The Influence of Thermal Stress on Cadmium Uptake in Arctic Charr (Salvelinus alpinus) and Its Effects on Indicators of Fish Health and Condition, with Implications for Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Design
2.2. Cadmium Analysis
2.3. Enzyme and Protein Assays
2.4. Statistical Analysis
3. Results
3.1. Variation in Biometric Variables and Fish Condition Across Exposure Treatments
3.2. Variation in Cd Uptake in Dorsal Muscle, Liver, and Kidney Tissues and Relationships with Biometric Variables
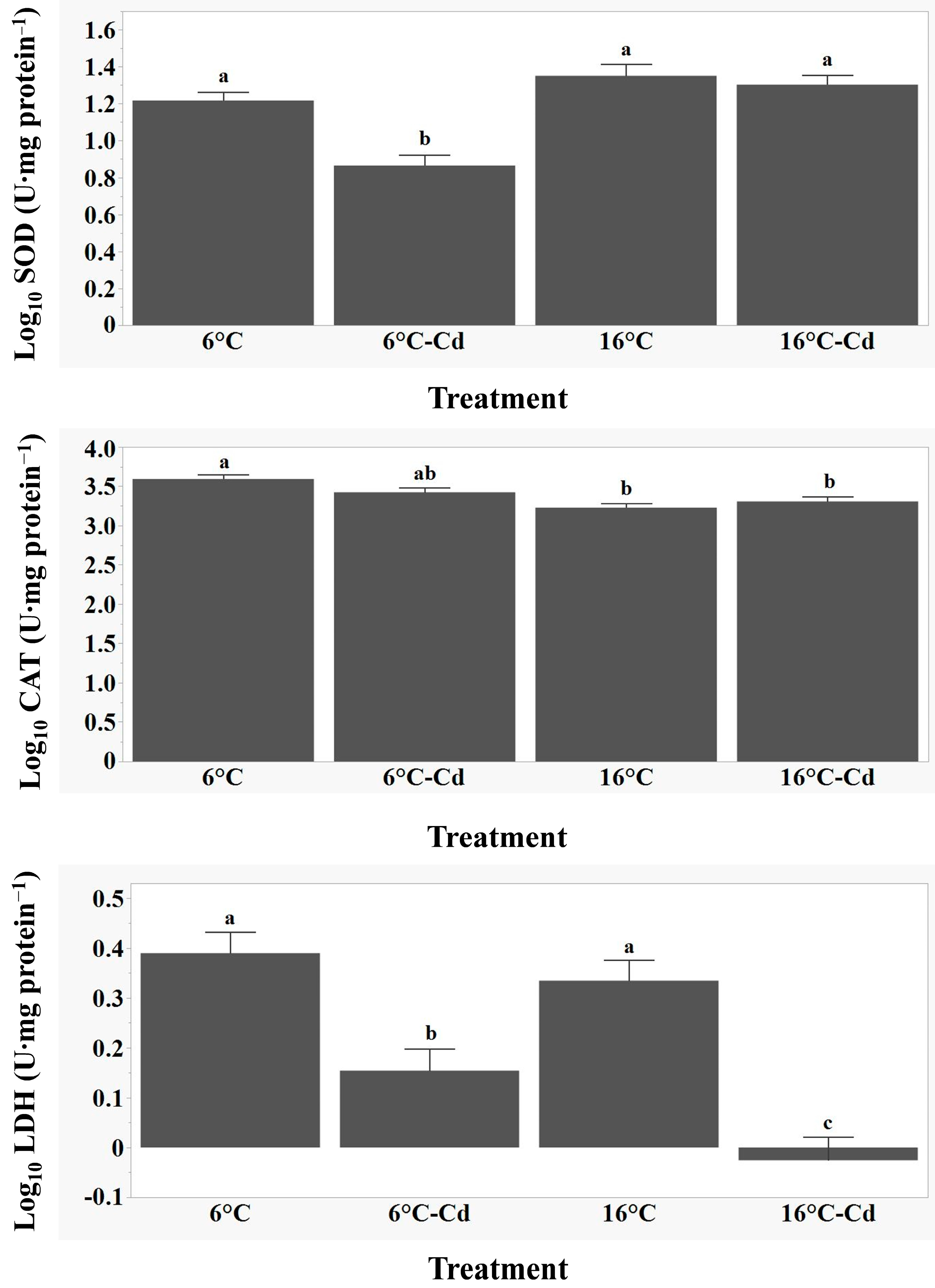
3.3. Variations in SOD, CAT, and LDH Activity and Relationships with Biometric Variables and Tissue Cd Concentrations
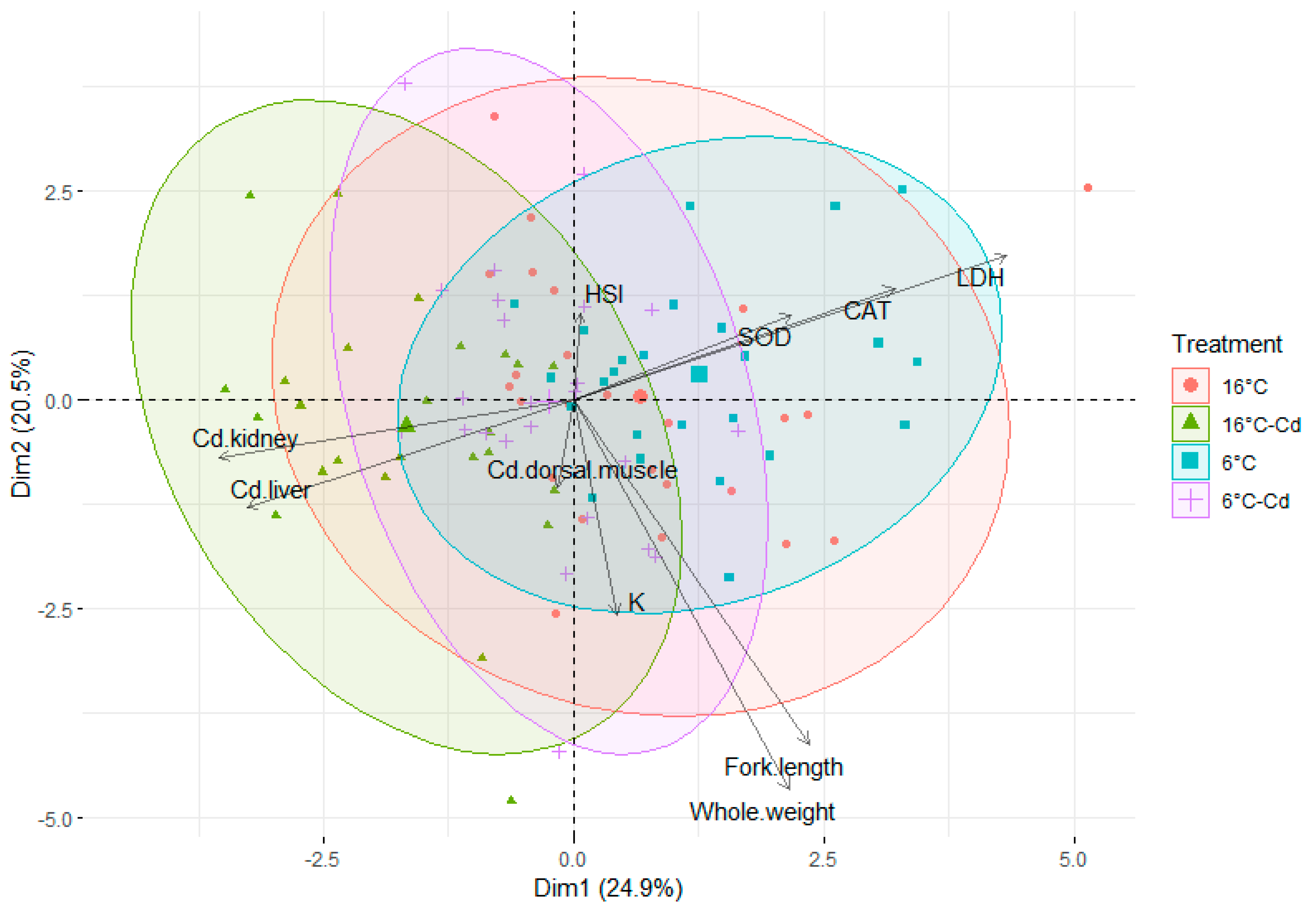
3.4. Principal Component Analysis
4. Discussion
4.1. Variation in Biometric Variables and Fish Condition Across Exposure Treatments
4.2. Variation in Cd Uptake in Dorsal Muscle, Liver, and Kidney Tissues and Relationships with Biometric Variables
4.3. Variation in SOD, CAT, and LDH Activity, as Well as Relationships with Biometric Variables and Tissue Cd Concentrations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reist, J.D.; Wrona, F.J.; Prowse, T.D.; Power, M.; Dempson, J.B.; Beamish, R.J.; King, J.R.; Carmichael, T.J.; Sawatzky, C.D. General effects of climate change on Arctic fishes and fish populations. Ambio J. Hum. Environ. 2006, 35, 370–380. [Google Scholar] [CrossRef]
- Murdoch, A.; Power, M. The effect of lake morphometry on thermal habitat use and growth in Arctic charr populations: Implications for understanding climate-change impacts. Ecol. Freshw. Fish 2013, 22, 453–466. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Marques, T.; Bispo, R.; Dionísio, G.; Faria, A.M.; Machado, J.; Peck, M.A.; Pörtner, H.; Pousão-Ferreira, P. Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim. Change 2016, 137, 495–509. [Google Scholar] [CrossRef]
- Anwar, S.B.; Cathcart, K.; Darakananda, K.; Gaing, A.N.; Shin, S.Y.; Vronay, X.; Wright, D.N.; Ellerby, D.J. The effects of steady swimming on fish escape performance. J. Comp. Physiol. A 2016, 202, 425–433. [Google Scholar] [CrossRef]
- Fenkes, M.; Shiels, H.A.; Fitzpatrick, J.L.; Nudds, R.L. The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 193, 11–21. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Grasset, J.; Ollivier, É.; Bougas, B.; Yannic, G.; Campbell, P.G.C.; Bernatchez, L.; Couture, P. Combined effects of temperature changes and metal contamination at different levels of biological organization in yellow perch. Aquat. Toxicol. 2016, 177, 324–332. [Google Scholar] [CrossRef]
- Aissia, E.; Blier, P.U.; Fadhlaoui, M.; Couture, P. Thermal modulation of mitochondrial function is affected by environmental nickel in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2023, 257, 106451. [Google Scholar] [CrossRef]
- Couture, P.; Pyle, G. Live fast and die young: Metal effects on condition and physiology of wild yellow perch from along two metal contamination gradients. Hum. Ecol. Risk Assess. 2008, 14, 73–96. [Google Scholar] [CrossRef]
- Smith, R.W.; Blaney, S.C.; Dowling, K.; Sturm, A.; Jönsson, M.; Houlihan, D.F. Protein synthesis costs could account for the tissue-specific effects of sub-lethal copper on protein synthesis in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 53, 265–277. [Google Scholar] [CrossRef]
- Sherwood, G.D.; Rasmussen, J.B.; Rowan, D.J.; Brodeur, J.; Hontela, A. Bioenergetic costs of heavy metal exposure in yellow perch (Perca flavescens): In situ estimates with a radiotracer (137Cs) technique. Can. J. Fish. Aquat. Sci. 2000, 57, 441–450. [Google Scholar] [CrossRef]
- Laflamme, J.-S.; Couillard, Y.; Campbell, P.G.; Hontela, A. Interrenal metallothionein and cortisol secretion in relation to Cd, Cu, and Zn exposure in yellow perch, Perca flavescens, from Abitibi lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 1692–1700. [Google Scholar] [CrossRef]
- Ikemoto, T.; Tu, N.P.; Okuda, N.; Iwata, A.; Omori, K.; Tanabe, S.; Tuyen, B.C.; Takeuchi, I. Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch. Environ. Con. Tox. 2008, 54, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Q.; Yang, F.; Yan, X.W. Biomagnification of trace elements in a benthic food web: The case study of Deer Island (Northern Yellow Sea). Chem. Ecol. 2013, 29, 197–207. [Google Scholar] [CrossRef]
- Blanchet, C.; Rochette, L. Nutrition and food consumption among the Inuit of Nunavik. Nunavik Inuit health survey 2004, Qanuippitaa? How are we? 2550526325; Institut national de santé publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS): Québec, QC, Canada, 2008. [Google Scholar]
- Huet, C.; Rosol, R.; Egeland, G.M. The prevalence of food insecurity is high and the diet quality poor in Inuit communities. J. Nutr. 2012, 142, 541–547. [Google Scholar] [CrossRef]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio J. Hum. Environ. 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Smith, A.H.; Steinmaus, C.M. Health effects of arsenic and chromium in drinking water: Recent human findings. Annu. Rev. Public Health 2009, 30, 107–122. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, C.A.; Belger, L.; Pelletier, E.; Rouleau, C. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environ. Res. 2002, 90, 217–225. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, C.A.; Rouleau, C.; Pelletier, E.; Audet, C.; Tjälve, H. Distribution kinetics of dietary methylmercury in the arctic charr (Salvelinus alpinus). Environ. Sci. Technol. 1999, 33, 902–907. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.; Nordberg, M.; Friberg, L. Cadmium. In Handblook on the Toxicology of Metals, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 446–486. [Google Scholar]
- Li, C.; Wang, H.; Liao, X.; Xiao, R.; Liu, K.; Bai, J.; Li, B.; He, Q. Heavy metal pollution in coastal wetlands: A systematic review of studies globally over the past three decades. J. Hazard. Mater. 2022, 424, 127312. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Saxena, G.; Mulla, S. Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1. [Google Scholar]
- Cesar Minga, J.; Elorza, F.J.; Rodriguez, R.; Iglesias, A.; Esenarro, D. Assessment of water resources pollution associated with mining activities in the Parac subbasin of the Rimac River. Water 2023, 15, 965. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Andrés, J.A.; Niyogi, S. Molecular evidence and physiological characterization of iron absorption in isolated enterocytes of rainbow trout (Oncorhynchus mykiss): Implications for dietary cadmium and lead absorption. Aquat. Toxicol. 2010, 99, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sloman, K.A.; Scott, G.R.; Diao, Z.; Rouleau, C.; Wood, C.M.; McDonald, D.G. Cadmium affects the social behaviour of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2003, 65, 171–185. [Google Scholar] [CrossRef]
- Messaoudi, I.; Barhoumi, S.; Saïd, K.; Kerken, A. Study on the sensitivity to cadmium of marine fish Salaria basilisca (Pisces: Blennidae). J. Environ. Sci. 2009, 21, 1620–1624. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Li, W.-Y. Chronic waterborne zinc and cadmium exposures induced different responses towards oxidative stress in the liver of zebrafish. Aquat. Toxicol. 2016, 177, 261–268. [Google Scholar] [CrossRef]
- Croteau, M.N.; Luoma, S.N.; Stewart, A.R. Trophic transfer of metals along freshwater food webs: Evidence of cadmium biomagnification in nature. Limnol. Oceanogr. 2005, 50, 1511–1519. [Google Scholar] [CrossRef]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Lipmann, M. Human Exposures and Their Health Effects, 2nd ed.; Wiley Intersciences: Hoboken, NJ, USA, 2000. [Google Scholar]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Defo, M.A.; Bernatchez, L.; Campbell, P.G.; Couture, P. Waterborne cadmium and nickel impact oxidative stress responses and retinoid metabolism in yellow perch. Aquat. Toxicol. 2014, 154, 207–220. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Couture, P. Combined effects of temperature and metal exposure on the fatty acid composition of cell membranes, antioxidant enzyme activities and lipid peroxidation in yellow perch (Perca flavescens). Aquat. Toxicol. 2016, 180, 45–55. [Google Scholar] [CrossRef]
- Wepener, V.; Van Vuren, J.; Chatiza, F.; Mbizi, Z.; Slabbert, L.; Masola, B. Active biomonitoring in freshwater environments: Early warning signals from biomarkers in assessing biological effects of diffuse sources of pollutants. Phys. Chem. Earth 2005, 30, 751–761. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, A.; Dounce, A.L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol. Rev. 1970, 50, 319–375. [Google Scholar] [CrossRef]
- Audet, D.; Couture, P. Seasonal variations in tissue metabolic capacities of yellow perch (Perca flavescens) from clean and metal-contaminated environments. Can. J. Fish. Aquat. Sci. 2003, 60, 269–278. [Google Scholar] [CrossRef]
- Garnier, C.; Blier, P.U.; Couture, P. Evaluation of the combined effects of manganese and thermal stress on the metabolic capacities of Arctic charr (Salvelinus alpinus). Ecotoxicol. Environ. Saf. 2025, 292, 117895. [Google Scholar] [CrossRef]
- Liu, X.J.; Luo, Z.; Xiong, B.X.; Liu, X.; Zhao, Y.H.; Hu, G.F.; Lv, G.J. Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol. Environ. Saf. 2010, 73, 1286–1291. [Google Scholar] [CrossRef]
- Carvalho, C.d.S.; Fernandes, M.N. Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 437–442. [Google Scholar] [CrossRef]
- Swanson, H.K.; Kidd, K.A. Mercury concentrations in Arctic food fishes reflect the presence of anadromous Arctic charr (Salvelinus alpinus), species, and life history. Environ Sci Technol 2010, 44, 3286–3292. [Google Scholar] [CrossRef]
- Maes, G.; Raeymaekers, J.; Pampoulie, C.; Seynaeve, A.; Goemans, G.; Belpaire, C.; Volckaert, F. The catadromous European eel Anguilla anguilla (L.) as a model for freshwater evolutionary ecotoxicology: Relationship between heavy metal bioaccumulation, condition and genetic variability. Aquat. Toxicol. 2005, 73, 99–114. [Google Scholar] [CrossRef]
- Eastwood, S.; Couture, P. Seasonal variations in condition and liver metal concentrations of yellow perch (Perca flavescens) from a metal-contaminated environment. Aquat Toxicol 2002, 58, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-C. The lead accumulation and hematological findings in juvenile rock fish Sebastes schlegelii exposed to the dietary lead (II) concentrations. Ecotoxicol. Environ. Saf. 2015, 115, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Luo, Z.; Chen, Q.-L.; Liu, X.; Liu, C.-X.; Zhao, Y.-H.; Gong, Y. Effect of waterborne zinc exposure on metal accumulation, enzymatic activities and histology of Synechogobius hasta. Ecotoxicol. Environ. Saf. 2011, 74, 1864–1873. [Google Scholar] [CrossRef]
- Gagnon, A.; Jumarie, C.; Hontela, A. Effects of Cu on plasma cortisol and cortisol secretion by adrenocortical cells of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2006, 78, 59–65. [Google Scholar] [CrossRef]
- Heath, A.G. Water Pollution and Fish Physiology; CRC press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Fadhlaoui, M.; Pierron, F.; Couture, P. Temperature and metal exposure affect membrane fatty acid composition and transcription of desaturases and elongases in fathead minnow muscle and brain. Ecotoxicol. Environ. Saf. 2018, 148, 632–643. [Google Scholar] [CrossRef]
- Larsson, S. Thermal preference of Arctic charr, Salvelinus alpinus, and brown trout, Salmo trutta—Implications for their niche segregation. Environ. Biol. Fishes 2005, 73, 89–96. [Google Scholar] [CrossRef]
- Larsson, S.; Berglund, I. Growth and food consumption of 0+ Arctic charr fed pelleted or natural food at six different temperatures. J. Fish Biol. 1998, 52, 230–242. [Google Scholar] [CrossRef]
- Rikardsen, A.H.; Diserud, O.H.; Elliott, J.M.; Dempson, J.B.; Sturlaugsson, J.; Jensen, A.J. The marine temperature and depth preferences of Arctic charr (Salvelinus alpinus) and sea trout (Salmo trutta), as recorded by data storage tags. Fish. Oceanogr. 2007, 16, 436–447. [Google Scholar] [CrossRef]
- Larsson, S.; Berglund, I. The effect of temperature on the energetic growth efficiency of Arctic charr (Salvelinus alpinus L.) from four Swedish populations. J. Therm. Biol. 2005, 30, 29–36. [Google Scholar] [CrossRef]
- Hansen, A.K.; Byriel, D.B.; Jensen, M.R.; Steffensen, J.F.; Svendsen, M.B.S. Optimum temperature of a northern population of Arctic charr (Salvelinus alpinus) using heart rate Arrhenius breakpoint analysis. Polar Biol. 2017, 40, 1063–1070. [Google Scholar] [CrossRef]
- Harris, L.N.; Yurkowski, D.J.; Gilbert, M.J.; Else, B.G.; Duke, P.J.; Ahmed, M.M.; Tallman, R.F.; Fisk, A.T.; Moore, J. Depth and temperature preference of anadromous Arctic char Salvelinus alpinus in the Kitikmeot Sea, a shallow and low-salinity area of the Canadian Arctic. Mar. Ecol. Prog. Ser. 2020, 634, 175–197. [Google Scholar] [CrossRef]
- Devaux, A.; Fiat, L.; Gillet, C.; Bony, S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus). Aquat. Toxicol. 2011, 101, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.L.; McGowan, C.R.; Cooper, G.A.; Koop, B.F.; Davidson, W.S. Identification of genes associated with heat tolerance in Arctic charr exposed to acute thermal stress. Physiol. Genom. 2011, 43, 685–696. [Google Scholar] [CrossRef]
- Crawford, A.; Stroeve, J.; Smith, A.; Jahn, A. Arctic open-water periods are projected to lengthen dramatically by 2100. Commun. Earth Environ. 2021, 2, 109. [Google Scholar] [CrossRef]
- Han, J.-S.; Park, H.-S.; Chung, E.-S. Projections of central Arctic summer sea surface temperatures in CMIP6. Environ. Res. Lett. 2023, 18, 124047. [Google Scholar] [CrossRef]
- Beuvard, C.; Imsland, A.K.; Thorarensen, H. The effect of temperature on growth performance and aerobic metabolic scope in Arctic charr, Salvelinus alpinus (L.). J. Therm. Biol. 2022, 104, 103117. [Google Scholar] [CrossRef]
- Stephenson, M.; Mackie, G. Total cadmium concentrations in the water and littoral sediments of central Ontario lakes. Water Air Soil Pollut. 1988, 38, 121–136. [Google Scholar] [CrossRef]
- Couture, P.; Busby, P.; Gauthier, C.; Rajotte, J.W.; Pyle, G.G. Seasonal and regional variations of metal contamination and condition indicators in yellow perch (Perca flavescens) along two polymetallic gradients. I. Factors influencing tissue metal concentrations. Hum. Ecol. Risk Assess. 2008, 14, 97–125. [Google Scholar] [CrossRef]
- Loon, J.C.V.; Beamish, R.J. Heavy-Metal Contamination by Atmospheric Fallout of Several Flin Flon Area Lakes and the Relation to Fish Populations. J. Fish. Res. Board Can. 1977, 34, 899–906. [Google Scholar] [CrossRef]
- Cao, L.; Huang, W.; Liu, J.; Yin, X.; Dou, S. Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp. Biochem. Physiol. C 2010, 151, 386–392. [Google Scholar] [CrossRef]
- Hollis, L.; McGeer, J.C.; McDonald, D.G.; Wood, C.M. Cadmium accumulation, gill Cd binding, acclimation, and physiological effects during long term sublethal Cd exposure in rainbow trout. Aquat. Toxicol. 1999, 46, 101–119. [Google Scholar] [CrossRef]
- Xie, D.; Li, Y.; Liu, Z.; Chen, Q. Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C 2019, 222, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Driessnack, M.K.; Jamwal, A.; Niyogi, S. Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol. Environ. Saf. 2017, 140, 65–75. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 154–155. [Google Scholar]
- Laurén, D.J.; Wails, D. Liver structural alterations accompanying chronic toxicity in fishes: Potential biomarkers of exposure. In Biomarkers of Environmental Contamination; CRC Press: Boca Raton, FL, USA, 2018; pp. 17–57. [Google Scholar]
- Larose, C.; Canuel, R.; Lucotte, M.; Di Giulio, R.T. Toxicological effects of methylmercury on walleye (Sander vitreus) and perch (Perca flavescens) from lakes of the boreal forest. Comp. Biochem. Physiol. C 2008, 147, 139–149. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lapointe, D.; Pierron, F.; Couture, P. Individual and combined effects of heat stress and aqueous or dietary copper exposure in fathead minnows (Pimephales promelas). Aquat. Toxicol. 2011, 104, 80–85. [Google Scholar] [CrossRef]
- Gauthier, C.; Campbell, P.G.C.; Couture, P. Physiological correlates of growth and condition in the yellow perch (Perca flavescens). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 526–532. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental design and data analysis for biologists; Cambridge University Press: London, UK, 2002. [Google Scholar]
- McCullagh, P. Generalized Linear Models; Routledge: London, UK, 2019. [Google Scholar]
- Kammerer, B.D.; Heppell, S.A. The effects of semichronic thermal stress on physiological indicators in steelhead. Trans. Am. Fish. Soc. 2013, 142, 1299–1307. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Mendonça, V.; Leal, M.C.; Flores, A.A.V.; Cabral, H.N.; Diniz, M.S.; Vinagre, C. Thermal stress, thermal safety margins and acclimation capacity in tropical shallow waters—An experimental approach testing multiple end-points in two common fish. Ecol. Indic. 2017, 81, 146–158. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Wilson, R.S.; Navas, C.A.; James, R.S. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 2003, 18, 234–240. [Google Scholar] [CrossRef]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Nilsson, G.E. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob. Change Biol. 2011, 17, 1712–1719. [Google Scholar] [CrossRef]
- Drąg-Kozak, E.; Pawlica-Gosiewska, D.; Gawlik, K.; Socha, M.; Gosiewski, G.; Łuszczek-Trojnar, E.; Solnica, B.; Popek, W. Cadmium-induced oxidative stress in Prussian carp (Carassius gibelio Bloch) hepatopancreas: Ameliorating effect of melatonin. Environ. Sci. Pollut. Res. 2019, 26, 12264–12279. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Andrés, J.A.; Niyogi, S. Effects of dietary cadmium exposure on tissue-specific cadmium accumulation, iron status and expression of iron-handling and stress-inducible genes in rainbow trout: Influence of elevated dietary iron. Aquat. Toxicol. 2011, 102, 1–9. [Google Scholar] [CrossRef]
- Çiftçi, N.; Ay, Ö.; Karayakar, F.; Cicik, B.; Erdem, C. Effects of zinc and cadmium on condition factor, hepatosomatic and gonadosomatic index of Oreochromis niloticus. Fresenius Environ. Bull. 2015, 24, 1–4. [Google Scholar]
- Bernal, M.A.; Donelson, J.M.; Veilleux, H.D.; Ryu, T.; Munday, P.L.; Ravasi, T. Phenotypic and molecular consequences of stepwise temperature increase across generations in a coral reef fish. Mol. Ecol. 2018, 27, 4516–4528. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kumar, R.; Sundaray, J.K.; Patnaik, S.T.; Mishra, C.; Rather, M.A. Structural damage in liver, gonads, and reduction in spawning performance and alteration in the haematological parameter of Anabas testudineus by glyphosate-a herbicide. Aquac. Res. 2021, 52, 1150–1159. [Google Scholar] [CrossRef]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Dallinger, R.; Egg, M.; Kock, G.; Hofer, R. The role of metallothionein in cadmium accumulation of Arctic char (Salvelinus alpinus) from high alpine lakes. Aquat. Toxicol. 1997, 38, 47–66. [Google Scholar] [CrossRef]
- Martyniuk, M.A.C.; Couture, P.; Tran, L.; Beaupré, L.; Urien, N.; Power, M. A seasonal comparison of trace metal concentrations in the tissues of Arctic charr (Salvelinus alpinus) in Northern Québec, Canada. Ecotoxicology 2020, 29, 1327–1346. [Google Scholar] [CrossRef]
- Köck, G.; Triendl, M.; Hofer, R. Seasonal patterns of metal accumulation in Arctic char (Salvelinus alpinus) from an oligotrophic Alpine lake related to temperature. Can. J. Fish. Aquat. Sci. 1996, 53, 780–786. [Google Scholar] [CrossRef]
- Douben, P.E. A mathematical model for cadmium in the stone loach (Noemacheilus barbatulus L.) from the River Ecclesbourne, Derbyshire. Ecotoxicol. Environ. Saf. 1990, 19, 160–183. [Google Scholar] [CrossRef]
- Olsson, P.-E.; Haux, C.; Förlin, L. Variations in hepatic metallothionen, zinc and copper levels during an annual reproductive cycle in rainbow trout, Salmo gairdneri. Fish Physiol. Biochem. 1987, 3, 39–47. [Google Scholar] [CrossRef]
- Overnell, J.; McIntosh, R.; Fletcher, T. The levels of liver metallothionein and zinc in plaice, Pleuronectes platessa L., during the breeding season, and the effect of oestradiol injection. J. Fish Biol. 1987, 30, 539–546. [Google Scholar] [CrossRef]
- Povlsen, A.F.; Korsgaard, B.; Bjerregaard, P. The effect of cadmium on vitellogenin metabolism in estradiol-induced flounder(Platichthys flesus(L.)) males and females. Aquat. Toxicol. 1990, 17, 253–262. [Google Scholar] [CrossRef]
- Giles, M.A. Accumulation of cadmium by rainbow trout, Salmo gairdneri, during extended exposure. Can. J. Fish. Aquat. Sci. 1988, 45, 1045–1053. [Google Scholar] [CrossRef]
- Harrison, S.; Klaverkamp, J. Uptake, elimination and tissue distribution of dietary and aqueous cadmium by rainbow trout (Salmo gairdneri Richardson) and lake whitefish (Coregonus clupeaformis Mitchill). Environ. Toxicol. Chem. Int. J. 1989, 8, 87–97. [Google Scholar] [CrossRef]
- Glynn, A.W.; Andersson, L.; Gabring, S.; Runn, P. Cadmium turnover in minnows, Phoxinus phoxinus, fed 109Cd-labeled Daphnia magna. Chemosphere 1992, 24, 359–368. [Google Scholar] [CrossRef]
- Varanasi, U.; Markey, D. Uptake and release of lead and cadmium in skin and mucus of Coho salmon (Oncorhynchus kisutch). Comp. Biochem. Physiol. C 1978, 60, 187–191. [Google Scholar] [CrossRef]
- Oronsaye, J. The uptake and loss of dissolved cadmium by the stickleback, Gasterosteus aculeatus L. Ecotoxicol. Environ. Saf. 1987, 14, 88–96. [Google Scholar] [CrossRef]
- Black, M.C.; Millsap, D.S.; McCarthy, J.F. Effects of acute temperature change on respiration and toxicant uptake by rainbow trout, Salmo gairdneri (Richardson). Physiol. Zool. 1991, 64, 145–168. [Google Scholar] [CrossRef]
- Douben, P.E.T. Metabolic rate and uptake and loss of cadmium from food by the fish Noemacheilus barbatulus L. (stone loach). Environ. Pollut. 1989, 59, 177–202. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.-X. Waterborne cadmium and zinc uptake in a euryhaline teleost Acanthopagrus schlegeli acclimated to different salinities. Aquat Toxicol 2007, 84, 173–181. [Google Scholar] [CrossRef]
- Ward, D.M.; Nislow, K.H.; Chen, C.Y.; Folt, C.L. Reduced trace element concentrations in fast-growing juvenile Atlantic salmon in natural streams. Environ. Sci. Technol. 2010, 44, 3245–3251. [Google Scholar] [CrossRef]
- Yi, Y.-J.; Zhang, S.-H. Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ. Sci. Pollut. Res. 2012, 19, 3989–3996. [Google Scholar] [CrossRef]
- Ramos-Osuna, M.; Patiño-Mejía, C.; Ruelas-Inzunza, J.; Escobar-Sánchez, O. Bioaccumulation of mercury in Haemulopsis elongatus and Pomadasys macracanthus from the SE Gulf of California: Condition indexes and health risk assessment. Environ. Monit. Assess. 2020, 192, 704. [Google Scholar] [CrossRef]
- Baroudy, E.; Elliott, J. The critical thermal limits for juvenile Arctic charr Salvelinus alpinus. J. Fish Biol. 1994, 45, 1041–1053. [Google Scholar] [CrossRef]
- Thyrel, M.; Berglund, I.; Larsson, S.; Näslund, I. Upper thermal limits for feeding and growth of 0+ Arctic charr. J. Fish Biol. 1999, 55, 199–210. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Kregel, K.C. Invited review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef]
- Abele, D. Toxic oxygen: The radical life-giver. Nature 2002, 420, 27. [Google Scholar] [CrossRef]
- Bachinski, N.; Koziol, C.; Batel, R.; Labura, Z.; Schröder, H.C.; Müller, W.E. Immediate early response of the marine sponge Suberites domuncula to heat stress: Reduction of trehalose and glutathione concentrations and glutathione S-transferase activity. J. Exp. Mar. Biol. Ecol. 1997, 210, 129–141. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp. Biochem. Physiol. C 2006, 143, 36–41. [Google Scholar] [CrossRef]
- Härtig, E.; Jahn, D. Regulation of the Anaerobic Metabolism in Bacillus subtilis. In Advances in Microbial Physiology, Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 61, pp. 195–216. [Google Scholar]
- Castro-Santos, T. Optimal swim speeds for traversing velocity barriers: An analysis of volitional high-speed swimming behavior of migratory fishes. J. Exp. Biol. 2005, 208, 421–432. [Google Scholar] [CrossRef]

| Exposure Treatment | Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 °C (101) | 6 °C-Cd (105) | 16 °C (99) | 16 °C-Cd (105) | Model | Temperature | Cd | Interaction | |
| Fork length | 165 ± 12 a 126;197 | 162 ± 13 a 117;191 | 159 ± 12 b 128;190 | 157 ± 11 b 125;192 | F(3,409)
= 9.158 p < 0.0001 | <0.0001 | 0.0642 | 0.6521 |
| Whole weight | 40.6 ± 9.8 a 19.0;78.0 | 38.3 ± 10.0 ab 12.0;71.0 | 35.5 ± 9.1 b 15.0;64.0 | 35.2 ± 7.8 b 16.0;65.0 | F(3,409)
= 7.600 p < 0.0001 | <0.0001 | 0.1640 | 0.2866 |
| K | 0.90 ± 0.07 a 0.69;1.08 | 0.88 ± 0.79 a 0.63;1.13 | 0.87 ± 0.09 a 0.67;1.09 | 0.90 ± 0.08 a 0.73;1.13 | F(2,407) = 0.6009 p = 0.5488 | 0.7314 | 0.2993 | - |
| HSI | 1.16 ± 0.22 a 0.55;2.00 | 1.22 ± 0.22 a 0.69;1.96 | 0.98 ± 0.16 c 0.44;1.38 | 1.04 ± 0.18 b 0.64;1.66 | F(3,409)
= 31.75 p < 0.0001 | <0.0001 | 0.0016 | 0.8906 |
| Dorsal Muscle Cd | ||||||||
|---|---|---|---|---|---|---|---|---|
| Linear Regressions | GLMs | |||||||
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | Model | Temperature | Cd | Interaction | |
| Fork length | r2(1,93) = 0.011 p = 0.2937 | r2(1,102) = 0.051 p = 0.0218 | r2(1,94) = 0.000 p = 0.4927 | r2(1,103) = 0.011 p = 0.2750 | X2(4,397)= 32.79 p < 0.0001 | 0.0543 | 0.0025 | 0.0028 |
| Whole weight | r2(1,93) = 0.020 p = 0.1690 | r2(1,102 = 0.027 p = 0.0975 | r2(1,94) = 0.001 p = 0.7898 | r2(1,103) = 0.016 p = 0.1930 | X2(4,397)= 27.14 p < 0.0001 | 0.0682 | 0.0049 | 0.0057 |
| K | r2(1,93) = 0.009 p = 0.3516 | r2(1,102) = 0.001 p = 0.7348 | r2(1,94) = 0.000 p = 0.8559 | r2(1,103) = 0.016 p = 0.1930 | X2(4,397) = 1.972 p = 0.7409 | 0.2266 | 0.2528 | - |
| HSI | r2(1,93) = 0.009 p = 0.3721 | r2(1,102) = 0.032 p = 0.0697 | r2(1,94) = 0.000 p = 0.1881 | r2(1,103) = 0.001 p = 0.7364 | X2(4,397)= 77.06 p < 0.0001 | 0.0006 | 0.1207 | 0.0973 |
| Liver Cd | ||||||||
| Linear regressions | GLMs | |||||||
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | Model | Temperature | Cd | Interaction | |
| Fork length | r2(1,98) = 0.113 p = 0.0006 | r2(1,103) = 0.085 p = 0.0025 | r2(1,96) = 0.005 p = 0.4591 | r2(1,101) = 0.006 p = 0.4503 | X2(4,404)= 23.61 p < 0.0001 | <0.0001 | 0.7704 | 0.4906 |
| Whole weight | r2(1,98) = 0.139 p = 0.0001 | r2(1,103) = 0.069 p= 0.0066 | r2(1,96) = 0.013 p = 0.2673 | r2(1,101) = 0.022 p = 0.1363 | X2(4,404)= 19.07 p < 0.0001 | <0.0001 | 0.9892 | 0.9681 |
| K | r2(1,98) = 0.073 p = 0.0066 | r2(1,103) = 0.007 p = 0.4063 | r2(1,96) = 0.013 p = 0.2649 | r2(1,101) = 0.036 p = 0.0549 | X2(4,404) = 7.573 p = 0.1085 | 0.9669 | 0.2252 | - |
| HSI | r2(1,98) = 0.224 p < 0.0001 | r2(1,103) = 0.056 p = 0.0151 | r2(1,96) = 0.059 p = 0.0153 | r2(1,101) = 0.000 p = 0.9893 | X2(4,404)= 80.60 p < 0.0001 | <0.0001 | 0.2390 | 0.2036 |
| Kidney Cd | ||||||||
| Linear regressions | GLMs | |||||||
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | Model | Temperature | Cd | Interaction | |
| Fork length | r2(1,97) = 0.219 p < 0.0001 | r2(1,102) = 0.028 p = 0.0896 | r2(1,97) = 0.000 p = 0.9144 | r2(1,103) = 0.000 p = 0.9902 | X2(4,404)= 25.17 p < 0.0001 | 0.1958 | 0.3226 | - |
| Whole weight | r2(1,97) = 0.293 p <0.0001 | r2(1,102) = 0.044 p= 0.0320 | r2(1,97) = 0.007 p = 0.4179 | r2(1,103) = 0.004 p = 0.5620 | X2(4,404)= 20.33 p = 0.0004 | 0.2603 | 0.3157 | - |
| K | r2(1,97) = 0.130 p = 0.0002 | r2(1,102) = 0.042 p = 0.0379 | r2(1,97) = 0.054 p = 0.0211 | r2(1,103) = 0.044 p = 0.0313 | X2(4,404)= 10.67 p = 0.0305 | 0.9181 | 0.9824 | - |
| HSI | r2(1,97) = 0.081 p = 0.0043 | r2(1,102) = 0.000 p = 0.9283 | r2(1,97) = 0.086 p = 0.0033 | r2(1,103) = 0.002 p = 0.6416 | X2(4,404)= 83.91 p < 0.0001 | <0.0001 | 0.0550 | 0.3410 |
| SOD | ||||
|---|---|---|---|---|
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | |
| Fork length | r2(1,24) = 0.025 p = 0.4475 | r2(1,24) = 0.022 p = 0.4832 | r2(1,24) = 0.019 p = 0.5109 | r2(1,24) = 0.050 p = 0.2832 |
| Whole weight | r2(1,24) = 0.068 p = 0.2089 | r2(1,24) = 0.031 p = 0.3691 | r2(1,24) = 0.050 p = 0.2837 | r2(1,24) = 0.052 p = 0.2724 |
| K | r2(1,24) = 0.030 p = 0.4077 | r2(1,24) = 0.004 p = 0.7668 | r2(1,24) = 0.036 p = 0.3618 | r2(1,24) = 0.001 p = 0.9135 |
| HSI | r2(1,24) = 0.027 p = 0.4288 | r2(1,24) = 0.017 p = 0.5395 | r2(1,24) = 0.001 p = 0.5709 | r2(1,24) = 0.111 p = 0.1032 |
| Cd dorsal muscle | r2(1,24) = 0.000 p = 0.9209 | r2(1,24) = 0.071 p = 0.1965 | r2(1,23) = 0.050 p = 0.2843 | r2(1,24) = 0.000 p = 0.9182 |
| Cd liver | r2(1,24) = 0.021 p = 0.4933 | r2(1,23) = 0.012 p = 0.6089 | r2(1,22) = 0.344 p = 0.0033 | r2(1,24) = 0.129 p = 0.0788 |
| Cd kidney | r2(1,24) = 0.007 p = 0.6845 | r2(1,24) = 0.003 p = 0.7834 | r2(1,24) = 0.079 p = 0.1722 | r2(1,24) = 0.031 p = 0.4016 |
| CAT | ||||
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | |
| Fork length | r2(1,24) = 0.006 p = 0.7180 | r2(1,24) = 0.010 p = 0.6351 | r2(1,24) = 0.032 p = 0.3957 | r2(1,24) = 0.034 p = 0.3776 |
| Whole weight | r2(1,24) = 0.002 p = 0.8427 | r2(1,24) = 0.002 p = 0.8489 | r2(1,24) = 0.078 p = 0.1772 | r2(1,24) = 0.078 p = 0.1752 |
| K | r2(1,24) = 0.001 p = 0.8595 | r2(1,24) = 0.144 p = 0.0612 | r2(1,24) = 0.079 p = 0.1791 | r2(1,24) = 0.004 p = 0.7767 |
| HSI | r2(1,24) = 0.011 p = 0.6189 | r2(1,24) = 0.053 p = 0.2574 | r2(1,24) = 0.003 p = 0.7791 | r2(1,24) = 0.044 p = 0.3128 |
| Cd dorsal muscle | r2(1,24) = 0.033 p = 0.3866 | r2(1,24) = 0.014 p = 0.5675 | r2(1,24) = 0.028 p = 0.4213 | r2(1,24) = 0.140 p = 0.0650 |
| Cd liver | r2(1,24) = 0.046 p = 0.3046 | r2(1,23) = 0.156 p = 0.0537 | r2(1,24) = 0.027 p = 0.4311 | r2(1,24) = 0.058 p = 0.2450 |
| Cd kidney | r2(1,24) = 0.081 p = 0.1684 | r2(1,24) = 0.174 p = 0.0382 | r2(1,24) = 0.080 p = 0.1720 | r2(1,24) = 0.000 p = 0.9896 |
| LDH | ||||
| 6 °C | 6 °C-Cd | 16 °C | 16 °C-Cd | |
| Fork length | r2(1,24) = 0.001 p = 0.6593 | r2(1,24) = 0.001 p = 0.8613 | r2(1,24) = 0.004 p = 0.7628 | r2(1,24) = 0.018 p = 0.5180 |
| Whole weight | r2(1,24) = 0.000 p = 0.8288 | r2(1,24) = 0.015 p = 0.5603 | r2(1,24) = 0.020 p = 0.4980 | r2(1,24) = 0.027 p = 0.4299 |
| K | r2(1,24) = 0.000 p = 0.9241 | r2(1,24) = 0.025 p = 0.4539 | r2(1,24) = 0.024 p = 0.4629 | r2(1,24) = 0.003 p = 0.7950 |
| HSI | r2(1,24) = 0.014 p = 0.5770 | r2(1,24) = 0.161 p = 0.0469 | r2(1,24) = 0.000 p = 0.9434 | r2(1,24) = 0.001 p = 0.8729 |
| Cd dorsal muscle | r2(1,24) = 0.059 p = 0.2398 | r2(1,24) = 0.027 p = 0.4293 | r2(1,23) = 0.000 p = 0.9629 | r2(1,24) = 0.179 p = 0.0353 |
| Cd liver | r2(1,24) = 0.021 p = 0.4920 | r2(1,23) = 0.152 p = 0.0601 | r2(1,24) = 0.025 p = 0.4471 | r2(1,24) = 0.000 p = 0.9223 |
| Cd kidney | r2(1,24) = 0.030 p = 0.4098 | r2(1,24) = 0.026 p = 0.4442 | r2(1,24) = 0.044 p = 0.3130 | r2(1,24) = 0.065 p = 0.2195 |
| Fork length | Whole weight | K | HSI | Dorsal muscle Cd | Liver Cd | Kidney Cd | SOD | CAT | LDH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fork length | 1.0000 - | |||||||||
| Whole weight | 0.9162 <0.0001 | 1.0000 - | ||||||||
| K | 0.1142 0.2578 | 0.4613 <0.0001 | 1.0000 - | |||||||
| HSI | −0.1047 0.3001 | −0.0894 0.3763 | 0.0294 0.7713 | 1.0000 - | ||||||
| Dorsal muscle Cd | 0.0891 0.3781 | 0.0724 0.4742 | −0.0261 0.7964 | −0.1695 0.0919 | 1.0000 - | |||||
| Liver Cd | −0.0736 0.4667 | −0.0029 0.9771 | 0.1344 0.1825 | 0.0313 0.7574 | −0.0588 0.5609 | 1.0000 - | ||||
| Kidney Cd | −0.2002 0.0458 | −0.1303 0.1963 | 0.1294 0.1995 | −0.0675 0.5045 | −0.0605 0.5496 | 0.6848 <0.0001 | 1.0000 - | |||
| SOD | −0.0396 0.6960 | −0.0029 0.9772 | 0.1283 0.2034 | −0.1456 0.1482 | −0.0198 0.8451 | −0.0635 0.5302 | 0.0377 0.7093 | 1.0000 - | ||
| CAT | 0.1099 0.2765 | 0.0612 0.5453 | −0.0283 0.7795 | 0.2034 0.0423 | −0.1924 0.0551 | −0.1290 0.2007 | −0.1615 0.1084 | 0.2543 0.0107 | 1.0000 - | |
| LDH | 0.0651 0.5201 | 0.0463 0.6474 | 0.0186 0.8541 | 0.0058 0.9544 | −0.0198 0.4636 | −0.4094 <0.0001 | −0.4365 < 0.0001 | 0.5535 <0.0001 | 0.6476 <0.0001 | 1.0000 - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martyniuk, M.A.C.; Garnier, C.; Couture, P. The Influence of Thermal Stress on Cadmium Uptake in Arctic Charr (Salvelinus alpinus) and Its Effects on Indicators of Fish Health and Condition, with Implications for Climate Change. Environments 2025, 12, 176. https://doi.org/10.3390/environments12060176
Martyniuk MAC, Garnier C, Couture P. The Influence of Thermal Stress on Cadmium Uptake in Arctic Charr (Salvelinus alpinus) and Its Effects on Indicators of Fish Health and Condition, with Implications for Climate Change. Environments. 2025; 12(6):176. https://doi.org/10.3390/environments12060176
Chicago/Turabian StyleMartyniuk, Mackenzie Anne Clifford, Camille Garnier, and Patrice Couture. 2025. "The Influence of Thermal Stress on Cadmium Uptake in Arctic Charr (Salvelinus alpinus) and Its Effects on Indicators of Fish Health and Condition, with Implications for Climate Change" Environments 12, no. 6: 176. https://doi.org/10.3390/environments12060176
APA StyleMartyniuk, M. A. C., Garnier, C., & Couture, P. (2025). The Influence of Thermal Stress on Cadmium Uptake in Arctic Charr (Salvelinus alpinus) and Its Effects on Indicators of Fish Health and Condition, with Implications for Climate Change. Environments, 12(6), 176. https://doi.org/10.3390/environments12060176







