Recirculation of Saline Concentrate in Spirulina Cultivation: A Promising Strategy for High Production of Biomass and Biomolecules in Semiarid Regions
Abstract
1. Introduction
2. Materials and Methods
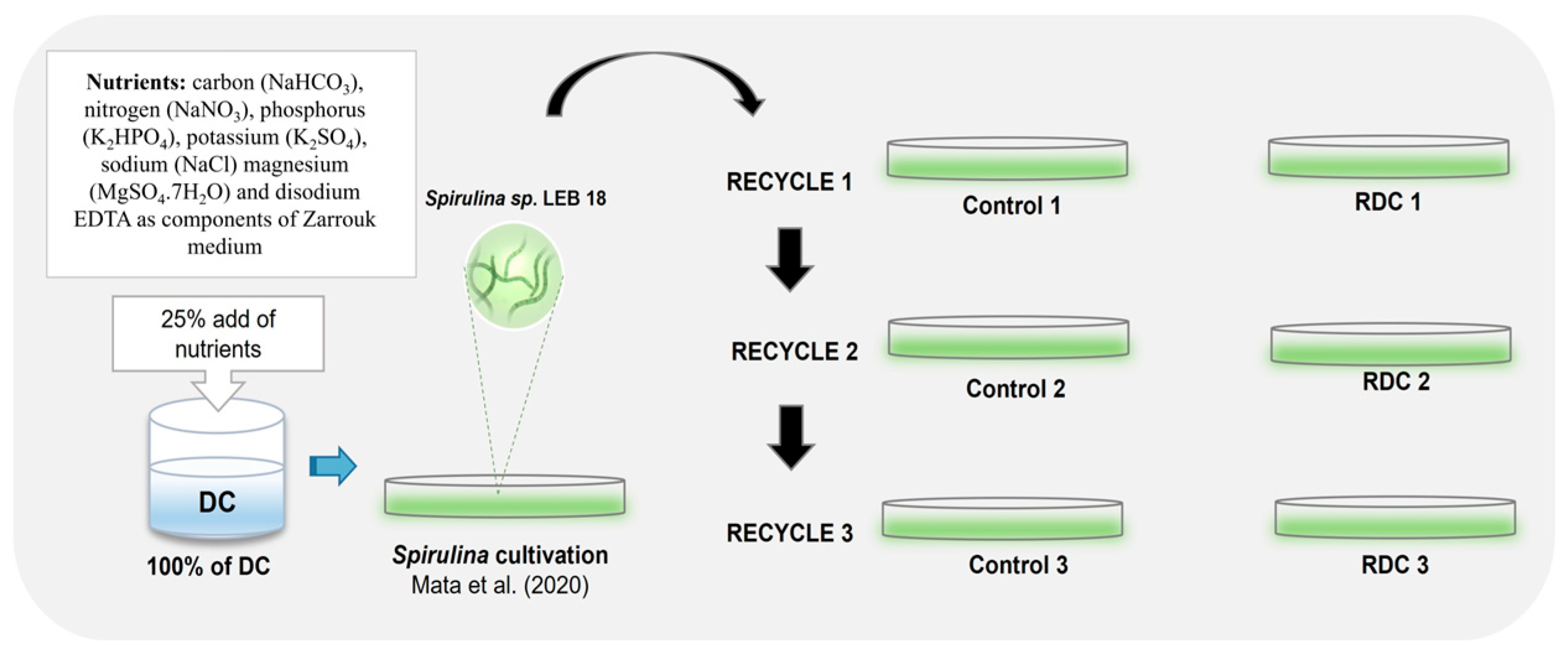
2.1. Microalgae and, Water Collection
2.2. Experimental Conditions
2.3. Biomass Concentration and pH
2.4. Determination of Growth Parameters
2.5. Recovery and Biochemical Characterization of Biomass
2.6. Quantification and Determination of Amino Acids
2.7. Fatty Acid Methyl Ester Composition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth Parameters
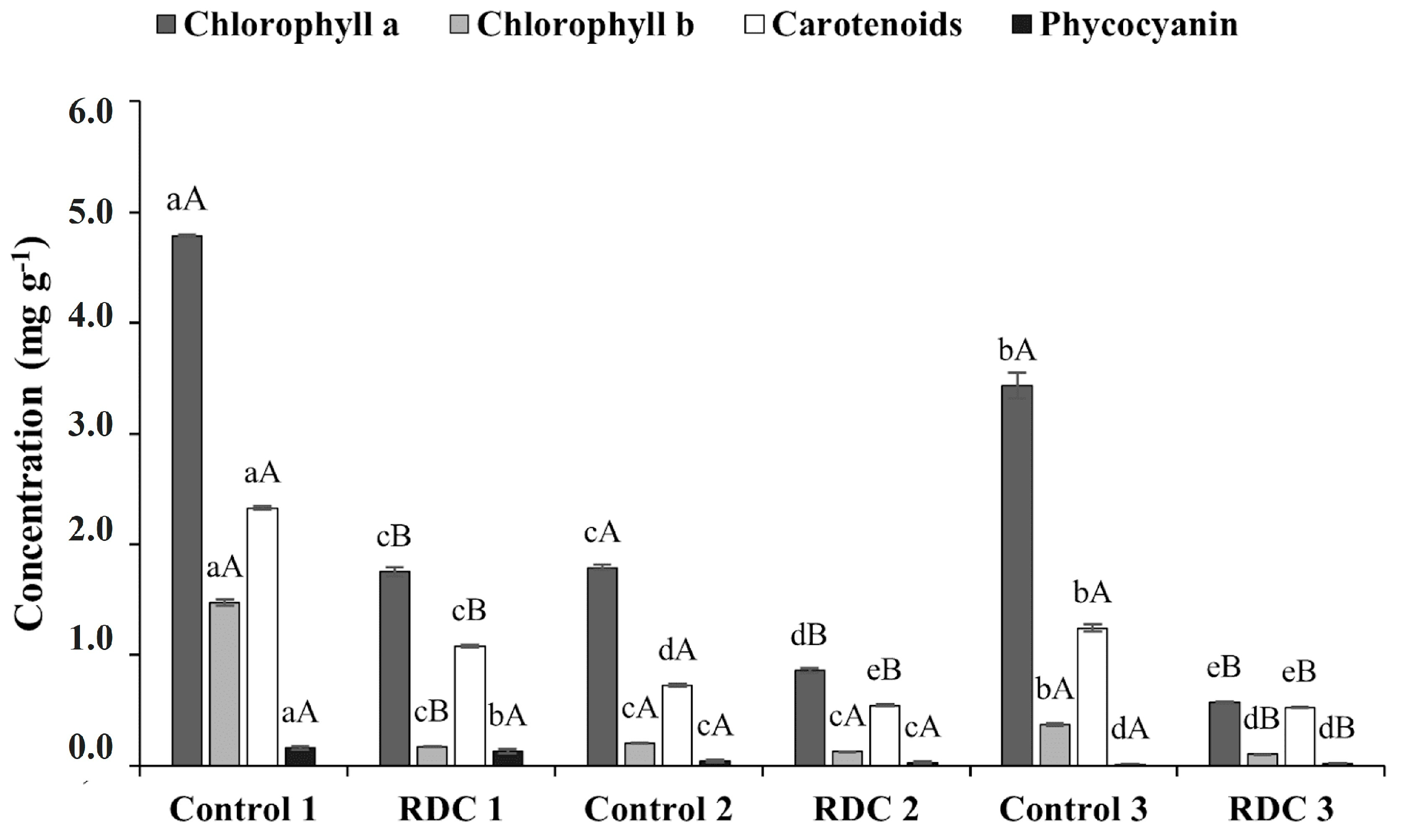
3.2. Determination of Chlorophylls A and B, Total Carotenoids, and Phycocyanin
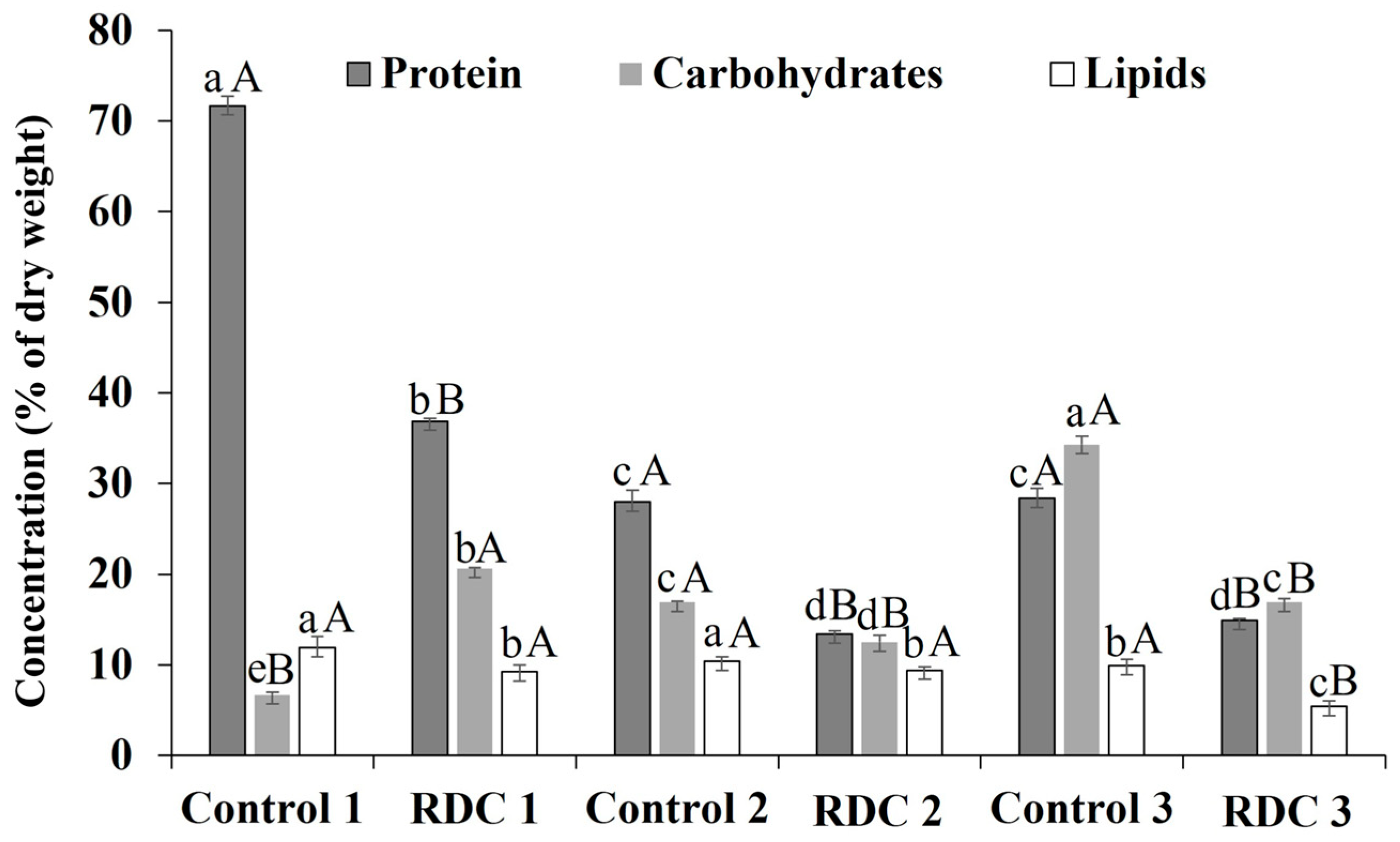
3.3. Biomass Biochemical Composition
3.4. Quantification and Determination of Amino Acids
3.5. FAME Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DC | Desalination concentrate |
| RDC | Reused desalination concentrate |
| TDS | Total Dissolved Solids |
| ROS | Reactive Oxygen Species |
| FAME | Fatty acid methyl ester |
| FAO | Food and Agriculture Organization |
| WHO | World Health Organization |
References
- U.N. Water. The United Nations World Water Development Report 2020: Water and Climate Change; UN-Water: Geneva, Switzerland, 2020; in press. [Google Scholar]
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; Facts, Figures and Action Examples; Koncagül, E., Connor, R., Eds.; UNESCO: Paris, France, 2023; pp. 1–158. [Google Scholar]
- Sánchez, A.S.; Nogueira, I.B.R.; Kalid, R.A. Uses of the reject brine from inland desalination for fish farming, Spirulina cultivation, and irrigation of forage shrub and crops. Desalination 2015, 364, 96–107. [Google Scholar] [CrossRef]
- Matos, Â.P.; Moecke, E.H.; Sant’Anna, E.S. The use of desalination concentrate as a potential substrate for microalgae cultivation in Brazil. Algal Res. 2017, 24, 505–508. [Google Scholar] [CrossRef]
- Andrade, B.B.; Cardoso, L.G.; Assis, D.J.; Costa, J.A.V.; Druzian, J.I.; Lima, S.T.C. Production and characterization of Spirulina sp LEB 18 cultured in reused Zarrouk’s médium in a raceway-type bioreactor. Bioresour. Technol. 2019, 284, 340–348. [Google Scholar] [CrossRef]
- Kumari, P.; Shukla, S.P.; Bhuvaneswari, G.R.; Kumar, S.; Xavier, M.; Kumar, M. High value pigment production and carbon sequestration through wastewater grown Spirulina (Arthrospira) platensis: A green technology for wastewater utilization. Waste Manag. Bull. 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Hung, C.M.; Chen, C.W.; Huang, C.P.; Dong, C.D. Bioremediation pretreatment of waste-activated sludge using microalgae Spirulina platensis derived biochar coupled with sodium sulfite: Performance and microbial community dynamics. Bioresour. Technol. 2022, 362, 127867. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Moraes, L.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Spirulina sp. LEB 18 cultivation in seawater and reduced nutrients: Bioprocess strategy for increasing carbohydrates in biomass. Bioresour. Technol. 2020, 316, 123883. [Google Scholar] [CrossRef]
- Mata, S.N.; de Souza Santos, T.; Cardoso, L.G.; Andrade, B.B.; Duarte, J.H.; Costa, J.A.V.; Druzian, J.I. Spirulina sp. LEB 18 cultivation in a raceway-type bioreactor using wastewater from desalination process: Production of carbohydrate-rich biomass. Bioresour. Technol. 2020, 311, 123495. [Google Scholar] [CrossRef]
- Matos, Â.P.; Ferreira, W.B.; de Oliveira Torres, R.C.; Morioka, L.R.I.; Canella, M.H.M.; Rotta, J.; Sant’Anna, E.S. Optimization of biomass production of Chlorella vulgaris grown in desalination concentrate. J. Appl. Phycol. 2015, 27, 1473–1483. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; Sant’Anna, E.S. Biomass, lipid productivities and fatty acids composition of marine Nannochloropsis gaditana cultured in desalination concentrate. Bioresour. Technol. 2015, 197, 48–55. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Colla, L.M.; Duarte Filho, P. Improving Spirulina platensis biomass yield using a fedbatch process. Bioresour. Technol. 2004, 92, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Rosa, G.M.; de Morais, M.G.; Costa, J.A.V. Green alga cultivation with monoethanolamine: Evaluation of CO2 fixation and macromolecule production. Bioresour. Technol. 2018, 261, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Machado, S.; Costa, A.S.; Pimentel, F.; Oliveira, M.B.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef]
- Henderson, J.W., Jr.; Brooks, A. Improved Amino Acid Methods Using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC instrumentation and Separation Goals (Application Note). Available online: https://www.agilent.com/cs/library/applications/5990-4547EN.pdf (accessed on 13 February 2025).
- Zheng, M.; Li, H.; Guo, X.; Chen, B.; Wang, M. A semi-continuous efficient strategy for removing phosphorus and nitrogen from eel aquaculture wastewater using the self-flocculating microalga Desmodesmus sp. PW1. J. Environ. Manag. 2023, 346, 118970. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Lelekov, A.S.; Avsiyan, A.L. Effect of specific irradiance on productivity and pigment and protein production of Porphyridium purpureum (Rhodophyta) semi-continuous culture. Bioresour. Technol. 2023, 374, 128771. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Vonshak, A. Spirulina platensis (Arthrospira): Physiology, Cell Biology and Biotechnology, 2nd ed.; Taylor and Francis: London, UK, 1997; pp. 1–304. [Google Scholar]
- Zhang, C.; Zhang, W.; Huang, Y.; Gao, X. Analysing the correlations of long-term seasonal water quality parameters, suspended solids and total dissolved solids in a shallow reservoir with meteorological factors. Environ. Sci. Pollut. Res. 2017, 24, 6746–6756. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Kumar, K.; Gross, M.; Kunetz, Z.; Wen, Z. Removal of total dissolved solids from wastewater using a revolving algal biofilm reactor. Water Environ. Res. 2020, 92, 766–778. [Google Scholar] [CrossRef]
- Mirzaei, M.; Jazini, M.; Aminiershad, G.; Refardt, D. Biodesalination of saline aquaculture wastewater with simultaneous nutrient removal and biomass production using the microalgae Arthrospira and Dunaliella in a circular economy approach. Desalination 2024, 581, 117564. [Google Scholar] [CrossRef]
- Church, J.; Hwang, J.H.; Kim, K.T.; McLean, R.; Oh, Y.K.; Nam, B.; Lee, W.H. Effect of salt type and concentration on the growth and lipid content of Chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour. Technol. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, M.F.; Li, T.L. Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci. 2012, 196, 8–17. [Google Scholar] [CrossRef]
- Jiang, Y.; Yoshida, T.; Quigg, A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol. Biochem. 2012, 54, 70–77. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Effects of phosphorus concentration and light intensity on the biomass composition of Arthrospira (Spirulina) platensis. World J. Microbiol. Biotechnol. 2012, 28, 2661–2670. [Google Scholar] [CrossRef]
- Affan, M.A.; Lee, D.W.; Jeon, S.M.; Noh, J.H.; Heo, S.J.; Oh, C.; Kang, D.H. Bituminous coal and sodium hydroxide-pretreated seawater stimulates Spirulina (Arthrospira) maxima growth with decreased production costs. Aquaculture 2015, 436, 121–126. [Google Scholar] [CrossRef]
- Leema, J.M.; Kirubagaran, R.; Vinithkumar, N.V.; Dheenan, P.S.; Karthikayulu, S. High value pigment production from Arthrospira (Spirulina) platensis cultured in seawater. Bioresour. Technol. 2010, 101, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Lamela, T.; Márquez-Rocha, F.J. Phycocyanin production in seawater culture of Arthrospira maxima. Cienc. Mar. 2000, 26, 607–619. [Google Scholar] [CrossRef][Green Version]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Maurya, R.; Trivedi, K.; Patidar, S.K.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Moraes, L.; Silva, T.N.M.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Costa, J.A.V. Innovative application of brackish groundwater without the addition of nutrients in the cultivation of Spirulina and Chlorella for carbohydrate and lipid production. Bioresour. Technol. 2022, 345, 126543. [Google Scholar] [CrossRef]
- Torre, F.; Cañas, R.A.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Yang, H.; Qi, D.; Si, X.; Yan, Z.; Guo, L.; Shao, C.; Yang, L. One novel Cd-MOF as a highly effective multi-functional luminescent sensor for the detection of Fe3+, Hg2+, CrVI, Aspartic acid and Glutamic acid in aqueous solution. J. Solid State Chem. 2022, 310, 123008. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, Z.; Duan, M.; Ma, Q.; Xu, H.; Liang, M. Responses to graded levels of leucine and branched-chain amino acid imbalance in tiger puffer Takifugu rubripes. Aquaculture 2022, 548, 737699. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, I.; Fatma, S.; Peres, H. Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquac. Nutr. 2021, 27, 1270–1289. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sust. Energ. Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Villadó, S.; García-Vaquero, M.; Morán, L.; Álvarez, C.; Cabral, E.M.; Lafarga, T. Effect of seawater on the biomass composition of Spirulina produced at a pilot-scale. New Biotech. 2023, 78, 173–179. [Google Scholar] [CrossRef]
| Assays | Xmax (g·L−1) | Xfinal (g·L−1) | Pmax (g·L−1·d−1) | Pfinal (g·L−1·d−1) | µmax (d−1) | pH | |
|---|---|---|---|---|---|---|---|
| Mata et al. (2020) [10] | Control | - | 1.25 ± 0.01 | - | 0.03 ± 0.02 | 0.02 ± 0.00 | 10.15 ± 0.12 |
| DC | - | 1.14 ± 0.01 | - | 0.05 ± 0.01 | 0.03 ± 0.00 | 9.75 ± 0.52 | |
| Recycle 1 | Control 1 | 1.21(30th) ± 0.24 cA | 1.20(30th) ± 0.02 bA | 0.04 ± 0.01 dA | 0.04 ± 0.01 dA | 0.02 ± <0.01 bA | 10.50 ± 0.17 aA |
| RDC 1 * | 1.24(29th) ± 0.15 bA | 1.13(30th) ± 0.05 cB | 0.04 ± <0.01 cA | 0.03 ± 0.01 cB | 0.02 ± <0.01 bA | 10.36 ± 0.24 cB | |
| Recycle 2 | Control 2 | 0.88(9th) ± 0.17 dB | 0.54(27th) ± 0.02 eB | 0.08 ± 0.02 eA | 0.01 ± 0.00 eB | 0.02 ± <0.01 bA | 10.45 ± 0.07 bA |
| RDC 2 * | 1.28(19th) ± 0.19 bA | 0.71(28th) ± 0.15 dB | 0.05 ± 0.01 bA | 0.01 ± 0.01 dB | 0.01 ±< 0.01 bA | 10.23 ± 0.11 dB | |
| Recycle 3 | Control 3 | 1.36(26th) ± 0.25 aA | 1.35(26th) ± 0.21 aA | 0.04 ± 0.02 aA | 0.04 ± 0.02 aA | 0.03 ± <0.01 aA | 9.50 ± 0.14 eA |
| RDC 3 * | 1.25 (12th) ± 0.33 bB | 0.57(24th) ± 0.17 eB | 0.09 ± <0.01 bA | 0.01 ± <0.01 eB | 0.02 ± <0.01 bA | 9.47 ± 0.25 fB | |
| ΣTotal | Control | 3.45 B | 3.09 A | 0.371 A | 0.06 | - | - |
| RDCs | 3.77 A | 2.41 B | 0.703 B | - | - | - |
| Amino Acid | Control 1 | RDC1 | Control 2 | RDC2 | Control 3 | RDC3 |
|---|---|---|---|---|---|---|
| Aspartic acid | 43.98 ± 2.35 a | 19.25 ± 1.02 b | 18.80 ± 0.40 bc | 11.53 ± 0.31 d | 17.54 ± 0.57 c | 11.35 ± 0.38 d |
| Glutamic acid | 56.81 ± 2.92 a | 25.05 ± 1.30 b | 24.56 ± 0.52 b | 14.87 ± 0.41 d | 21.56 ± 0.66 c | 14.70 ± 0.44 d |
| Serine | 22.80 ± 1.18 a | 10.05 ± 0.52 b | 7.71 ± 0.16 c | 5.15 ± 0.16 d | 8.01 ± 0.26 c | 5.08 ± 0.16 d |
| Histidine | 7.49 ± 0.35 a | 2.61 ± 0.13 b | 2.64 ±0.05 b | 1.40 ± 0.07 c | 2.26 ± 0.07 b | 1.38 ± 0.08 c |
| Glycin | 26.63 ± 1.36 a | 11.62 ± 0.68 bc | 10.04 ± 0.21 c | 7.31 ± 0.20 d | 10.84 ± 0.40 c | 7.25 ± 0.21 d |
| Threoninae * | 19.89 ± 0.97 a | 8.71 ± 0.42 b | 7.45 ± 0.13 bc | 5.27 ± 0.14 d | 6.87 ± 0.21 c | 5.21 ± 0.14 d |
| Arginine | 36.46 ± 1.81 a | 15.25 ± 0.91 b | 15.25 ± 0.51 b | 8.54 ± 0.31 d | 12.86 ± 0.24 c | 8.35 ± 0.41 d |
| Alanine | 36.73 ± 1.71 a | 15.92 ± 0.83 b | 13.11 ± 0.32 c | 9.06 ± 0.25 d | 13.77 ± 0.43 c | 8.93 ± 0.27 d |
| Tirosine | 19.91 ± 1.15 a | 7.74 ± 0.36 b | 5.07 ± 0.13 c | 3.36 ± 0.13 d | 2.60 ± 0.14 e | 3.23 ± 0.29 d |
| Valine * | 26.69 ± 1.35 a | 11.66 ± 0.62 b | 10.28 ± 0.26 b | 6.87 ± 0.16 c | 10.65 ± 0.31 b | 6.70 ± 0.22 c |
| Methionine * | 9.26 ± 0.50 a | 3.82 ± 0.38 b | 2.15 ± 0.06 c | 1.42 ± 0.06 d | 1.08 ± 0.06 d | 1.26 ± 0.14 d |
| Tryptophan * | 2.48 ±0.18 a | 0.84 ± 0.11 b | 0.85 ± 0.04 b | 0.50 ± 0.01 c | 0.87 ± 0.06 b | 0.49 ± 0.02 c |
| Fenilalanine * | 26.82 ±1.31 a | 11.14 ± 0.55 b | 9.78 ± 0.28 c | 6.67 ± 0.18 d | 10.19 ± 0.30 b | 6.51 ± 0.22 d |
| Isoleucine * | 22.37 ± 1.12 a | 9.44 ± 0.47 b | 7.86 ± 0.19 d | 5.14 ± 0.12 e | 8.67 ± 0.26 c | 5.06 ± 0.14 e |
| Leucine * | 48.70 ± 2.38 a | 20.87 ± 0.98 b | 17.54 ± 0.42 d | 11.66 ± 0.29 e | 18.62 ± 0.55 c | 11.48 ± 0.32 e |
| Lysine * | 21.71 ± 1.01 a | 9.88 ± 0.43 b | 7.48 ± 0.07 c | 5.56 ± 0.16 d | 7.60 ± 0.22 c | 5.61 ± 0.12 d |
| Hidroxiproline | 0.32 ± 0.01 a | 0.18 ± <0.01 b | 0.17 ± <0.01 b | 0.18 ± <0.01 b | 0.17 ± <0.01 b | 0.18 ± <0.01 b |
| Proline | 17.06 ± 0.74 a | 7.48 ± 0.34 b | 6.32 ± 0.20 c | 4.56 ± 0.11 d | 6.69 ± 0.17 c | 4.47 ± 0.16 d |
| Essentials | 177.92 a | 76.36 b | 63.38 c | 43.07 d | 64.54 c | 42.30 d |
| Non essentials | 268.19 a | 115.14 b | 103.67 c | 65.96 e | 96.29 d | 64.90 e |
| Total | 446.10 a | 191.51 b | 167.06 c | 109.04 d | 160.83 c | 107.21 d |
| Treatments | |||||
|---|---|---|---|---|---|
| Fatty Acid | Nomenclature | Control 1 | RDC1 | Control 2 | RDC2 |
| C10:0 | Capric acid | 5.78 ± 0.15 b | 6.46 ± 0.69 a | 4.27 ± 0.05 c | 3.51 ± 0.04 d |
| C12:0 | Lauric acid | 1.35 ± 0.29 | - | - | - |
| C13:0 | Tridecylic acid | 1.84 ± 0.02 | - | - | - |
| C15:0 | Pentadecylic acid | 0.97 ± 0.01 | - | - | - |
| C15:1 | Pentadecenoic acid | 44.5 ± 0.14 c | 42.96 ± 0.42 d | 50.23 ± 0.40 a | 48.87 ± 0.18 b |
| C16:0 | Palmitic acid | 3.06 ± 0.02 b | 2.89 ± 0.20 c | 7.12 ± 0.07 a | 7.62 ± 0.05 a |
| C17:1 | Heptadecanoic acid | 1.9 ± 0.13 d | 3.58 ± 0.39 c | 6.59 ± 0.37 b | 7.80 ± 0.07 a |
| C18:0 | Stearic acid | 6.86 ± 0.31 b | 8.93 ± 2.30 a | 3.11 ± 0.05 c | 3.45 ± 0.44 c |
| C18:1n9c | Oleic acid | 14.5 ± 0.03 b | 13.89 ± 0.37 c | 15.19 ± 0.57 b | 17.17 ± 0.07 a |
| C18:2n6t | Linolelaidic acid | 19.24 ± 0.10 a | 18.03 ± 0.98 b | 13.49 ± 0.15 c | 11.57 ± 0.03 d |
| C18:3n6 | α-linolenic acid | - | 3.25 ± 0.36 | - | - |
| Σ saturated | 19.86 | 18.28 | 14.49 | 14.58 | |
| Σ monounsaturated | 60.90 | 78.72 | 72.01 | 73.84 | |
| Σ polyunsaturated | 19.24 | 21.29 | 13.49 | 11.57 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, S.N.; Cardoso, L.G.; Bezerra, P.Q.M.; Andrade, B.B.; Oliveira, M.B.P.P.; Machado, S.; de Almeida Medeiros, R.M.; Miranda, N.H.; de Jesus Silva, J.S.; Costa, J.A.V.; et al. Recirculation of Saline Concentrate in Spirulina Cultivation: A Promising Strategy for High Production of Biomass and Biomolecules in Semiarid Regions. Environments 2025, 12, 134. https://doi.org/10.3390/environments12050134
Mata SN, Cardoso LG, Bezerra PQM, Andrade BB, Oliveira MBPP, Machado S, de Almeida Medeiros RM, Miranda NH, de Jesus Silva JS, Costa JAV, et al. Recirculation of Saline Concentrate in Spirulina Cultivation: A Promising Strategy for High Production of Biomass and Biomolecules in Semiarid Regions. Environments. 2025; 12(5):134. https://doi.org/10.3390/environments12050134
Chicago/Turabian StyleMata, Saulo Nascimento, Lucas Guimarães Cardoso, Priscilla Quenia Muniz Bezerra, Bianca Bomfim Andrade, Maria Beatriz Prior Pinto Oliveira, Susana Machado, Ravena Maria de Almeida Medeiros, Natália Hlavnicka Miranda, Jamila Sueira de Jesus Silva, Jorge Alberto Vieira Costa, and et al. 2025. "Recirculation of Saline Concentrate in Spirulina Cultivation: A Promising Strategy for High Production of Biomass and Biomolecules in Semiarid Regions" Environments 12, no. 5: 134. https://doi.org/10.3390/environments12050134
APA StyleMata, S. N., Cardoso, L. G., Bezerra, P. Q. M., Andrade, B. B., Oliveira, M. B. P. P., Machado, S., de Almeida Medeiros, R. M., Miranda, N. H., de Jesus Silva, J. S., Costa, J. A. V., de Jesus Assis, D., da Silva, J. B. A., & Souza, C. O. d. (2025). Recirculation of Saline Concentrate in Spirulina Cultivation: A Promising Strategy for High Production of Biomass and Biomolecules in Semiarid Regions. Environments, 12(5), 134. https://doi.org/10.3390/environments12050134









