-agency (accessed on 23 January 2025).
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for Single Laboratory Validation of Methods of Analysis. (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. Available online: https://publications.iupac.org/pac/2002/pdf/7405x0835.pdf (accessed on 23 January 2025). [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education Limited: London, UK, 2005. [Google Scholar]
- Fu, J.; Zhao, C.; Luo, Y.; Liu, C.; Kyzas, G.Z.; Luo, Y.; Zhao, D.; An, S.; Zhu, H. Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J. Hazard. Mater. 2014, 270, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Vane, C.H.; Kim, A.W.; Beriro, D.J.; Cave, M.R.; Knights, K.; Moss-Hayes, V.; Nathanail, P.C. Polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in urban soils of Greater London, UK. Appl. Geochem. 2014, 51, 303–314. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Lube, G.V.; Jarcovis, R.; De, L.; da Silva, M.J.; de Souza, A.C. Navigating the PAH maze: Bioaccumulation, risks, and review of the quality guidelines in marine ecosystems with a spotlight on the Brazilian coastline. Mar. Pollut. Bull. 2023, 197, 115764. [Google Scholar] [CrossRef]

| Soil Parameters for SV | Soil Parameters for FC | |||||
|---|---|---|---|---|---|---|
| Location | pH | EC (μS/cm) | OM (%) | pH | EC (μS/cm) | OM (%) |
| FJ | 7.02 | 566 | 7.04 | 7.12 | 824 | 8.00 |
| BN | 7.37 | 850 | 8.27 | 7.53 | 588 | 7.07 |
| SN | 7.45 | 386 | 7.94 | 7.27 | 504 | 8.30 |
| NS | 7.45 | 549 | 8.28 | 7.39 | 196 | 9.29 |
| O | 7.08 | 351 | 7.00 | 7.77 | 362 | 5.98 |
| S | 6.46 | 267 | 8.73 | 7.45 | 982 | 14.28 |
| BJ | 7.37 | 508 | 9.45 | 7.06 | 284 | 10.56 |
| K | 7.39 | 518 | 5.78 | 7.67 | 378 | 7.57 |
| G | 6.55 | 240 | 5.42 | 7.05 | 392 | 6.87 |
| Compound | Rt (min) | Quantifier Ion | LOD (µg/kg) | LOQ (µg/kg) | Calibration Curve Equation | Correlation Coefficient | Recoverys (%) | Recoveryp (%) | RSDs (%) | RSDp (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nap | 11.792 | 128.0 | 0.78 | 2.60 | y = 0.0343x | 0.99 | 113 | 102 | 10.8 | 5.6 |
| Acy | 17.721 | 152.0 | 0.29 | 0.96 | y = 0.0056x | 0.99 | 100 | 99 | 6.37 | 4.17 |
| Ace | 18.460 | 153.0 | 0.19 | 0.65 | y = 0.0627x | 0.99 | 94 | 110 | 2.74 | 5.37 |
| Flr | 20.380 | 166.0 | 0.48 | 1.60 | y = 0.004x | 0.99 | 95 | 88 | 7.38 | 6.74 |
| Phe | 24.000 | 178.0 | 1.50 | 5.00 | y = 0.298x | 0.99 | 96 | 95 | 9.11 | 7.44 |
| Ant | 24.177 | 178.0 | 0.39 | 1.30 | y = 0.109x | 0.98 | 100 | 111 | 4.79 | 9.54 |
| Flt | 28.575 | 202.0 | 0.51 | 1.70 | y = 0.069x | 0.98 | 96 | 93 | 6.34 | 7.45 |
| Pyr | 29.383 | 202.0 | 0.60 | 2.00 | y = 0.104x | 0.99 | 96 | 89 | 4.76 | 6.54 |
| CHR | 34.072 | 228.0 | 1.02 | 3.40 | y = 0.053x | 0.98 | 116 | 106 | 4.97 | 5.42 |
| BaA | 34.238 | 228.0 | 0.50 | 1.67 | y = 0.029x | 0.99 | 112 | 95 | 7.98 | 8.42 |
| BbF+BkF | 38.103 | 253.0 | 0.05 | 0.18 | y = 1.495x | 0.99 | 91 | 98 | 3.87 | 2.97 |
| BaP | 39.039 | 253.0 | 0.60 | 2.00 | y = 0.0225x | 0.99 | 92 | 98 | 6.54 | 5.56 |
| IcP | 42.760 | 277.0 | 0.63 | 2.10 | y = 0.076x | 0.99 | 99 | 84 | 4.79 | 5.45 |
| DhA | 42.878 | 279.0 | 1.17 | 3.90 | y = 0.0037x | 0.99 | 90 | 107 | 7.86 | 5.87 |
| BgP | 43.637 | 276.0 | 0.90 | 2.00 | y = 0.626x | 0.99 | 96 | 100 | 8.93 | 6.87 |
| Location/Plant | LMW PAHs | |||||||
|---|---|---|---|---|---|---|---|---|
| Nap | Acy | Ace | Flr | Ant | Phe | |||
| FJ | SV | 106 ± 14 | 12 ± 1 | 3.6 ± 0.6 | 11.9 ± 0.3 | 3.4 ± 0.7 | 49 ± 4 | |
| FC | 149 ± 3 | 17.8 ± 0.5 | 4.3 ± 0.5 | 12.0 ± 0.1 | 2.3 ± 0.3 | 69 ± 4 | ||
| BN | SV | 178 ± 4 | 52 ± 4 | 38 ± 5 | 36 ± 3 | 166 ± 6 | 395 ± 13 | |
| FC | 68 ± 1 | 16.8 ± 0.8 | 5.8 ± 0.3 | 10.0 ± 0.8 | 14 ± 1 | 65 ± 8 | ||
| SN | SV | 165 ± 3 | 31.0 ± 0.2 | 15.8 ± 0.7 | 24 ± 3 | 9.2 ± 0.6 | 154 ± 13 | |
| FC | 158 ± 13 | 35 ± 3 | 16.4 ± 0.7 | 28 ± 2 | 8.0 ± 0.6 | 119 ± 15 | ||
| NS | SV | 64 ± 2 | 32 ± 1 | 4.6 ± 0.1 | 10.1 ± 0.1 | 17 ± 2 | 96 ± 2 | |
| FC | 165 ± 10 | 36 ± 2 | 10.2 ± 0.6 | 20 ± 2 | 11.5 ± 0.4 | 116 ± 8 | ||
| O | SV | 187 ± 26 | 29 ± 4 | 16.1 ± 0.8 | 33 ± 1 | 4.7 ± 0.3 | 109 ± 17 | |
| FC | 85 ± 6 | 14.6 ± 0.9 | 9.0 ± 0.2 | 26 ± 3 | nd | 102 ± 9 | ||
| S | SV | 147 ± 8 | 18.4 ± 0.4 | 8.2 ± 0.6 | 33 ± 1 | nd | 189 ± 11 | |
| FC | 111 ± 11 | 46 ± 1 | 24.3 ± 0.4 | 37 ± 2 | 53 ± 4 | 160 ± 4 | ||
| BJ | SV | 106 ± 5 | 21 ± 1 | 37 ± 2 | 27 ± 3 | nd | 255 ± 18 | |
| FC | 190 ± 17 | 35 ± 5 | 13 ± 1 | 20 ± 3 | nd | 86 ± 8 | ||
| K | SV | 66 ± 5 | 6.2 ± 0.7 | 13.6 ± 0.4 | 11 ± 2 | nd | 221 ± 13 | |
| FC | 86 ± 4 | 9 ± 1 | 7.5 ± 0.3 | 14 ± 2 | nd | 71 ± 3 | ||
| G | SV | 108 ± 7 | 15 ± 1 | 9.4 ± 0.1 | 19.3 ± 0.9 | nd | 73 ± 5 | |
| FC | 143 ± 8 | 22 ± 2 | 8.7 ± 0.3 | 15.5 ± 0.9 | 5.6 ± 0.9 | 96 ± 2 | ||
| USEPA RSL a (mg/kg) | ||||||||
| Residential | 2.0 b,c | ne b,c | 3600 b/360 c | 2400 b/240 c | 18,000 b/1800 c | ne b,c | ||
| Industrial | 8.6 b,c | ne b,c | 45,000 b/4500 c | 30,000 b/3000 c | 230,000 b/23,000 c | ne b,c | ||
| Location/Plant | HMW PAHs | |||||||
| Flt | Pyr | BaA | CHR | BaP | BbF+BkF | DhA | ||
| FJ | SV | 67 ± 4 | 35.5 ± 0.3 | 83 ± 9 | 17 ± 2 | 8 ± 2 | 12 ± 1 | 30 ± 2 |
| FC | 99 ± 4 | 52 ± 4 | 71 ± 5 | 14.6 ± 0.8 | 7.7 ± 0.9 | 10.0 ± 0.1 | 24.7 ± 0.7 | |
| BN | SV | 1504 ± 70 | 831 ± 39 | 560 ± 41 | 270 ± 25 | 6.5 ± 0.5 | 5.0 ± 0.6 | 70 ± 7 |
| FC | 199 ± 10 | 119 ± 13 | 107 ± 5 | 39 ± 4 | 5.8 ± 0.6 | nd | nd | |
| SN | SV | 232 ± 6 | 131 ± 2 | 165 ± 20 | 28 ± 5 | 9.4 ± 0.3 | 11.1 ± 0.3 | nd |
| FC | 147 ± 13 | 81 ± 4 | 61 ± 6 | 18 ± 1 | 9.5 ± 0.3 | 7.4 ± 0.5 | nd | |
| NS | SV | 256 ± 3 | 194 ± 3 | 108 ± 6 | 34 ± 2 | 7.0 ± 0.8 | nd | nd |
| FC | 307 ± 37 | 260 ± 20 | 248 ± 13 | 75 ± 3 | 9.7 ± 0.1 | 50 ± 4 | 128 ± 6 | |
| O | SV | 68 ± 8 | 30 ± 2 | 59 ± 7 | nd | 9.3 ± 0.2 | 10.1 ± 0.3 | nd |
| FC | 58 ± 5 | 23 ± 3 | 63 ± 5 | 6.3 ± 0.5 | 9.8 ± 0.4 | 13 ± 2 | nd | |
| S | SV | 271 ± 12 | 132 ± 9 | 99 ± 9 | 42 ± 4 | 9.3 ± 0.7 | 8.2 ± 0.3 | 69 ± 5 |
| FC | 401 ± 15 | 230 ± 6 | 309 ± 33 | 44 ± 3 | 9.4 ± 0.8 | nd | 109 ± 6 | |
| BJ | SV | 38.5 ± 0.6 | 25 ± 3 | 42 ± 5 | 10.0 ± 0.5 | 10.7 ± 0.5 | nd | nd |
| FC | 102 ± 14 | 65 ± 7 | 155 ± 8 | 14 ± 1 | 8.7 ± 0.6 | 8.7 ± 0.4 | nd | |
| K | SV | 21 ± 2 | 12.8 ± 0.4 | nd | nd | 8.2 ± 0.7 | nd | nd |
| FC | 19.5 ± 0.6 | 11.0 ± 0.6 | nd | nd | 9.3 ± 0.3 | nd | nd | |
| G | SV | 24 ± 3 | 13.2 ± 0.4 | 38 ± 5 | nd | 9.0 ± 0.6 | nd | nd |
| FC | 177 ± 1 | 117 ± 2 | 68 ± 17 | 28.6 ± 0.4 | 9.5 ± 0.6 | nd | nd | |
| USEPA RSL a (mg/kg) | BbF/BkF | |||||||
| Residential | 2400 b/240 c | 1800 b/180 c | 1.1 b,c | 110 b,c | 0.11 b,c | 1.1 b,c/11 b,c | 0.11 b,c | |
| Industrial | 30,000 b/3000 c | 23,000 b/2300 c | 21.0 b,c | 2100 b,c | 2.1 b,c | 21 b,c/210 b,c | 2.1 b,c | |
| Location/Plant | LMW PAHs | |||||||
|---|---|---|---|---|---|---|---|---|
| Nap | Acy | Ace | Flr | Ant | Phe | |||
| FJ | SV | 32 ± 2 | 35 ± 6 | 60 ± 7 | 68 ± 8 | 21 ± 2 | 301 ± 34 | |
| FC | 31 ± 1 | 25.7 ± 0.7 | 38 ± 3 | 143 ± 9 | 14 ± 1 | 269 ± 16 | ||
| BN | SV | 23.4 ± 0.9 | 3.9 ± 0.1 | 94 ± 8 | 127 ± 6 | nd | 335 ± 12 | |
| FC | 129 ± 3 | 185 ± 9 | 10.4 ± 0.4 | 51 ± 3 | nd | 138.4 ± 0.7 | ||
| SN | SV | 25 ± 3 | 4.39 ± 0.05 | 10 ± 1 | 403 ± 24 | 19.68 ± 0.05 | 157 ± 8 | |
| FC | 59 ± 2 | 30 ± 2 | 15.4 ± 0.1 | 131 ± 5 | nd | 133 ± 1 | ||
| NS | SV | 27 ± 2 | nd | 10 ± 1 | 222 ± 4 | nd | 426 ± 19 | |
| FC | 99 ± 5 | nd | 16 ± 2 | 85 ± 5 | nd | 145 ± 5 | ||
| O | SV | 24 ± 3 | 183 ± 17 | 65 ± 1 | 331 ± 54 | nd | 52 ± 2 | |
| FC | 29.6 ± 0.7 | 2.91 ± 0.05 | 21.2 ± 0.8 | 81.4 ± 0.8 | nd | 108.2 ± 0.4 | ||
| S | SV | 52 ± 5 | nd | 29 ± 1 | 279 ± 40 | nd | nd | |
| FC | 114 ± 7 | nd | 15 ± 2 | 300 ± 28 | nd | 101 ± 3 | ||
| BJ | SV | 20 ± 3 | nd | 15.0 ± 0.7 | 627 ± 33 | nd | 16 ± 2 | |
| FC | 89 ± 4 | 3.1 ± 0.2 | 5.2 ± 0.6 | 64 ± 5 | nd | nd | ||
| K | SV | 18 ± 2 | nd | 111 ± 8 | 5592 ± 90 | nd | nd | |
| FC | 82 ± 8 | nd | 9.4 ± 0.8 | 98 ± 8 | nd | 141 ± 4 | ||
| G | SV | 15.0 ± 0.9 | nd | 55 ± 3 | 4990 ± 156 | nd | 79 ± 9 | |
| FC | 79 ± 4 | 2.3 ± 0.3 | 7.7 ± 0.8 | 189.7 ± 0.5 | nd | 149 ± 8 | ||
| Location/Plant | HMW PAHs | |||||||
| Flt | Pyr | BaA | CHR | BaP | BbF+BkF | DhA | ||
| FJ | SV | 36 ± 5 | 14 ± 1 | nd | nd | nd | 2.8 ± 0.5 | nd |
| FC | 124 ± 4 | 12.5 ± 0.6 | 77 ± 3 | nd | nd | 4.5 ± 0.3 | 90 ± 2 | |
| BN | SV | 14.2 ± 0.1 | 5.6 ± 0.4 | nd | nd | nd | 7.4 ± 0.4 | 58 ± 5 |
| FC | nd | nd | 192 ± 4 | nd | nd | 20 ± 1 | 146 ± 33 | |
| SN | SV | 385 ± 52 | 78 ± 13 | 50 ± 2 | nd | nd | nd | nd |
| FC | nd | nd | 8 ± 1 | nd | nd | nd | 97 ± 6 | |
| NS | SV | 36 ± 4 | 7.2 ± 0.2 | nd | nd | nd | 8.3 ± 0.2 | 901 ± 16 |
| FC | nd | nd | 816 ± 52 | nd | nd | nd | nd | |
| O | SV | 2.5 ± 0.3 | 2.6 ± 0.1 | nd | nd | nd | 4.7 ± 0.5 | 285 ± 26 |
| FC | nd | nd | nd | nd | nd | nd | 172.8 ± 0.3 | |
| S | SV | 7.1 ± 0.7 | 6.4 ± 0.8 | nd | nd | nd | nd | 220 ± 2 |
| FC | nd | nd | 148 ± 27 | nd | nd | 9.7 ± 0.5 | 129 ± 3 | |
| BJ | SV | 231 ± 4 | 39.5 ± 0.5 | nd | nd | nd | 8.3 ± 0.3 | 329 ± 42 |
| FC | nd | nd | 47 ± 6 | nd | nd | 7.4 ± 0.4 | 29 ± 4 | |
| K | SV | 20 ± 3 | 6.7 ± 0.5 | nd | nd | nd | 3.7 ± 0.2 | 191 ± 5 |
| FC | nd | nd | nd | nd | nd | nd | 48.7 ± 0.9 | |
| G | SV | 17 ± 4 | 4.3 ± 0.2 | nd | nd | nd | 2.6 ± 0.3 | 278 ± 27 |
| FC | nd | nd | nd | nd | nd | 7.3 ± 0.4 | 55 ± 6 | |
| Location/Plant | LMW PAHs | |||||||
|---|---|---|---|---|---|---|---|---|
| Nap | Acy | Ace | Flr | Ant | Phe | |||
| FJ | SV | 0.30 | 2.77 | 16.88 | 5.71 | 6.10 | 6.12 | |
| FC | 0.21 | 1.44 | 8.91 | 11.92 | 6.30 | 3.90 | ||
| BN | SV | 0.13 | 0.08 | 2.45 | 3.48 | nc | 0.85 | |
| FC | 1.90 | 11.02 | 1.81 | 5.12 | nc | 2.14 | ||
| SN | SV | 0.15 | 0.14 | 0.66 | 16.60 | 2.14 | 1.01 | |
| FC | 0.37 | 0.88 | 0.94 | 4.74 | nc | 1.12 | ||
| NS | SV | 0.42 | nc | 2.09 | 21.94 | nc | 4.42 | |
| FC | 0.60 | nc | 1.55 | 4.18 | nc | 1.24 | ||
| O | SV | 0.13 | 6.26 | 4.05 | 10.07 | nc | 0.48 | |
| FC | 0.35 | 0.20 | 2.34 | 3.20 | nc | 1.06 | ||
| S | SV | 0.35 | nc | 3.54 | 8.52 | nc | nc | |
| FC | 1.03 | nc | 0.62 | 8.01 | nc | 0.63 | ||
| BJ | SV | 0.19 | nc | 0.41 | 23.14 | nc | 0.06 | |
| FC | 0.47 | 0.09 | 0.40 | 3.23 | nc | nc | ||
| K | SV | 0.27 | nc | 8.15 | 499.55 | nc | nc | |
| FC | 0.47 | 0.09 | 0.40 | 3.23 | nc | nc | ||
| G | SV | 0.14 | nc | 5.85 | 258.51 | nc | 1.08 | |
| FC | 0.55 | 0.10 | 0.89 | 12.27 | nc | 1.55 | ||
| Location/Plant | HMW PAHs | |||||||
| Flt | Pyr | BaA | CHR | BaP | BbF+BkF | DhA | ||
| FJ | SV | 0.54 | 0.39 | nc | nc | nc | 0.24 | nc |
| FC | 1.26 | 0.24 | 1.09 | nc | nc | 0.45 | 3.63 | |
| BN | SV | 0.01 | 0.01 | nc | nc | nc | 1.47 | 0.84 |
| FC | nc | nc | 1.79 | nc | nc | nc | nc | |
| SN | SV | 1.65 | 0.59 | 0.31 | nc | nc | nc | nc |
| FC | nc | nc | 0.13 | nc | nc | nc | nc | |
| NS | SV | 0.14 | 0.04 | nc | nc | nc | nc | nc |
| FC | nc | nc | 3.29 | nc | nc | nc | nc | |
| O | SV | 0.04 | 0.09 | nc | nc | nc | 0.46 | nc |
| FC | nc | nc | nc | nc | nc | nc | nc | |
| S | SV | 0.03 | 0.05 | nc | nc | nc | nc | 3.19 |
| FC | nc | nc | 0.48 | nc | nc | nc | 1.18 | |
| BJ | SV | 5.98 | 1.59 | nc | nc | nc | nc | nc |
| FC | nc | nc | 0.30 | nc | nc | 0.85 | nc | |
| K | SV | 0.95 | 0.52 | nc | nc | nc | nc | nc |
| FC | nc | nc | nc | nc | nc | nc | nc | |
| G | SV | 0.71 | 0.32 | nc | nc | nc | nc | nc |
| FC | nc | nc | nc | nc | nc | nc | nc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papludis, A.D.; Alagić, S.Č.; Milić, S.M.; Nikolić, J.S.; Jevtović, S.Č.; Stankov Jovanović, V.P.; Stojanović, G.S. Chemometric Evaluation of 16 Priority PAHs in Soil and Roots of Syringa vulgaris and Ficus carica from the Bor Region (Serbia): An Insight into the Natural Plant Potential for Soil Phytomonitoring and Phytoremediation. Environments 2025, 12, 256. https://doi.org/10.3390/environments12080256
Papludis AD, Alagić SČ, Milić SM, Nikolić JS, Jevtović SČ, Stankov Jovanović VP, Stojanović GS. Chemometric Evaluation of 16 Priority PAHs in Soil and Roots of Syringa vulgaris and Ficus carica from the Bor Region (Serbia): An Insight into the Natural Plant Potential for Soil Phytomonitoring and Phytoremediation. Environments. 2025; 12(8):256. https://doi.org/10.3390/environments12080256
Chicago/Turabian StylePapludis, Aleksandra D., Slađana Č. Alagić, Snežana M. Milić, Jelena S. Nikolić, Snežana Č. Jevtović, Vesna P. Stankov Jovanović, and Gordana S. Stojanović. 2025. "Chemometric Evaluation of 16 Priority PAHs in Soil and Roots of Syringa vulgaris and Ficus carica from the Bor Region (Serbia): An Insight into the Natural Plant Potential for Soil Phytomonitoring and Phytoremediation" Environments 12, no. 8: 256. https://doi.org/10.3390/environments12080256
APA StylePapludis, A. D., Alagić, S. Č., Milić, S. M., Nikolić, J. S., Jevtović, S. Č., Stankov Jovanović, V. P., & Stojanović, G. S. (2025). Chemometric Evaluation of 16 Priority PAHs in Soil and Roots of Syringa vulgaris and Ficus carica from the Bor Region (Serbia): An Insight into the Natural Plant Potential for Soil Phytomonitoring and Phytoremediation. Environments, 12(8), 256. https://doi.org/10.3390/environments12080256







