Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review
Abstract
1. Introduction
2. Chemical Properties
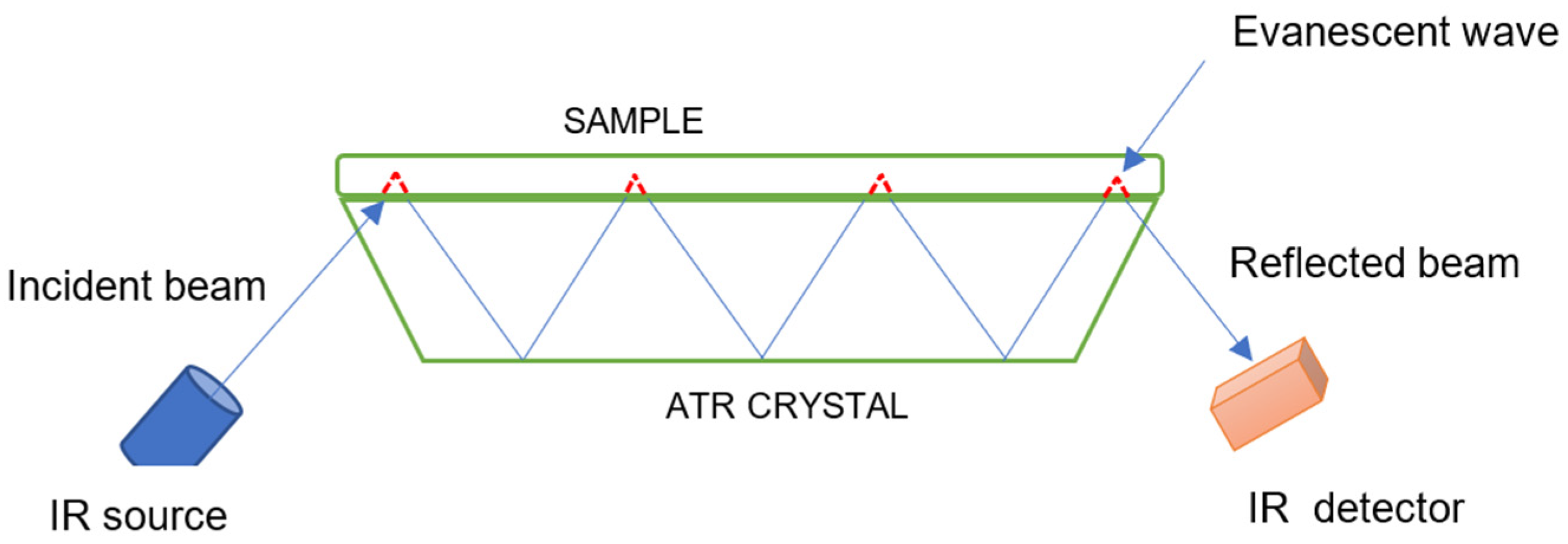
2.1. Fourier Transform Infrared Spectroscopy (FTIR)
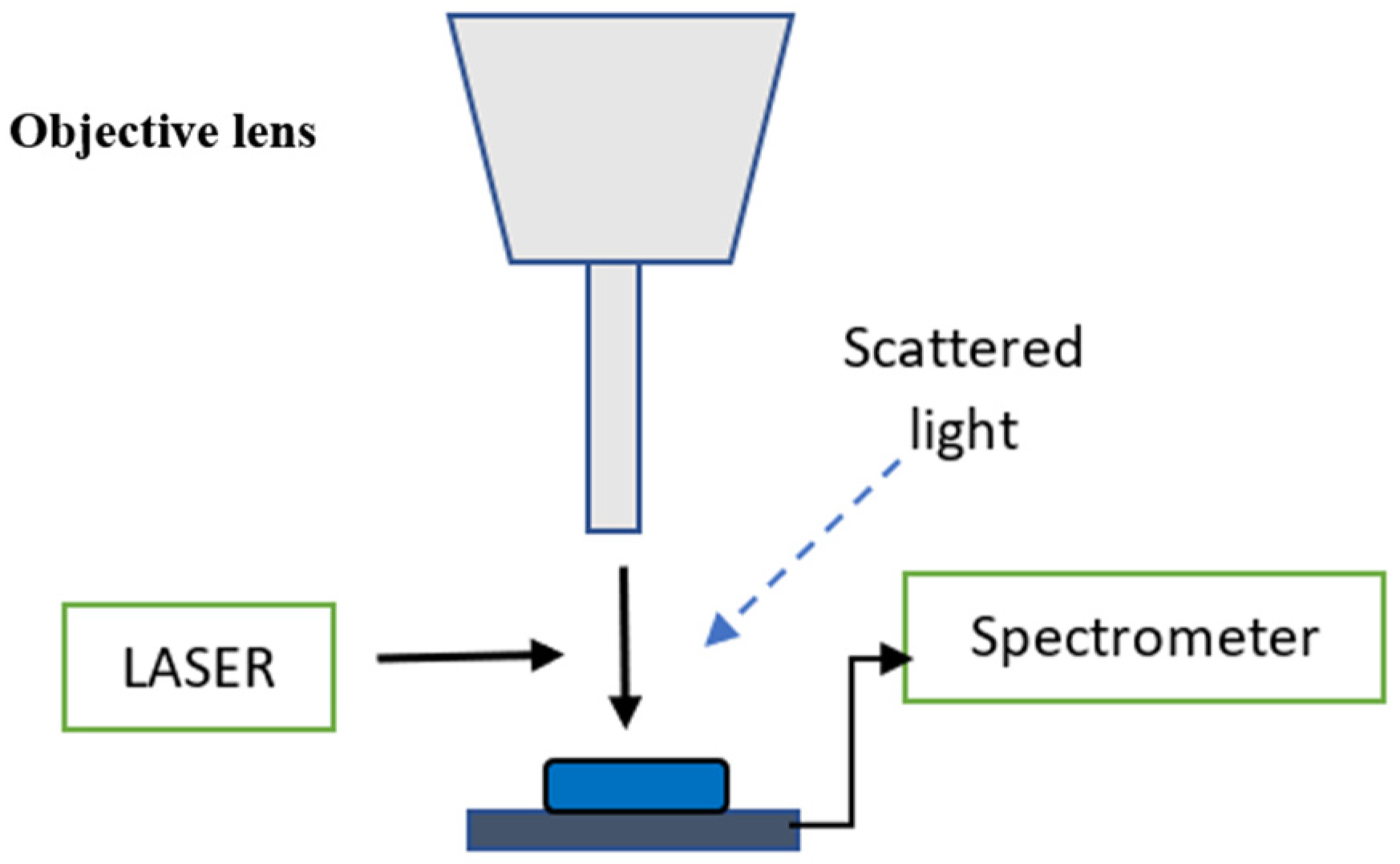
2.2. Micro-Raman Spectroscopy
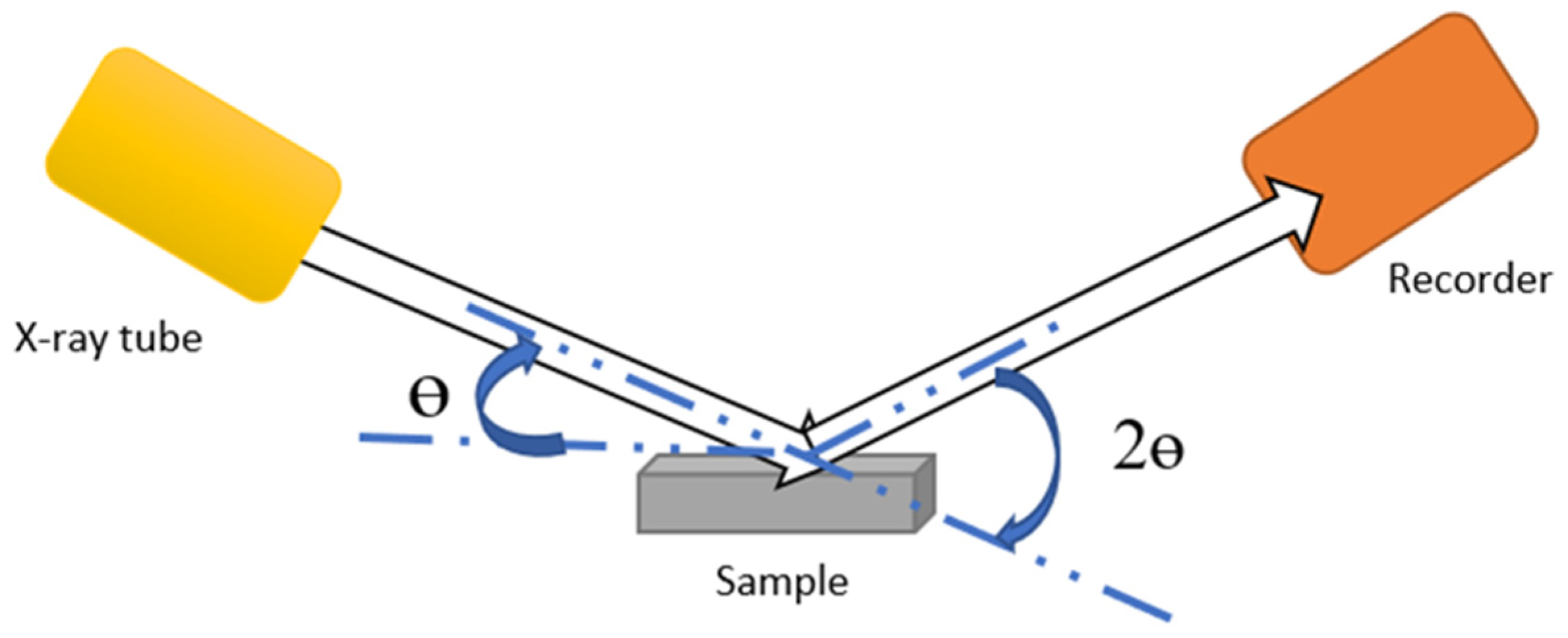
2.3. X-Ray Fluorescence (XRF)
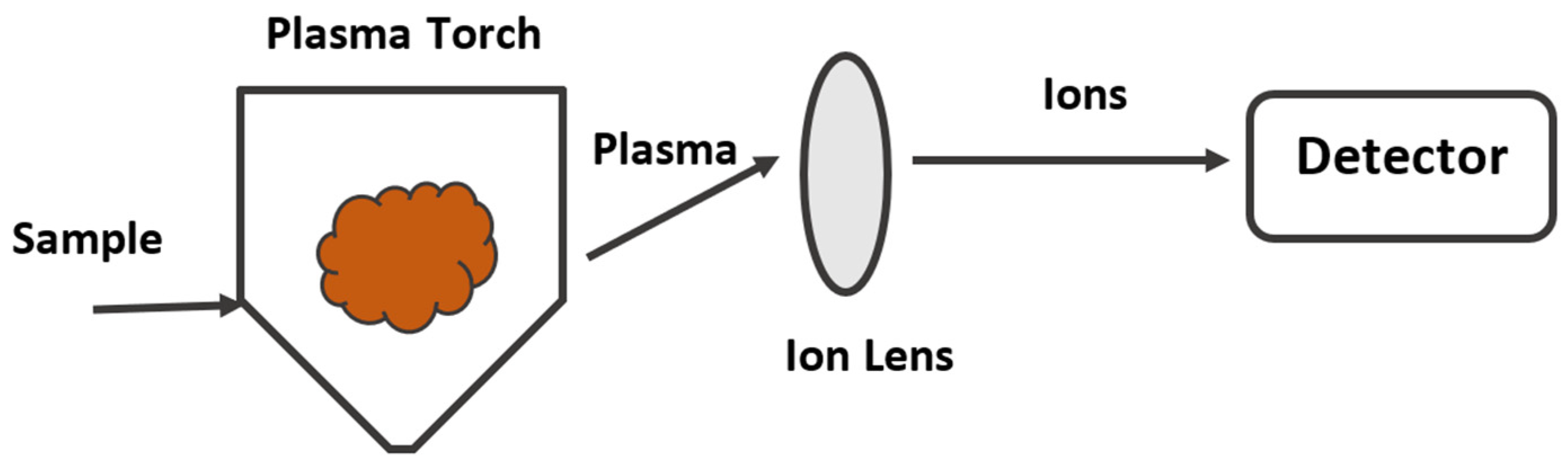
2.4. Inductively Coupled Plasma (ICP)
3. Structural Properties
X-Ray Powder Diffraction (XRPD)
4. Morphological Properties
Scanning Electron Microscopy (SEM)
5. Physical Properties
Differential Scanning Calorimetry (DSC)
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uekert, T.; Singh, A.; DesVeaux, J.S.; Ghosh, T.; Bhatt, A.; Yadav, G.; Afzal, S.; Walzberg, J.; Knauer, K.M.; Nicholson, S.R.; et al. Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics. ACS Sustain. Chem. Eng. 2023, 11, 965–978. [Google Scholar] [CrossRef]
- Plastics Europe. The Circular Economy for Plastics—A European Analysis 2024; Plastics Europe: Brussels, Belgium, 2024. [Google Scholar]
- Shen, L.; Worrell, E. Plastic Recycling. In Handbook of Recycling: State-of-the-art for Practitioners, Analysts, and Scientists; Elsevier: Amsterdam, The Netherlands, 2014; pp. 497–510. [Google Scholar]
- Yap, P.P.X.; Yen, Z.; Salim, T.; Lim, H.C.; Chung, C.K.; Lam, Y.M. The Impact of Mechanical Recycling on the Degradation of Polyamide. Polym. Degrad. Stab. 2024, 225, 110773. [Google Scholar] [CrossRef]
- He, L.; Ding, J.; Yang, S.-S.; Zang, Y.-N.; Pang, J.-W.; Xing, D.; Zhang, L.-Y.; Ren, N.; Wu, W.-M. Molecular-Weight-Dependent Degradation of Plastics: Deciphering Host–Microbiome Synergy Biodegradation of High-Purity Polypropylene Microplastics by Mealworms. Environ. Sci. Technol. 2024, 58, 6647–6658. [Google Scholar] [CrossRef]
- Cusano, I.; Campagnolo, L.; Aurilia, M.; Costanzo, S.; Grizzuti, N. Rheology of Recycled PET. Materials 2023, 16, 3358. [Google Scholar] [CrossRef]
- Carniel, A.; dos Santos, N.F.; Buarque, F.S.; Resende, J.V.M.; Ribeiro, B.D.; Marrucho, I.M.; Coelho, M.A.Z.; Castro, A.M. From Trash to Cash: Current Strategies for Bio-Upcycling of Recaptured Monomeric Building Blocks from Poly(Ethylene Terephthalate) (PET) Waste. Green Chem. 2024, 26, 5708–5743. [Google Scholar] [CrossRef]
- Pierri, E.; Egle, L.; Milios, L.; Saveyn, H. EU-Wide End-of-Waste Criteria for Plastic Waste; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Johansson, O. The End-of-Waste for the Transition to Circular Economy: A Legal Review of the European Union Waste Framework Directive. Environ. Policy Law 2023, 53, 167–179. [Google Scholar] [CrossRef]
- EN 15344; Plastics—Recycled Plastics—Characterization of Polyethylene (PE) Recyclates. iTeh Standards: Newark, DE, USA, 2021.
- EN 15348; Plastics—Recycled Plastics—Characterization of Polyethyleneterephthalate (PET) Recyclates. iTeh Standards: Newark, DE, USA, 2024.
- EN 15346; Plastics—Recycled Plastics—Characterization of Polyvinylchloride (PVC) Recyclates. iTeh Standards: Newark, DE, USA, 2024.
- EN 15345; Plastics—Recycled Plastics—Characterization of Polypropylene (PP) Recyclates. iTeh Standards: Newark, DE, USA, 2007.
- EN 15342; Plastics—Recycled Plastics—Characterization of Polystyrene (PS) Recyclates. iTeh Standards: Newark, DE, USA, 2007.
- EN 15347-1; Plastics—Sorted Plastics Wastes—Part 1: General Characterisation. iTeh Standards: Newark, DE, USA, 2024.
- Mihelčič, M.; Oseli, A.; Huskić, M.; Perše, L.S. Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene. Polymers 2022, 14, 5438. [Google Scholar] [CrossRef]
- Ghosh, A. Performance modifying techniques for recycled thermoplastics. Resour. Conserv. Recycl. 2021, 175, 105887. [Google Scholar] [CrossRef]
- Di Duca, F.; Montuori, P.; De Rosa, E.; De Simone, B.; Scippa, S.; Dadà, G.; Triassi, M. Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements. Foods 2024, 13, 2716. [Google Scholar] [CrossRef]
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175. [Google Scholar] [CrossRef]
- Szeredai, B.D.; Frentiu, T.; Ponta, M.; Muntean, N.; Covaci, E. High-Resolution Continuum Source Quartz Tube/Flame Atomic Absorption Spectrometry Method with Broad Applicability for the Comprehensive Assessment of Selected Toxic Elements Content in Recyclable (bio)Plastic Materials. Spectrochim. Acta Part B At. Spectrosc. 2024, 218, 106995. [Google Scholar] [CrossRef]
- Acquafredda, P. XRF technique. Phys. Sci. Rev. 2019, 4, 20180171. [Google Scholar] [CrossRef]
- Klingenberg, P.; Schirmeister, C.G.; Kappeler, M.; Calean, A.; Biester, H.; Licht, E.; Rapp, B. Quantification of Regulated METALS in Recycled Post-Consumer Polypropylene Through Comparative ICP-MS, AAS and LIBS Analyses. Polym. Test. 2024, 136, 108480. [Google Scholar] [CrossRef]
- Castro, G.; Cobo, M.; Rodríguez, I. Identification of Hazardous Organic Compounds in e-Waste Plastic Using Non-Target and Suspect Screening Approaches. Chemosphere 2024, 356, 141946. [Google Scholar] [CrossRef]
- Jang, M.; Yang, H.; Park, S.-A.; Sung, H.K.; Koo, J.M.; Hwang, S.Y.; Jeon, H.; Oh, D.X.; Park, J. Analysis of Volatile Organic Compounds Produced During Incineration of Non-Degradable and Biodegradable Plastics. Chemosphere 2022, 303, 134946. [Google Scholar] [CrossRef]
- Enders, A.A.; North, N.M.; Fensore, C.M.; Velez-Alvarez, J.; Allen, H.C. Functional Group Identification for FTIR Spectra Using Image-Based Machine Learning Models. Anal. Chem. 2021, 93, 9711–9718. [Google Scholar] [CrossRef]
- Nel, H.A.; Chetwynd, A.J.; Kelly, C.A.; Stark, C.; Valsami-Jones, E.; Krause, S.; Lynch, I. An Untargeted Thermogravimetric Analysis-Fourier Transform Infrared-Gas Chromatography-Mass Spectrometry Approach for Plastic Polymer Identification. Environ. Sci. Technol. 2021, 55, 8721–8729. [Google Scholar] [CrossRef]
- da Silva, D.J.; Wiebeck, H. ATR-FTIR Spectroscopy Combined with Chemometric Methods for the Classification of Polyethylene Residues Containing Different Contaminants. J. Polym. Environ. 2022, 30, 3031–3044. [Google Scholar] [CrossRef]
- Rivera, J.G.; Gámez, J.F.H.; Ramos, J.J.B.; Hernández, M.S.; Martínez, D.I.S. Effects of Residual Wax Content on the Thermal, Rheological, and Mechanical Properties of High-Density Polyethylene. Polym. Eng. Sci. 2022, 62, 1867–1875. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, J.; Tang, N.; He, Y.; Fan, L. Deep Learning Analysis for Rapid Detection and Classification of Household PLASTICS Based on Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123854. [Google Scholar] [CrossRef]
- Peller, J.R.; Durlam, N.; Flaherty, Y.; Valicevic, A.; Davis, C.M.; Watson, S.; Tournebise, J.E.; Medina-Garcia, J.A.; Dadmun, M.; Mezyk, S.P. Incognito Forms of Polyethylene Small Micro and Nanoplastics in Solvents: Changes in Molecular Vibrations. Sci. Total Environ. 2025, 968, 178923. [Google Scholar] [CrossRef]
- Bowen, N.; Guyer, C.; Rippon, T.; Daly, M.; Gao, P.; Galati, V.; Lograsso, S.; Johnston, S.; Masato, D. Mechanical and Crystallization Properties of hot Runner Injection Molded Virgin and Recycled Polypropylene. Polym. Eng. Sci. 2024, 64, 2241–2255. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, H.; Li, Z.; Hu, C.; Wang, Z. Effect of Mechanical Recycling on Crystallization, Mechanical, and Rheological Properties of Recycled High-Density Polyethylene and Reinforcement Based on Virgin High-Density Polyethylene. J. Appl. Polym. Sci. 2023, 141, 55097. [Google Scholar] [CrossRef]
- Seier, M.; Archodoulaki, V.-M.; Koch, T. The Morphology and Properties of Recycled Plastics Made from Multi-Layered Packages and the Consequences for the Circular Economy. Resour. Conserv. Recycl. 2023, 202, 107388. [Google Scholar] [CrossRef]
- Chialanza, M.R.; Sierra, I.; Parada, A.P.; Fornaro, L. Identification and Quantitation of Semi-Crystalline Microplastics Using Image Analysis and Differential Scanning Calorimetry. Environ. Sci. Pollut. Res. 2018, 25, 16767–16775. [Google Scholar] [CrossRef]
- House, K.L.; Christian, K.H.; Emge, T.J.; Pacheco, H.; Haber, R.A.; O’Carroll, D.M. Characterization of Nanoscale Morphology and Mechanical Properties of Conjugated Polymer thin Films Dynamically Exposed to a Secondary Solvent. Polymer 2023, 293, 126625. [Google Scholar] [CrossRef]
- Yavuzyegit, B.; Avcu, E.; Smith, A.D.; Donoghue, J.M.; Lunt, D.; Robson, J.D.; Burnett, T.L.; da Fonseca, J.Q.; Withers, P.J. Mapping Plastic Deformation Mechanisms in AZ31 Magnesium Alloy at the Nanoscale. Acta Mater. 2023, 250, 118876. [Google Scholar] [CrossRef]
- Bashirgonbadi, A.; Ureel, Y.; Delva, L.; Fiorio, R.; Van Geem, K.M.; Ragaert, K. Accurate Determination of Polyethylene (PE) and Polypropylene (PP) Content in Polyolefin Blends Using Machine Learning-Assisted Differential Scanning Calorimetry (DSC) analysis. Polym. Test. 2024, 131, 108353. [Google Scholar] [CrossRef]
- Lynch, J.M.; Corniuk, R.N.; Brignac, K.C.; Jung, M.R.; Sellona, K.; Marchiani, J.; Weatherford, W. Differential Scanning Calorimetry (DSC): An Important Tool for Polymer Identification and Characterization of Plastic Marine Debris. Environ. Pollut. 2024, 346, 123607. [Google Scholar] [CrossRef]
- Periasamy, D.; Manoharan, B.; Niranjana, K.; Aravind, D.; Krishnasamy, S.; Natarajan, V. Recycling of Thermoset Waste/High-density Polyethylene Composites: Examining the Thermal Properties. Polym. Compos. 2023, 45, 2739–2748. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jamal, M.; Biligiri, K.P.; Giustozzi, F. Effect of Various Compatibilizers on the Storage Stability, Thermochemical and Rheological Properties of Recycled Plastic-Modified Bitumen. Int. J. Pavement Res. Technol. 2023, 17, 854–867. [Google Scholar] [CrossRef]
- Zhang, J.; Hirschberg, V.; Goecke, A.; Wilhelm, M.; Yu, W.; Orfgen, M.; Rodrigue, D. Effect of Mechanical Recycling on Molecular Structure and Rheological Properties of High-Density Polyethylene (HDPE). Polymer 2024, 297, 126866. [Google Scholar] [CrossRef]
- Kneidinger, C.; Wagner, E.; Längauer, M.; Zitzenbacher, G. Estimation of the Shear Viscosity of Mixed-Polymer Materials for Screw Extrusion-Based Recycling Process Modeling. Polymers 2024, 16, 1339. [Google Scholar] [CrossRef]
- Primpke, S.; Cross, R.K.; Mintenig, S.M.; Simon, M.; Vianello, A.; Gerdts, G.; Vollertsen, J. Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (siMPle) for Spectroscopic Analysis. Appl. Spectrosc. 2020, 74, 1127–1138. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.-P.; Chu, X.-L. Quantitative Analysis of Plastic blends Based on Virtual Mid-Infrared Spectroscopy Combined with Chemometric Methods. Talanta 2025, 292, 128006. [Google Scholar] [CrossRef]
- Ati, E.M.; Hano, S.H.; Abbas, R.F.; Ajmi, R.N.; Latif, A.S. Laser Induced Spectroscopy (LIBS) Technology and Environmental Risk Index (RI) to Detect Microplastics in Drinking Water in Baghdad, Iraq. Nat. Environ. Pollut. Technol. 2024, 23, 2441–2446. [Google Scholar] [CrossRef]
- Takkalkar, P.; Jatoi, A.S.; Jadhav, A.; Jadhav, H.; Nizamuddin, S. Thermo-Mechanical, Rheological, and Chemical Properties of Recycled Plastics. In Plastic Waste for Sustainable Asphalt Roads; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–42. ISBN 9780323857895. [Google Scholar]
- Alqaheem, Y.; Alomair, A.A. Microscopy and Spectroscopy Techniques for Characterization of Polymeric Membranes. Membranes 2020, 10, 33. [Google Scholar] [CrossRef]
- Xiao, T.; Yuan, H.; Ma, Q.; Guo, X.; Wu, Y. An Approach for In Situ Qualitative and Quantitative Analysis of Moisture Adsorption in Nanogram-Scaled Lignin by Using Micro-FTIR Spectroscopy and Partial Least Squares Regression. Int. J. Biol. Macromol. 2019, 132, 1106–1111. [Google Scholar] [CrossRef]
- Bruno, C.; Blasi, M.F.; Mattei, D.; Martellone, L.; Brancaleone, E.; Savoca, S.; Favero, G. Polymer Composition Analysis of plastic debris Ingested by Loggerhead Turtles (Caretta caretta) in Southern Tyrrhenian Sea Through ATR-FTIR Spectroscopy. Mar. Environ. Res. 2022, 179, 105676. [Google Scholar] [CrossRef] [PubMed]
- Rosalina, R.; Kamwilaisak, K.; Sutthanut, K.; Srisongkram, T.; Weerapreeyakul, N. Probing the Stability and Quality of the Cellulose-Based Pickering Emulsion Containing Sesamolin-Enriched Sesame Oil by Chemometrics-Assisted ATR-FTIR Spectroscopy. Food Chem. 2024, 452, 139555. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Popescu, D.; Cojocaru, F.D.; Aradoaei, M.; Ciobanu, R.C.; Mihai, C.T. Use of Fourier-Transform Infrared Spectroscopy for DNA Identification on Recycled PET Composite Substrate. Appl. Sci. 2022, 12, 4371. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Guo, X.; Wang, K.; Jia, J.; Zhao, J.; Zhang, J. Comprehensive Investigation into the Impact of Degradation of Recycled Polyethylene and Recycled Polypropylene on the Thermo-Mechanical Characteristics and Thermal Stability of Blends. Molecules 2024, 29, 4499. [Google Scholar] [CrossRef] [PubMed]
- Mwanzia, M.N.; Mugo, W.; Soitah, T.N. Correlation of Mechanical and Optical Properties of Polypropylene Plastic Waste for Application in Composite Panels. J. Agric. Sci. Technol. 2023, 23, 125–130. [Google Scholar] [CrossRef]
- Audy, R.; Enfrin, M.; Boom, Y.J.; Giustozzi, F. Selection of Recycled Waste Plastic for Incorporation in Sustainable Asphalt Pavements: A Novel Multi-Criteria Screening Tool Based on 31 Sources of Plastic. Sci. Total Environ. 2022, 829, 154604. [Google Scholar] [CrossRef] [PubMed]
- Maria, C. Application of FTIR Spectroscopy in Environmental Studies. In Advanced Aspects of Spectroscopy; InTech: Singapore, 2012. [Google Scholar]
- Nguyen, T.; Shamsabadi, A.A.; Bavarian, M. Coupling ATR-FTIR Spectroscopy with Multivariate Analysis for Polymers Manufacturing and Control of Polymers’ Molecular Weight. Digit. Chem. Eng. 2023, 7, 100089. [Google Scholar] [CrossRef]
- Munoz, L.P.; Baez, A.G.; McKinney, D.; Garelick, H. Characterisation of “flushable” and “non-flushable” Commercial Wet Wipes Using microRaman, FTIR Spectroscopy and Fluorescence Microscopy: To Flush or Not to Flush. Environ. Sci. Pollut. Res. 2018, 25, 20268–20279. [Google Scholar] [CrossRef]
- Cialla-May, D.; Schmitt, M.; Popp, J. Theoretical principles of Raman spectroscopy. Phys. Sci. Rev. 2019, 4, 20170040. [Google Scholar] [CrossRef]
- Unal, M.; Ahmed, R.; Mahadevan-Jansen, A.; Nyman, J.S. Compositional Assessment of Bone by Raman Spectroscopy. Anal. 2021, 146, 7464–7490. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, Z.; Zhu, L.; Hou, Y.; Qiu, Y. Direct Observation of the Release of Nanoplastics from Commercially Recycled Plastics with Correlative Raman Imaging and Scanning Electron Microscopy. ACS Nano 2020, 14, 7920–7926. [Google Scholar] [CrossRef]
- Marica, I.; Aluaș, M.; Pînzaru, S.C. Raman Technology Application for Plastic Waste Management Aligned with FAIR Principle to Support the Forthcoming Plastic and Environment Initiatives. Waste Manag. 2022, 144, 479–489. [Google Scholar] [CrossRef]
- Giese, A.; Kerpen, J.; Weber, F.; Prediger, J. A Preliminary Study of Microplastic Abrasion from the Screw Cap System of Reusable Plastic Bottles by Raman Microspectroscopy. ACS ES&T Water 2021, 1, 1363–1368. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; He, Z.; Song, Y.; Fu, X.; Rommel, M.; Luo, X.; Hartmaier, A.; Zhang, J.; Fang, F. Topic Review: Application of Raman Spectroscopy Characterization in Micro/Nano-Machining. Micromachines 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Chibwe, L.; De Silva, A.O.; Spencer, C.; Teixera, C.F.; Williamson, M.; Wang, X.; Muir, D.C.G. Target and Nontarget Screening of Organic Chemicals and Metals in Recycled Plastic Materials. Environ. Sci. Technol. 2023, 57, 3380–3390. [Google Scholar] [CrossRef]
- Maurya, A.K. Novel Application of Silicon Multi-Vacancy Satellite Peaks for Silicate Minerals Analysis in Igneous Rocks Using WD-XRF Coupled with Chemometrics Analysis. J. Anal. At. Spectrom. 2024, 39, 2543–2550. [Google Scholar] [CrossRef]
- Kajiwara, N.; Matsukami, H.; Malarvannan, G.; Chakraborty, P.; Covaci, A.; Takigami, H. Recycling Plastics Containing Decabromodiphenyl Ether Into New Consumer Products Including Children’s Toys Purchased in Japan and Seventeen Other Countries. Chemosphere 2022, 289, 133179. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Lappas, A.A.; Achilias, D.S. Thermo-Chemical Recycling of Plastics Retrieved from Waste Electric and Electronic Equipment (WEEE) by Pyrolysis: Identification of the Polymer Type, Removal of Bromine Compounds from PLASTICS Based on an Environmentally-Friendly Process and Characterization of the Pyrolysates. Sustain. Chem. Pharm. 2023, 35, 101210. [Google Scholar] [CrossRef]
- Jandric, A.; Olscher, C.; Zafiu, C.; Lielacher, R.; Lechner, C.; Lassenberger, A.; Part, F. Adding Rare Earth Oxide Markers to Polyoxymethylene to Improve Plastic Recycling through Tracer-Based Sorting. Polymers 2024, 16, 2591. [Google Scholar] [CrossRef]
- Holub, D.; Buday, J.; Pořízka, P.; Kaiser, J. Determination of Pb Content in Recycled Plastic Debris by Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2023, 207, 106752. [Google Scholar] [CrossRef]
- Núñez, S.S.; Moltó, J.; Conesa, J.A.; Fullana, A. Heavy metals, PAHs and POPs in Recycled Polyethylene Samples of Agricultural, Post-Commercial, Post-Industrial and Post-Consumer Origin. Waste Manag. 2022, 144, 113–121. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.g.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2014, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Gopi, S.; Jayaraj, K.; Pius, A. Characterization Studies of Polymer-Based Composites Related to Functionalized Filler-Matrix Interface. In Interfaces in Particle and Fibre Reinforced Composites: Current Perspectives on Polymer, Ceramic, Metal and Extracellular Matrices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–250. [Google Scholar]
- Epp, J. X-Ray Diffraction (XRD) Techniques for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 81–124. [Google Scholar]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental Methods in Chemical Engineering: X-Ray Diffraction Spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Panda, D.; Hota, S.S.; Choudhary, R.N.P. Investigation of the Structural, Surface Topographical, Fractal, Capacitive, and Electrical Properties of a Defect Brownmillerite Perovskite Material KBiFeMnO5 for Electronic Devices. RSC Adv. 2024, 14, 3400–3412. [Google Scholar] [CrossRef]
- Świetlicki, M.; Chocyk, D.; Klepka, T.; Prószyński, A.; Kwaśniewska, A.; Borc, J.; Gładyszewski, G. The Structure and Mechanical Properties of the Surface Layer of Polypropylene Polymers with Talc Additions. Materials 2020, 13, 698. [Google Scholar] [CrossRef]
- Kane, S.; Thane, A.; Espinal, M.; Lunday, K.; Armağan, H.; Phillips, A.; Heveran, C.; Ryan, C. Biomineralization of Plastic Waste to Improve the Strength of Plastic-Reinforced Cement Mortar. Materials 2021, 14, 1949. [Google Scholar] [CrossRef] [PubMed]
- Mekprasart, W.; Thongpradith, T.; Pecharapa, W.; Ishihara, K.N. Photocatalytic Properties and Plastic Degradation of TiO2 Nanocomposite with Synthetic-rutile from Natural Ore. J. Jpn. Soc. Powder Powder Met. 2018, 65, 719–724. [Google Scholar] [CrossRef]
- Saleh, M.; Anwar, S.; AlFaify, A.Y.; Al-Ahmari, A.M.; Elgawad, A.E.E.A. Development of PLA/Recycled-Desized Carbon Fiber Composites for 3D Printing: Thermal, Mechanical, and Morphological Analyses. J. Mater. Res. Technol. 2024, 29, 2768–2780. [Google Scholar] [CrossRef]
- Karaagac, E.; Koch, T.; Archodoulaki, V.-M. The effect of PP Contamination in Recycled High-Density Polyethylene (rPE-HD) from Post-Consumer Bottle Waste and Their Compatibilization with Olefin Block Copolymer (OBC). Waste Manag. 2021, 119, 285–294. [Google Scholar] [CrossRef]
- Fang, C.; Yu, J.; Gopalan, S.; Naidu, R. Investigating MICROPLASTICS and Nanoplastics Released from Food Bag Ziplock Using SEM and Raman Imaging. Nano Express 2024, 5, 025025. [Google Scholar] [CrossRef]
- de Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of Sterilization by Electron Beam, Gamma Radiation and X-Rays on Electrospun poly-(ε-caprolactone) Fiber Mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Gravgaard, D.P.; Henriksen, M.L.; Hinge, M. Dissolution Recycling for Recovery of Polypropylene and Glass Fibres. J. Mater. Cycles Waste Manag. 2024, 26, 961–969. [Google Scholar] [CrossRef]
- Pavlyuchkova, E.A.; Malkin, A.Y.; Kornev, Y.V.; Simonov-Emel’yanov, I.D. Distribution of Filler in Polymer Composites. Role of Particle Size and Concentration. Polym. Sci. Ser. A 2024, 66, 113–120. [Google Scholar] [CrossRef]
- Sanchez-Olivares, G.; Rockel, D.; Calderas, F.; Schartel, B. Utilizing Leather Fibers from Industrial Wastes as Bio-Filler to Improve Flame Retardancy in Polypropylene. J. Ind. Eng. Chem. 2023, 132, 148–160. [Google Scholar] [CrossRef]
- Zeng, S.; Guo, P.; Hu, C.; Wang, Z. Effects of Mechanical Recycling on Optical Properties and Microstructure of Recycled High-density Polyethylene Pellets and Bottles. J. Appl. Polym. Sci. 2022, 140, 53446. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Yang, X.; Ali, H.M.; Said, Z.; Liu, C. Fabrication of Shape-Stabilized Phase Change Materials Based on Waste Plastics for Energy Storage. J. Energy Storage 2022, 52, 104973. [Google Scholar] [CrossRef]
- Al Maamori, M.H.; Habeeb, S.A.; Moslem, M.A. Enhancing the Physical Properties of Recycled Low-Density Polyethylene and Virgin Low-Density Polyethylene Blend Using Octanoate Starch. Prog. Rubber Plast. Recycl. Technol. 2024, 40, 445–457. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M. Differential Scanning Calorimetry of Polymers. In Polymer Morphology: Principles, Characterization, and Processing; Wiley: Hoboken, NJ, USA, 2016; pp. 72–99. [Google Scholar]
- Gliński, T.; Tański, T.; Bilewicz, M.; Smok, W. Comparison of the Standard and Recycled PE 3-Layered Films. Adv. Mater. Sci. 2024, 24, 57–66. [Google Scholar] [CrossRef]
- Šudomová, L.; Weissmannová, H.D.; Steinmetz, Z.; Řezáčová, V.; Kučerík, J. A Differential Scanning Calorimetry (DSC) Approach for Assessing the Quality of Polyethylene Terephthalate (PET) Waste for Physical Recycling: A Proof-of-Concept Study. J. Therm. Anal. Calorim. 2023, 148, 10843–10855. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, S.; Yang, H.; Wu, X.; Xiao, Y. An Analysis of the Influence of DSC Parameters on the Measurement of the Thermal Properties of Phase-Change Material. Materials 2024, 17, 5689. [Google Scholar] [CrossRef]
- Blennerhassett, L.C.; Guyett, P.C.; Tomlinson, E.L. Tephra Identification Without Pre-Separation in Ashed Peat. J. Quat. Sci. 2024, 39, 816–830. [Google Scholar] [CrossRef]
- Petrovič, A.; Čolnik, M.; Prša, A.; Fan, Y.; Škerget, M.; Knez, Ž.; Klemeš, J.J.; Čuček, L. Comparative Analysis of Virgin and Recycled Thermoplastic Polymer Based on Thermochemical Characteristics. Chem. Eng. Trans. 2022, 94, 1321–1326. [Google Scholar] [CrossRef]
- Juan, R.; Paredes, B.; García-Muñoz, R.A.; Domínguez, C. Quantification of PP Contamination in Recycled PE by TREF Analysis for Improved the Quality and Circularity of Plastics. Polym. Test. 2021, 100, 107273. [Google Scholar] [CrossRef]
- Scoppio, A.; Cavallo, D.; Müller, A.J.; Tranchida, D. Temperature Modulated DSC for Composition Analysis of Recycled Polyolefin Blends. Polym. Test. 2022, 113, 107656. [Google Scholar] [CrossRef]



| Properties | Characteristic | Analytical Method |
|---|---|---|
| Chemical | Elemental composition | X-ray Fluorescence (XRF) |
| Trace metal concentrations | Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | |
| Organic compound concentrations | Gas Chromatography–Mass Spectrometry (GC-MS) | |
| Functional groups | Fourier Transform Infrared Spectroscopy (FTIR) | |
| Nonpolar molecular structures and conjugation | Raman Spectroscopy | |
| Structural | Crystalline and amorphous phase quantification | X-ray Powdered Diffraction (XRPD) |
| Molecular weight distribution | Gel Permeation Chromatography (GPC) | |
| Morphological | Surface morphology and micro-void formation | Scanning Electron Microscopy (SEM) |
| Nanoscale mechanical property mapping | Atomic Force Microscopy (AFM) | |
| Physical | Melting/crystallisation behaviour | Differential Scanning Calorimetry (DSC) |
| Thermal stability and decomposition temperature | Thermogravimetric Analysis (TGA) | |
| Detailed viscosity profile | Rotational Rheometry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzoh Fonkou, J.P.; Beggio, G.; Salviulo, G.; Lavagnolo, M.C. Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review. Environments 2025, 12, 154. https://doi.org/10.3390/environments12050154
Dzoh Fonkou JP, Beggio G, Salviulo G, Lavagnolo MC. Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review. Environments. 2025; 12(5):154. https://doi.org/10.3390/environments12050154
Chicago/Turabian StyleDzoh Fonkou, Joseph Patrick, Giovanni Beggio, Gabriella Salviulo, and Maria Cristina Lavagnolo. 2025. "Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review" Environments 12, no. 5: 154. https://doi.org/10.3390/environments12050154
APA StyleDzoh Fonkou, J. P., Beggio, G., Salviulo, G., & Lavagnolo, M. C. (2025). Analytical Methods for In-Depth Assessment of Recycled Plastics: A Review. Environments, 12(5), 154. https://doi.org/10.3390/environments12050154






