Abstract
This study investigated possible associations between dietary patterns and blood heavy metal levels in Korean adults, using data from the Korea National Health and Nutrition Examination Survey (2012–2016). To explore these associations, demographic, physical activity, anthropometric, biochemical, and dietary data, including a food frequency questionnaire, were analyzed. Foods were categorized into 19 groups, and principal component factor analysis identified three dietary patterns: Meat and processed food-enriched diet (MPD), vegetables and milk-enriched diet (VMD), and fermented and fish-enriched diet (FFD). Multivariate logistic regression was used to evaluate the association between dietary patterns and high levels of heavy metals in the blood. The results showed that a high MPD score had a positive association with high levels of blood Pb (OR = 1.470, 95% CI = 1.173–1.842) and Hg (OR = 1.559, 95% CI = 1.259–1.932); a high FFD score also showed a positive association with high levels of blood Pb (OR = 1.492, 95% CI = 1.227–1.814) and Cd (OR = 1.276, 95% CI = 1.045–1.559). In contrast, VMD score was negatively associated with high levels of blood Pb (OR = 0.760, 95% CI = 0.628–0.920) and Cd (OR = 0.948, 95% CI = 0.781–1.151). Moreover, the effect of each dietary pattern on blood heavy metal levels showed differences by sex. Some dietary patterns, such as a high intake of meat, processed foods, fermented foods, and fish, can increase blood heavy metal levels, whereas other dietary patterns, such as vegetables and milk, have a protective effect against heavy metal concentrations.
1. Introduction
Heavy metals are a group of metals and metalloids with an atomic density higher than the 4000 kg/m3. Among them, lead (Pb), mercury (Hg), and cadmium (Cd) are known as hazardous heavy metals that humans are commonly exposed to through contaminated environmental systems, food consumption, drinking water, inhalation in the air, and contact with skin [1,2]. Heavy metals that enter the body replace other useful elements and accumulate in various tissues, such as those found in blood vessels, bones, muscles, and joints, and organs, interfering with biological functions and potentially causing irreversible changes in the body [1,3]. These effects can cause serious health issues in human beings, even at a very low concentration. Previous epidemiologic studies and systematic reviews have reported an association between exposure to heavy metals and cardiovascular disease [3,4,5], metabolic syndrome [6,7,8], neurodevelopment [9,10,11], fatty liver disease [12,13], kidney function impairment [14,15], and breast cancer [16]. Notably, exposure to heavy metals during pregnancy and childbirth can result in these metals being transmitted to the fetus or newborn through the placenta and breastfeeding, resulting in a shortened chromosomal telomere length, decreased birth weight, and impaired neurodevelopment [17,18,19]. Therefore, given their non-biodegradable nature, accumulation in various parts of the body, long biological half-lives, and health effects, it is essential to investigate the sources of exposure to heavy metals.
In addition to occupational exposure, dietary exposure is a major source of heavy metal exposure [20,21]. A total dietary survey of 2976 residents of Guangzhou, China, revealed that rice, seaweed, vegetables, and raw marine products accounted for 89% of Cd exposure [22]. A study that analyzed the relationship between blood Hg (Hg-B) and Pb (Pb-B) concentrations and diet in 1026 adolescents aged 8–17 years reported that fish and fruit dietary patterns and high fast food intake patterns were significantly associated with high levels of Hg and Pb, respectively [23]. However, a protective effect against heavy metal concentrations in the blood can be obtained via dietary intake of, for example, vitamins, selenium, zinc, iron, calcium, magnesium, green tea, and jujube [24,25,26]. This suggests that dietary interventions, such as encouraging or limiting specific diets, can contribute to reducing heavy metal exposure. For example, in an intervention study targeting pregnant women and women of childbearing age, a low frequency of fish intake (12 ounces or less per week) and the consumption of specific fish species (fish from lower trophic levels in the food chain) were found to be associated with decreased levels of methylmercury [27,28].
Given the complex nature of diet and the human body, dietary interventions to control heavy metal exposure and subsequent health effects need to be based on more elaborate evidence. While studies exist on metal exposure through the consumption of individual food items [29,30,31], knowledge about the role of the overall diet is limited [32,33,34,35]. Therefore, there is a growing interest in using dietary patterns as a realistic and comprehensive approach to assessing the complex health effects of food consumption [36]. In addition, the concentrations of heavy metals such as Pb and Cd continue to be higher in Korea than in other major countries [37]. In this study, we aimed to identify dietary patterns that contribute to blood Cd (Cd-B), Pb-B, and Hg-B concentrations based on data from the National Health and Nutrition Examination Survey (KNHANES) targeting the entire Korean population.
2. Materials and Methods
2.1. Study Source and Population
This study used data from the KNHANES, conducted from 2012 to 2016 (2014–2015 data were excluded because heavy metals were not measured). The KNHANES is a cross-sectional, population-based national survey conducted by the Korea Disease Control and Prevention Agency that aims to assess the health and nutritional status of the Korean population. The KNHANES started in 1998 and is conducted annually. The key contents of this survey are as follows: a health status assessment, a nutritional status assessment, health behaviors, healthcare utilization, and an accessibility assessment. To ensure representativeness, a stratified multistage probability sampling method was adopted. A total of 16,712 adults with measured blood heavy metal levels were included in the trend analysis.
Among the 24,226 subjects who participated in the 2012, 2013, and 2016 KNHANES, we excluded subjects aged <20 years because dietary survey data were unavailable. We also excluded participants who did not have blood heavy metal levels (n = 11,750) or semi-quantitative food frequency questionnaire (SQ-FFQ) data (n = 2495). After excluding subjects who were pregnant or diagnosed with cerebrovascular disease, cancer, or diabetes mellitus, we finally included 4024 subjects in the analysis (Figure 1).
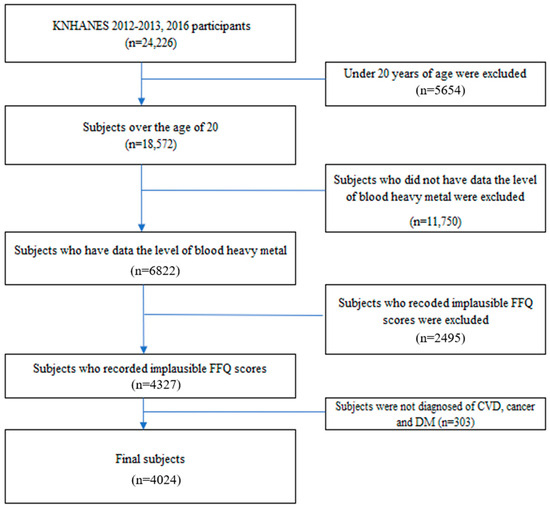
Figure 1.
Flow chart of study populations.
2.2. General Characteristics of Subjects
The KNHANES contains information on general characteristics, physical activity, and clinical data. General characteristics included current smoking status, region (urban or metropolitan, rural), occupation (administrators or professionals, office workers, service workers, agricultural or fishing workers, technicians or simple labor workers, and joblessness or other missing values). The physical activity of the subjects was divided into two levels: exercise (walking for at least 30 min at least 5 days per week or performing exercises such as swimming or badminton for more than 30 min at least 5 times per week) and no exercise. The following anthropometric measurement data were collected by the trained experts: body weight (kg), height (cm), body mass index (BMI) (kg/m2), waist circumference (WC) (cm), systolic blood pressure (SBP) (mmHg), and diastolic blood pressure (DBP) (mmHg). To collect clinical biochemical data, blood was sampled after 12 hours of fasting. Fasting blood glucose (mg/L), total cholesterol (mg/dL), low-density lipoprotein cholesterol (LDL-C) (mg/dL), high-density lipoprotein cholesterol (HDL-C) (mg/dL), triglycerides (mg/dL), aspartate aminotransferase (IU/L), and alanine aminotransferase (IU/L) were quantified in whole blood samples. In addition to occupation and physical activity data, we included data on sex, BMI, region, and smoking status, which could be potential covariates, for a more detailed analysis [38,39,40].
2.3. Heavy Metal Concentration in Blood
In KNHANES, to assess the concentration of heavy metals in whole blood, 3 mL of venous blood was collected from each participant using standard evacuated tubes containing sodium heparin (Vacutainer; Becton, Dickinson & Co., Franklin Lakes, NJ, USA). All analyses were performed by the Neodin Medical Institute, a laboratory accredited by the Korean Ministry of Health and Welfare and designated by the Ministry of Employment and Labor for trace metal analysis [41]. All sample collection and handling procedures were performed under standardized trace metal-free conditions to minimize external contamination. Blood Pb and Cd concentrations were determined using graphite furnace atomic absorption spectrometry (GFAAS) equipped with Zeeman background correction (AAnalyst 600; Perkin Elmer, Turku, Finland). Blood Hg levels were measured using the gold amalgamation method with thermal decomposition atomic absorption spectrophotometry (DMA-80; Milestone, Bergamo, Italy), which allows for direct analysis without chemical pre-treatment. To ensure analytical precision and accuracy, the laboratory participated in both internal and external quality-assurance programs. Internal quality control was conducted using commercial reference materials (Lyphochek Whole Blood Metals Control; Bio-Rad, Hercules, CA, USA), and the coefficients of variation for Pb, Cd, and Hg were maintained within acceptable ranges throughout the analysis period. For external validation, the laboratory regularly participated in the German External Quality Assessment Scheme (G-EQUAS) operated by Friedrich-Alexander University and the Quality Assurance Program of the Korea Occupational Safety and Health Agency. While the exact detection limits varied slightly depending on instrument sensitivity and matrix conditions, the method detection limits (MDLs) applied in the national monitoring protocol were approximately 0.12 µg/dL for Pb, 0.056 µg/L for Cd, and 0.158 µg/L for Hg. All analyzed samples in the present study had concentrations above these detection thresholds. The results were shown to have acceptable bias ranges, and inter-laboratory comparison testing indicated correlation coefficients of 0.921 for Pb and 0.932 for Cd, with equivalence confirmed at medical decision levels. For Hg, reproducibility was similarly ensured, with a correlation coefficient of 0.932 and equivalence at clinical thresholds [42].
2.4. Dietary Patterns
A total of 112 food items in the SQ-FFQ were categorized into 19 food groups based on the Korean nutrient database [43]: grains, noodles, instant noodles, fast food, potatoes, vegetables, fermented vegetables, fermented beans, fruits, red meat, white meat, processed meat, fish, fish soup, processed fish, seaweed, dairy products, alcohol, and soda (Table S1). The frequency of consumption for each food category was determined on a 9-point scale: 3 times per day, twice per day, once per day, 5–6 times per week, 2–4 times per week, once per week, 2–3 times per month, once per month, and nearly never. The amount consumed each time was estimated using the 3-point scale: 0.5 portion, 1 portion, and 1.5 portions. For the analysis, we calculated the average annual consumption by multiplying the frequency of consumption by the amount consumed each time for each item.
Factor analysis was used to identify the major dietary patterns of Korean adults. Dietary patterns were extracted using exploratory principal component factor analysis. Eigenvalues larger than 1.3 (Kaiser criterion) were applied to determine the number of factors. Scree plots were rotated using the varimax transformation to achieve a more interpretable result. After rotation, the factor scores for each dietary pattern were calculated by summing the intake of each food group weighted by the factor loading values. As shown in Table 1, three distinct patterns were identified based on the factor loading values. Each pattern was named according to the degree of factor loading. Pattern 1 is characterized by the consumption of meat and processed or instant food content as a meat and processed food-enriched diet (MPD); pattern 2 is characterized by the consumption of fruits, vegetables, and dairy products as a vegetables and milk-enriched diet (VMD); and pattern 3 is characterized by the consumption of typical foods in Korea, including grains, fermented foods, and fish, as a fermented and fish-enriched diet (FFD). Patterns 1, 2, and 3 displayed 17.47%, 9.88%, and 7.75% variance in the total dietary intake, respectively.

Table 1.
Factor loading matrix for major factors in the dietary patterns of Korean adults.
2.5. Statistical Analyses
Continuous variables were presented as means ± standard error, while categorical variables were expressed as numbers and percentages of subjects. The subjects were divided into tertiles based on their dietary pattern scores, and their nutrient intake and serum heavy metal levels were compared. Differences among the tertiles of dietary pattern groups were assessed using chi-square test, analysis of variance, and general linear model (GLM), adjusting for age and sex, followed by post hoc analysis (Bonferroni method). Using multivariate logistic regression models, odds ratios (ORs) and 95% confidence intervals (CIs) for the highest tertile group of serum heavy metal concentrations across tertiles of dietary pattern scores were compared with the lowest tertile group after adjusting for covariates, including age, sex, body mass index, region, occupation, smoking status, and physical activity. Statistical significance was set at the p-value < 0.05 level. All statistical analyses were performed using SPSS version 26.0 (SPSS Corp., Armonk, NY, USA) and Stata/SE, version 13.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. General Characteristics, Heavy Metal Concentration, and Biochemical Values According to Dietary Patterns
Table S2 presents the general characteristics, blood heavy metal concentration levels, and biochemical markers of the study participants according to the tertiles of the three dietary patterns. In MPD, the highest tertile of score had a significantly higher proportion of males and a lower mean age, both before and after adjusting for age and sex (p < 0.001). Hg-B levels were significantly higher in the highest tertile of MPD score (p < 0.001). WC and triglyceride levels tended to increase as MPD score increased. In VMD, the proportion of male subjects increased as the tertile of the VMD score increased. The Pb-B concentration and DBP tended to decrease as VMD score increased before and after adjusting for age and sex. In FFD, the proportion of female subjects and the mean age tended to increase as the tertile of FFD score increased, even after adjusting for age and sex (p < 0.001). Pb-B and Cd-B concentrations, WC, and BMI showed an increasing pattern as the FFD score increased, even after adjustment.
3.2. Association Between Blood Heavy Metal Concentrations and Dietary Patterns
Figure 2 shows a forest plot of the logistic regression results for the association between high blood heavy metal levels and tertiles of dietary patterns. The ORs for highest tertile of Pb-B and Hg-B were significantly higher in the highest tertile of the MPD score than in the lowest tertile after adjusting the models. In model 4, adjusted for covariates including age, sex, BMI, region, occupation, current smoking status, and physical activity, a high MPD score was positively associated with high levels of Pb-B (OR = 1.470, 95% CI = 1.173–1.842) and Hg-B (OR = 1.559, 95% CI = 1.259–1.932), but not with Cd-B. The increasing tertile of the VMD pattern score showed a significant association with Pb-B (OR = 0.760, 95% CI = 0.628–0.920) and Hg-B (OR = 1.327, 95% CI = 1.104–1.596), but not with Cd-B, in model 4. In the highest tertile of the FFD score, Pb-B and Cd-B concentrations showed positive associations both before and after adjustment.
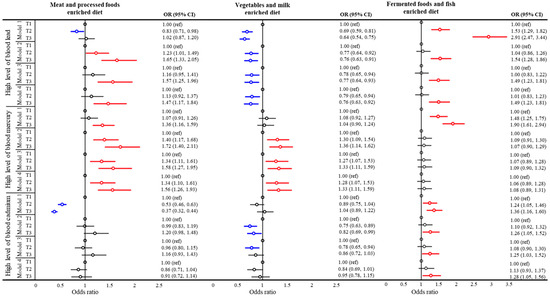
Figure 2.
Forest plot of association between blood heavy metal levels and dietary pattern. Statistical significance is presented in red (p < 0.05). High heavy metal level was defined as the upper tertile group. Model 1: crude; model 2: adjusted for age, sex, and body mass index; model 3: same adjustments as model 2 plus region and occupation; model 4: same adjustments as model 3 plus smoking status and physical activity.
Table 2 shows the odds ratios for high heavy metal levels according to tertile of dietary pattern scores by sex and p for interactions between sex and dietary pattern scores. The effect of dietary patterns on heavy metal concentrations in blood showed significant differences between the sexes. The association between MPD patterns and Pb-B was only significant for male subjects. In male, high VMD scores were significantly negatively associated with high levels of Pb-B, while in women there were positively associated, but did not show a significant association (all p for interaction <0.005).

Table 2.
Multivariate adjusted odds ratios for high heavy metal levels according to tertiles of score for dietary patterns.
4. Discussion
In this study, we investigated the associations between three heavy metal levels and dietary patterns in the Korean population. Our results indicate that the MPD pattern was significantly associated with increased Pb-B and Hg-B concentrations, and the FFD pattern was significantly associated with increased Pb-B and Cd-B concentrations. Interestingly, the VMD pattern was associated with lower concentrations of Pb-B and Cd-B. These results are consistent with previous studies conducted in Korea [20,23,37,44] and in other populations [45,46,47,48,49,50]. A prior study investigating the association between Korean dietary patterns and heavy metals identified the ‘balanced diet pattern’, ‘grain and kimchi pattern’, and ‘alcohol and noodle pattern’. Each of these patterns shared major food groups with our VMD, FFD, and MPD, but some food components were considered differently. The groups associated with meat and fish consumption, which were considered the main food groups in a balanced diet pattern, along with vegetables and fruits, had similar consumption patterns to those associated with the ‘alcohol and noodle’ and ‘grain and kimchi (fermented vegetables)’ groups, respectively, in our study. This difference may be caused by the rapid change in dietary patterns in Korea over the past decade due to industrial development and the westernization of lifestyles [51,52]. Additionally, the consumption of cereals, represented by rice, which has been the staple food of Koreans, and vegetables and fruits, have decreased, while meat consumption has increased dramatically, and seafood consumption has increased and then decreased [52]. The changes in the consumption of these foods, which are a major source of heavy metal exposure, may explain the declining trend in blood heavy metals in Koreans (Figure 3). The difference between our results and those of previous studies may also be explained by the fact that, unlike previous studies that used only the frequency of food intake, this study used the SQ-FFQ to reflect the average intake over a year. More importantly, the present study identified practical and current dietary risk factors for heavy metals, such as processed foods and fermented vegetables, which have become increasingly problematic in the Asian population.
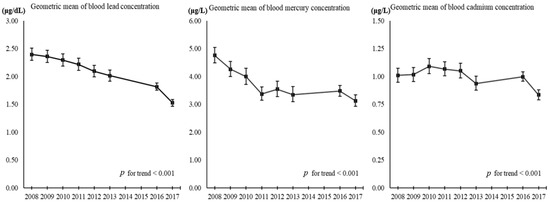
Figure 3.
Trend in blood heavy metals levels in Korean adults over time. KNHANES, Korean National Health and Nutrition Examination Survey; KoNEHS, Korean National Environmental Health Survey; KNHANES and KoNEHS data were extracted only for adults (≥19 years old) and non-pregnant population; KoNHAENS cycle 5 (2012, n = 5900), cycle 6 (2013, n = 5685), and cycle 7 (2016, n = 6029); KoNEHS cycle 1 (2009–2011, n = 6311), cycle 2 (2012–2014, n = 6478), cycle 3 (2015–2017, n = 3787), and cycle 4 (2018–2020, n = 4239).
To further evaluate the potential health risks associated with heavy metals detected in the blood, we reviewed the Health-based Guidance Values (HbGVs) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Although these are not the main findings of our study, they are presented as reference points to aid reader comprehension. The HbGVs for Pb, Cd, and Hg recommended by JECFA are as follows: 0.6 µg/kg body weight/day for Pb, associated with a loss of one IQ point in children; a provisional tolerable monthly intake (PTMI) of 25 µg/kg bw/month for Cd; a provisional tolerable weekly intake (PTWI) of 4.0 µg/kg bw/week for inorganic Hg, based on kidney weight changes [53].
Additionally, the international Codex standards for food safety set maximum levels for certain heavy metals in specific foods. For Pb, the limits include 0.2 mg/kg in polished rice, 0.1 mg/kg in potatoes, apples, and sweet potatoes, 0.2 mg/kg in bovine offal, and 0.1 mg/kg in poultry offal. For Cd, the limits are 0.4 mg/kg in polished rice, 2.0 mg/kg in squid, 0.1 mg/kg in brown rice, 0.2 mg/kg in spinach, and 2.0 mg/kg in 100% cocoa powder. Methylmercury is regulated at 1.0 mg/kg in large predatory fish and other deep-sea fish. However, international standards have not yet been established for many foods [53].
The association with dietary patterns varied according to the type of heavy metal. Pb is absorbed from the gastrointestinal tract into the blood, soft tissues, and bones; most of it is excreted through urine, but its biological half-life is approximately 27 years [54] and high blood Pb concentrations are known to be associated with an increased risk of death [55]. In the present study, increased MPD and FFD score was associated with a high Pb-B concentration, and increased VMD score was negatively associated with Pb-B concentration. Previous data in Korea also showed that high fast food consumption, low milk and tofu consumption, and alcohol and noodle consumption had the strongest associations with Pb-B [20,23]. According to the Korean Ministry of Food and Drug Safety’s heavy metal re-evaluation report, the food that makes the highest contribution to Pb levels is grains (16.8%) [53]. Their contribution to MPD is 47.1% and FFD is 31.4%. These results are consistent with those of previous studies on other populations [47,48,49]. A possible explanation for the increase in the concentration of Pb-B is that some nutrients, such as calcium and vitamin C, can interfere with the absorption of Pb. For example, Pb absorption may be reduced when calcium levels are sufficient because Pb competes with other heavy metals for the same gastrointestinal transporters [56,57]. A study targeting children reported that for every 100 mg increase in calcium, Pb-B concentration decreased by 3.8% [58]. Vitamin C (ascorbic acid) is an antioxidant that reduces oxygen-, nitrogen-, and sulfur-centered radicals and acts as a chelating agent to reduce Pb concentration in the blood [59,60]. Therefore, the U.S. Centers for Disease Control and Prevention recommend that vitamin C is used for the nutritional management of children with high Pb and B levels [60]. A recent review reported that a mixture of garlic and vitamins E and C can increase collagen formation, bone density, and excretion, thereby lowering the concentration of Pb in the body [61]. Previous studies have also shown that sex affects Pb concentration. Male respondents are known to have higher Pb-B levels than female respondents; moreover, female respondents are more vulnerable to Pb immunotoxicity, while male respondents are more vulnerable to neurotoxicity [21,62]. In the current study, the association between Pb-B concentration and dietary pattern showed a significant interaction effect with sex. Therefore, to reduce Pb-B in the body, nutrients with evidence of a chelating effect must be utilized in the daily diet considering sex differences.
Cd enters the human body via food in the general population and accumulates in all tissues and organs, particularly the liver and kidneys, with a biological half-life of >26 years [63,64,65,66]. The biomarker for Cd is the concentration of Cd in whole Cd-B and urine Cd; Cd-B reflects short-term exposure over 2–3 months and urine Cd reflects the long-term storage of Cd. In the present study, an increased FFD score was associated with increased Cd-B concentrations, and this result is similar to that of previous studies [37,44]. The FFD included grains (white rice), fermented foods (kimchi and soybean paste), and sea products (fish, mollusks, and shellfish), which are representative Korean foods. According to the Korean Ministry of Food and Drug Safety’s heavy metal re-evaluation report, foods contaminated with Cd include grains, seafood, and fermented foods, accounting for 45.2% of the total food exposure [53]. In the current study, an increased VMD score was associated with decreased Cd-B levels, which is consistent with the results of previous studies [37,45,46,50]. As dietary fiber from green vegetables passes through the intestines quickly, it may reduce the absorption of Cd due to changes in the intestinal microbial community [50]. In practice, a British cohort study conducted on pregnant women confirmed that when green leafy vegetables were consumed four or more times per week, Cd-B concentration decreased by 72% [50]. Calcium, which is present in large quantities in milk and dairy products, inhibits Cd’s low competition for absorption binding sites; as a result, a high-calcium diet lowers the concentration of Cd-B [50,67]. Studies conducted on women of childbearing age in Asian countries reported that a dairy diet and high-calcium vitamin diet lowered Cd-B levels by 14% [46]. A study of pregnant women in Canada also reported an association between increased calcium and vitamin D diets and decreased Cd-B [45]. Although the pattern of dietary fiber and calcium in VMD seems to have a Cd-chelation effect, further follow-up studies with more elaborate designs are needed. Sex differences were noted in Cd concentration. Cd retention rates are generally higher in women than in men, and this trend was similar to that of the VMD pattern in the current study [21,62].
Hg exists in elemental, inorganic, and organic forms [68]. The biological half-life of Hg is approximately 60 days. Biomarkers include Hg-B, which indicates short-term exposure, and urine Hg, which indicates long-term exposure [68,69]. In the present study, MPD and VMD scores were significantly associated with increased Hg-B. Although there is relatively little research on the association among MPD, VMD, and Hg, a relationship between alcohol consumption in MPD and Hg-B has been reported [70]. As alcohol increases the permeability of cell membranes, it can increase Pb absorption in the gastrointestinal tract and its excretion from the bone, thereby increasing Pb-B [20,71]. In previous studies, current alcohol drinkers had higher Hg-B levels than past alcohol drinkers and non-drinkers [70]. However, in the current study, FFD intake was not significantly associated with an increase in Hg-B, which is contrary to the results of previous studies. Many studies in Korea and other populations have reported an association between fish intake and Hg-B levels [20,21,23,28,70,72,73]. This difference is believed to result from the differing food groupings among studies; the FFD group in the present study included only fish, without shellfish. However, shellfish in Korea are highly contaminated with heavy metals [53]. The FFD group included fermented foods, such as kimchi and soybean paste, in addition to fish. Fermented foods are produced by the advanced conversion of food ingredients through controlled microbial growth and enzyme action, with potential probiotic effects, and can lead to the production of biogenic amines and bioactive peptides through fermentation, increasing the biological activity of phenolic compounds and their anti-inflammatory properties [74]. Considering these benefits, fermented foods may reduce Hg absorption from seafood. Although fish and seafood are the main sources of methylmercury, their health benefits should also be considered. Fish are a source of high-quality proteins and omega-3 polyunsaturated fatty acids (PUFA), such as docosahexaenoic acid and eicosapentaenoic acid, which cannot be efficiently synthesized in the body [70,75]. In particular, there is scientific evidence of an association between PUFA and cardiovascular disease, Alzheimer’s disease, and fetal neurological development [76,77,78]. Additionally, processed and fermented foods contain a large amount of sodium, which is known to be highly correlated with obesity [79,80,81], consistent with the current study, which showed that the MPD and FFD groups had positive associations with health indicators such as WC, triglycerides, and BMI. Therefore, although foods with high concentrations of Hg should be avoided, the additional health considerations of certain foods should be considered when determining the risk of heavy metal exposure.
This study had some limitations. We clustered various types of foods into three categories through factor analysis and were unable to examine the associations between individual foods and heavy metal concentrations in the blood. Recently, owing to the increase in the detection and risk of Cd, the European Commission and Codex Alimentarius have enforced maximum allowable Cd concentrations in chocolate and cocoa powders [82,83]. The Korea Ministry of Food and Drug Safety (KMFD) also concluded that standards need to be set for cocoa powder and chocolates, considering that most processed cocoa products are imported and that they are popular among sensitive population groups [53]. In the present study, cacao and chocolate were not included in the SQ-FFQ, as they were not considered major food groups; however, this distinction should be considered in future studies. In addition, because the SQ-FFQ relies on participants’ memory and fixed food categories, it may be prone to recall bias and misclassification. Considering that heavy metal exposure can vary significantly depending on food preparation methods and the specific parts of the food consumed, this approach may introduce measurement uncertainty. To enhance the accuracy of dietary data and minimize uncertainty, future research should consider the use of alternative dietary assessment tools, such as 24 h dietary recall methods. On the other hand, the use of dietary patterns derived from major food groups may still offer value, as it provides a broader perspective on the relationship between diet and blood heavy metal concentrations, accounting for the potential interactions between foods and their role in metal absorption and the metabolism.
Second, we presented only a cross-sectional study on the relationship between heavy metal blood concentrations and dietary patterns in adults because we analyzed data from the National Health and Nutrition Examination Survey conducted on the general population in Korea. As this study employs a cross-sectional observational design, it does not allow for causal inference. However, some of the conclusions imply causality without adequately addressing potential confounding factors. The authors should explicitly acknowledge the limitations of a cross-sectional approach and discuss the possibility of reverse causality or residual confounding.
Moreover, infants, young children, and pregnant women may have different dietary patterns than the general adult population, and may experience more severe and long-term health effects from heavy metals. In fact, long-term exposure to heavy metals can affect fetal health, as Pb accumulated in the bones may enter the bloodstream owing to metabolic changes during pregnancy [84]. In the future, it will be necessary to conduct long-term research targeting environmentally vulnerable groups such as pregnant women and infants. Despite these limitations, this study is significant in that it identified the dietary groups contributing to heavy metal concentrations in adults based on representative population data in Korea.
5. Conclusions
This study examined the associations between the dietary patterns and blood concentrations of heavy metals in a nationally representative sample of Korean adults. Through principal component analysis, three distinct dietary patterns were identified, each showing different relationships with internal heavy metal levels. Higher adherence to meat and processed food-based diets, as well as fermented and fish-enriched diets, was associated with increased levels of Pb, Hg, and Cd in the blood. In contrast, an increased consumption of vegetables and dairy products was linked to lower levels of Pb and Cd, suggesting a potential protective role. Notably, the associations varied by sex, indicating that dietary influences on heavy metal exposure may differ between men and women. These findings contribute novel insights into how common dietary behaviors can influence chronic exposure to environmental toxicants. By utilizing multi-year data from the Korea National Health and Nutrition Examination Survey, this study reflects temporal trends and ensures broad population representativeness. The results align with the international evidence and support the relevance of dietary patterns as modifiable risk factors for internal contamination.
From a public health perspective, our findings underscore the need for dietary recommendations that emphasize plant-based and dairy-rich eating habits while discouraging the excessive intake of certain animal-based and fermented foods, particularly in populations vulnerable to environmental exposure. These data may serve as a valuable reference for the development of national nutritional guidelines and inform policies aimed at mitigating heavy metal exposure through diet. Future longitudinal and mechanistic studies are needed to confirm causal pathways and to refine intervention strategies tailored to population-specific dietary practices and environmental conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12040125/s1, Table S1: Food groups from the food frequency questionnaire used in dietary pattern analysis. Table S2. General characteristic and biochemical markers according to tertiles of score for dietary patterns.
Author Contributions
D.P.: Investigation, validation, data curation, formal analysis, writing—original draft, visualization, writing—review, and editing; N.M.: investigation, validation, data curation, writing—original draft, visualization, and writing—review and editing; H.J.J.: data curation, formal analysis, writing—original draft, writing—review and editing; S.J.H.: data curation, writing—original draft, writing—review and editing; S.P.: conceptualization, supervision, writing—original draft, and writing—review and editing. M.-J.S.: writing—review and editing. J.H.K.: conceptualization, investigation, methodology, data curation, supervision, funding acquisition, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (24192MFDS077) from the Ministry of Food and Drug Safety in the Republic of Korea (2024). This work was also supported by a National Research Foundation of Korea grant funded by the Government of Korea (RS-2024-00355637).
Institutional Review Board Statement
This study was conducted using secondary data from the KNHANES provided by the Korea Disease Control and Prevention Agency (KDCA). Since the data are publicly available and anonymized, IRB approval was waived in accordance with institutional requirements.
Informed Consent Statement
This study involved secondary analysis of publicly available data, and informed consent was not directly obtained.
Data Availability Statement
Data available in a publicly accessible repository. The original data presented in the study are openly available in [Korea National Health and Nutrition Examination Survey, Korea Disease Control and Prevention Agency] at https://knhanes.kdca.go.kr/knhanes/postSendPage.do?url=/rawDataDwnld/rawDataDwnld.do&postparam=%7B%22menuId%22:%2210031001%22%7D (accessed on 10 March 2025).
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered potential competing interests: Ju Hee Kim reports that financial support was provided by the Ministry of Food and Drug Safety in the Republic of Korea (24192MFDS077). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
Pb, lead; Cd, cadmium; Hg, mercury; KoFDS, Korea Ministry of Food and Drug Safety; KNHANES, Korean National Health and Nutrition Examination Survey; SQ-FFQ, Semi-Quantitative Food Frequency Questionnaire; BMI, body mass index; WC, waist circumference; Pb-B, blood lead; Cd-B, blood cadmium; Hg-B, blood mercury; GLM, general linear model; OR, odds ratio; MPD, meat and process food-enriched diet; VMD, vegetable and milk-enriched diet; FFD, fermented food and fish-enriched diet.
References
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Ardila, P.A.R.; Alonso, R.Á.; Valsero, J.J.D.; García, R.M.; Cabrera, F.Á.; Cosío, E.L.; Laforet, S.D. Assessment of heavy metal pollution in marine sediments from southwest of Mallorca Island, Spain. Environ. Sci. Pollut. Res. 2023, 30, 16852–16866. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, A.; Rahimpouran, S.; Rad, H.M.; Eghlimi, E.; Zandian, H.; Hosseinkhani, A.; Vosoughi, M.; Valizadeh, F.; Hossinzadeh, R. Investigation of the link between the type and concentrations of heavy metals and other elements in blood and urinary stones and their association to the environmental factors and dietary pattern. J. Trace Elem. Med. Biol. 2023, 80, 127270. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gong, T.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, L.; Yang, Z.; Shen, P.; Lin, H.; Shui, L.; Tang, M.; Jin, M.; Chen, K.; Wang, J. Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients 2022, 14, 4038. [Google Scholar] [CrossRef]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Deng, L.; Liu, N. Non-linear associations between metabolic syndrome and four typical heavy metals: Data from NHANES 2011–2018. Chemosphere 2022, 291 Pt 2, 132953. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Forsthuber, M.; Szigeti, T.; Kakucs, R.; Mustieles, V.; Fernandez, M.F.; Bengtsen, E.; Vogel, U.; Hougaard, K.S.; Saber, A.T. Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int. J. Hyg. Environ. Health 2021, 238, 113855. [Google Scholar] [CrossRef]
- Farias, P.; Hernandez-Bonilla, D.; Moreno-Macias, H.; Montes-Lopez, S.; Schnaas, L.; Texcalac-Sangrador, J.L.; Rios, C.; Riojas-Rodriguez, H. Prenatal Co-Exposure to Manganese, Mercury, and Lead, and Neurodevelopment in Children during the First Year of Life. Int. J. Environ. Res. Public Health 2022, 19, 13020. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, L.; Huang, S.; Wei, L.; Cao, D.; Zan, G.; Tan, Y.; Wang, S.; Yang, M.; Tian, L.; et al. Association of both prenatal and early childhood multiple metals exposure with neurodevelopment in infant: A prospective cohort study. Environ. Res. 2022, 205, 112450. [Google Scholar] [CrossRef] [PubMed]
- Sadighara, P.; Abedini, A.H.; Irshad, N.; Ghazi-Khansari, M.; Esrafili, A.; Yousefi, M. Association Between Non-alcoholic Fatty Liver Disease and Heavy Metal Exposure: A Systematic Review. Biol. Trace Elem. Res. 2023, 201, 5607–5615. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Ding, R.; Xing, H.; Wang, R.; Zhang, M. Lead exposure as a causative factor for metabolic associated fatty liver disease (MAFLD) and a lead exposure related nomogram for MAFLD prevalence. Front. Public Health 2022, 10, 1000403. [Google Scholar] [CrossRef]
- Kulathunga, M.; Wijayawardena, M.A.A.; Naidu, R. Dietary heavy metal(loid)s exposure and prevalence of chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Environ. Geochem. Health 2022, 44, 3863–3874. [Google Scholar] [CrossRef]
- Sanders, A.P.; Mazzella, M.J.; Malin, A.J.; Hair, G.M.; Busgang, S.A.; Saland, J.M.; Curtin, P. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ. Int. 2019, 131, 104993. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef]
- Heng, Y.Y.; Asad, I.; Coleman, B.; Menard, L.; Benki-Nugent, S.; Hussein Were, F.; Karr, C.J.; McHenry, M.S. Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS ONE 2022, 17, e0265536. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; McCullough, L.E.; Tzeng, J.Y.; Darrah, T.; Vengosh, A.; Maguire, R.L.; Maity, A.; Samuel-Hodge, C.; Murphy, S.K.; Mendez, M.A.; et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 2017, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef]
- Chung, H.K.; Park, J.Y.; Cho, Y.; Shin, M.J. Contribution of dietary patterns to blood heavy metal concentrations in Korean adults: Findings from the Fifth Korea National Health and Nutrition Examination Survey 2010. Food Chem. Toxicol. 2013, 62, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Suomi, J.; Valsta, L.; Tuominen, P. Dietary Heavy Metal Exposure among Finnish Adults in 2007 and in 2012. Int. J. Environ. Res. Public Health 2021, 18, 10581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Liu, Y.; Liang, B.; Zhou, H.; Li, Y.; Zhang, Y.; Huang, J.; Yu, C.; Chen, K. An Assessment of Dietary Exposure to Cadmium in Residents of Guangzhou, China. Int. J. Environ. Res. Public Health 2018, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.W.; Kim, B.; Joshi, P.; Kwon, S.O.; Kim, Y.; Oh, J.S.; Kim, J.; Oh, S.Y.; Lim, J.A.; Choi, B.S.; et al. Effect of dietary patterns on the blood/urine concentration of the selected toxic metals (Cd, Hg, Pb) in Korean children. Food Sci. Biotechnol. 2018, 27, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Hasanghaliaei, N.; Poursafa, P.; Keikha, M.; Ghannadi, A.; Yazdi, M.; Rahimi, E. A randomized controlled trial on the effects of jujube fruit on the concentrations of some toxic trace elements in human milk. J. Res. Med. Sci. 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hossain, K.F.B.; Banik, S.; Sikder, M.T.; Akter, M.; Bondad, S.E.C.; Rahaman, M.S.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review. Ecotoxicol. Environ. Saf. 2019, 168, 146–163. [Google Scholar] [CrossRef]
- Sun, H.; Chen, J.; Xiong, D.; Long, M. Detoxification of Selenium Yeast on Mycotoxins and Heavy Metals: A Review. Biol. Trace Elem. Res. 2023, 201, 5441–5454. [Google Scholar] [CrossRef]
- Carrington, C.D.; Montwill, B.; Bolger, P.M. An intervention analysis for the reduction of exposure to methylmercury from the consumption of seafood by women of child-bearing age. Regul. Toxicol. Pharmacol. 2004, 40, 272–280. [Google Scholar] [CrossRef]
- Naess, S.; Kjellevold, M.; Dahl, L.; Nerhus, I.; Midtbo, L.K.; Bank, M.S.; Rasinger, J.D.; Markhus, M.W. Effects of seafood consumption on mercury exposure in Norwegian pregnant women: A randomized controlled trial. Environ. Int. 2020, 141, 105759. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- Hassan, F.I.; Niaz, K.; Khan, F.; Maqbool, F.; Abdollahi, M. The relation between rice consumption, arsenic contamination, and prevalence of diabetes in South Asia. EXCLI J. 2017, 16, 1132. [Google Scholar] [PubMed]
- Signes-Pastor, A.J.; Punshon, T.; Cottingham, K.L.; Jackson, B.P.; Sayarath, V.; Gilbert-Diamond, D.; Korrick, S.; Karagas, M.R. Arsenic exposure in relation to apple consumption among infants in the new hampshire birth cohort study. Expo. Health 2020, 12, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Queirolo, E.I.; Mañay, N.; Peregalli, F.; Hsiao, P.Y.; Lu, Y.; Vahter, M. Low-level arsenic exposure: Nutritional and dietary predictors in first-grade Uruguayan children. Environ. Res. 2016, 147, 16–23. [Google Scholar] [CrossRef]
- Burganowski, R.; Vahter, M.; Queirolo, E.I.; Peregalli, F.; Baccino, V.; Barcia, E.; Mangieri, S.; Ocampo, V.; Mañay, N.; Kordas, K. A cross-sectional study of urinary cadmium concentrations in relation to dietary intakes in Uruguayan school children. Sci. Total Environ. 2019, 658, 1239–1248. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Tan, Z.; Dai, Y. Potential dietary factors for reducing lead burden of Chinese preschool children. Environ. Sci. Pollut. Res. 2019, 26, 22922–22928. [Google Scholar] [CrossRef] [PubMed]
- Notario-Barandiaran, L.; Irizar, A.; Begoña-Zubero, M.; Soler-Blasco, R.; Riutort-Mayol, G.; Fernández-Somoano, A.; Tardón, A.; Casas, M.; Vrijheid, M.; Signes-Pastor, A.J. Association between mediterranean diet and metal (loid) exposure in 4-5-year-old children living in Spain. Environ. Res. 2023, 233, 116508. [Google Scholar] [CrossRef]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines2. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef]
- Moon, M.K.; Lee, I.; Lee, A.; Park, H.; Kim, M.J.; Kim, S.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Lead, mercury, and cadmium exposures are associated with obesity but not with diabetes mellitus: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Res. 2022, 204 Pt A, 111888. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Knol, L.L.; Yang, X.; Kong, L. Dietary fiber intake is inversely related to serum heavy metal concentrations among US adults consuming recommended amounts of seafood: NHANES 2013–2014. Food Front. 2021, 3, 142–149. [Google Scholar] [CrossRef]
- Laouali, N.; Benmarhnia, T.; Lanphear, B.P.; Weuve, J.; Mascari, M.; Boutron-Ruault, M.-C.; Oulhote, Y. Association between blood metals mixtures concentrations and cognitive performance, and effect modification by diet in older US adults. Environ. Epidemiol. 2022, 6, e192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Luo, W.; Jia, S.; Liu, D.; Ma, W.; Gu, H.; Wei, X.; He, Y.; Cao, S.; et al. Relationship between maternal heavy metal exposure and congenital heart defects: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 55348–55366. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. Quality Control of the Clinical Laboratory for the Korea National Health and Nutrition Examination Survey (KNHANES). 2017. Available online: https://knhanes.kdca.go.kr/knhanes/archive/wsiQcRpt.do# (accessed on 10 February 2025.).
- Park, S.; Lee, B.K. Body fat percentage and hemoglobin levels are related to blood lead, cadmium, and mercury concentrations in a Korean adult population (KNHANES 2008–2010). Biol. Trace Elem. Res. 2013, 151, 315–323. [Google Scholar] [CrossRef]
- The Korean Nutrition Society. Dietary Reference Intake for Koreans, 1st ed.; The Korean Nutrition Society: Seoul, Republic of Korea, 2010. [Google Scholar]
- Park, S.; Lee, B.K. Strong positive association of traditional Asian-style diets with blood cadmium and lead levels in the Korean adult population. Int. J. Environ. Health Res. 2013, 23, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; MIREC Study Group. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Dix-Cooper, L.; Kosatsky, T. Blood mercury, lead and cadmium levels and determinants of exposure among newcomer South and East Asian women of reproductive age living in Vancouver, Canada. Sci. Total Environ. 2018, 619–620, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Chun, O.K.; Song, W.O. Determinants of the blood lead level of US women of reproductive age. J. Am. Coll. Nutr. 2005, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alica Pizent, J.J.; Telišman, S. Blood pressure in relation to dietary calcium intake, alcohol consumption, blood lead, and blood cadmium in female nonsmokers. J. Trace Elem. Med. Biol. 2001, 15, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, S.A.; Derkho, M.A.; Gizatullina, F.G.; Sereda, T.I. Leukocytes as Indicators of the Accumulation of Metals in the Body of Growing Heifers. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2022, 13, 134. [Google Scholar]
- Taylor, C.M.; Doerner, R.; Northstone, K.; Kordas, K. Maternal diet during pregnancy and blood cadmium concentrations in an observational cohort of British women. Nutrients 2020, 12, 904. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.; Park, D.; Lee, J.; Kim, R.; Subramanian, S.V.; Oh, H.; Shin, M.J. Trends in Diet Quality and Cardiometabolic Risk Factors Among Korean Adults, 2007–2018. JAMA Netw. Open 2022, 5, e2218297. [Google Scholar] [CrossRef]
- Park, D.; Shin, M.J.; Després, J.P.; Eckel, R.H.; Tuomilehto, J.; Lim, S. 20-year trends in metabolic syndrome among Korean adults from 2001 to 2020. JACC Asia 2023, 3 Pt 2, 491–502. [Google Scholar] [CrossRef]
- Korea Ministry of Food and Drug Safety. Reevaluation Report on Standards and Specifications for Heavy Metals in Food. 2024. Available online: https://www.mfds.go.kr (accessed on 10 February 2025.).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead; US Government Printing: Atlanta, GA, USA, 2005.
- Byun, G.; Kim, S.; Kim, S.Y.; Park, D.; Shin, M.J.; Oh, H.; Lee, J.T. Blood lead concentrations and mortality in Korean adults: The Korea National Health and Nutrition Examination Survey with mortality follow-up. Int. J. Environ. Res. Public Health 2020, 17, 6898. [Google Scholar] [CrossRef] [PubMed]
- Shah-Kulkarni, S.; Ha, M.; Kim, B.M.; Kim, E.; Hong, Y.C.; Park, H.; Kim, Y.; Kim, B.N.; Chang, N.; Oh, S.Y.; et al. Neurodevelopment in Early Childhood Affected by Prenatal Lead Exposure and Iron Intake. Medicine 2016, 95, e2508. [Google Scholar] [CrossRef] [PubMed]
- Leroux, I.N.; Ferreira, A.; Silva, J.; Bezerra, F.F.; da Silva, F.F.; Salles, F.J.; Luz, M.S.; de Assuncao, N.A.; Cardoso, M.R.A.; Olympio, K.P.K. Lead exposure from households and school settings: Influence of diet on blood lead levels. Environ. Sci. Pollut. Res. Int. 2018, 25, 31535–31542. [Google Scholar] [CrossRef] [PubMed]
- Turgeon O’Brien, H.; Gagne, D.; Vaissiere, E.; Blanchet, R.; Lauziere, J.; Vezina, C.; Ayotte, P. Effect of dietary calcium intake on lead exposure in Inuit children attending childcare centres in Nunavik. Int. J. Environ. Health Res. 2014, 24, 482–495. [Google Scholar] [CrossRef]
- Ghanwat, G.; Patil, A.J.; Patil, J.; Kshirsagar, M.; Sontakke, A.; Ayachit, R.K. Effect of Vitamin C Supplementation on Blood Lead Level, Oxidative Stress and Antioxidant Status of Battery Manufacturing Workers of Western Maharashtra, India. J. Clin. Diagn. Res. 2016, 10, BC08–BC11. [Google Scholar] [CrossRef]
- Yin, N.; Han, Z.; Jia, W.; Fu, Y.; Ma, J.; Liu, X.; Cai, X.; Li, Y.; Chen, X.; Cui, Y. Effect of vitamin C supplement on lead bioaccessibility in contaminated soils using multiple in vitro gastrointestinal assays: Mechanisms and health risks. Ecotoxicol. Environ. Saf. 2022, 243, 113968. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Khan, R.; Shakir, H.A.; Tahir, H.M.; Mumtaz, S.; Andleeb, S. Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ. Sci. Pollut. Res. Int. 2020, 27, 8953–8964. [Google Scholar] [CrossRef]
- Vahter, M.; Akesson, A.; Liden, C.; Ceccatelli, S.; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C.; SRC Inc. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Gao, L.; Chang, J.; Chen, R.; Li, H.; Lu, H.; Tao, L.; Xiong, J. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Nakatsuka, H.; Watanabe, T.; Shimbo, S. Estimation of dietary intake of cadmium from cadmium in blood or urine in East Asia. J. Trace Elem. Med. Biol. 2018, 50, 24–27. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Mackenzie, B. Interaction of calcium with the human divalent metal-ion transporter-1. Biochem. Biophys. Res. Commun. 2010, 393, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Dart, R.C. (Ed.) Medical Toxicology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Kim, S.A.; Kwon, Y.; Kim, S.; Joung, H. Assessment of Dietary Mercury Intake and Blood Mercury Levels in the Korean Population: Results from the Korean National Environmental Health Survey 2012–2014. Int. J. Environ. Res. Public Health 2016, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko-Jakoniuk, J.; Jurczuk, M.; Galazyn-Sidorczuk, M.; Brzóska, M.M. Lead turnover and changes in the body status of chosen micro-and macroelements in rats exposed to lead and ethanol. Pol. J. Environ. Stud. 2003, 12, 335–344. [Google Scholar]
- Basta, P.C.; de Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.; de Aguiar, D.S.; de Souza, C.C.; Oliveira-da-Costa, M. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef]
- Seo, J.W.; Kim, B.G.; Hong, Y.S. The Relationship between Mercury Exposure Indices and Dietary Intake of Fish and Shellfish in Women of Childbearing Age. Int. J. Environ. Res. Public Health 2020, 17, 4907. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Laye, S. Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lv, X.; Wang, R.; Li, X.; Xu, W.; Wang, N.; Ma, S.; Huang, H.; Niu, Y.; Kong, X. Association of marine PUFAs intakes with cardiovascular disease, all-cause mortality, and cardiovascular mortality in American adult male patients with dyslipidemia: The U.S. National Health and Nutrition Examination Survey, 2001 to 2016. Nutr. J. 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. Metabolism: High salt intake as a driver of obesity. Nat. Rev. Nephrol. 2018, 14, 285. [Google Scholar] [CrossRef]
- Oh, S.W.; Koo, H.S.; Han, K.H.; Han, S.Y.; Chin, H.J. Associations of sodium intake with obesity, metabolic disorder, and albuminuria according to age. PLoS ONE 2017, 12, e0188770. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 488/2014 of 12 May 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014, 138, 75. [Google Scholar]
- Vanderschueren, R.; Doevenspeck, J.; Goethals, L.; Andjelkovic, M.; Waegeneers, N.; Smolders, E. The contribution of cacao consumption to the bioaccessible dietary cadmium exposure in the Belgian population. Food Chem. Toxicol. 2023, 172, 113599. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).