Virgin and Photoaged Polyethylene Microplastics Have Different Effects on Collembola and Enchytraeids
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastics
2.2. Experimental Soil
2.3. Model Organisms
2.4. MP Exposure
2.5. Statistical Analyses
3. Results
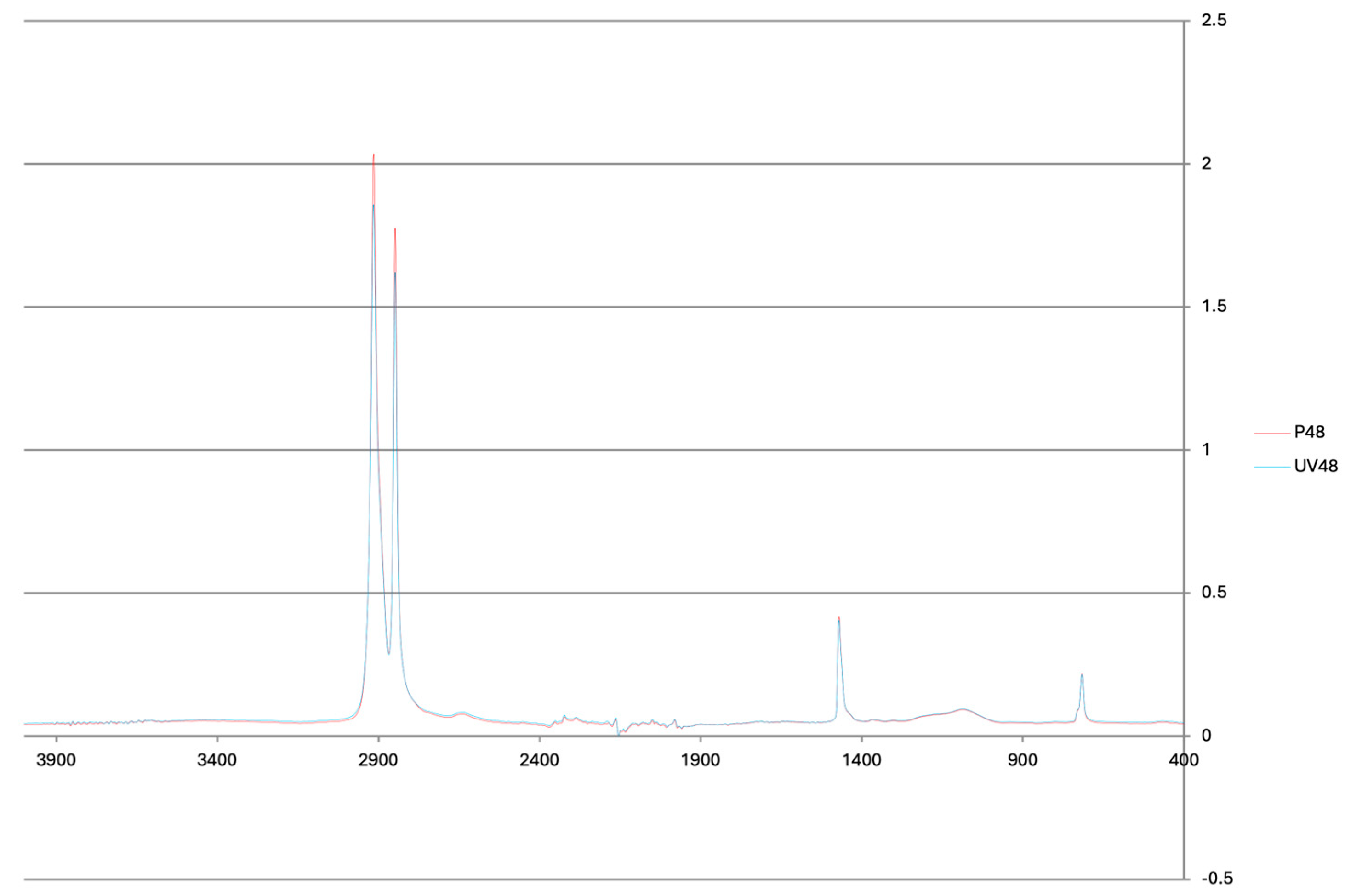
3.1. Chemical Changes from Virgin to Photoaged PE MPs
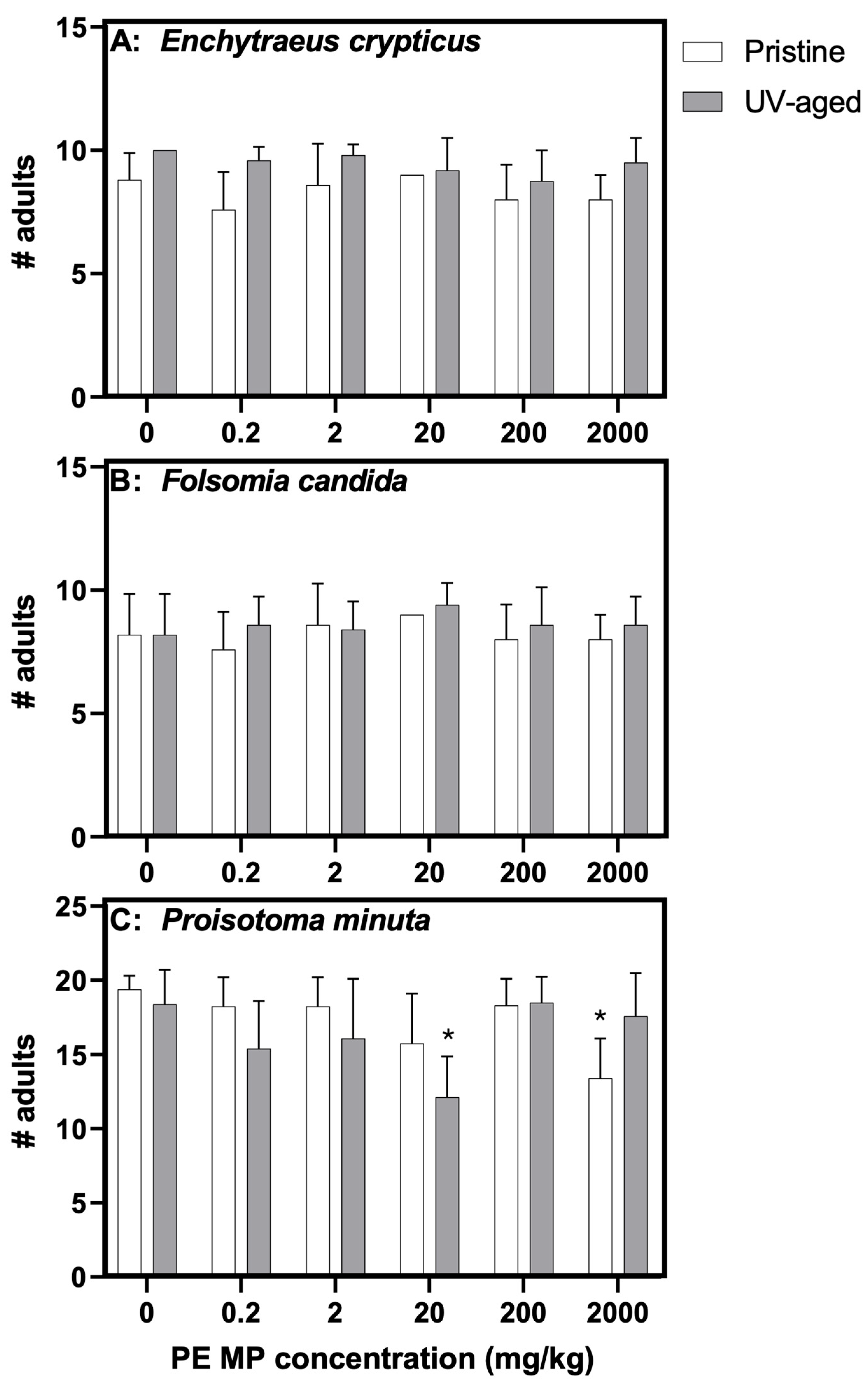
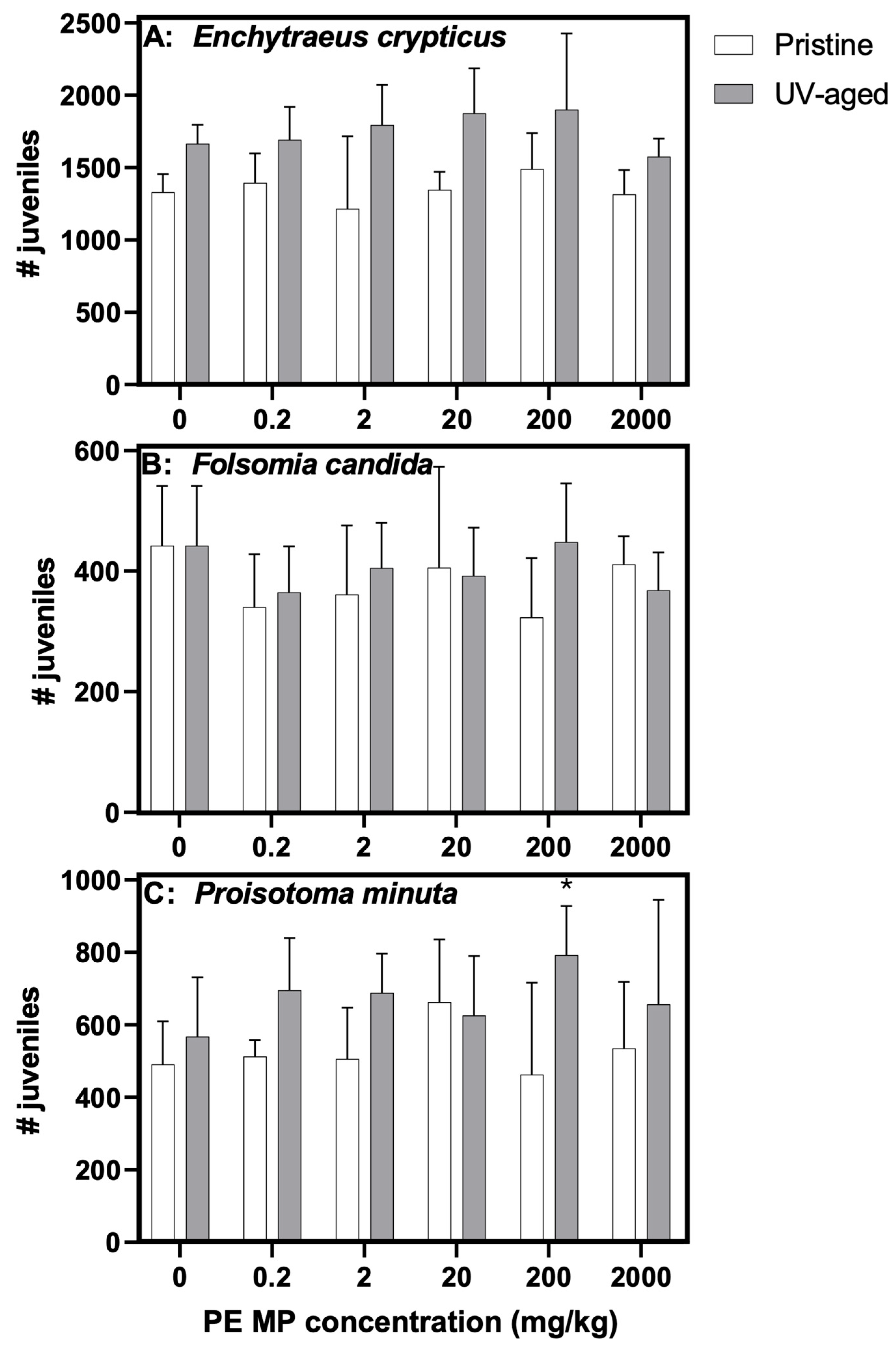
3.2. Effects of Virgin and Photoaged PE MPs on Mesofauna Survival and Reproduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| PE | Polyethylene |
| UV OM | Ultraviolet Organic Matter |
| FTIR PS | Fourier-transform infrared spectroscopy Polystyrene |
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. ANNEX XV Restriction Report Proposal for a Restriction Substance Name(s): Intentionally Added Microplastics; European Chemicals Agency: Helsinki, Finland, 2019. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Büks, F.; Kaupenjohann, M. Global Concentrations of Microplastics in Soils—A Review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic Mulching in Agriculture. Trading Short-Term Agronomic Benefits for Long-Term Soil Degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Liu, Y.; Zhang, Y.; Qu, J. Non-Negligible Effects of UV Irradiation on Transformation and Environmental Risks of Microplastics in the Water Environment. J. Xenobiot. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of Microplastics and Interaction with Other Coexisting Constituents in Terrestrial and Aquatic Environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Liu, W.; Zeb, A.; Shi, R.; Lian, Y.; Wang, Q.; Wang, J.; Li, J.; Zheng, Z.; Liu, J.; et al. Environmental Fate, Aging, Toxicity and Potential Remediation Strategies of Microplastics in Soil Environment: Current Progress and Future Perspectives. Sci. Total Environ. 2024, 906, 167785. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Wang, C.; Hua, X.; Li, H.; Xie, D.; Xiang, M.; Yu, Y. Reproductive Toxicity of UV-Photodegraded Polystyrene Microplastics Induced by DNA Damage-Dependent Cell Apoptosis in Caenorhabditis Elegans. Sci. Total Environ. 2022, 811, 152350. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, J.; Ye, Z.; Klobučar, G.; Li, M. Microplastics—Back to Reality: Impact of Pristine and Aged Microplastics in Soil on Earthworm Eisenia Fetida under Environmentally Relevant Conditions. Environ. Sci. Technol. 2023, 57, 16788–16799. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, L.; Song, W.; Jiang, C.; Li, B.; Du, Z.; Wang, J.; Wang, J.; Li, D.; Zhang, K. Combined Effects of Mulch Film-Derived Microplastics and Atrazine on Oxidative Stress and Gene Expression in Earthworm (Eisenia Fetida). Sci. Total Environ. 2020, 746, 141280. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yang, G.; Dou, P.; Qian, S.; Zhao, L.; Yang, Y.; Fanin, N. Microplastics Negatively Affect Soil Fauna but Stimulate Microbial Activity: Insights from a Field-Based Microplastic Addition Experiment. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201268. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jia, H.; Gao, Q.; Han, S.; Yu, Y.; Sun, L. Influence of Aged and Pristine Polyethylene Microplastics on Bioavailability of Three Heavy Metals in Soil: Toxic Effects to Earthworms (Eisenia Fetida). Chemosphere 2023, 311, 136833. [Google Scholar] [CrossRef]
- Boughattas, I.; Hattab, S.; Zitouni, N.; Mkhinini, M.; Missawi, O.; Bousserrhine, N.; Banni, M. Assessing the Presence of Microplastic Particles in Tunisian Agriculture Soils and Their Potential Toxicity Effects Using Eisenia Andrei as Bioindicator. Sci. Total Environ. 2021, 796, 148959. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Huang, Y.; Feng, Y.; Johansen, A.; Xue, J.; Tremblay, L.A.; Li, Z. Effects of Pristine Microplastics and Nanoplastics on Soil Invertebrates: A Systematic Review and Meta-Analysis of Available Data. Sci. Total Environ. 2021, 788, 147784. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A.; Hendrix, P.F. Secondary Production: Activities of Heterotrophic Organisms—The Soil Fauna. In Fundamentals of Soil Ecology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 79–185. [Google Scholar]
- Castro-Ferreira, M.P.; Roelofs, D.; van Gestel, C.A.M.; Verweij, R.A.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeus Crypticus as Model Species in Soil Ecotoxicology. Chemosphere 2012, 87, 1222–1227. [Google Scholar] [CrossRef]
- Filser, J. The Role of Collembola in Carbon and Nitrogen Cycling in Soil. Pedobiologia 2002, 46, 234–245. [Google Scholar] [CrossRef]
- Potapov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting Taxonomy and Ecology: Trophic Niches of Collembolans as Related to Taxonomic Identity and Life Forms. Soil. Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Wolters, V. Effects of Mesenchytraeus Glandulosus (Oligochaeta, Enchytraeidae) on Decomposition Processes. Pedobiologia 1988, 32, 387–398. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Maaß, S.; Daphi, D.; Lehmann, A.; Rillig, M.C. Transport of Microplastics by Two Collembolan Species. Environ. Pollut. 2017, 225, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-K.; Fang, Y.-M.; Zhu, D.; Christie, P.; Ke, X.; Zhu, Y.-G. Exposure to Nanoplastics Disturbs the Gut Microbiome in the Soil Oligochaete Enchytraeus Crypticus. Environ. Pollut. 2018, 239, 408–415. [Google Scholar] [CrossRef]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in Agricultural Soils: Sources, Effects, and Their Fate. Curr. Opin. Environ. Sci. Health 2022, 25, 100311. [Google Scholar] [CrossRef]
- Liu, Y.; Rillig, M.C.; Liu, Q.; Huang, J.; Khan, M.A.; Li, X.; Liu, Q.; Wang, Q.; Su, X.; Lin, L.; et al. Factors Affecting the Distribution of Microplastics in Soils of China. Front. Environ. Sci. Eng. 2023, 17, 110. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic Fertilizer as a Vehicle for the Entry of Microplastic into the Environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and Fragmentation of Plastics in a Changing Environment; from UV-Radiation to Biological Degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Beriot, N.; Corradini, F.; Silva, V.; Yang, X.; Baartman, J.; Rezaei, M.; van Schaik, L.; Riksen, M.; Geissen, V. Review of Microplastic Sources, Transport Pathways and Correlations with Other Soil Stressors: A Journey from Agricultural Sites into the Environment. Chem. Biol. Technol. Agric. 2022, 9, 20. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; An, Y.-J. Species Sensitivity Distributions of Micro- and Nanoplastics in Soil Based on Particle Characteristics. J. Hazard. Mater. 2023, 452, 131229. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Silva, A.L.P.; Gravato, C.; Pestana, J.L.T. Ingestion of Small-Sized and Irregularly Shaped Polyethylene Microplastics Affect Chironomus Riparius Life-History Traits. Sci. Total Environ. 2019, 672, 862–868. [Google Scholar] [CrossRef] [PubMed]
- ISO 16387:2013; Soil Quality—Effects of Contaminants on Enchytraeidae (Enchytraeus sp.) Determination of Effects on Reproduction. International Organization for Standardization: Geneva, Switzerland, 2013.
- Organization for Economic Cooperation and Development. OECD 220: Guidelines for Testing of Chemicals—Enchytraeid Reproduction Test; Organization for Economic Cooperation and Development: Paris, France, 2004. [Google Scholar]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in Soils: A Review of Methods, Occurrence, Fate, Transport, Ecological and Environmental Risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef] [PubMed]
- ISO 11267:2014; Soil Quality: Inhibition of Reproduction of Collembola (Folsomia Candida) by Soil Contaminants. International Organization for Standardization: Geneva, Switzerland, 2014.
- Ju, H.; Zhu, D.; Qiao, M. Effects of Polyethylene Microplastics on the Gut Microbial Community, Reproduction and Avoidance Behaviors of the Soil Springtail, Folsomia Candida. Environ. Pollut. 2019, 247, 890–897. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.-J. Soil Microplastics Inhibit the Movement of Springtail Species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.L.; An, X.L.; Yang, X.R.; Christie, P.; Ke, X.; Wu, L.H.; Zhu, Y.G. Exposure of Soil Collembolans to Microplastics Perturbs Their Gut Microbiota and Alters Their Isotopic Composition. Soil. Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Amorim, M.J.B.; Scott-Fordsmand, J.J. Plastic Pollution—A Case Study with Enchytraeus Crypticus—From Micro-to Nanoplastics. Environ. Pollut. 2021, 271, 116363. [Google Scholar] [CrossRef]
- Kim, S.W.; Waldman, W.R.; Kim, T.-Y.; Rillig, M.C. Effects of Different Microplastics on Nematodes in the Soil Environment: Tracking the Extractable Additives Using an Ecotoxicological Approach. Environ. Sci. Technol. 2020, 54, 13868–13878. [Google Scholar] [CrossRef]
- Jemec Kokalj, A.; Dolar, A.; Titova, J.; Visnapuu, M.; Škrlep, L.; Drobne, D.; Vija, H.; Kisand, V.; Heinlaan, M. Long Term Exposure to Virgin and Recycled LDPE Microplastics Induced Minor Effects in the Freshwater and Terrestrial Crustaceans Daphnia Magna and Porcellio Scaber. Polymers 2021, 13, 771. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Mansoor, B.; Ayoub, G.; Colin, X.; Benzerga, A.A. Effect of UV-Aging on the Mechanical and Fracture Behavior of Low Density Polyethylene. Polym. Degrad. Stab. 2020, 180, 109185. [Google Scholar] [CrossRef]
- Mouallif, I.; Latrach, A.; Chergui, M.; Benali, A.; Barbe, N. FTIR Study of HDPE Structural Changes, Moisture Absorption and Mechanical Properties Variation When Exposed to Sulphuric Acid Aging in Various Temperatures. In Proceedings of the CFM 2011-20ème Congrès Français de Mécanique, Besançon, France, 29 August–2 September 2011. [Google Scholar]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Yao, C.; Xia, W.; Dou, M.; Du, Y.; Wu, J. Oxidative Degradation of UV-Irradiated Polyethylene by Laccase-Mediator System. J. Hazard. Mater. 2022, 440, 129709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The Toxicity of Virgin and UV-Aged PVC Microplastics on the Growth of Freshwater Algae Chlamydomonas Reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus Moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged Microplastics Polyvinyl Chloride Interact with Copper and Cause Oxidative Stress towards Microalgae Chlorella Vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Huang, H.; Wang, H.; Shi, Y.; Gao, S. Simulation of Natural Aging Property of Microplastics in Yangtze River Water Samples via a Rooftop Exposure Protocol. Sci. Total Environ. 2021, 785, 147265. [Google Scholar] [CrossRef]
- Song, W.; Fu, C.; Fang, Y.; Wang, Z.; Li, J.; Zhang, X.; Bhatt, K.; Liu, L.; Wang, N.; Liu, F.; et al. Single and Combined Toxicity Assessment of Primary or UV-Aged Microplastics and Adsorbed Organic Pollutants on Microalga Chlorella Pyrenoidosa. Environ. Pollut. 2023, 318, 120925. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Guo, M.; Cao, X.; Zheng, X.; Bao, D. UV-Induced Microplastics (MPs) Aging Leads to Comprehensive Toxicity. Mar. Pollut. Bull. 2023, 189, 114745. [Google Scholar] [CrossRef]
- Ding, P.; Xiang, C.; Li, X.; Chen, H.; Shi, X.; Li, X.; Huang, C.; Yu, Y.; Qi, J.; Li, A.J.; et al. Photoaged Microplastics Induce Neurotoxicity via Oxidative Stress and Abnormal Neurotransmission in Zebrafish Larvae (Danio Rerio). Sci. Total Environ. 2023, 881, 163480. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, Y.; Wu, X.; Wang, H.; Huang, H.; Guo, X.; Gao, S. Review of the Artificially-Accelerated Aging Technology and Ecological Risk of Microplastics. Sci. Total Environ. 2021, 768, 144969. [Google Scholar] [CrossRef]
- Ding, J.; Liang, Z.; Lv, M.; Li, X.; Lu, S.; Ren, S.; Yang, X.; Li, X.; Tu, C.; Zhu, D.; et al. Aging in Soil Increases the Disturbance of Microplastics to the Gut Microbiota of Soil Fauna. J. Hazard. Mater. 2024, 461, 132611. [Google Scholar] [CrossRef] [PubMed]
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Skalar, T.; Parramon Dolcet, L.; Hurley, R.; van Gestel, C.A.M. Exploring the Impacts of Plastics in Soil—The Effects of Polyester Textile Fibers on Soil Invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- Buch, A.C.; Niemeyer, J.C.; Fernandes Correia, M.E.; Silva-Filho, E.V. Ecotoxicity of Mercury to Folsomia Candida and Proisotoma Minuta (Collembola: Isotomidae) in Tropical Soils: Baseline for Ecological Risk Assessment. Ecotoxicol. Environ. Saf. 2016, 127, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Filser, J.; Wiegmann, S.; Schröder, B. Collembola in Ecotoxicology—Any News or Just Boring Routine? Appl. Soil. Ecol. 2014, 83, 193–199. [Google Scholar] [CrossRef]
- Salmon, S.; Ponge, J.F.; Gachet, S.; Deharveng, L.; Lefebvre, N.; Delabrosse, F. Linking Species, Traits and Habitat Characteristics of Collembola at European Scale. Soil. Biol. Biochem. 2014, 75, 73–85. [Google Scholar] [CrossRef]
- Büks, F.; Loes van Schaik, N.; Kaupenjohann, M. What Do We Know about How the Terrestrial Multicellular Soil Fauna Reacts to Microplastic? Soil 2020, 6, 245–267. [Google Scholar] [CrossRef]
- Greenslade, P.; Vaughan, G.T. A Comparison of Collembola Species for Toxicity Testing of Australian Soils. Pedobiologia 2003, 47, 171–179. [Google Scholar] [CrossRef]
- Forbes, V.E. Is Hormesis an Evolutionary Expectation? Funct. Ecol. 2000, 14, 12–24. [Google Scholar] [CrossRef]
- Chelinho, S.; Domene, X.; Andrés, P.; Natal-da-Luz, T.; Norte, C.; Rufino, C.; Lopes, I.; Cachada, A.; Espíndola, E.; Ribeiro, R.; et al. Soil Microarthropod Community Testing: A New Approach to Increase the Ecological Relevance of Effect Data for Pesticide Risk Assessment. Appl. Soil. Ecol. 2014, 83, 200–209. [Google Scholar] [CrossRef]
- Crommentuijn, T.; Stab, J.A.; Doornekamp, A.; Estoppey, O.; van Gestel, C.A.M. Comparative Ecotoxicity of Cadmium, Chlorpyrifos and Triphenyltin Hydroxide for Four Clones of the Parthenogenetic Collembolan Folsomia Candida in an Artificial Soil. Funct. Ecol. 1995, 9, 734. [Google Scholar] [CrossRef]
- Menta, C.; Maggiani, A.; Vattuone, Z. Effects of Cd and Pb on the Survival and Juvenile Production of Sinella Coeca and Folsomia Candida. Eur. J. Soil. Biol. 2006, 42, 181–189. [Google Scholar] [CrossRef]
- Nakamori, T.; Yoshida, S.; Kubota, Y.; Ban-nai, T.; Kaneko, N.; Hasegawa, M.; Itoh, R. Sensitivity to Cadmium of the Standard Test Species Folsomia Candida Compared to Two Other Species, Onychiurus Yodai and Sinella Umesaoi (Collembola). Eur. J. Soil. Biol. 2008, 44, 266–270. [Google Scholar] [CrossRef]
- Briones, M.J.I. The Serendipitous Value of Soil Fauna in Ecosystem Functioning: The Unexplained Explained. Front. Environ. Sci. 2018, 6, 149. [Google Scholar] [CrossRef]
| pH | WC | ||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Pristine | C0 | 4.97 ± 0.02 | 4.94 ± 0.05 | 18.31 ± 1.15 | 17.68 ± 0.97 |
| C1 | 5.01 ± 0.69 | 4.93 ± 0.07 | 20.14 ± 2.54 | 18.5 ± 0.78 | |
| C2 | 5.07 ± 0 | 5.07 ± 0.16 | 16.72 ± 0.71 | 16.67 ± 0.5 | |
| C3 | 4.88 ± 0.07 | 4.85 ± 0.13 | 17.93 ± 1.2 | 17.59 ± 2.8 | |
| C4 | 4.97 ± 0.05 | 4.87 ± 0.03 | 18.18 ± 2.02 | 16.82 ± 1.56 | |
| C5 | 5 ± 0.03 | 4.92 ± 0.05 | 17.98 ± 1.02 | 18.96 ± 2.83 | |
| UV | C0 | 4.96 ± 0.06 | 4.85 ± 0.06 | 19.39 ± 0 | 15.48 ± 2.36 |
| C1 | 4.92 ± 0.006 | 4.83 ± 0.005 | 18.39 ± 0 | 15.29 ± 1.06 | |
| C2 | 4.95 ± 0.03 | 4.88 ± 0.03 | 20.21 ± 0 | 16.75 ± 1.06 | |
| C3 | 4.94 ± 0.03 | 4.88 ± 0.03 | 24.73 ± 0 | 18.12 ± 0.75 | |
| C4 | 4.92 ± 0.01 | 4.85 ± 0.01 | 20.99 ± 0 | 16.36 ± 1.46 | |
| C5 | 4.96 ± 0.04 | 4.9 ± 0.04 | 18.95 ± 0 | 16.6 ± 1.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quigley, E.; Patrício Silva, A.L.; Chelinho, S.; Briones, M.J.I.; Sousa, J.P. Virgin and Photoaged Polyethylene Microplastics Have Different Effects on Collembola and Enchytraeids. Environments 2025, 12, 175. https://doi.org/10.3390/environments12060175
Quigley E, Patrício Silva AL, Chelinho S, Briones MJI, Sousa JP. Virgin and Photoaged Polyethylene Microplastics Have Different Effects on Collembola and Enchytraeids. Environments. 2025; 12(6):175. https://doi.org/10.3390/environments12060175
Chicago/Turabian StyleQuigley, Elise, Ana L. Patrício Silva, Sónia Chelinho, Maria J. I. Briones, and Jose P. Sousa. 2025. "Virgin and Photoaged Polyethylene Microplastics Have Different Effects on Collembola and Enchytraeids" Environments 12, no. 6: 175. https://doi.org/10.3390/environments12060175
APA StyleQuigley, E., Patrício Silva, A. L., Chelinho, S., Briones, M. J. I., & Sousa, J. P. (2025). Virgin and Photoaged Polyethylene Microplastics Have Different Effects on Collembola and Enchytraeids. Environments, 12(6), 175. https://doi.org/10.3390/environments12060175







