Abstract
This study explores the bioaccumulation behavior of heavy metals (cadmium (Cd), lead (Pb), and zinc (Zn)) in lavender (Lavandula spp.) cultivated under controlled greenhouse conditions to assess its potential in sustainable phytoremediation. The plants were grown in pots filled with either unpolluted soil or soil artificially enriched with cadmium, lead, or zinc at concentrations exceeding the normal (Cd 1 mg/kg d.w.; Pb 20 mg/kg d.w.; Zn 100 mg/kg d.w.), alert (Cd 3 mg/kg d.w.; Pb 50 mg/kg d.w.; Zn 300 mg/kg d.w.), and intervention (Cd 5 mg/kg d.w.; Pb 100 mg/kg d.w.; Zn 600 mg/kg d.w.) thresholds set for sensitive land use. A comparative analysis of two lavender varieties (lavender and lavandin) over a four-month period revealed an accumulation trend of Pb > Cd > Zn. Empirical modeling indicated that cadmium uptake followed a linear pattern, lead accumulation conformed closely to the Mitscherlich model, while zinc uptake did not align well with any of the tested models. Overall, the results emphasize the potential of lavender species in developing biomimetic approaches for heavy metal remediation and contribute valuable insights into sustainable soil decontamination practices.
1. Introduction
Across the globe, the rapid pace of industrialization, urban sprawl, and agricultural expansion has resulted in heavy metal accumulation in soils, often surpassing established regulatory thresholds [1]. To mitigate such contamination, various remediation techniques have been introduced, some of which also contribute to improving air quality in densely populated urban areas. In cities, vehicular traffic remains the predominant source of heavy metal pollutants, with fuel combustion identified as a significant contributor. These emissions settle on the surfaces of plants and soils [1,2,3,4]. Meanwhile, in rural regions or on the outskirts of urban zones, facilities such as wastewater treatment plants, waste incinerators, and composting centers also play a major role in the buildup of heavy metals in the soil [5,6,7,8,9].
Heavy metals present in soil have become a widespread environmental pollutant and pose a significant threat to public health due to their inherent toxicity. They tend to bioaccumulate in the human body by moving through the food chain. Unlike many organic contaminants that are eventually broken down through biodegradation, heavy metals persist in the environment, causing long-term toxic effects since they cannot degrade and are readily absorbed by plants, ultimately impacting both human and animal health [10,11,12]. In natural systems, toxic metal ions only undergo changes in oxidation state [13]. These ionic forms can interact with the soil matrix, becoming mobile when environmental conditions shift [14]. Moreover, heavy metals exert toxic effects on soil microbial communities [15,16]. Sustaining soil health and quality over time is essential to ensure environmental sustainability [16,17,18].
Heavy metals are typically classified as either essential or non-essential. Essential heavy metals, including the ions of Co, Cu, Fe, Mn, Ni, and Zn, act as micronutrients or trace elements vital for biological functions at low concentrations, although they become toxic when present in excess. By contrast, non-essential heavy metals and metalloids—such as As, Pb, Cd, Cr, and Hg—are known for their high toxicity, even at minimal exposure levels [10,13,17]. Essential metals play crucial roles in supporting the biochemical and physiological processes necessary for a plant’s development throughout its life cycle [11,12,15]. The bioavailability and phytotoxic effects of these toxic metals are influenced by a range of factors, including plant species, soil pH, organic matter content, and fertilization regimes. Moreover, the extent of metal accumulation within or on the surface of leaves is largely determined by exposure duration, metal concentration, and climatic conditions [1,2,10,17,18]. According to the existing literature, metals and metalloids such as Cd, Pb, As, Cr, Cu, Hg, Ni, Se, and Zn are associated with DNA damage and carcinogenic outcomes in both animals and humans, primarily due to their mutagenic properties [15,17]. Certain toxic metals promote the formation of free radicals, triggering oxidative stress, carcinogenicity, mutagenicity, teratogenicity, and endocrine disruption, while others are linked to neurological and behavioral disorders, particularly in children [11,12,17,19]. The experiments conducted in this study utilized heavy metal ions commonly detected at considerable concentrations in soils and road dust due to vehicular emissions [20], as well as in sewage sludge originating from wastewater treatment plants [21,22,23]. For instance, in road dust samples collected near Kuala Lumpur, lead concentrations were measured at 239.3 mg/kg, cadmium at 5.03 mg/kg, and zinc at 505 mg/kg [20]. Even higher levels were reported in Thessaloniki, where cadmium reached 4.95 mg/kg, lead 429 mg/kg, and zinc 1075 mg/kg [24]. Among heavy metals present in sewage sludge, zinc exhibited the highest concentration, with values of 446.6 mg/kg in Beijing (Pb 13.1 mg/kg, Cd below detection limits [21]) and 723 mg/kg in Poland (Pb 20.42 mg/kg, Cd 1.13 mg/kg [23]). Previous research has also documented significant accumulations of cadmium and lead in agricultural soils situated near urban or industrial zones [25]. Cadmium is a non-essential element for humans, known to accumulate primarily in the kidneys and liver. Long-term exposure has been associated with an increased risk of cancer development [18], as well as other health issues such as osteoporosis [2], renal dysfunction, anemia, and hypertension [18]. Acute cadmium poisoning may result in symptoms including chest pain, nausea, vomiting, abdominal cramps, shortness of breath, and muscle weakness [2,19]. The World Health Organization (WHO) has classified cadmium as a human carcinogen [18,26,27,28,29,30]. Among the metal ions studied, lead is recognized as the most toxic. Predominantly present in atmospheric particulate matter, lead impacts virtually every organ and physiological system. It is classified as a carcinogen and is linked to a range of health issues, including loss of appetite, fatigue, anemia, impaired hemoglobin synthesis, elevated blood pressure, and dysfunctions of the kidney, cardiovascular, and nervous systems [2,19,31]. Although zinc is an essential nutrient, it can become toxic when present in high concentrations. While typically regarded as non-toxic, excessive zinc intake has been associated with serious health conditions, including liver and kidney failure, anemia, jaundice, vomiting, diarrhea, and hematuria [2,17,18,19]. Remediating soils contaminated with toxic metals is essential for minimizing associated risks and preserving environmental health [16]. Traditional remediation methods, however, are often expensive and can cause additional environmental harm [13,16,32]. In contrast, phytoremediation—a technique that employs plants and microorganisms to cleanse polluted soils—provides an eco-friendly, cost-effective, and sustainable alternative [13,16,19,32,33,34,35,36,37]. Through their natural uptake mechanisms, plants absorb and accumulate heavy metals, thereby mitigating soil contamination [19,32]. Depending on their interactions with the soil, plants are generally categorized into three distinct groups [38]:
- (i)
- Accumulators, which concentrate heavy metals in their above-ground parts.
- (ii)
- Indicators, which reflect the concentration of heavy metals present in the soil.
- (iii)
- Excluders, which maintain low internal metal concentrations, despite high external levels.
Phytoremediation can be further classified into several strategies, including phytostabilization, phytostimulation, phytotransformation, phytofiltration, and phytoextraction, with the latter being the most extensively applied for soil remediation purposes [12,13,16,19,32,33,34,35,39,40]. One of the primary advantages of phytoremediation is that it preserves soil structure while simultaneously enhancing microbial activity and increasing carbon content, ultimately improving soil fertility [13,19,36,39]. In phytoextraction, plants absorb metals from the soil and accumulate them in harvestable tissues [40].
Medicinal and aromatic plants have emerged as promising candidates for the phytoremediation of soils contaminated with heavy metals [3,26,27,29,41,42,43,44,45,46,47,48]. These plants are capable of absorbing and accumulating metals in various tissues, including roots, stems, leaves, and inflorescences [47,48,49,50], although some species limit metal translocation to their aerial parts [19,27,29,30,43,44,45,46,47,48,49,51]. The bioavailability of metals in soil is largely determined by a combination of factors, such as soil physicochemical properties (pH, organic matter content, redox potential, carbonate concentration, and texture), climatic conditions, metal species, and oxidation states, as well as the type of root system [27,29,39,43,44,45,46,47,48,49,51]. The literature data indicate that Lamiaceae family plants (thyme, mint, hyssop, basil, oregano, sage, etc.) are hyperaccumulators of heavy metals [26,29,32,39,40,41,43,44,45,49,50,51,52].
In this study, the biological material consisted of lavender plants (Lavandula angustifolia and Lavandula x intermedia Emeric ex Loisel: lavender and lavandin) [26,41,42,44,49,53,54]. Lavender is an important aromatic and medicinal plant with significant economic value in the pharmaceutical, cosmetic, aromatherapy, and food industries, as well as in honey production. Its biological activity is due to active compounds such as tannins, anthocyanins, minerals, saponins, flavonoids, polyphenols, and essential oils. Metal ion retention in plants occurs through deposition, biosorption, and bioaccumulation [48]. The bioconcentration factor (BCF) and translocation factor (TF) are key indices for evaluating phytoremediation potential, with plants exhibiting both TF and BCF values above 1 considered suitable for phytoextraction [40,47].
This experimental study focused on the bioaccumulation of Cd, Pb, and Zn ions in two lavender species from artificially polluted soil. The aim was to assess lavender’s ability to accumulate metals and translocate them from the roots to the aerial parts, with BCF and TF being calculated [29,47,51]. While BCF evaluates metal retention in the roots, TF measures the transfer to other plant parts (e.g., stems, leaves).
The objective of this research was to investigate heavy metal accumulation in lavender, evaluating its potential for phytoremediation in contaminated soils and its safety for therapeutic or cosmetic uses in polluted areas (e.g., near highways or industrial zones). Experiments analyzed the evolution of metal ion concentrations over time and determined the Cd, Pb, and Zn transfer factors. Comparisons were made between plants grown in unpolluted (control) and single-metal-contaminated soils, with amounts exceeding normal values, alert thresholds, and intervention thresholds for sensitive land use according to environmental legislation [29,47,54,55,56,57].
To our knowledge, this work presents novel insights into lavender’s phytoremediation potential. In this context, our work brings a novel contribution by evaluating the response of two lavender taxa (Lavandula angustifolia and Lavandula x intermedia) to Cd, Pb, and Zn stress. What sets this study apart is the integration of simple but informative modeling tools—such as linear regressions and Mitscherlich-type functions—to describe the uptake behavior of metals, offering a useful predictive approach [58,59]. Beyond their phytoremediation capacity [60], lavender species have also been explored as eco-friendly agents in the green synthesis of metallic nanoparticles due to the bioactive compounds present in their extracts [61,62] or their essential oils, which continues to be an economically relevant use for lavender [63]. These findings suggest that lavender biomass, even after being exposed to contaminants, can still display practical applications, which adds further value to the species studied. By bringing together these different perspectives—species comparison, metal uptake modeling, and potential valorization—our study aims to provide a more integrated understanding of lavender’s role in sustainable remediation strategies. While previous studies have demonstrated lavender’s general tolerance to heavy metals [63], comparative analyses between lavender and lavandin under controlled contamination scenarios remain scarce. Moreover, few reports incorporate empirical modeling tools to predict metal uptake patterns in aromatic plants, despite their practical utility in designing phytoremediation strategies [64,65].
2. Materials and Methods
2.1. Analytical Reagents and Certified Reference Materials
Nitric acid (65%) and hydrogen peroxide (30%) of ultrapure quality (Sigma-Aldrich, Germany) were used for the digestion of plant tissues. Hydrochloric acid (37%) and nitric acid (65%) of ultrapure quality (Merck, Darmstadt, Germany) were used for soil digestion.
For calibration, a certified reference material (CRM), Multi-element 21 solution at 100 mg/L (CPAChem, Bogomilovo, Bulgaria), was used to prepare the calibration curve for the target metal ions (Cd, Pb, Zn). Analytical control was performed using a reference material (RM), Quality Control Standard 21 Multi-element, 100 mg/L (LGC Quality, Wesel, Germany).
For soil enrichment with metal ions, single-element standard solutions of 10 g/L Cd, Pb, and Zn (CPAChem, Bogomilovo, Bulgaria) were used. Argon and nitrogen, purity grade 5.0 (Linde-Gas), were employed during the analyses.
2.2. Equipment
Lavender and soil samples from the experiments were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) [29,47]. For the experimental study, an ICP-OES Avio 500 spectrometer (PerkinElmer, Shelton, CT, USA) was employed.
High-quality water was obtained using an Ultrapure Water System, ELIX Technology Milli-Q (Merck KGaA, Darmstadt, Germany).
The following equipment was used for sample preparation of the plant tissues and soils: a lyophilizer (Christ Alpha 1–2 LD, Martin Christ GmbH, Darmstadt, Germany), a mortar grinder (RM 200, Retsch, Darmstadt, Germany), a laboratory oven (Binder GmbH, Darmstadt, Germany), and an analytical balance (Kern ABT 220-50M, Darmstadt, Germany).
2.3. Plants and Soil Characteristics
The preparation of lavender plant tissue samples was carried out in the vegetation room. The plant material consisted of lavender seedlings (Lavandula angustifolia) and lavandin (Lavandula x intermedia Emeric ex Loisel) sourced from local agriculture.
A universal soil amendment for plant cultivation, produced locally (Florisol Product), was used.
Initially, before planting, physico-chemical analyses were performed on the soil in which the seedlings were planted (Table 1), the metal content of the water used for irrigation throughout the experiment, and the plant samples (roots, stems, leaves) collected from the lavender and lavandin seedlings prior to planting (Table 2) [47,54,55,57]. The initial physico-chemical properties of the soil and water ensured proper control over experimental conditions.

Table 1.
Physico-chemical properties of the soil used in the experimental study.

Table 2.
Composition of the soil (prior to the beginning of the experiments), irrigation water, lavender seedlings, and lavandin seedlings.
2.4. Experiment Characteristics
The experiments were conducted in a 6 m2 polycarbonate greenhouse under controlled conditions (temperature, humidity, and light), and the plants were grown in plastic pots containing 5000 g of soil. The purchased seedlings were initially grown in uncontaminated soil. Subsequently, to simulate intensely polluted soil, the soil was enriched with solutions of Cd, Pb, and Zn ions. The concentrations selected for the tests are presented in Table 3. The pots, except for the control samples (soil without added metals), were contaminated monthly.

Table 3.
Metal ion concentrations in control soil and corresponding normal, alert, and intervention threshold values (mg/kg d.w.) [55].
The amount of metal added in each experiment was calculated to reach the regulated toxic concentrations corresponding to either the normal value limit, the alert threshold, or the intervention threshold for sensitive land use (Table 3). These thresholds are defined by the legislation currently in force [55].
The water used for irrigation during the experiments did not contain the toxic metals of interest, namely Cd and Pb, or other toxic metals, except for zinc, which displayed a concentration of 15.1 µg/L.
Realization of the Metal (Zn, Cd, and Pb) Enrichment Solutions
To prepare the solutions enriched with Zn, Cd, and Pb, the required amount of each metal for soil enrichment was calculated, considering the initial soil concentration, the soil mass, and the final concentration to be achieved. The pots were contaminated by watering monthly. The soil enrichment solutions were prepared from standard solutions of Cd, Pb, and Zn dissolved in 5% HNO3 in ultrapure water.
The experiment was designed to highlight the separate effects of the toxic metals Cd, Pb, and Zn on lavender. Soil and plant samples (roots, stems, leaves, and flowers—where applicable) were collected after each month of the experiment (30, 60, 90, and 120 days). All experiments were conducted from June to September (four months) at an ambient temperature of 24 ± 5 °C.
The initial soil composition analysis indicated normal values for Cd, Pb, and Zn contents (Table 3).
The experiments were performed in duplicate. The standard deviations were lower than 10% of the average measurements, indicating that the results are highly reproducible.
A total of 40 pots with plants were prepared: two control experiments for each plant species, without added metal ions (M0 and M1); and six pots for each single-contaminant study on both lavender and lavandin plants, i.e., (1) zinc experiment, (2) cadmium experiment, and (3) lead experiment. Additionally, each main experiment included three sub-experiments corresponding to three different metal ion concentrations, in accordance with the thresholds stipulated by Order 756/1997 [55].
The phytotoxic concentrations of the metal content in the plants are provided in Table 4, and the numbering of the experiments is summarized in Table 5.

Table 4.
Phytotoxic concentrations of metals in plants (mg/kg) [40].

Table 5.
Experimental design and description of metal enrichment conditions for lavender and lavandin species.
The structure and numbering of the experimental setup are detailed in Table 5.
2.5. Soil and Vegetation Analysis
Pretreatment of plant tissue and soil samples in an open system (digestion method): open system digestion is still widely used for the decomposition of samples and the destruction of organic matter [56].
The extraction of metals from lavender plants was performed by an acid digestion procedure as follows: approximately 1 g of the lyophilized and crushed plant sample was used. The sample was mineralized with a mixture of ultrapure HNO3 and H2O2 in a ratio of 10:3 (v/v) at room temperature for 24 h to ensure the destruction of organic matter (cold digestion). The acid extracts were then filtered and diluted with ultrapure water to a final volume of 25 mL [57].
The metal content in the soil was determined from approximately 1.5 g of sieved, oven-dried (maximum 50 °C), and homogenized soil. A mixture of 5 mL ultrapure nitric acid (65%) and 15 mL ultrapure hydrochloric acid (37%) (aqua regia extraction) was added to the sample. The digests were filtered and diluted with ultrapure water to a final volume of 50 mL [54].
After harvesting, the plant tissues were washed with tap water followed by distilled water. Subsequently, the plants were separated into components (roots, stems, leaves, and flowers) and dried in a lyophilizer (Christ Alpha 1-2 LD lyophilizer). The samples were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES, Avio 500, PerkinElmer, USA) [29,40,47,54,55,56,57].
2.6. Data Analysis
The analysis of metals in the soil and lavender plants was performed on two samples for each type, with the reported value representing the average of the two measurements. The results were correlated and compared with blank sample values and reference values for soil and plant quality.
The assessment of metal accumulation from the soil to the lavender plants, as well as their transfer from the roots to the aerial parts, was carried out by calculating the transfer index, defined as the ratio between the metal concentration in the plant and its concentration in the soil [29].
2.7. Evaluation of Metal Uptake and Accumulation
The evaluation of metal uptake and accumulation in lavender plants was based on the quantification of Cd, Pb, and Zn concentrations in different plant organs (roots, stems, leaves, flowers), as determined by ICP-OES analysis after acid digestion. The accumulation potential was assessed using the transfer index (TI), calculated as the ratio between the metal concentration in the plant tissues (mg/kg dry weight) and its concentration in the corresponding soil (mg/kg dry weight). This index reflects the plant’s ability to absorb and translocate metals from the soil to the aboveground biomass. TI values greater than 1 indicate effective uptake and translocation, while values below 1 suggest limited mobility or retention in the roots.
All data were collected over a 120-day period at four exposure intervals (30, 60, 90, and 120 days) for both the control and contaminated samples, allowing for the temporal analysis of accumulation dynamics. This method enables the identification of trends and thresholds relevant to phytoremediation efficiency.
2.8. Biometrical Measurements
Biometrical measurements were performed. Plant height (h) was measured both before the exposure of lavender and lavandin plants to toxic metals and at the end of the experiments (120 days). The results depicted in Figure 1 highlight the following relevant aspects:
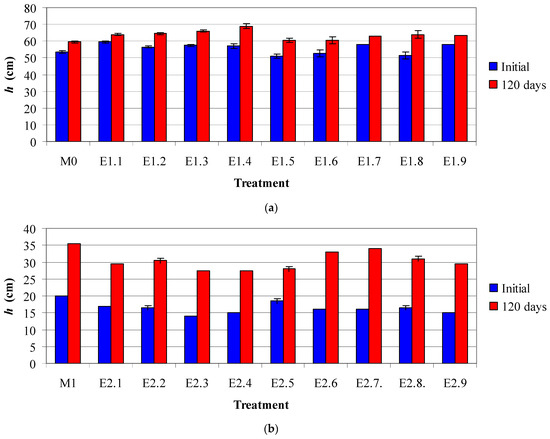
Figure 1.
Plant height (mean value ± standard deviation) for lavender (a) and lavandin (b) under different treatments.
- (i)
- Compared to the initial mean values of h (51.0–59.5 cm for lavender and 14.0–20.0 cm for lavandin), the mean values of h at 120 days were 1.09–1.24 times higher for lavender (59.5–69.0 cm) and 1.51–2.13 times higher for lavandin (27.5–35.5 cm).
- (ii)
- Compared to the control M0, for which the mean values of h for lavender plants increased by 11% over 4 months, the treatments E1.4–E1.6 with Cd and E1.8 with Pb exhibited positive effects, with mean height values increasing by 15–24%, whereas the other treatments showed similar effects.
- (iii)
- Compared to the control M1, in which the mean values of h for lavandin plants increased by 78% over 4 months, the treatments E2.3 with Zn, E2.6 with Cd, and E2.7 and E2.9 with Pb exhibited positive effects, with mean height values increasing more than twofold, whereas the other treatments showed similar effects.
Figure 1 illustrates the changes in plant height (mean ± SD) for lavender and lavandin subjected to different metal treatments.
Lavender plants chosen and prepared for the beginning of the experiments are depicted in Figure 2 and Figure 3.

Figure 2.
Lavender plants used in control (M0) (left) and experimental setups for metal accumulation studies (right).

Figure 3.
Lavandin plants used in control (M1) (left) and experimental setups for the study of metal uptake (right).
3. Results and Discussion
This experimental study explored the bioaccumulation of toxic metals—Cd, Pb, and Zn—from contaminated soils enriched with metal ions in two lavender species (Lavandula spp.) recognized for their medicinal and aromatic properties. The research assessed the temporal variation in contaminant concentrations and determined the transfer factors of these metals from the soil to the plant tissues, providing insights into the uptake efficiency of lavender under heavy metal stress.
3.1. Zinc Uptake and Accumulation Dynamics
The distribution of zinc in the plant and soil compartments showed a clear temporal pattern, reflecting enhanced accumulation over time, as shown in Figure 4 and Figure 5. The bioaccumulation behavior of zinc in lavender plants appears to be influenced by the duration of the exposure. The transfer index (TI), calculated as the ratio between the zinc concentration in plant tissues and that in the corresponding soil, is shown in Figure 6. TI values exceeding 1 suggest active zinc bioaccumulation; however, across all six experimental conditions, the highest TI was recorded at 60 days in lavandin (E2.1). Over time, TI values declined and remained below 1, indicating a limited accumulation capacity. A TI value of 1 marks the threshold for bioaccumulation, and under these conditions, both lavender species predominantly acted as excluders for zinc. Additionally, the zinc concentrations in plant tissues did not surpass the normal value (VN = 150 mg/kg [40]) during the entire experimental period (Figure 4).
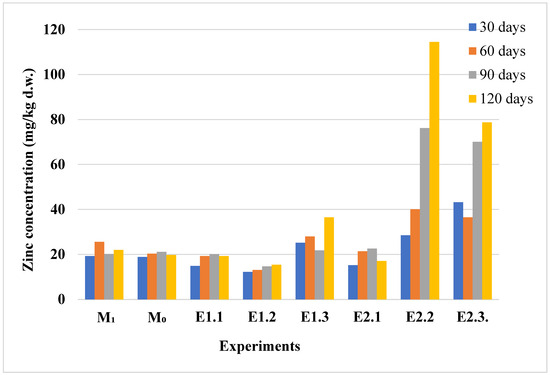
Figure 4.
Zinc concentration (mg/kg d.w.) in aerial parts of lavender and lavandin plants exposed to control and Zn-contaminated soils at 30, 60, 90, and 120 days. Experimental codes (M0, M1, E1.1–E2.3) correspond to the treatment conditions described in Table 1.
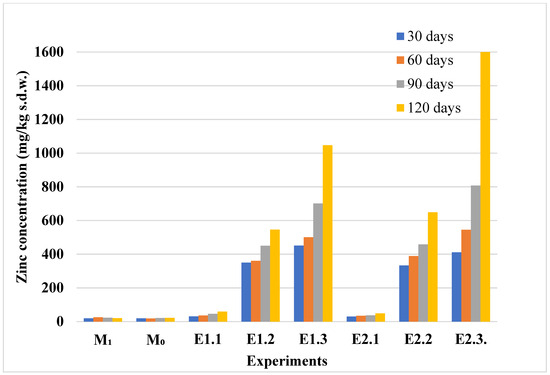
Figure 5.
Zinc concentration in soils from control and Zn-contaminated experiments, measured at 30, 60, 90, and 120 days. Experimental codes (e.g., M0, M1, E1.1–E2.3) correspond to specific contamination levels, as described in Table 1.
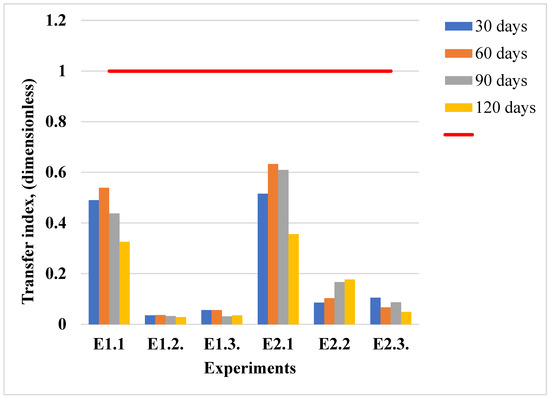
Figure 6.
Transfer index values for zinc translocation from soil to plants in the conducted experiments. The red line at TI = 1.0 serves as a reference threshold: values above 1 indicate efficient zinc movement from roots to aerial parts, while values below 1 reflect limited translocation and higher root retention of zinc.
As illustrated in Figure 4, zinc accumulation in lavender plants remained relatively low during the initial contamination stages, with minimal variations between the control (M0, M1) and low-level contamination experiments (E1.1, E1.2). A noticeable increase in zinc concentration was observed in experiments E2.2 and E2.3, particularly after 90 and 120 days, indicating that significant zinc uptake occurs only beyond the alert threshold in the soil. These findings suggest that lavender species may act as zinc excluders under moderate contamination levels, shifting towards accumulation only at higher soil zinc concentrations. To complement the observed zinc accumulation patterns in the plant tissues, the corresponding zinc concentrations measured in the soil samples are presented in Figure 5.
Zinc concentrations in the soil samples varied consistently with the level and duration of contamination, revealing a clear pattern of accumulation under increasing stress conditions (Figure 5). In the control and low-contamination experiments (M0, M1, E1.1, E1.2), soil zinc levels remained relatively stable and below the alert thresholds. A significant increase in soil zinc concentration was observed in experiments E1.3, E2.2, and E2.3, particularly after 90 and 120 days. These findings correlate with the plant accumulation patterns seen in Figure 4, suggesting that higher soil zinc levels are required to trigger notable bioaccumulation in lavender species.
In addition to zinc content measurements, the transfer index from the soil to the plants was calculated to evaluate bioaccumulation efficiency (Figure 6).
The calculated transfer index values indicate how efficiently zinc was translocated from the soil to the aerial parts in lavender plants under different contamination scenarios, as detailed in Figure 6. Throughout the study period, the TI values remained below 1 under all conditions, indicating the limited translocation of zinc from the soil to plant tissues. Higher TI values were recorded in E1.1 and E2.1, yet none exceeded the bioaccumulation threshold. These results support the hypothesis that lavender acts as a zinc excluder, restricting zinc uptake even at elevated soil concentrations.
3.2. Cadmium Uptake and Accumulation Dynamics
Cadmium accumulation patterns in lavender species under varying contamination levels and exposure times are depicted in Figure 7 and Figure 8. Specifically, Figure 7 illustrates cadmium concentrations in plant tissues, while Figure 8 presents the corresponding concentrations in soil samples. During exposure periods shorter than 90 days, cadmium accumulates in both lavender species at levels exceeding the normal range (VN = 0.1–1 mg/kg [40]), yet remaining below the phytotoxicity threshold (VF = 10 mg/kg [40]). However, as exposure approaches 120 days, lavandin plants subjected to the highest cadmium concentrations surpass the VF limit (Figure 7). The transfer index values from the soil to plant tissues are presented in Figure 9. Overall, the TI values are relatively high, often exceeding the threshold of 1. Particularly, lavender plants exposed to cadmium concentrations near the intervention threshold exhibit significantly elevated TI values, indicating an active bioaccumulation of cadmium.
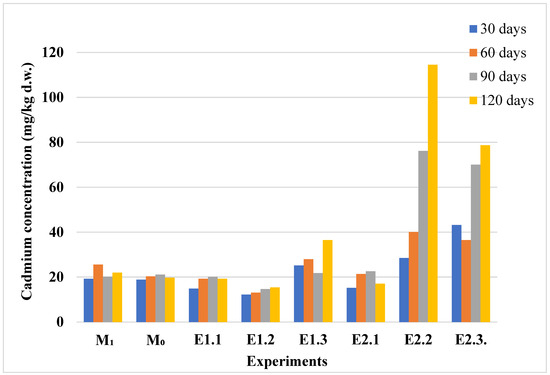
Figure 7.
Cadmium content in control and Cd-contaminated lavender plants. Experimental treatments are labeled according to Table 1: M1 and M0 (controls), E1.x (lavender under increasing Cd contamination: 1–5 mg/kg d.w.), and E2.x (lavandin under same conditions). Bars show cadmium concentrations (mg/kg dry weight) measured in plant tissues at 30, 60, 90, and 120 days.
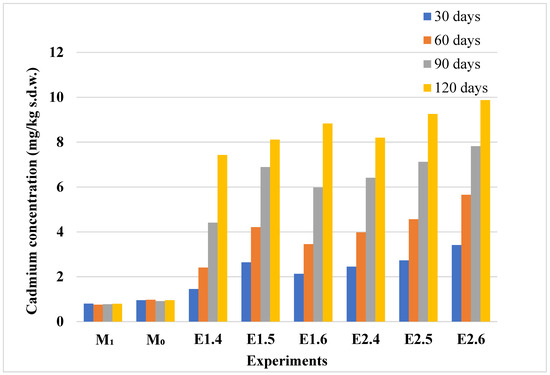
Figure 8.
Cadmium content in control and Cd-contaminated soils. Experimental conditions are coded as follows (see Table 1): M1 and M0—control soils; E1.4–E1.6—soils used for lavender experiments with increasing Cd contamination (1–5 mg/kg d.w.); E2.4–E2.6—soils for lavandin under the same contamination levels. Bars indicate cadmium concentrations in soils at 30, 60, 90, and 120 days.
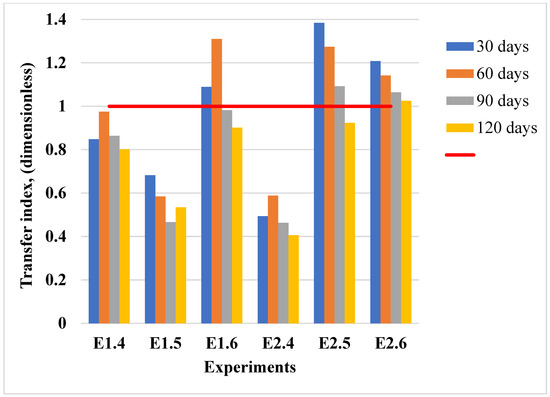
Figure 9.
Transfer index values for cadmium translocation from soil to plants in the conducted experiments. The red horizontal line at TI = 1.0 marks the threshold for efficient metal transfer: values above 1 suggest cadmium movement from roots to aerial tissues, while values below 1 indicate preferential retention of cadmium in root tissues.
Under control and low-contamination conditions (M0, M1, E1.1, E1.2), cadmium concentrations in lavender tissues remained low and relatively stable, showing limited uptake from the soil. These findings are supported by the data shown in Figure 7. A significant increase in cadmium accumulation was observed in experiments E2.2 and E2.3, particularly after 90 and 120 days of exposure. These findings suggest that lavender plants have the capacity to accumulate cadmium more readily than zinc, especially under higher soil contamination conditions. To better understand the cadmium uptake behavior observed in plants, the corresponding cadmium concentrations measured in the soil samples are presented in Figure 8.
In the soil samples associated with the lavender experiments, cadmium concentrations in the control groups (M0, M1) remained consistently low throughout the study, indicating minimal background contamination. These values are detailed in Figure 8. In contrast, a significant and progressive increase in soil cadmium concentration was observed in the contaminated experiments (E1.4–E2.6), particularly after 90 and 120 days. The steady accumulation of cadmium in soil correlates with the higher cadmium uptake observed in lavender plants, highlighting the strong dependence of plant bioaccumulation on soil contamination levels.
The transfer index (TI) values calculated for cadmium reveal the extent to which this metal was translocated from the soil to th eaerial parts in lavender plants under different contamination conditions, as shown in Figure 9. While TI values remained below 1 in some experiments (E1.4, E1.5, E2.4), indicating limited cadmium translocation, values exceeded the threshold of 1 in experiments E1.6, E2.5, and E2.6, particularly after 30 and 60 days. These results suggest that lavender plants exhibit cadmium accumulation behavior under higher soil contamination levels and early exposure periods, with a tendency towards stabilization at prolonged durations.
3.3. Lead Uptake and Accumulation Dynamics
As illustrated in Figure 10, lead concentrations in lavender plants exceeded the normal range (VN: 1–5 mg/kg [40]) as early as 60 days into the experiment, although they remained below the phytotoxic threshold (VF: 20 mg/kg [40]). With prolonged exposure, lead accumulation continued to increase, and by the end of the experimental period, Pb concentrations in both lavender species surpassed the phytotoxic limit
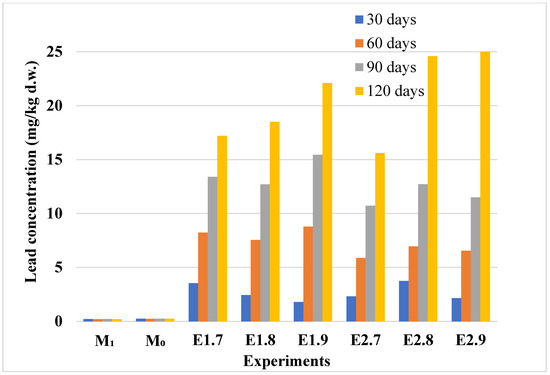
Figure 10.
Lead content in control and Pb-contaminated lavender and lavandin plants. Experimental groups include M0 (lavender control), M1 (lavandin control), E1.7–E1.9 (lavender plants exposed to Pb at 20, 50, and 100 mg/kg d.w.), and E2.7–E2.9 (lavandin plants exposed to Pb at the same concentrations). Experimental details are provided in Table 1. Bars represent Pb concentrations measured in plant tissues after 30, 60, 90, and 120 days.
Lead concentrations in lavender plants increased proportionally with soil contamination levels, reflecting a clear uptake trend across experimental conditions. These results are summarized in Figure 10. In the control samples (M0, M1), lead content remained negligible throughout the exposure period. However, significant lead accumulation was observed in the contaminated samples (E1.7–E2.9), with a marked increase in concentration over time, particularly after 90 and 120 days. The results highlight the high affinity of lavender plants for lead uptake under elevated soil contamination conditions. The lead accumulation trends in plants are further supported by the soil concentration profiles shown in Figure 11.
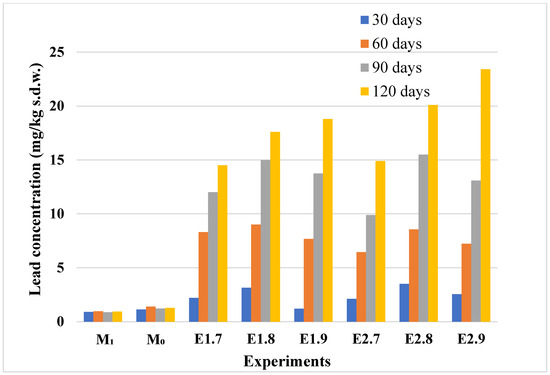
Figure 11.
Lead content in control and Pb-contaminated soils used in lavender and lavandin experiments. Treatments include M0 (lavender control soil), M1 (lavandin control soil), E1.7–E1.9 (soils for lavender contaminated with 20, 50, and 100 mg Pb/kg d.w.), and E2.7–E2.9 (soils for lavandin contaminated at the same levels). Full experimental setup is described in Table 1. Bars indicate Pb concentrations in soil samples after 30, 60, 90, and 120 days.
The lead concentrations in the soil samples mirrored the experimental contamination design, with values increasing progressively across treatments. These measurements are presented in Figure 11. Lead levels remained minimal in the control samples (M0, M1) throughout the experimental period. In the contaminated samples (E1.7–E2.9), a consistent and substantial increase in lead concentration was observed over time, particularly after 90 and 120 days. These results align with the trends observed in plant tissues, suggesting efficient lead transfer from soil to plant under elevated contamination levels. To complement the soil concentration data, the transfer indices for lead uptake are presented in Figure 12. Combined with the concentration profiles depicted in Figure 10 and Figure 11, these results highlight that lavender species accumulate lead at a significantly higher rate compared to that of cadmium.
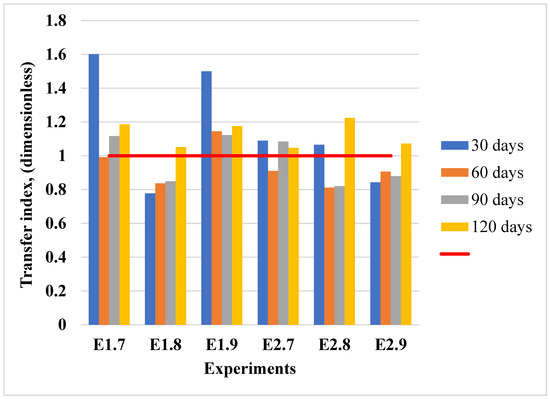
Figure 12.
Transfer index values for lead translocation from soil to plants in the conducted experiments. The red horizontal line at TI = 1.0 serves as a reference threshold: values above 1 indicate efficient translocation of metals from roots to shoots, whereas values below 1 suggest metal retention primarily in root tissues.
The transfer index (TI) values for lead uptake indicate low translocation efficiency from the soil to the aerial tissues in lavender plants, even at higher contamination levels. These patterns are detailed in Figure 12. The TI values exceeded 1 in most experiments, particularly at earlier exposure times (30 days), indicating an active bioaccumulation of lead. Even though the TI values slightly decreased over time in some experiments, they generally remained close to or above 1, confirming the capacity of lavender plants to accumulate lead from contaminated soils.
Our results indicate that both Lavandula angustifolia and Lavandula x intermedia exhibit promising potential for phytoremediation, particularly in soils contaminated with cadmium, lead, and zinc. These findings are in line with those of previous studies that have assessed lavender species in similar contexts. For example, Angelova et al. (2015) reported that Lavandula vera was capable of accumulating significant concentrations of Cd and Pb under field conditions, classifying it as a potential hyperaccumulator for lead [66]. Likewise, Pirsarandib et al. (2022) demonstrated enhanced heavy metal uptake by L. angustifolia under greenhouse conditions, particularly when the plants were inoculated with arbuscular mycorrhizal fungi, although they noted a reduction in overall biomass at higher contamination levels [67]. Compared to these references, our data showed comparable bioconcentration and transfer factors, especially for cadmium and zinc, while the biomass remained relatively stable under the tested metal doses. This suggests that, under moderate contamination, lavender can maintain physiological integrity and metal translocation efficiency—an essential criterion for sustainable phytoremediation strategies. The consistency between our findings and existing reports strengthens the argument for integrating lavender into site-specific remediation plans, particularly when biomass valorization is also envisioned.
An important aspect that emerged from our findings is the potential for the sustainable valorization of lavender biomass after metal uptake. Since the aerial parts (flowers and leaves) tended to accumulate lower concentrations of Cd, Pb, and Zn compared to that in the roots, their selective use could align with circular economy strategies aiming to reduce phytoremediation-derived waste. One practical valorization pathway is the extraction of essential oils. Although cultivated on contaminated soils, Lavandula angustifolia has been shown to produce essential oils free of detectable levels of heavy metals, due to their poor volatility and low affinity for oil fractions during steam distillation [64]. This suggests that such biomass could still be safely exploited in industrial or cosmetic applications, provided that appropriate purification and analytical screening are performed.
Another promising route is the green synthesis of metallic nanoparticles, an area in which lavender extracts have shown high potential as both reducing and capping agents. Studies have demonstrated the successful biosynthesis of silver and zinc oxide nanoparticles using Lavandula angustifolia extracts, with notable antimicrobial properties and structural stability [61,62]. These eco-friendly methods avoid the use of harmful chemicals and rely on the phytochemical richness of the plant—particularly flavonoid, terpenoid, and polyphenol content. This supports the feasibility of repurposing lavender biomass post-remediation for nanotechnological applications, while maintaining safety and environmental integrity.
Building upon the experimental results, the following section focuses on modeling the heavy metal accumulation patterns in lavender plants to better describe and predict their uptake behavior.
3.4. Modeling of the Experimental Data
This section presents an integrated discussion of the experimental results obtained for heavy metal accumulation in lavender and lavandin, followed by empirical modeling aimed at describing the uptake behavior and translocation dynamics under varying contamination levels. The mechanisms underlying heavy metal uptake from soil remain only partially understood. However, it is generally accepted that the accumulation process involves several key steps:
- (a)
- Mass transfer of heavy metal ions within the soil layer, influenced by factors such as concentration gradients, soil pH, temperature, and moisture;
- (b)
- Diffusion-driven transport of ions toward the plant root surface;
- (c)
- Uptake of metal ions by plant roots, followed by their translocation to stems, leaves, shoots, and flowers.
Due to the complexity of these processes, the specific role of soil microorganisms in facilitating heavy metal uptake by roots has not yet been fully clarified. Another significant challenge in developing a mechanistic model lies in accurately quantifying the root surface area, which is inherently dynamic—capable of growth, degradation, and alteration through the adhesion of dry soil particles or sand, potentially restricting ion access.
Given these complexities, empirical mathematical models are often favored to correlate heavy metal concentrations in plant tissues with those in the surrounding soil. In this study, three empirical models were tested to describe the accumulation behavior of heavy metals in lavender plants (Table 6).

Table 6.
Empirical models used for data fitting in the experimental study.
Linear fitting has previously been applied to correlate cadmium uptake in crops such as soybeans and ryegrass, yielding satisfactory results [66,67,68,69]. However, some studies reported poor agreement between experimental and predicted data when using a linear model. To address this limitation, the Mitscherlich model was introduced [70]. The Mitscherlich function has been traditionally employed to relate the nutrient content in plant tissues to the nutrient availability in soil [71], and was initially developed to optimize the application rates of NPK fertilizers [72,73,74]. Furthermore, the modified Mitscherlich model has been found to best describe cadmium accumulation in ryegrass, maize, and soybean plants [75]. Based on the experimental results from E1.1, E1.2, and E2.1, it can be concluded that both lavender and lavandin plants act as excluders for zinc within the tested concentration range in soil. Consequently, these data sets were excluded from the modeling study. The experimental data used for model fitting corresponded to the E1.3, E2.2, and E2.3 experiments. Zinc accumulation in plant tissues, within the concentration range at which uptake was observed, was best described by a linear model. However, the correlation coefficient obtained was not satisfactory (Figure 13).
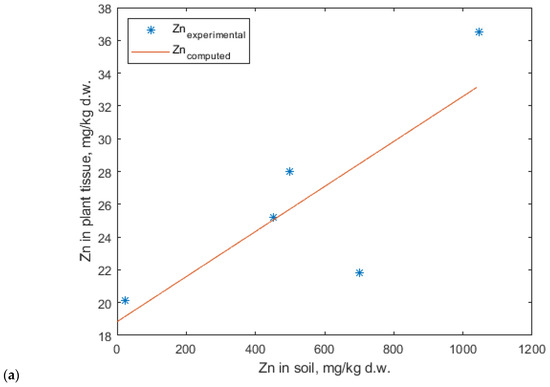
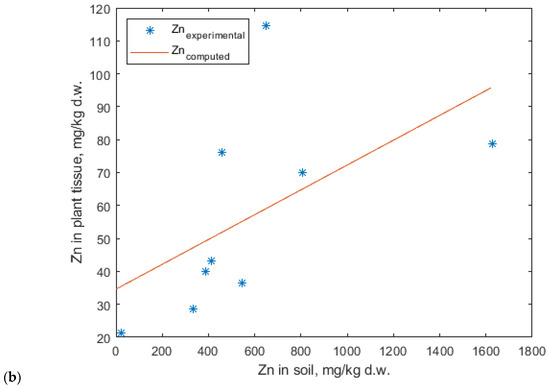
Figure 13.
Linear regression model describing zinc accumulation in (a) lavender and (b) lavandin.
The experimental data for cadmium accumulation were best fitted by the linear model (Figure 14), whereas the Mitscherlich models provided unsatisfactory results.
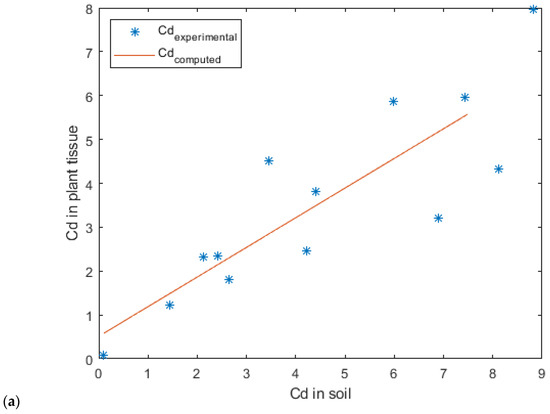
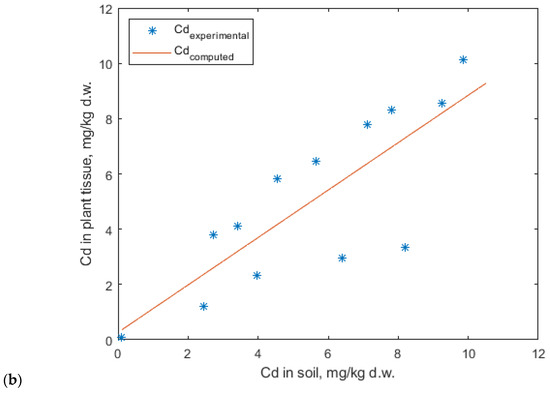
Figure 14.
Linear regression model for cadmium uptake in (a) lavender and (b) lavandin.
The experimental data for lead accumulation were best fitted by the Mitscherlich model (Figure 15). Although the linear model also provided a relatively good fit, the graphical representations suggest that the Mitscherlich model offers a more accurate description of the experimental data (Figure 15 and Figure 16).
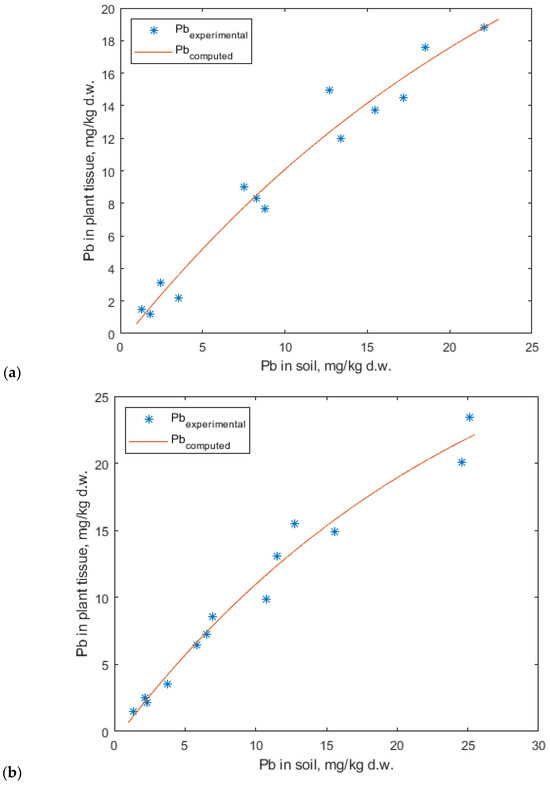
Figure 15.
Mitscherlich fitting model for lead accumulation in (a) lavender and (b) lavandin plants.
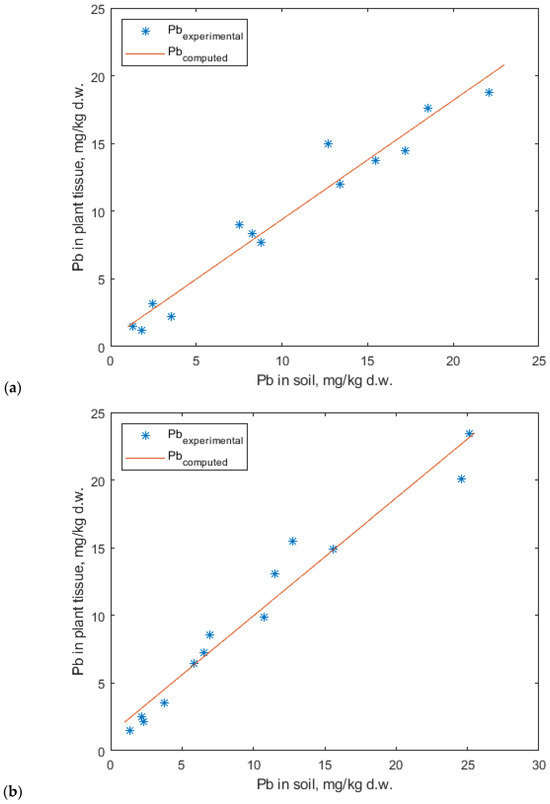
Figure 16.
Linear regression model for lead uptake in lavender (a) and lavandin (b).
The modified Mitscherlich model provided poor fitting results across all experimental data sets tested. The values of the model parameters and the corresponding determination coefficients (R2) are summarized in Table 7. From the fitting results, it can be observed that notable similarities exist between the two lavender species. Although the model parameters differ slightly and the plants accumulate somewhat different amounts of heavy metals, the empirical models that yield the best fit for lavender also perform the best for lavandin.

Table 7.
Regression coefficients obtained from soil and plant data fitting.
4. Conclusions
This study demonstrated that lavender plants have the capacity to accumulate lead and cadmium from contaminated soils, while zinc uptake remained limited, with both lavender species acting as excluders at concentrations below the alert threshold. Notably, zinc accumulation commenced only when soil concentrations exceeded the alert level. The bioaccumulation efficiency for the tested metals followed the order: Pb > Cd > Zn.
Empirical modeling further revealed that cadmium accumulation is best described by a linear model, whereas lead uptake aligns more closely with the Mitscherlich model. The modified Mitscherlich model, however, did not provide satisfactory fits for any of the tested data sets.
These findings highlight the potential application of lavender species in phytoremediation strategies targeting cadmium- and lead-contaminated soils. Additionally, the results provide a valuable framework for assessing the safety of cultivating lavender for pharmaceutical and cosmetic purposes in moderately polluted environments.
Future research could focus on expanding the scope to multi-metal contaminated soils and evaluating the influence of soil microbiota on the bioaccumulation dynamics in lavender species to enhance the understanding and application of phytoremediation techniques.
Author Contributions
Conceptualization, methodology, validation, laboratory analysis: E.A.Ș., D.S.Ș., M.B., C.U., and M.Ș.; resources: D.S.Ș.; data curation: E.A.Ș. and M.B.; software: M.B.; writing—original draft preparation: E.A.Ș., D.S.Ș., M.B., M.Ș., and C.U.; writing—review and editing D.S.Ș. and M.Ș.; funding acquisition D.S.Ș. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the contracting authority through the EPCEcoA project PN-IV-INO-PTE-2024. C.U. and D.S.S. gratefully acknowledge the financial support provided by this project.
Data Availability Statement
The data can be made available by addressing a request to the corresponding author.
Acknowledgments
The authors dedicate this work to the memory of their esteemed colleague, Ecaterina Anca Șerban.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.S.; Serbanescu, C. Plants Biosensors for Monitoring the Heavy Metals from Urban Pollution; Éditions Universitaires Européennes: Saarbrücken, Germany, 2017; ISBN 978-3-639-56066-4. [Google Scholar]
- Mishra, B.; Chandra, M. Evaluation of phytoremediation potential of aromatic plants: A systematic review. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100405. [Google Scholar] [CrossRef]
- Alharbi, M.; Aljeddani, G. Heavy metals phytoremediation and its impact on photosynthetic pigments and metabolic content in some plant species grown in the streets of Jeddah Governorate, Saudi Arabia. J. Environ. Prot. 2022, 13, 557–574. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; de Figueiredo, C.C.; da Silva, J.; Jorge Paz-Ferreiro, J. The residual effect of sewage sludge biochar on soil availability and bioaccumulation of heavy metals: Evidence from a three-year field experiment. J. Environ. Manag. 2021, 279, 111824. [Google Scholar] [CrossRef] [PubMed]
- Feizi, M.; Jalali, M. Leaching of Cd, Cu, Ni and Zn in a sewage sludge-amended soil in presence of geo- and nano-materials. J. Clean. Prod. 2021, 297, 126506. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256, 56–66. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Zhan, J.; Guo, X. Variation and factors on heavy metal speciation during co-composting of rural sewage sludge and typical rural organic solid waste. J. Environ. Manag. 2022, 306, 114418. [Google Scholar] [CrossRef]
- Chu, Z.; Fan, X.; Wang, W.; Huang, W.C. Quantitative evaluation of heavy metals’ pollution hazards and estimation of heavy metals’ environmental costs in leachate during food waste composting. Waste Manag. 2019, 84, 119–128. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Bharti, K.R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Qasim, A.; Zahir, A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Qishlaqi, A.; Moore, F. Statistical analysis of accumulation and sources of heavy metals occurrence in agricultural soils of Khoshk River banks, Shiraz, Iran. Am. Eurasian J. Agric. Environ. Sci. 2007, 2, 565–573. [Google Scholar]
- Revathi, S.; Subhashree, V. Physiological and biochemical mechanisms of heavy metal tolerance. Int. J. Environ. Sci. 2013, 3, 1339–1354. [Google Scholar]
- Aghili, S.; Golzary, A. Greening the earth, healing the soil: A comprehensive life cycle assessment of phytoremediation for heavy metal contamination. Environ. Technol. Innov. 2023, 32, 103241. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Americo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Li, G.; Yan, L.; Chen, X.; Lam, S.S.; Rinklebe, J.; Yu, Q.; Yang, Y.; Peng, W.; Sonne, C. Phytoremediation of cadmium from soil, air and water. Chemosphere 2023, 320, 138058. [Google Scholar] [CrossRef]
- Alsafran, M.; Saleem, M.H.; Jabri, H.A.; Rizwan, M.; Usman, K. Principles and applicability of integrated remediation strategies for heavy metal removal/recovery from contaminated environments. J. Plant Growth Regul. 2023, 42, 3419–3440. [Google Scholar] [CrossRef]
- Han, N.M.M.; Latif, M.T.; Othman, M.; Dominick, D.; Mohamad, N.; Juahir, H.; Tahir, N.M. Composition of selected heavy metals in road dust from Kuala Lumpur city centre. Environ. Earth Sci. 2014, 72, 849–859. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, J.; Zheng, X.; Li, S.; Lv, Q.; Liang, C. Removal of heavy metals from sewage sludge by chemical leaching with biodegradable chelator methyl glycine diacetic acid. Chemosphere 2022, 300, 134496. [Google Scholar] [CrossRef]
- Achkir, A.; Aouragh, A.; El Mahi, M.; Lotfi, E.M.; Labjar, N.; Bouch, M.E.; Ouahidi, M.L.; Badza, T.; Farhan, H.; Moussaoui, T.E. Implication of sewage sludge increased application rates on soil fertility and heavy metals contamination risk. Emerg. Contam. 2023, 9, 100200. [Google Scholar] [CrossRef]
- Urbaniak, M.; Baran, A.; Giebułtowicz, J.; Bednarek, A.; Serwecinska, L. The occurrence of heavy metals and antimicrobials in sewage sludge and their predicted risk to soil—Is there anything to fear? Sci. Total Environ. 2024, 912, 168856. [Google Scholar] [CrossRef] [PubMed]
- Bourliva, A.; Christophoridis, C.; Papadopoulou, L.; Giouri, K.; Papadopoulos, A.; Mitsika, E.; Fytianos, K. Characterization, heavy metal content and health risk assessment of urban road dusts from the historic center of the city of Thessaloniki, Greece. Environ. Geochem. Health 2017, 39, 611–634. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Chen, T.; Xu, Y.; Qi, M.; Sun, G.; Wu, X.; Chen, M.; Xu, W.; Liu, C. Combining Cd and Pb isotope analyses for heavy metal source apportionment in facility agricultural soils around typical urban and industrial areas. J. Hazard. Mater. 2024, 466, 133568. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.B.; Momayezi, M.; Taleei, D. Biochar effect on cadmium accumulation and phytoremediation factors by lavender (Lavandula stoechas L.). Open J. Ecol. 2017, 7, 447–459. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- Aibibu, N.; Liu, Y.; Zeng, G.; Wang, X.; Chen, B.; Song, H.; Xu, L. Cadmium accumulation in vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresour. Technol. 2010, 101, 6297–6303. [Google Scholar] [CrossRef]
- Serban, E.A.; Vasile, G.-G.; Gheorghe, S.; Stoica, C.; Ene, C. Effects of toxic metals Cd, Ni and Pb on Matricaria chamomilla L. growth in a laboratory study. Rev. Chim. 2020, 71, 325–335. [Google Scholar] [CrossRef]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M.; et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef]
- Arshad, M.; Naqvi, N.; Gul, I.; Yaqoob, K.; Bilal, M.; Kallerhoff, J. Lead phytoextraction by Pelargonium hortorum: Comparative assessment of EDTA and DIPA for Pb mobility and toxicity. Sci. Total Environ. 2020, 748, 141496. [Google Scholar] [CrossRef]
- Muttaleb, W.H.; Ali, Z.H. Bioremediation an eco-friendly method for administration of environmental contaminants. Int. J. Appl. Sci. Technol. 2022, 4, 22–32. [Google Scholar] [CrossRef]
- Ilinskiy, A.; Vinogradov, D.; Politaeva, N.; Badenko, V.; Ilin, I. Features of the phytoremediation by agricultural crops of heavy metal contaminated soils. Agronomy 2023, 13, 127. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Intern. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Deepika; Haritash, A.K. Phytoremediation potential of ornamental plants for heavy metal removal from contaminated soil: A critical review. Hortic. Environ. Biotechnol. 2023, 64, 709–734. [Google Scholar]
- Hussain, M.M.; Farooqi, Z.U.R.; Mohy-Ud-Din, W.; Younas, F.; Shahzad, M.T.; Ghani, M.U.; Ayub, M.A.; Qadeer, A. Application of Bioremediation as sustainable approach to remediate heavy metal and pesticide polluted environments. Plant Environ. 2021, 1, 62–92. [Google Scholar]
- Baker, A.J.M. Accumulators and Excluders Strategies in Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Lone, I.A.; Gaffar, M. Phytoremediation potential of medicinal and aromatic plants. Medicinal and Aromatic Plants, 1st ed.; Aftab, T., Hakeem, K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 741–760. [Google Scholar]
- Hosseinniaee, S.; Jafari, M.; Tavili, A.; Zare, S.; Cappai, G.; De Giudici, G. Perspectives for phytoremediation capability of native plants growing on Angouran Pb–Zn mining complex in northwest of Iran. J. Environ. Manag. 2022, 315, 115184. [Google Scholar] [CrossRef]
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of lavender (Lavandula vera L.) for phytoremediation of soils contaminated with heavy metals. Int. J. Agric. Biosyst. Eng. 2015, 9, 522–529. [Google Scholar]
- Tăbărasu, A.M.; Anghelache, D.N.; Găgeanu, I.; Biris, S.S.; Vlădut, N.V. Considerations on the use of active compounds obtained from lavender. Sustainability 2023, 15, 8879. [Google Scholar] [CrossRef]
- Stancheva, I.; Geneva, M.; Markovska, Y.; Tzvetkova, N.; Mitova, I.; Todorova, M.; Petrov, P. A comparative study on plant morphology, gas exchange parameters, and antioxidant response of Ocimum basilicum L. and Origanum vulgare L. grown on industrially polluted soil. Turk. J. Biol. 2014, 38, 89–102. [Google Scholar] [CrossRef]
- Angelova, V. Potential of some medicinal and aromatic plants for phytoremedation of contaminated with heavy metals soils. In Proceedings of the XV Balkan Mineral Processing Congress, Sozopol, Bulgaria, 12–16 June 2013; pp. 1045–1048. [Google Scholar]
- Sarma, H.; Deka, S.; Deka, H.; Saikia, R.R. Accumulation of heavy metals in selected medicinal plants. Rev. Environ. Contam. Toxicol. 2011, 214, 63–86. [Google Scholar] [PubMed]
- Refaz, A.D.; Mohd, S.; Parvaiz, H.Q. Overview of medicinal plants spread and their uses in Asia. J. Phytopharmacol. 2017, 6, 349–351. [Google Scholar]
- Dinu, C.; Gheorghe, S.; Tenea, A.G.; Stoica, C.; Vasile, G.-G.; Popescu, R.L.; Serban, E.A.; Pascu, L.F. Toxic metals (As, Cd, Ni, Pb) impact in the most common medicinal plant (Mentha piperita). Int. J. Environ. Res. Public Health 2021, 18, 3904. [Google Scholar] [CrossRef]
- Singh, S.K.; Biswojit, B. Bioavailability of heavy metals (Cd, Cr, Ni, Pb) to french marigold (Tagetes patula) in relation to soil properties. Trends Tech. Sci. Res. 2018, 1, 94–96. [Google Scholar]
- Maharia, R.S.; Dutta, R.K.; Acharya, R.; Reddy, A.V. Heavy metal bioaccumulation in selected medicinal plants collected from Khetri copper mines and comparison with those collected from fertile soil in Haridwar. India J. Environ. Sci. Health Part B 2010, 45, 174–181. [Google Scholar] [CrossRef]
- Mihalașcu, C.; Bolohan, C.; Tudor, V.; Mihalache, M.; Teodorescu, R.I. Research on the growth and development of some varieties of Lavandula angustifolia (Mill.) in the South-East of Romania. Rom. Biotechnol. Lett. 2020, 25, 2180–2187. [Google Scholar] [CrossRef]
- Nescu, V.; Ciulca, S.; Sumalan, R.M.; Berbecea, A.; Velicevici, G.; Negrea, P.; Gaspar, S.; Sumalan, R.L. Physiological aspects of absorption, translocation, and accumulation of heavy metals in Silphium perfoliatum L. plants grown in a mining-contaminated soil. Minerals 2022, 12, 334. [Google Scholar] [CrossRef]
- Mussali-Galante, P.; Santoyo-Martínez, M.; Castrejón-Godínez, M.L.; Breton-Deval, L.; Rodríguez-Solis, A.; Valencia-Cuevas, L.; Tovar-Sánchez, E. The bioaccumulation potential of heavy metals by Gliricidia sepium (Fabaceae) in mine tailings. Environ. Sci. Pollut. Res. 2023, 30, 38982–38999. [Google Scholar] [CrossRef] [PubMed]
- Crisan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current trends for lavender (Lavandula angustifolia Mill.) crops and products with emphasis on essential oil quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- ISO 11466; Soil Quality. Extraction of Trace Elements Soluble in Aqua Regia. International Organization for Standardization: Geneva, Switzerland, 1995.
- MAPPM. Ordin 756 al Ministerului Apelor, Padurilor si Protectiei Mediului (MAPPM) Pentru Aprobarea Reglementarii Privind Evaluarea Poluarii Mediului, M. of Romania 303 bis. 1997. Available online: http://www.mmediu.ro/app/webroot/uploads/files/OM-184-1997-bilant-de-mediu-si-OM-756-1997-evaluarea-poluarii-mediului.pdf (accessed on 18 September 2023).
- Serban, E.A.; Stefan, D.S.; Peticila, A.; Rau, I.; Boșomoiu, M. Comparative study of the bioaccumulation of heavy metals in lavender plants. U.P.B. Sci. Bull. Ser. B 2023, 85, 145–156. [Google Scholar]
- ISO 11464; Soil Quality. Pretreatment of Samples for Physical-Chemical Analysis. International Organization for Standardization: Geneva, Switzerland, 2006.
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M. Phytoextraction of toxic metals: A review of biological mechanisms. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Suica-Bunghez, I.-R.; Fierascu, R.; Dumitrescu, O.; Fierascu, I.; Ion, R.-M. Characterization of Silver Nanoparticles Obtained by Lavandula angustifolia Extract. Rev. Roum. Chim. 2015, 60, 515–519. [Google Scholar]
- Fakhirah, D.; Magfira, T.A.; Hutama, A.S.; Septama, A.W.; Maryani, F.; Krismastuti, F.S.H. Synthesis, Characterization, and Antibacterial Activity of Plant Derived Zinc Oxide Nanostructure Using Lavandula angustifolia and Phyllanthus niruri Extracts. Indones. J. Chem. 2024, 24, 865–875. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Nielsen, N.E. Studies on the Effect of Heavy Metals (Cd, Pb, Cu, Mn, Zn and Fe) upon the Growth, Productivity and Quality of Lavandula angustifolia Mill. Production. J. Essent. Oil Res. 1996, 8, 259–274. [Google Scholar] [CrossRef]
- Bukar, L.I.; Hati, S.S.; Dimari, G.A.; Waziri, M. Applicability of Linear Regression Model for the Estimation of Mobility Factor of Heavy Metal in Contaminated Soil against Native Concentrations. Adv. Anal. Chem. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Shi, L.; Li, J.; Palansooriya, K.N.; Chen, Y.; Hou, D.; Meers, E.; Tsang, D.C.W.; Wang, X.; Ok, Y.S. Modeling phytoremediation of heavy metal contaminated soils through machine learning. J. Hazard. Mater. 2023, 441, 129904. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Madjar, R.; Dobre, T.; Egri, D.; Moț, A.; Iliescu, L.M.; et al. Effects of biochar on soil properties and tomato growth. Agronomy 2022, 12, 1824. [Google Scholar] [CrossRef]
- Ion, V.A.; Pârvulescu, O.C.; Velcea, D.; Popa, O.; Ahmadi, M. Physico-chemical parameters and antioxidant activity of Romanian sea buckthorn berries. Rev. Chim. 2019, 70, 4187–4192. [Google Scholar]
- Bell, M.J.; McLaughlin, M.J.; Wright, G.C.; Cruickshank, A. Inter and intra-specific variation in accumulation of cadmium by peanut, soyabean, and navybean. Austr. J. Agric. Res. 1997, 48, 1151–1160. [Google Scholar]
- Eriksson, J.E. The influence of pH, soil type and time on adsorption and uptake by plants of cadmium added to soil. Water Air Soil Pollut. 1989, 48, 317–335. [Google Scholar] [CrossRef]
- Logan, T.J.; Lindsay, B.J.; Goins, L.E.; Ryan, J.A. Field assessment of sludge metal bioavailability to crops: Sludge rate response. J. Environ. Qual. 1997, 26, 534–550. [Google Scholar] [CrossRef]
- Sorensen, R.C. Teaching the characteristics of yield response with the Mitscherlich equation using computers. J. Agric. Educ. 1983, 12, 21–25. [Google Scholar] [CrossRef]
- Ferreira, I.E.P.; Zocchi, S.S.; Baron, D. Reconciling the Mitscherlich’s law of diminishing returns with Liebig’s law of the minimum. Some results on crop modeling. Math. Biosci. 2017, 293, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nievergelt, Y. On the existence of best Mitscherlich, Verhulst, and West growth curves for generalized least-squares regression. J. Comput. Appl. Math. 2013, 248, 31–46. [Google Scholar] [CrossRef]
- Liao, Z.Q.; Zheng, J.; Fan, J.L.; Pei, S.Z.; Dai, Y.L.; Zhang, F.C.; Li, Z.J. Novel models for simulating maize growth based on thermal time and photothermal units: Applications under various mulching practices. J. Integr. Agric. 2023, 22, 1381–1395. [Google Scholar] [CrossRef]
- Tudoreanu, L.; Phillips, C.J.C. Empirical models of cadmium accumulation in maize, rye grass and soya bean plants. J. Food Agric. 2004, 84, 845–852. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).