Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania
Abstract
1. Introduction
2. Materials and Methods
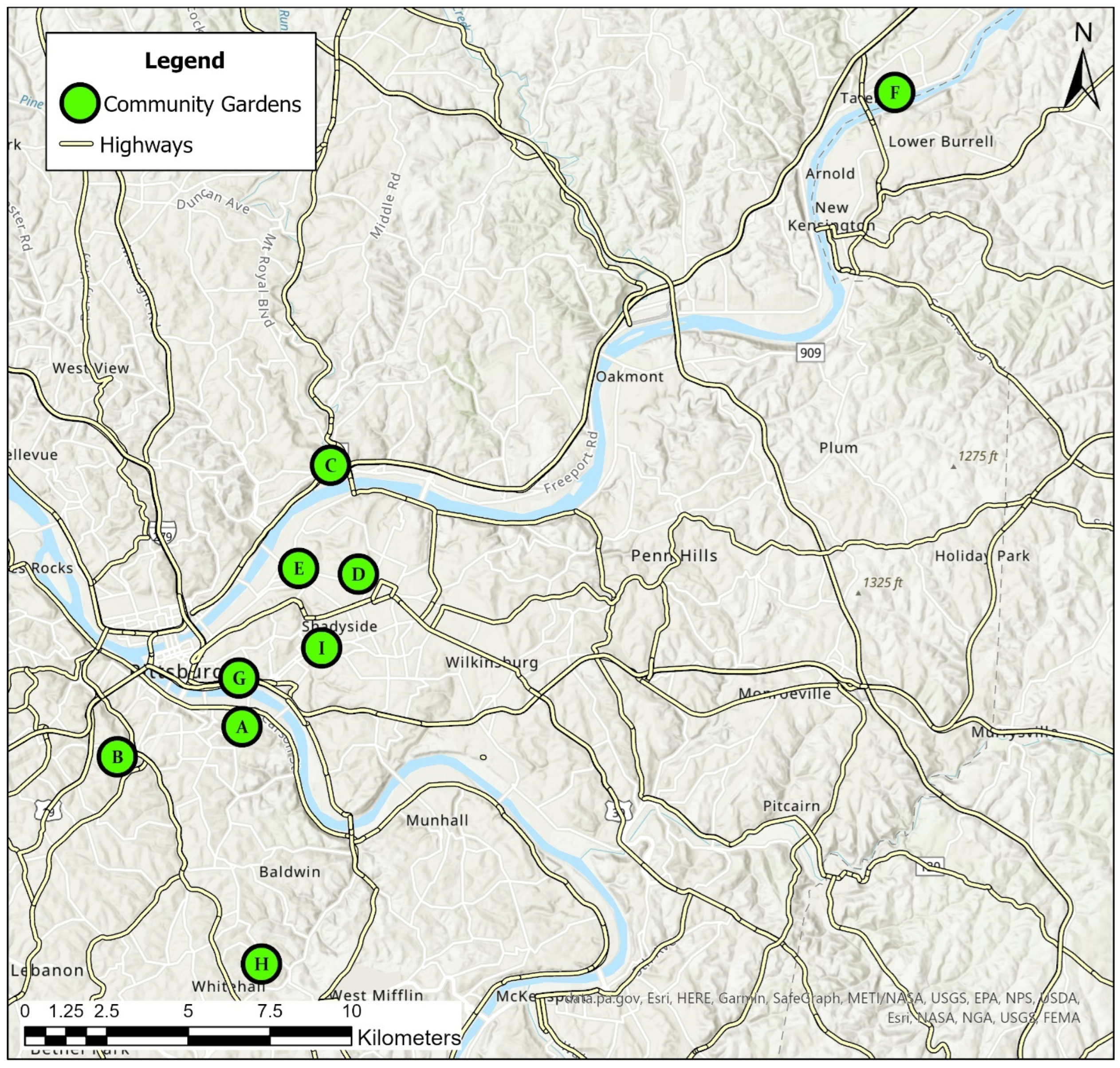
2.1. Soil Sampling
2.2. Sample Preparation
2.3. Trace Metal Analysis
2.3.1. XRF Analysis
2.3.2. ICP-MS Analysis
2.4. Enrichment Factor
2.5. Statistical Analysis
2.6. Limitations
3. Results and Discussion
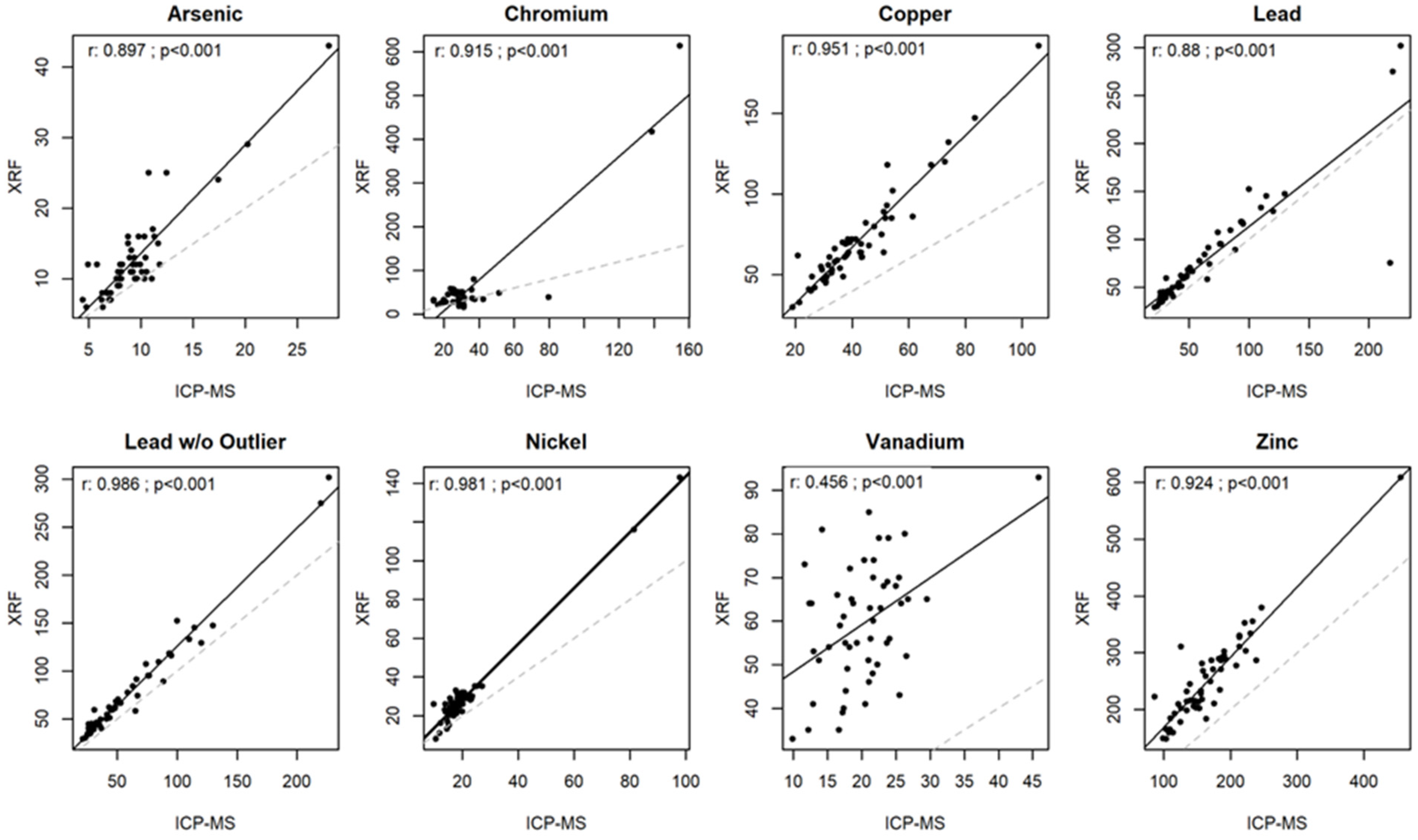
3.1. Comparison of Two Measurement Methods: ICP-MS and XRF Concentrations
3.2. Comparison with Other Studies
3.3. Standards Relevant to Community Garden Soils
3.4. Raised-Bed vs. Ground Samples
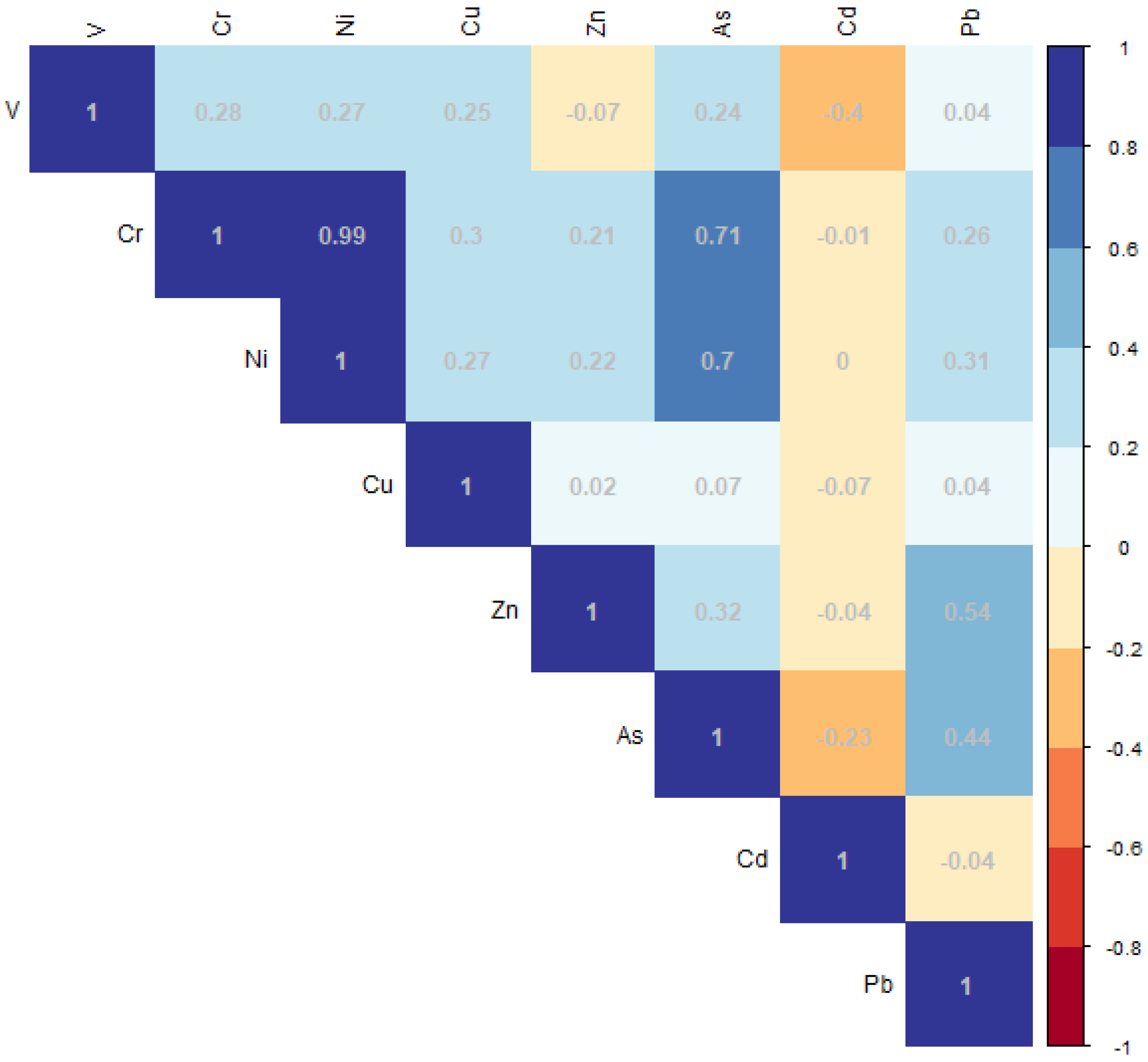
3.5. Correlation Analysis
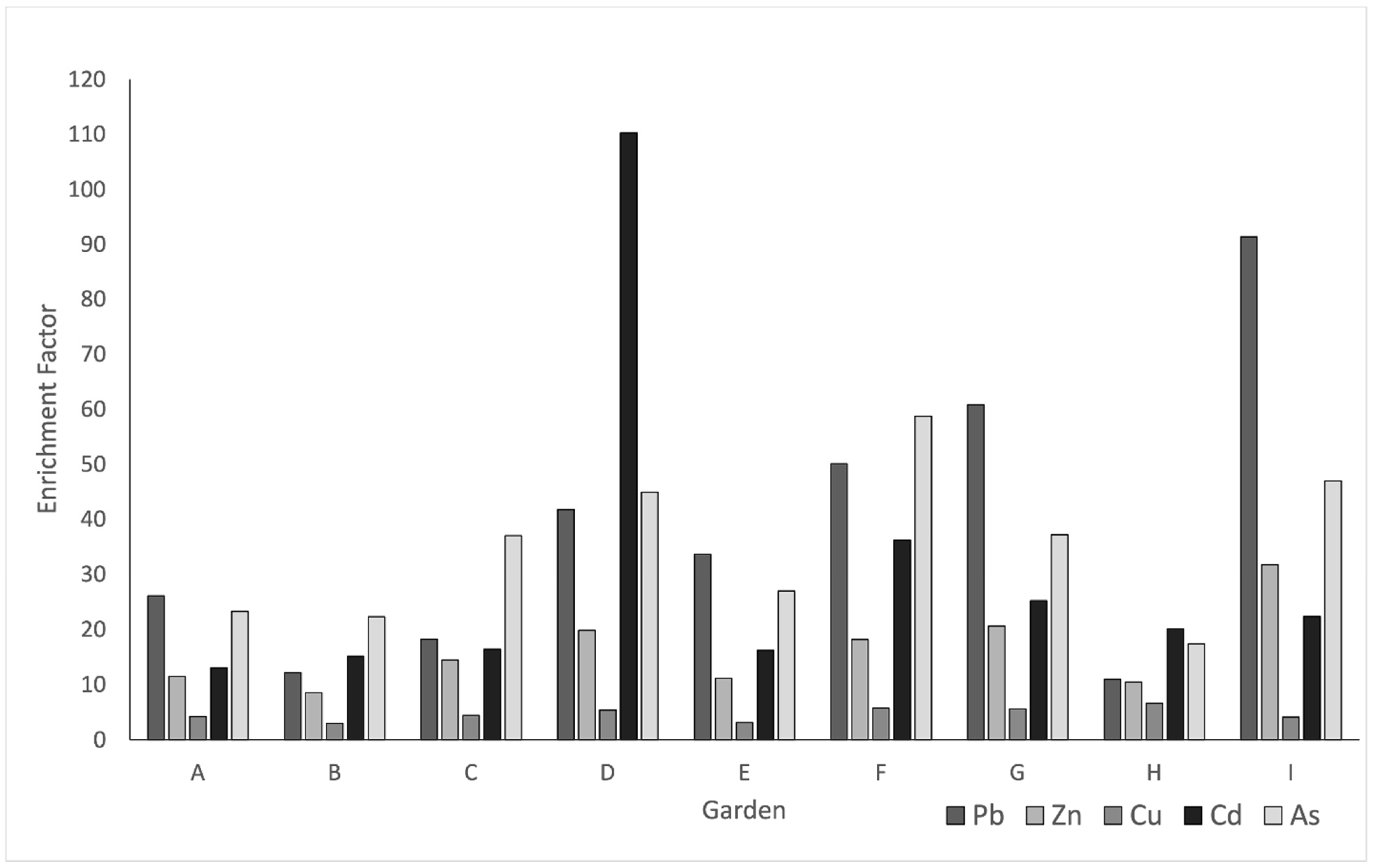
3.6. Trace Metal Enrichment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrigan, M.P. Growing What You Eat: Developing Community Gardens in Baltimore, Maryland. Appl. Geogr. 2011, 31, 1232–1241. [Google Scholar] [CrossRef]
- Ploeg, M.V.; Breneman, V.; Farrigan, T.; Hamrick, K.; Hopkins, D.; Kaufman, P.; Lin, B.-H.; Nord, M.; Smith, T.A.; Williams, R.; et al. Access to Affordable and Nutritious Food-Measuring and Understanding Food Deserts and Their Consequences: Report to Congress. Available online: http://www.ers.usda.gov/publications/pub-details/?pubid=42729 (accessed on 7 May 2023).
- Ferdinand, K.C.; Mahata, I. Limited Healthy Foods in the Land of Plenty. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004131. [Google Scholar] [CrossRef] [PubMed]
- Ober Allen, J.; Alaimo, K.; Elam, D.; Perry, E. Growing Vegetables and Values: Benefits of Neighborhood-Based Community Gardens for Youth Development and Nutrition. J. Hunger Environ. Nutr. 2008, 3, 418–439. [Google Scholar] [CrossRef]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Felix, D.; Alcantara, F.; Zaslavsky, I.; Work, A.; Watson, P.L.; Pezzoli, K.; Yu, Q.; Zhu, D.; Scavo, A.J.; et al. Monitoring and Mitigation of Toxic Heavy Metals and Arsenic Accumulation in Food Crops: A Case Study of an Urban Community Garden. Plant Direct 2020, 4, e00198. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.; Viscarra Rossel, R.; McBratney, A. Diagnostic Screening of Urban Soil Contaminants Using Diffuse Reflectance Spectroscopy. Aust. J. Soil Res. 2009, 47, 433–442. [Google Scholar] [CrossRef]
- Clarke, L.W.; Jenerette, G.D.; Bain, D.J. Urban Legacies and Soil Management Affect the Concentration and Speciation of Trace Metals in Los Angeles Community Garden Soils. Environ. Pollut. 2015, 197, 1–12. [Google Scholar] [CrossRef]
- Thornton, I. Metal Contamination of Soils in Urban Areas. In Soils in the Urban Environment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1991; pp. 47–75. [Google Scholar] [CrossRef]
- Cheng, Z.; Paltseva, A.; Li, I.; Morin, T.; Huot, H.; Egendorf, S.; Su, Z.; Yolanda, R.; Singh, K.; Lee, L.; et al. Trace Metal Contamination in New York City Garden Soils. Soil Sci. 2015, 180, 167–174. [Google Scholar] [CrossRef]
- Cheng, Z.; Lee, L.; Dayan, S.; Grinshtein, M.; Shaw, R. Speciation of Heavy Metals in Garden Soils: Evidences from Selective and Sequential Chemical Leaching. J. Soils Sediments 2011, 11, 628–638. [Google Scholar] [CrossRef]
- Misenheimer, J.; Nelson, C.; Huertas, E.; Medina-Vera, M.; Prevatte, A.; Bradham, K. Total and Bioaccessible Soil Arsenic and Lead Levels and Plant Uptake in Three Urban Community Gardens in Puerto Rico. Geosciences 2018, 8, 43. [Google Scholar] [CrossRef]
- Sage, L.; Bassetti, O.; Johnson, E.; Shakya, K.; Weston, N. Assessment of Heavy Metal Contamination in Soil and Produce of Philadelphia Community Gardens. Environ. Pollut. Bioavailab. 2023, 35, 2209283. [Google Scholar] [CrossRef]
- Malone, M.; Shakya, K.M. Trace Metal Contamination in Community Garden Soils across the United States. Sustainability 2024, 16, 1831. [Google Scholar] [CrossRef]
- Cassidy, B.M. A 94-Year Lake Sediment Record of Industrial Pollutants in the Pittsburgh Metropolitan Area. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2008. [Google Scholar]
- Youger, J.D.; Mitsch, W.J. Heavy Metal Concentrations in Ohio River Sediments—Longitdunal and Temporal Patterns. Ohio J. Sci. 1989, 89, 172–175. [Google Scholar]
- Baraff, R.; Emig, T. Rivers of Steel—Steel Footprints. Rivers Steel National Heritage Area System. Available online: https://riversofsteel.com/programs/heritage-area/ (accessed on 20 November 2022).
- Maxim, A.; Bain, D.J.; Burgess, J. Urban Soils in a Historically Industrial City: Patterns of Trace Metals in Pittsburgh, Pennsylvania. Environ. Res. Commun. 2022, 4, 075004. [Google Scholar] [CrossRef]
- Shefsky, S. Comparing Field Portable X-Ray Fluorescence (XRF) To Laboratory Analysis Of Heavy Metals In Soil. In Proceedings of the International Symposium of Field Screening Methods for Hazardous Wastes and Toxic Chemicals, Las Vegas, NV, USA, 29–31 January 1997. [Google Scholar]
- Al Maliki, A.; Al-lami, A.K.; Hussain, H.M.; Al-Ansari, N. Comparison between Inductively Coupled Plasma and X-Ray Fluorescence Performance for Pb Analysis in Environmental Soil Samples. Environ. Earth Sci. 2017, 76, 433. [Google Scholar] [CrossRef]
- Bassetti, O.G.; McDonough, R.A.; Shakya, K.M. Soil Contamination in Community Gardens of Philadelphia and Pittsburgh, Pennsylvania. Environ. Monit. Assess. 2023, 195, 782. [Google Scholar] [CrossRef]
- Grow Pittsburgh Grower’s Map. Available online: https://www.growpittsburgh.org/garden-and-farm-resources/growers-map/ (accessed on 20 November 2022).
- OREAS 70b Nickel Sulphide Ore Reference Material. 2011. Available online: https://www.oreas.com/downloads/?fileId=817 (accessed on 6 May 2025).
- US EPA METHOD 6020A; Inductively Coupled Plasma—Mass Spectrometry. Revision 1; US Environmental Protection Agency: Washington DC, USA, 1998.
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of Chemical Elements in the Continental Crust: A New Table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Gallhofer, D.; Lottermoser, B.G. The Influence of Spectral Interferences on Critical Element Determination with Portable X-Ray Fluorescence (pXRF). Minerals 2018, 8, 320. [Google Scholar] [CrossRef]
- Campbell, M.J.; Demesmay, C.; Ollé, M. Determination of Total Arsenic Concentrations in Biological Matrices by Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 1994, 9, 1379–1384. [Google Scholar] [CrossRef]
- Mielke, H.W.; Anderson, J.C.; Berry, K.J.; Mielke, P.W.; Chaney, R.L.; Leech, M. Lead Concentrations in Inner-City Soils as a Factor in the Child Lead Problem. Am. J. Public Health 1983, 73, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Stilwell, D.E.; Rathier, T.M.; Musante, C.L.; Ranciato, J.F. Lead and Other Heavy Metals in Community Garden Soils in Connecticut. Bulletin 1019. The Connecticut Agricultural Experiment Station, New Haven. Available online: https://portal.ct.gov/-/media/caes/documents/publications/bulletins/b1019pdf.pdf (accessed on 30 August 2022).
- Witzling, L.; Wander, M.; Phillips, E. Testing and Educating on Urban Soil Lead: A Case of Chicago Community Gardens. J. Agric. Food Syst. Community Dev. 2010, 1, 167–185. [Google Scholar] [CrossRef]
- Holmes, C.; Hall, S.J.; Heavenrich, H.; Shock, E. Trace Metal Content of Community Garden Soils and Plants in Metropolitan Phoenix, AZ. Arizona State University. Available online: https://halllab.asu.edu/wp-content/uploads/2018/10/PhxCommunityGardenMetals_June-2018.pdf (accessed on 28 March 2023).
- Chander, K.; Hartmann, G.; Joergensen, R.G.; Khan, K.S.; Lamersdorf, N. Comparison of Methods for Measuring Heavy Metals and Total Phosphorus in Soils Contaminated by Different Sources. Arch. Agron. Soil Sci. 2008, 54, 413–422. [Google Scholar] [CrossRef]
- Akbulut, S.; Cevik, U.; Van, A.A.; De Wael, K.; Van Grieken, R. Precision and Accuracy of ST-EDXRF Performance for As Determination Comparing with ICP-MS and Evaluation of As Deviation in the Soil Media. Chemosphere 2014, 96, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.J.; Slemmons, A.K.; Canavan, H.E. Energy-Dispersive X-Ray Fluorescence Methods for Environmental Characterization of Soils. Environ. Sci. Technol. 1996, 30, 2318–2321. [Google Scholar] [CrossRef]
- Piercey, S.; Devine, M. Analysis of Powdered Reference Materials and Known Samples with a Benchtop, Field Portable X-Ray Fluorescence (pXRF) Spectrometer: Evaluation of Performance and Potential Applications for Exploration Lithogeochemistry. Geochem. Explor. Environ. Anal. 2014, 14, 139–148. [Google Scholar] [CrossRef]
- Lupolt, S.N.; Santo, R.E.; Kim, B.F.; Green, C.; Codling, E.; Rule, A.M.; Chen, R.; Scheckel, K.G.; Strauss, M.; Cocke, A.; et al. The Safe Urban Harvests Study: A Community-Driven Cross-Sectional Assessment of Metals in Soil, Irrigation Water, and Produce from Urban Farms and Gardens in Baltimore, Maryland. Environ. Health Perspect. 2021, 129, 117004. [Google Scholar] [CrossRef]
- ALS Heavy Metal Guidelines in Soil. Available online: https://www.alsenvironmental.co.uk/media-uk/pdf/datasheets/contaminated-land/als_cl_heavy-metals-guidelines-in-soil_uk_feb_17_v2.pdf (accessed on 15 February 2023).
- Canadian Council of Ministers of the Environment (CCME). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; Update 7.0. September 2007; Environment Canada: Gatineau, QC, Canada, 1998.
- Duke University. How Can Soil Contaminants Impact My Health? Available online: https://sites.nicholas.duke.edu/superfundcec/gardens/health-effects-soil-soil-contam/ (accessed on 11 February 2023).
- Grubinger, V.; Ross, D.; Faulkner, J. Interpreting the Results of Soil Tests for Heavy Metals. Available online: https://www.uvm.edu/vtvegandberry/factsheets/interpreting_heavy_metals_soil_tests.pdf (accessed on 11 February 2023).
- New Jersey Department of Environmental Protection (NJ DEP). Soil Remediation Standards for the Ingestion-Dermal Exposure Pathway. Basis and Background. 2021. Available online: https://www.nj.gov/dep/srp/guidance/rs/bb_ingestion_dermal.pdf (accessed on 11 February 2023).
- Pesnnsylvania Department of Environmental Protection (PA DEP). Statewide Health Standards. Available online: https://www.dep.pa.gov/Business/Land/LandRecycling/Standards-Guidance-Procedures/Pages/Statewide-Health-Standards.aspx (accessed on 11 February 2023).
- United States Environmental Protection Agency (US EPA). EPA Strengthens Safeguards to Protect Families and Children from Lead in Contaminated Soil at Residential Sites in Region 7. Available online: https://www.epa.gov/newsreleases/epa-strengthens-safeguards-protect-families-and-children-lead-contaminated-soil (accessed on 12 February 2024).
- Mitchell, R.G.; Spliethoff, H.M.; Ribaudo, L.N.; Lopp, D.M.; Shayler, H.A.; Marquez-Bravo, L.G.; Lambert, V.T.; Ferenz, G.S.; Russell-Anelli, J.M.; Stone, E.B.; et al. Lead (Pb) and Other Metals in New York City Community Garden Soils: Factors Influencing Contaminant Distributions. Environ. Pollut. 2014, 187, 162–169. [Google Scholar] [CrossRef]
- Clark, H.F.; Hausladen, D.M.; Brabander, D.J. Urban Gardens: Lead Exposure, Recontamination Mechanisms, and Implications for Remediation Design. Environ. Res. 2008, 107, 312–319. [Google Scholar] [CrossRef]
- Salvagio Manta, D.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy Metals in Urban Soils: A Case Study from the City of Palermo (Sicily), Italy. Sci. Total Environ. 2003, 300, 229–243. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, G.D.; Dwivedi, B.S.; Khatik, S.K. Chromium: As a polluant. J. Ind. Pollut. Control 2007, 23, 197–203. [Google Scholar]
- Iyaka, Y.A. Scientific Research and Essays—Nickel in Soils: A Review of Its Distribution and Impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar] [CrossRef]
- Flem, B.; Reimann, C.; Fabian, K. Excess Cr and Ni in Top Soil: Comparing the Effect of Geology, Diffuse Contamination, and Biogenic Influence. Sci. Total Environ. 2022, 843, 157059. [Google Scholar] [CrossRef] [PubMed]
- IARC. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993; Volume 58. [Google Scholar]
- ATSDR. Toxicological Profile for Arsenic. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf (accessed on 25 March 2023).
- US EPA. Inorganic Arsenic Emissions from Glass Manufacturing Plants—Background Information for Proposed Standards; United States Environmental Protection Agency: Washington DC, USA, 1983.
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Not. 2011, 2011, e402647. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential Sources of Anthropogenic Copper Inputs to European Agricultural Soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef]
- Aytop, H.; Koca, Y.K.; Senol, S. The Importance of Using Soil Series-Based Geochemical Background Values When Calculating the Enrichment Factor in Agricultural Areas. Env. Geochem. Health 2023, 45, 6215–6230. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Xu, J.; Li, Y.; Wang, S.; Long, S.; Wu, Y.; Chen, Z. Sources, Transfers and the Fate of Heavy Metals in Soil-Wheat Systems: The Case of Lead (Pb)/Zinc (Zn) Smelting Region. J. Hazard. Mater. 2023, 441, 129863. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, J.; Wang, X.; Pan, M.; Lin, Q.; Khan, K.Y.; Yan, B.; Li, T.; He, Z.; Yang, X.; et al. A Review on the Thermal Treatment of Heavy Metal Hyperaccumulator: Fates of Heavy Metals and Generation of Products. J. Hazard. Mater. 2021, 405, 123832. [Google Scholar] [CrossRef]
- Chen, C.-W.; Kao, C.-M.; Chen, C.-F.; Dong, C.-D. Distribution and Accumulation of Heavy Metals in the Sediments of Kaohsiung Harbor, Taiwan. Chemosphere 2007, 66, 1431–1440. [Google Scholar] [CrossRef]
- Odat, S. Application of Geoaccumulation Index and Enrichment Factors on the Assessment of Heavy Metal Pollution along Irbid/Zarqa Highway-Jordan. J. Appl. Sci. 2015, 15, 1318–1321. [Google Scholar] [CrossRef][Green Version]
- O’Shea, M.J.; Toupal, J.; Caballero-Gómez, H.; McKeon, T.P.; Howarth, M.V.; Pepino, R.; Gieré, R. Lead Pollution, Demographics, and Environmental Health Risks: The Case of Philadelphia, USA. Int. J. Environ. Res. Public Health 2021, 18, 9055. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Hossain Mondal, M.; Bhattarai, A.; Saha, B. A Comprehensive Review on the Sources, Essentiality and Toxicological Profile of Nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Cornell University. Understanding Your Test Results: Metals in Garden Soils and Vegetables Healthy Soils, Healthy Communities. Available online: https://cwmi.css.cornell.edu/UnderstandingTestResultsMetalsSoilsVeg.pdf (accessed on 1 March 2023).

| City | Concentration (mg/kg) | Contamination Source | |
|---|---|---|---|
| Pittsburgh, PA (n = 54) Metric: Mean ± standard deviation (Range) (This Study) | Raised Bed As: 8.58 ± 0.73 (7.83–10.24) Cd: 0.71 ± 0.16 (0.48–0.99) Cr: 31.10 ± 8.06 (21.92–48.84) Cu: 43.05 ± 10.76 (27.11–59.80) Ni: 18.61 ± 2.08 (14.64–21.78) Pb: 53.67 ± 23.34 (27.00–99.31) V: 20.34 ± 2.91 (15.56–24.85) Zn: 161.68 ± 31.84 (109.27–227.62) | Ground soil As: 9.74 ± 3.29 (5.50–17.53) Cd: 0.87 ± 0.62 (0.42–2.40) Cr: 31.57 ± 29.05 (14.24–107.74) Cu: 41.28 ± 12.89 (26.13–63.69) Ni: 22.13 ± 17.52 (11.56–68.16) Pb: 72.72 ± 41.21 (24.06–131.61) V: 19.32± 6.11 (10.49–30.42) Zn: 173.17 ± 49.90 (114.64–256.11) | Smelting, Transportation, Industry |
| Pittsburgh, PA (n = 20) Metric: Range (Bassetti et al., 2023) [21] | As: 5.50–17.53, Cu: 11.6–77.9, Cd: 0.2–12.5, Pb: 83.1–232.9 | Anthropogenic Sources | |
| Philadelphia, PA (n = 33) Metric: Mean (Bassetti et al., 2023) [21] | Raised Bed As: 3.18 Cd: 0.45 Cr: 39.34 Cu: 109.87 Ni: 25.78 Pb: 64.25 V: 33.33 Zn: 473.10 | Ground soil As: 4.58 Cd: 0.79 Cr: 31.22 Cu: 102.41 Ni: 15.93 Pb: 120.86 V: 27.38 Zn: 298.66 | Smelting |
| New York, NY (n = 106) Metric: Median (Cheng et al., 2015) [10] | As: 7.6, Cd: 1.1, Co: 8, Cr: 39, Cu: 55, Ni: 21, Pb: 140, Zn: 169 | Leaded Paint and Gasoline, Refuse Incineration | |
| Baltimore, MD (n = 422) Metric: Mean (Mielke et al., 1983) [29] | Cd: 0.56, Cu: 17, Ni: 2.8, Pb: 100, Zn: 92 | Industry, Incineration, Paints, Leaded Gasoline | |
| Connecticut (n = 174) Metric: Mean (Stilwell et al., 2008) [30] | As: 4.2, Cd: <0.5, Cr: 14, Cu: 40 Ni: 12, Pb: 176, Zn: 163 | Industrial Sites, Transportation, Manufacturing | |
| Chicago, IL (n = 86) Metric: Mean (Witzling et al., 2010) [31] | Raised Bed Cu: 8.99 Pb: 60.7 Zn: 38.4 | Ground soil Cu: 19.3 Pb: 224 Zn: 69.1 | Undefined |
| Phoenix, AZ (n = 28) Metric: Mean (Holmes et al., 2018) [32] | Cd: 0.9–4.4, Pb: 39.8–127.9 | Industry, Fertilizer | |
| Soil Metal Concentrations for Residential Gardening Soils (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard | V | Cr | Ni | Cu | Zn | As | Cd | Pb |
| 550 | 390 | 1600 | -- | 23,000 | 0.4 | 78 | 200 * |
| 1100 | 37 | 4400 | 7200 | 66,000 | 12 | 110 | 500 |
| -- | 22 ** | 140 | 270 | 2200 | 16 | 2.5 | 400 |
| 390 | -- | 1600 | 3100 | 23,000 | 19 | 71 | 200 |
| 130 | 64 | 50 | 63 | 200 | 12 | 10 | 140 |
| -- | 130 | 130 | -- | -- | 37 | 22 | 200 |
| Most Stringent Standard (mg/kg) | CCME | PA DEP | CCME | CCME | CCME | PA DEP & CCME | NY DEC | CCME |
|---|---|---|---|---|---|---|---|---|
| 130 | 37 | 50 | 63 | 200 | 12 | 2.5 | 140 | |
| Garden | Concentration (mg/kg) | |||||||
| V | Cr | Ni | Cu | Zn | As | Cd | Pb | |
| A | 23.83 | 32.27 * | 21.72 | 50.04 | 174.18 | 9.07 | 0.56 | 70.58 |
| B | 22.47 * | 31.65 * | 19.42 * | 55.67 * | 166.69 * | 8.38 * | 0.72 * | 44.03 * |
| C | 19.88 * | 48.84 * | 16.86 * | 43.54 * | 178.07 * | 8.60 | 0.72 * | 32.43 * |
| D | 22.62 * | 22.04 * | 18.66 * | 32.11 | 150.99 | 8.80 | 2.40 | 56.82 |
| E | 22.83 | 23.13 | 17.68 | 46.38 * | 172.90 | 9.86 | 0.66 | 85.52 |
| F | 20.47 | 107.74 | 68.16 | 52.19 | 210.22 | 17.53 | 1.20 | 103.77 |
| G | 18.82 | 27.35 | 19.29 | 59.80 * | 234.32 | 10.87 | 0.87 * | 123.59 |
| H | 30.42 | 33.63 * | 20.56 | 63.69 | 148.98 * | 7.87 * | 0.73 * | 27.00 * |
| I | 20.75 * | 33.38 | 17.98 * | 40.03 * | 203.11 | 9.75 | 0.68 * | 131.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonough, R.; Shakya, K.M. Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania. Environments 2025, 12, 159. https://doi.org/10.3390/environments12050159
McDonough R, Shakya KM. Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania. Environments. 2025; 12(5):159. https://doi.org/10.3390/environments12050159
Chicago/Turabian StyleMcDonough, Rebecca, and Kabindra M. Shakya. 2025. "Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania" Environments 12, no. 5: 159. https://doi.org/10.3390/environments12050159
APA StyleMcDonough, R., & Shakya, K. M. (2025). Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania. Environments, 12(5), 159. https://doi.org/10.3390/environments12050159









